Abstract
Recently, our laboratory has shown that the neural mechanisms for encoding lexico-semantic information in adults operate functionally by 12–18 months of age within left frontotemporal cortices (Travis et al., 2011. Spatiotemporal neural dynamics of word understanding in 12- to 18-month-old-infants. Cereb Cortex. 8:1832–1839). However, there is minimal knowledge of the structural changes that occur within these and other cortical regions important for language development. To identify regional structural changes taking place during this important period in infant development, we examined age-related changes in tissue signal properties of gray matter (GM) and white matter (WM) intensity and contrast. T1-weighted surface-based measures were acquired from 12- to 19-month-old infants and analyzed using a general linear model. Significant age effects were observed for GM and WM intensity and contrast within bilateral inferior lateral and anterovental temporal regions, dorsomedial frontal, and superior parietal cortices. Region of interest (ROI) analyses revealed that GM and WM intensity and contrast significantly increased with age within the same left lateral temporal regions shown to generate lexico-semantic activity in infants and adults. These findings suggest that neurophysiological processes supporting linguistic and cognitive behaviors may develop before cellular and structural maturation is complete within associative cortices. These results have important implications for understanding the neurobiological mechanisms relating structural to functional brain development.
Keywords: brain development; infants; language, structural MRI
Introduction
Many important behavioral milestones are achieved in the second year of life. Sensorimotor, linguistic, and cognitive skills rapidly mature in these months (Herschkowitz et al. 1999), and it is typically at these ages when the infant begins to speak and walk. Coinciding with the emergence of these behaviors are many notable neurobiological events, including region-specific changes in synaptogenesis (Huttenlocher and Dabholkar 1997), myelination (Yakovlev and Lecours 1967; Brody et al. 1987; Kinney et al. 1988), and glucose metabolism (Chugani 1998). Furthermore, this can also be a period when the behavioral symptoms of neurodevelopmental disorders such as autism first become apparent (Wetherby et al. 2004; Zwaigenbaum et al. 2005; Landa and Garrett-Mayer 2006). Despite the importance of these months for healthy behavioral and brain development, only a few neuroimaging studies have attempted to characterize the structural changes that occur in the months following an infant's first birthday (Gao et al. 2008; Knickmeyer et al. 2008; Gilmore et al. 2011).
Most morphometric measures used to assess maturational changes in brain structure have been acquired from 1- to 2-year-old infants as part of either cross-sectional or longitudinal neuroimaging studies of brain development (Schaefer et al. 1990; Pfefferbaum et al. 1994; Courchesne et al. 2000; Almli et al. 2007; Gilmore et al. 2007; Knickmeyer et al. 2008; Gilmore et al. 2011). These, and a few additional studies which have included infants between the ages of 12–24 months, have either characterized the development of a specific cortical or subcortical area (Pfluger et al. 1999; Utsunomiya et al. 1999), white matter (WM) development (Gao et al. 2008) or have acquired global volumetric measures of ventricular, GM and WM regions (Schaefer et al. 1990; Pfefferbaum et al. 1994; Courchesne et al. 2000; Gilmore et al. 2007; Knickmeyer et al. 2008; Choe et al. 2012).
Aside from knowledge of the gross neuroanatomical changes that occur during this important period in human development, very little is known of the structural maturation of specific cortical regions that support emerging sensory, cognitive, and linguistic behaviors. In older children and adolescents, region-specific structural changes have reliably been obtained using morphometric measures of cortical thickness (Sowell, Thompson, Leonard, et al. 2004; Sowell, Thompson, Toga, et al. 2004; O'Donnell et al. 2005; Lu et al. 2007; Sowell, Peterson et al. 2007; Shaw et al. 2008; Brown et al. 2012). More recently, tissue signal properties, including GM and WM T1-weighted signal intensity and contrast, have also been used to investigate structural neurodevelopment (Salat et al. 2009; Westlye et al. 2010; Brown et al. 2012). Regional changes in signal intensity have been shown to occur independent of variations in cortical thickness, and so may index different underlying neurobiological processes contributing to the structural changes observed during brain development and aging (Salat et al. 2009; Westlye et al. 2010). Specifically, GM and WM tissue intensities are related to proton-relaxation times, and so are sensitive to the degrees of tissue myelination (Walters et al. 2003; Eickhoff et al. 2005; Westlye et al. 2010). Both measures of cortical thickness and tissue signal intensities are likely to be more sensitive to subtle morphological changes than gross volumetric measures (Sowell et al. 2001); however, neither measure has been widely used to assess regional development of cortical structures in children younger than 3 years of age.
