Abstract
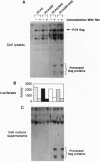
In human immunodeficiency virus type 1-infected cells, the efficient expression of viral proteins from unspliced and singly spliced RNAs is dependent on two factors: the presence in the cell of the viral protein Rev and the presence in the viral RNA of the Rev-responsive element (RRE). We show here that the HIV-1 Rev/RRE system can increase the expression of avian leukosis virus (ALV) structural proteins in mammalian cells (D-17 canine osteosarcoma) and promote the release of mature ALV virions from these cells. In this system, the Rev/RRE interaction appears to facilitate the export of full-length unspliced ALV RNA from the nucleus to the cytoplasm, allowing increased production of the ALV structural proteins. Gag protein is produced in the cytoplasm of the ALV-transfected cells even in the absence of a Rev/RRE interaction. However, a functional Rev/RRE interaction increases the amount of Gag present intracellularly and, more strikingly, results in the release of mature ALV particles into the supernatant. RCAS virus containing an RRE is replication-competent in chicken embryo fibroblasts; however, we have been unable to determine whether the particles produced in D-17 cells are as infectious as the particles produced in chicken embryo fibroblasts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo S. J., Chen I. S. Rev is necessary for translation but not cytoplasmic accumulation of HIV-1 vif, vpr, and env/vpu 2 RNAs. Genes Dev. 1991 May;5(5):808–819. doi: 10.1101/gad.5.5.808. [DOI] [PubMed] [Google Scholar]
- Arrigo S., Yun M., Beemon K. cis-acting regulatory elements within gag genes of avian retroviruses. Mol Cell Biol. 1987 Jan;7(1):388–397. doi: 10.1128/mcb.7.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich S. L., Macias M., Zhang L., Turek L. P., Stoltzfus C. M. Comparison of Rous sarcoma virus RNA processing in chicken and mouse fibroblasts: evidence for double-spliced RNA in nonpermissive mouse cells. J Virol. 1990 Sep;64(9):4313–4320. doi: 10.1128/jvi.64.9.4313-4320.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M., Prasad S., Dubay J. W., Hunter E., Jeang K. T., Rekosh D., Hammarskjöld M. L. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. H., Borg K. T., Haines J. K., Moon R. T., Schoenberg D. R., Arrigo S. J. Human immunodeficiency virus type 1 Rev is required in vivo for binding of poly(A)-binding protein to Rev-dependent RNAs. J Virol. 1994 Sep;68(9):5433–5438. doi: 10.1128/jvi.68.9.5433-5438.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Sharp P. A. Regulation by HIV Rev depends upon recognition of splice sites. Cell. 1989 Dec 1;59(5):789–795. doi: 10.1016/0092-8674(89)90602-8. [DOI] [PubMed] [Google Scholar]
- Cook K. S., Fisk G. J., Hauber J., Usman N., Daly T. J., Rusche J. R. Characterization of HIV-1 REV protein: binding stoichiometry and minimal RNA substrate. Nucleic Acids Res. 1991 Apr 11;19(7):1577–1583. doi: 10.1093/nar/19.7.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Copeland N. G., Zelenetz A. D., Krontiris T. Transformation of NIH-3T3 mouse cells by avian retroviral DNAs. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1169–1176. doi: 10.1101/sqb.1980.044.01.126. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Human immunodeficiency virus as a prototypic complex retrovirus. J Virol. 1991 Mar;65(3):1053–1056. doi: 10.1128/jvi.65.3.1053-1056.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Kopchick J. J., Stacey D. W. Effect of intron size on splicing efficiency in retroviral transcripts. Nucleic Acids Res. 1982 Oct 11;10(19):6177–6190. doi: 10.1093/nar/10.19.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino D. M., Felber B. K., Harrison J. E., Pavlakis G. N. The Rev protein of human immunodeficiency virus type 1 promotes polysomal association and translation of gag/pol and vpu/env mRNAs. Mol Cell Biol. 1992 Mar;12(3):1375–1386. doi: 10.1128/mcb.12.3.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C. T., Stehelin D., Bishop J. M., Varmus H. E. Characteristics of virus-specific RNA in avian sarcoma virus-transformed BHK-21 cells and revertants. Virology. 1977 Jan;76(1):313–330. doi: 10.1016/0042-6822(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Eisenman R. N., Vogt V. M. The biosynthesis of oncovirus proteins. Biochim Biophys Acta. 1978 Apr 6;473(3-4):187–239. doi: 10.1016/0304-419x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Emerman M., Vazeux R., Peden K. The rev gene product of the human immunodeficiency virus affects envelope-specific RNA localization. Cell. 1989 Jun 30;57(7):1155–1165. doi: 10.1016/0092-8674(89)90053-6. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Hadzopoulou-Cladaras M., Cladaras C., Copeland T., Pavlakis G. N. rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1495–1499. doi: 10.1073/pnas.86.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber B. K., Pavlakis G. N. Molecular biology of HIV-1: positive and negative regulatory elements important for virus expression. AIDS. 1993;7 (Suppl 1):S51–S62. [PubMed] [Google Scholar]
- Fu X. D., Katz R. A., Skalka A. M., Maniatis T. The role of branchpoint and 3'-exon sequences in the control of balanced splicing of avian retrovirus RNA. Genes Dev. 1991 Feb;5(2):211–220. doi: 10.1101/gad.5.2.211. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Fine D. L., Gregg M. Squirrel monkey retrovirus: electron microscopy of a virus from New World monkeys and comparison with Mason-Pfizer monkey virus. Arch Virol. 1978;56(4):297–307. doi: 10.1007/BF01315280. [DOI] [PubMed] [Google Scholar]
- Greene W. C. Regulation of HIV-1 gene expression. Annu Rev Immunol. 1990;8:453–475. doi: 10.1146/annurev.iy.08.040190.002321. [DOI] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Heimer J., Hammarskjöld B., Sangwan I., Albert L., Rekosh D. Regulation of human immunodeficiency virus env expression by the rev gene product. J Virol. 1989 May;63(5):1959–1966. doi: 10.1128/jvi.63.5.1959-1966.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaphy S., Dingwall C., Ernberg I., Gait M. J., Green S. M., Karn J., Lowe A. D., Singh M., Skinner M. A