Uncovering the structural changes that occur within functional brain areas during early development has important implications for improving understandings of both the neurobiological underpinnings of cognitive development and the underlying cellular processes that give rise to the structural changes observed throughout the lifespan. Evidence from structural neuroimaging studies of older children (Giedd et al. 1996; Reiss et al. 1996; Thompson et al. 2000; Sowell et al. 2002; Shaw et al. 2008; Brown et al. 2012), and post-mortem research, consistently demonstrate regional differences in rates of cellular, structural, and functional development (Casey et al. 2005; Toga et al. 2006). Primary sensory areas, including visual cortices, are some of the first regions to mature, attaining peak cortical thinness (Shaw et al. 2008), myelination (Flechsig 1920; Conel 1955; Yakovlev and Lecours 1967; Brody et al. 1987; Kinney et al. 1988), synaptic density (Huttenlocher and de Courten 1987), and adult-like dendritic morphology (Travis et al. 2005) before higher order associative cortices. Sensory abilities appear to rapidly develop in parallel with many of these neurobiological changes (Zilles et al. 1986) and do so much before maturation is observed for high-order cortices and the cognitive behaviors that these regions support (Casey et al. 2005; Toga et al. 2006). This temporal coincidence suggests that functional maturity may depend on the structural and cellular maturation of a given functional area. However, we have recently demonstrated that the neurophysiological processes thought to index semantic processing in adults (Kutas and Federmeier 2011) appear to operate functionally by 12–18 months of age (Travis et al. 2011). These new findings imply that the functional development of the neurophysiological processes important for word understanding occur before cellular and structural maturation is complete within associative cortices.
To explore this possibility further, we examined age-related changes in tissue signal properties of GM and WM intensity and tissue contrast within a population of 12- to 19-month-old infants. We specifically selected GM and WM intensity and gray–white contrast to index changes in brain structure since these measures are likely to be sensitive to alterations in the myelin composition that is known to occur within this age-range (Yakovlev and Lecours 1967; Brody et al. 1987; Kinney et al. 1988). Using these measures, we were able to examine whether age-related changes in tissue structure occur within the same left lateral temporal areas that have been demonstrated to generate lexico-semantic activity in both infants and adults (Travis et al. 2011).
Materials and Methods
Participants
Data from 17 typically developing infants aged 12–19 months (mean age = 15.4 months ± 2.3, 8M) are presented here. Infant subjects were initially recruited to participate in a magnetoencephalography (MEG) study of typical language development and structural T1-weighted MR images were acquired as part of participation in the MEG study. All infants were born full-term (>39 weeks), and had no known neurological or developmental impairments. Written parental consent was obtained for all infant subjects. This study was approved by the UCSD Institutional Review Board (Project #070254).
MRI acquisition
Imaging was performed during the evening hours around infants' typical bedtime. All scans were performed at the UCSD Radiology Imaging Laboratory on a General Electric 1.5T EXCITE HD scanner with an 8-channel phased-array head coil (General Electric, Waukesha, WI, USA). Image acquisitions included a conventional 3-plane localizer, GE calibration scan, and a T1-weighted volume acquisition pulse sequence (TE = 2.8 ms, TR = 6.5 ms, TI = 600 ms, flip angle = 12 deg, bandwidth = 31.25 Hz/pixel, FOV = 25.6 cm, matrix = 256 × 162, slice thickness = 1.2 mm). Acquisition parameters were optimized for increased GM/WM image contrast. In some cases, infants moved significantly during the acquisition or were woken by scanner noises and so only 1 T1-image could be acquired (n = 2). Scanning began once the child fell naturally to sleep in the scanner. Noise-cancelling headphones (INNOMED, Germany) were used to minimize and protect infants from scanner sounds.
MRI Processing
Raw data sets in DICOM format were transferred to Linux workstations for image processing and analyses located at the Multimodal Imaging Laboratory, University of California, San Diego. Images were corrected for nonlinear warping and intensity non-uniformities using methods developed through the Morphometry Biomedical Informatics Research Network (mBIRN). To obtain measures of tissue signal intensities, cortical surface reconstructions were performed using the open-source FreeSurfer software package (http://surfer.nmr.mgh.harvard.edu) version 4.05. Reconstruction of the cortical surface requires a series of steps which include: 1) segmentation of the WM, 2) tessellation of the GM/WM boundary, 3) inflation of the folded surface tessellation, and 4) automatic correction of topological defects. These steps are described in greater technical detail elsewhere (Dale et al. 1999; Fischl et al. 1999; Fischl and Dale 2000). Following initial surface cortical reconstruction steps, data were additionally processed using an automated-labeling system that subdivides the cerebral cortex on volumetric MRI into 32 neuroanatomical regions per hemisphere [4 medial temporal, 5 lateral temporal, 10 frontal, 5 parietal, 4 occipital, and 4 cingulate; Desikan et al. (2006)] and is available as part of the FreeSurfer package. The parcellation atlases derived from this processing step were inspected for each individual infant in order to visually assess the overall accuracy of the surface-based reconstructions (Fig. 1B). Cortical reconstructions were also manually inspected by trained technicians who assessed the accuracy of pial and WM boundary estimation. In most cases, significant manual editing was required in order to properly estimate GM/WM boundaries (Supplementary Fig. S1). Editing was done blind to subject age. The addition of control points were used to improve both pial and GM/WM boundaries (5933.47 ± 2224.36 points per subject). Post-hoc analysis confirmed that there was no relationship between age and number of control points (R2 = 0.0004, P < 0.93). Visual inspection revealed general improvement in GM/WM boundaries following the addition of control points (Supplementary Fig. S1).
Figure 1.
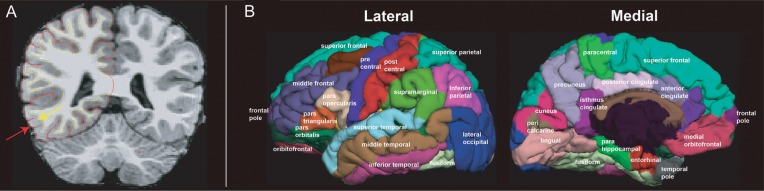
Tissue sampling method and cortical surface parcellations. (A) Tissue signal intensity values were selected 0.2 mm in either direction of the GM/WM surface, indicated by yellow line and arrow. Pial surface is represented as the red line and is indicated by a red arrow. (B) Surface parcellations derived from an automated labeling system (Desikan et al. 2006) and displayed on the reconstructed pial surface of an individual infant subject. Individual ROIs are displayed on the left lateral and medial surface as separate colors.
GM tissue intensities were sampled 0.2 mm from the GM/WM boundary in the direction of the pial surface and WM intensities were sampled 0.2 mm in the opposing direction (Fig. 1A). GM and WM intensity were expected to primarily reflect T1 and proton density (PD), as effects of T2 on GM/WM intensity were expected to be minimal given the short TE (∼3 ms; Deoni 2011). Signal intensity contrast, measured as the GM to WM ratio (GWR), was computed as the ratio of the difference of WM to GM to the average GM and WM intensity ((W−G)/(W + G/2)). Based on this notation, the ratio values closer to 0 signify poorer cortical contrast between tissue compartments whereas values closer to 1 indicate improved contrast. Between-subject intensity variations due to radio frequency (RF) coil sensitivities were considered to be negligible considering the current <1 mm scale of GWR. In general, between-subject variations in signal intensities were not expected to influence GWR which was computed as a within-subject ratio.
Statistical Analyses
The relationship amongst age and tissue signal properties was examined with a vertex-by-vertex general linear model (GLM). The results of these analyses can be displayed on the cortical surface as statistical parametric maps. These maps can be thresholded to show the distribution of P-values for pairwise comparisons between age and each of the 3 tissue intensity measures. The minimum P-value threshold for each map was set at (Fig. 2C) or below (Fig. 2A,B) the 5% criterion-level commonly used false discovery rate (FDR; Genovese et al. 2002). It is important to note that this FDR will determine different P-value thresholds for each comparison and hemisphere. Regional effects between age and all 3 tissue signal measures were also assessed using a Pearson's correlation from values derived from both specific lateral temporal ROIs (Fig. 3A–C) that were shown in a recent study (Travis et al. 2011) to exhibit lexico-semantic activity in 12- to 18-month-old infants and adults.
Figure 2.
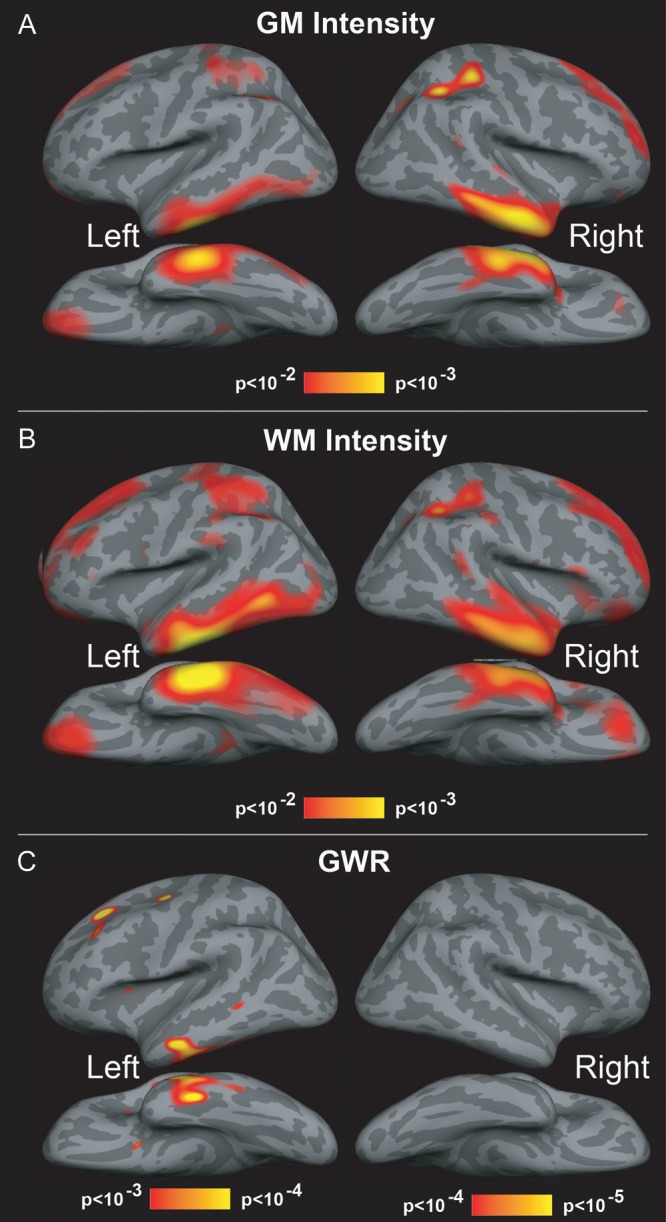
Effects of age of on GM and WM signal intensity, and contrast GWR. Results of GLM analysis displayed on the lateral, medial, and ventral surfaces of the brain. Heated colors (red to yellow) indicate regions where cortical GM and WM intensity and contrast were observed to significantly increase with age. (A,B) Significant effects of age in GM and WM signal intensity are observed bilaterally within lateral and anteroventral temporal regions, as well as dorsal and medial portions of superior frontal cortices and also lateral superior parietal cortices. (C) Contrast is observed to significantly increase with age in anteroventral temporal and dorsal prefrontal regions. Minimal to no changes in contrast are observed within regions of occipital and cingulate cortices. Images are thresholded using at or below 5% FDR criterion.
Figure 3.
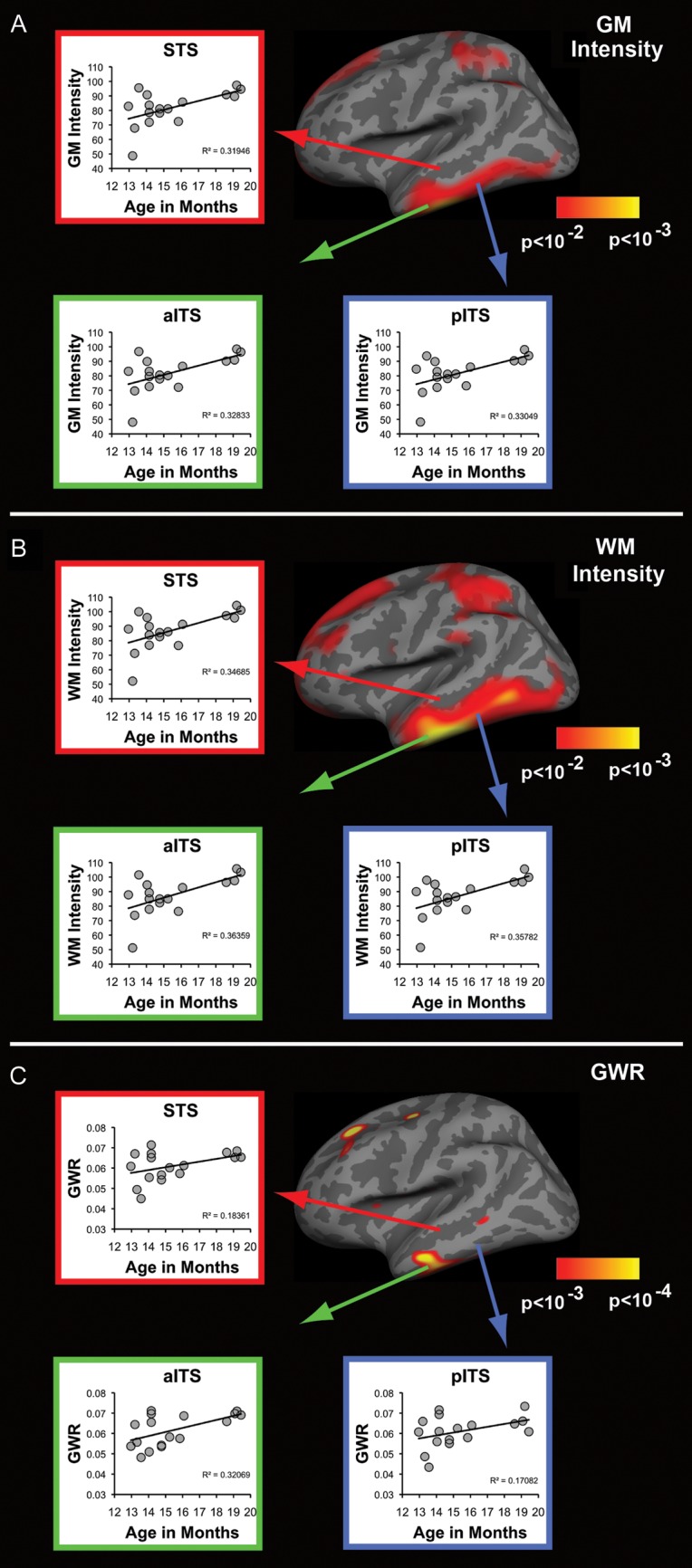
Tissue intensity and contrast significantly change with age within regions shown to generate lexico-semantic activity in infants and adults. (A–C) Results of correlations performed between age and all 3 tissue signal intensity measures for 3 left lateral temporal ROIs. (A,B) WM and GM signal intensity significantly increases with age for all 3 ROIs. (C) Contrast significantly increases with age within left anterior inferior temporal sulcus, with trends observed in the superior temporal sulcus and posterior inferior temporal areas. STS, superior temporal sulcus; aITS, anterior inferior temporal sulcus; p, posterior. GLM maps from Figure 2 are reproduced for convenience.
Results
Effects of Age on Tissue Signal Properties: GLM Analyses
Results of GLM analysis were first evaluated to examine age-related changes in tissue signal properties. Results of GLM analyses were displayed on the cortical surface and revealed significant regional effects of age for all 3 tissue signal properties (GM and WM intensity, GWR; Fig. 2A–C). Both GM and WM signal intensities appeared to show similar patterns of significant increases with age within lateral and anteroventral temporal regions bilaterally (Fig. 2A,B). In both hemispheres, significant age effects also obtained for both GM and WM intensity within dorsal aspects of superior frontal and superior parietal cortices (Fig. 2A,B). Consistent with these patterns, tissue contrast, measured as the GWR, also exhibited similar but more focal effects of age in left lateral and anteroventral temporal cortices and also superior frontal areas. No regions within the right hemisphere demonstrated significant effects of age for contrast measures. Clear effects of age were not apparent within occipital and cingulate cortices for any tissue intensity measure (Fig. 2A–C).
Effects of Age on Tissue Signal Properties: ROI Analyses
We also performed Pearson's correlations between age and tissue intensity values extracted from the exact same left lateral inferior and superior temporal ROIs (Fig. 3A–C) found to exhibit lexico-semantic activity in 12- to 18-month-old infants (Travis et al. 2011). It was decided a priori to exclude right hemisphere ROIs since these areas did not reliably demonstrate lexico-semantic activity in both infants and adults (Travis et al. 2011). The results of these correlations are summarized in Table 1. Significant effects of age were observed for both GM intensity and WM intensity in all 3 lateral temporal ROIs (Table 1, Fig. 3A,B). These results are consistent with age-related increases in GM and WM signal intensity that were observed with GLM analyses (Fig. 2A,B). In addition, GWR was observed to significantly increase with age within left anterior inferior temporal regions. Increases in contrast were observed to trend with age within left superior and posterior inferior temporal ROIs but did not reach significance. To rule out the possibility that these effects were driven by an outlying subject, post-hoc ROI analyses were repeated by removing this subject as well as the subject with the highest average GM/ WM intensity and GWR. Removing these 2 subjects from ROI analyses maintained significant effects of age for GWR within the anterior inferior temporal sulcus (R2 = 0.30, P < 0.031). Significant effects of age were maintained for WM intensity within both anterior (R2 = 0.27, P < 0.045) and posterior inferior temporal sulcus (R2 = 0.27, P < 0.048), and a trend of age remained in the superior temporal sulcus (R2 = 0.25, P < 0.057). Effects of age for GM intensity were no longer found to be significant after removing these 2 subjects; however, all regions continued to demonstrate trends with age for all ROIs [anterior (R2 = 0.23, P < 0.070) and posterior inferior temporal sulcus (R2 = 0.24, P < 0.066), and superior temporal sulcus (R2 = 0.22, P < 0.079)].
Table 1.
Results of correlation analyses performed between age and tissue signal properties in left lateral temporal ROIs
| R2 | P | |
|---|---|---|
| GM intensity | ||
| STS | 0.32 | 0.018 |
| aITS | 0.33 | 0.016 |
| pITS | 0.33 | 0.016 |
| WM intensity | ||
| STS | 0.35 | 0.012 |
| aITS | 0.36 | 0.010 |
| pITS | 0.36 | 0.010 |
| GWR | ||
| STS | 0.18 | 0.086a |
| aITS | 0.32 | 0.018 |
| pITS | 0.17 | 0.09a |
aRegions in which trends between age and GWR were observed.
Discussion
The present study examined age-related changes in cortical tissue signal properties, including those of GM and WM intensity and gray–white contrast. Until now, these measures have not been used to study cortical maturation in 12- to 19-month-old infants. GM/WM intensity and contrast significantly correlated with age within bilateral anteroventral and lateral temporal areas, dorsomedial superior frontal, and also superior parietal cortices (Fig. 2A–C). However, no significant changes were observed within bilateral inferior parietal, occipital, or cingulate regions. These measures were additionally employed to assess evidence for structural development within areas functionally associated with word understanding. Indeed, age effects were localized within precisely the same left lateral temporal regions previously found to generate lexico-semantic N400m activity in 12- to 18-month-old infants and adults (Fig. 3A–C; Travis et al. 2011). Taken together, the present findings have important implications for understanding the neurobiological mechanisms relating structural to functional brain development.
Previous neuroimaging studies of early brain development have identified some of the important volumetric changes that occur within cortical and subcortical areas functionally associated with infants' developing sensorimotor and cognitive abilities (Knickmeyer et al. 2008; Ortiz-Mantilla et al. 2010; Gilmore et al. 2011). However, to our knowledge, the present study is the first to observe direct evidence for structural changes within cortical areas specifically demonstrated to support functional activity (i.e. N400m) associated with word understanding in similarly aged infants (Travis et al. 2011). Evidence for structural changes in these specific brain areas suggests that the neural processes indexed by the N400m emerge and operate functionally despite incomplete structural maturation. This itself is a rather remarkable finding considering that the protracted structural development of associative cortices has, in part, been thought to account for the gradual emergence of language and other cognitive abilities (Casey et al. 2005; Toga et al. 2006). Taken together, these findings provide reasonable evidence to suggest that there may be sufficient structural maturation within lateral temporal areas for supporting higher order abilities like word understanding very early during early infant development.
Here, evidence for rapid structural changes within lateral temporal areas during infancy is consistent with previous neurodevelopmental studies demonstrating rapid increases in the amounts of synaptogenesis–spinogenesis (Huttenlocher and Dabholkar 1997; Petanjek et al. 2012), dendritic arborization (Petanjek et al. 2008), and cytoarchitectural maturation (Moore and Guan 2001; Zilles et al. 1986) within frontal and temporal associative cortices within the first 2 years after birth. It is possible that these rapid maturational changes reflect some of the neurobiological mechanisms important for supporting infants' developing cognitive and language skills (Tau and Peterson 2010; Bates et al. 1992) as well as the functional maturation of N400 activity. However, such relationships cannot be assumed without further evidence directly relating such neurodevelopmental processes to the maturation of cognitive and linguistic behaviors. In future neuroimaging studies, it will be interesting to examine whether measures of GM/WM intensity may be related to the maturation of the N400 response, as well as the vocabulary skills that emerge during the second year (Fenson et al. 1994). Establishing evidence in support of such relationships may improve understandings of how both structural and functional brain development contribute to language abilities at these ages and throughout the lifespan. Unfortunately, it was not possible to directly examine these questions in the present study because measures of functional brain activity and language abilities could not be acquired for all infant subjects. In general, the present findings help to increase knowledge of the structural changes taking place amongst cortical areas between 12 and 19 months of age. Consistent with our initial predictions, age-related increases in GM/WM intensity were spatially distributed amongst cortical areas showing evidence for myelination between the first and second year (Flechsig 1920; Conel 1955; Yakovlev and Lecours 1967; Brody et al. 1987; Kinney et al. 1988; Gao et al. 2008). Specifically, age-effects were most apparent within superior frontal, parietal, and later temporal associative cortices, but were not observed within primary motor or primary somatosensory, visual, and auditory regions (Fig. 2). Rapid myelination of these cortices within the first year of life may possibly account for the lack of significant effects within these regions (Gao et al. 2008). However, the cellular processes contributing to the observed changes may develop more gradually and in differing proportions within these regions (Brody et al. 1987; Kinney et al. 1988), and were thus not possible to detect with the limited size and age distribution of the present subject population. These same factors may also account for the lack of significant changes in GM/WM intensity and contrast within other associative cortices also implicated in semantic processing, particularly inferior aspects of the frontal and parietal cortex (Binder et al. 2009).
At present, the neurobiological mechanisms mediating changes in T1-weighted signal properties are not well established. However, there is considerable evidence to suggest that greater amounts of proton–lipid interactions within myelinated tissues contribute to the intensity and contrast changes seen both here and in other neurodevelopmental imaging studies (Barkovich et al. 1988; Barkovich 2000). Alternatively, T1-weighted proton-relaxation rates (i.e. longitudinal relaxation R1 = 1/T1) are also observed to correspond to tissue concentrations of both water and non-heme iron (Vymazal, Hajek et al. 1995; Vymazal, Brooks et al. 1996; Ogg and Steen 1998; Gelman et al. 2001). Since both GM/WM intensity measures sampled here are positively correlated to these rates (Westlye et al. 2010), these measures may instead reflect regional increases in cortical non-heme iron (Hallgren and Sourander 1958) and the loss of unbound water during the second year (Dobbing and Sands 1973). However, neuroimaging evidence in support of these possibilities has been inconsistent (Ogg and Steen 1998; Steen et al. 2000) and has yet to be directly examined in this age range. It is also unlikely that both water loss and iron deposition occur entirely independent of myelination (Barkovich et al. 1988; Connor and Menzies 1996). Thus, it appears reasonable to presume that present intensity measures primarily reflect developmental changes in tissue properties directly related to cortical myelination (Glasser and Van Essen 2011). However, it is important that further research establish the contribution of these various tissue properties to the MRI signal both at these ages and throughout development. This will likely be best achieved by acquiring measures of tissue properties from T1-, T2-, and diffusion-weighted imaging.
Taken together, the present study demonstrates the potential for GM/WM intensities to serve as an additional structural marker of the regional structural changes taking place during the infant and toddler years. Between 12 and 19 months of age, the tissue properties affecting GM and WM intensity appear to change most significantly within lateral and ventral temporal areas bilaterally. Finally, evidence that intensity and contrast measures significantly increase with age within the same left lateral temporal areas previously demonstrated to support lexico-semantic activity indicates that neurophysiological activity supporting higher order abilities may develop before structural maturation is achieved in associative cortices.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
Funding sources include Kavli Institute for Brain and Mind, UCSD http://kibm.ucsd.edu/ and NIH/NINDA R01 NS018741-23A1, R21 HD066364 funding of E.H. K.E.T and M.K.L. have been supported by NIH pre-doctoral training grants and the Chancellor's Collaboratories Award, UCSD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Material
Notes
We thank M. Erhart, for his generous advice. Conflict of Interest. E.H. has equity interest in CorTechs Labs, Inc. A.M.D. is a founder and holds equity in CorTechs Labs, Inc and also serves on the Scientific Advisory Board. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
References
- Almli CR, Rivkin MJ, McKinstry RC, Group BDC. The NIH MRI study of normal brain development Objective-2: newborns, infants, toddlers, and preschoolers. Neuroimage. 2007;35:308–325. doi: 10.1016/j.neuroimage.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ. Concepts of myelin and myelination in neuroradiology. Am J Neuroradiol. 2000;21:1099–1109. [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Kjos BO, Jackson DE, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166:173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- Bates E, Thal D, Finlay B, Clancy B. Early language development and its neural correlates. In: Rapin I, Segalowitz S, editors. Handbook of neuropsychology, child neurology. 2nd ed. Amsterdam: Elsevier: 1992. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody B, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropath Exp Neurol. 1987;46:283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- Brown TT, Kuperman JM, Chung Y, Erhart M, McCabe C, Hagler D, Venkatraman VK, Akshoomoff N, Amaral DG, Bloss CS, et al. Neuroanatomical assessment of biological maturity. Curr Biol. 2012;22:1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: What have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Choe MS, Ortiz-Mantilla S, Makris N, Gregas M, Bacic J, Haehn D, Pienaar R, Caviness VS, Jr, Benasich AA, Grant PE. Regional infant brain development: An MRI-based morphometric analysis in 3 to 13 month olds. Cereb Cortex. 2013;23:2100–2117. doi: 10.1093/cercor/bhs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani H. A critical period of brain development: Studies of cerebral glucose utilization with PET* 1. Prev Med. 1998;27:184–188. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- Conel JL. The post natal development of the human cerebral cortex. Cambridge, MA: Harvard University Press; 1955. The cortex of a fifteen month old infant. [Google Scholar]
- Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Deoni SC. Magnetic resonance relaxation and quantitative measurement in the brain. Method Mol Biol. 2011;711:65–108. doi: 10.1007/978-1-61737-992-5_4. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S, Walters NB, Schleicher A, Kril J, Egan GF, Zilles K, Watson JDG, Amunts K. High-resolution MRI reflects myeloarchitecture and cytoarchitecture of human cerebral cortex. Hum Brain Mapp. 2005;24:206–215. doi: 10.1002/hbm.20082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson L, Dale P, Reznick J, Bates E, Thal D. Variability in early communicative development. 1994. Monographs of the Society for Research in Child Development. 59. Wiley. [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno M, Dale A. Cortical Surface-Based Analysis* 1: II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;2:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Flechsig PE. Anatomic des menschlichen gehirns und ruckenmarks auf myelogenetischer gundlange. Vol. 1. Leipzig: Thieme; 1920. [Google Scholar]
- Gao W, Lin W, Chen Y, Gerig G, Smith JK, Jewells V, Gilmore JH. Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. Am J Neuroradiol. 2008;30:290–296. doi: 10.3174/ajnr.A1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman N, Ewing JR, Gorell JM, Spickler EM, Solomon EG. Interregional variation of longitudinal relaxation rates in human brain at 3.0 T: Relation to estimated iron and water contents. Magn Reson Med. 2001;45:71–79. doi: 10.1002/1522-2594(200101)45:1<71::aid-mrm1011>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Genovese C, Lazar N, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;4:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gilmore J, Shi F, Woolson S, Knickmeyer RC, Short sJ, Lin W, Zhu H, Hamer RM, Styner M, Shen D. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex. 2011;22:2478–2485. doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YSK, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Van Essen DC. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J. Neurol. 2011;31:11597–11616. doi: 10.1523/JNEUROSCI.2180-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Herschkowitz N, Kagan J, Zilles K. Neurobiological bases of behavioral development in the second year. Neuropediatrics. 1999;30:221–230. doi: 10.1055/s-2007-973495. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6:1–9. [PubMed] [Google Scholar]
- Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: Finding meaning in the N400 component of the event-related brain potential ERP. Annu Rev Psychol. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. J Child Psychol Psyc. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Leonard C, Thompson P, Kan E, Jolley J, Welcome S, Toga A, Sowell E. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- Moore J, Guan Y-L. Cytoarchitectural and axonal maturation in human auditory cortex. JARO. 2001;2:297–311. doi: 10.1007/s101620010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell S, Noseworthy MD, Levine B, Dennis M. Cortical thickness of the frontopolar area in typically developing children and adolescents. Neuroimage. 2005;24:948–954. doi: 10.1016/j.neuroimage.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Ogg RJ, Steen RG. Age-related changes in brain T1 are correlated with iron concentration. Magn Reson Med. 1998;40:749–753. doi: 10.1002/mrm.1910400516. [DOI] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Choe M-s, Flax J, Grant PE, Benasich AA. Associations between the size of the amygdala in infancy and language abilities during the preschool years in normally developing children. Neuroimage. 2010;49:2791–2799. doi: 10.1016/j.neuroimage.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Kostović I, Uylings HBM. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: A layer-specific pattern. Cereb Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HBM, Rakic P, Kostović I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2012;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfluger T, Weil S, Weis S, Vollmar C, Heiss D, Egger J, Scheck R, Hahn K. Normative volumetric data of the developing hippocampus in children based on magnetic resonance imaging. Epilepsia. 1999;40:414–423. doi: 10.1111/j.1528-1157.1999.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Salat DH, Lee SY, van der Kouwe AJ, Greve DN, Fischl B, Rosas HD. Age-associated alterations in cortical gray and white matter signal intensity and gray to white matter contrast. Neuroimage. 2009;48:21–28. doi: 10.1016/j.neuroimage.2009.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer GB, Thompson JN, Bodensteiner JB, Hamza M, Tucker RR, Marks W, Gay C, Wilson D. Quantitative morphometric analysis of brain growth using magnetic resonance imaging. J Child Neurol. 1990;5:127–130. doi: 10.1177/088307389000500211. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson P, Leonard C, Welcome S. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson P, Tessner K, Toga A. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;22:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner D, Gamst A, Jernigan T. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Steen RG, Reddick WE, Ogg RJ. More than meets the eye: Significant regional heterogeneity in human cortical T1. Magn Reson Imaging. 2000;18:361–368. doi: 10.1016/s0730-725x(00)00123-5. [DOI] [PubMed] [Google Scholar]
- Tau G, Peterson BS. Normal development of brain circuits. Neuropsychopharmacol Rev. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis K, Ford K, Jacobs B. Regional dendritic variation in neonatal human cortex: A quantitative Golgi study. Dev Neurosci. 2005;27:277–287. doi: 10.1159/000086707. [DOI] [PubMed] [Google Scholar]
- Travis KE, Leonard MK, Brown TT, Hagler DJ, Curran M, Dale AM, Elman JL, Halgren E. Spatiotemporal neural dynamics of word understanding in 12- to 18-month-old-infants. Cereb Cortex. 2011;8:1832–1839. doi: 10.1093/cercor/bhq259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya H, Takano K, Okazaki M, Mitsudome A. Development of the temporal lobe in infants and children: Analysis by MR-based volumetry. Am J of Neuroradiol. 1999;20:717–723. [PMC free article] [PubMed] [Google Scholar]
- Vymazal J, Brooks RA, Baumgarner C, Tran V, Katz D, Bulte JW, Bauminger R, Di Chiro G. The relation between brain iron and NMR relaxation times: An in vitro study. Magn Reson Med. 1996;35:56–61. doi: 10.1002/mrm.1910350108. [DOI] [PubMed] [Google Scholar]
- Vymazal J, Hajek M, Patronas N, Giedd JN, Bulte JW, Baumgarner C, Tran V, Brooks RA. The quantitative relation between T1-weighted and T2-weighted MRI of normal gray matter and iron concentration. J Magn Reson Imaging. 1995;5:554–560. doi: 10.1002/jmri.1880050514. [DOI] [PubMed] [Google Scholar]
- Walters N, Egan G, Kril J, Kean M, Waley P, Jenkinson M, Watson J. In vivo identification of human cortical areas using high-resolution MRI: An approach to cerebral structure–function correlation. Proc Natl Acad Sci USA. 2003;100:2981–2986. doi: 10.1073/pnas.0437896100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Østby Y, Fjell AM. Differentiating maturational and aging-related changes of the cerebral cortex by use of thickness and signal intensity. Neuroimage. 2010;52:172–185. doi: 10.1016/j.neuroimage.2010.03.056. [DOI] [PubMed] [Google Scholar]
- Wetherby AM, Woods J, Allen L, Cleary J, Dickinson H, Lord C. Early indicators of autism spectrum disorders in the second year of life. J Autism Dev Disord. 2004;34:473–493. doi: 10.1007/s10803-004-2544-y. [DOI] [PubMed] [Google Scholar]
- Yakovlev P, Lecours A. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell; 1967. pp. 3–70. [Google Scholar]
- Zilles K, Werner R, Büschig U, Schleicher A. Ontogenesis of the laminar structure in areas 17 and 18 of the human visual cortex. Anat Embyrol. 1986;174:329–353. doi: 10.1007/BF00698784. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


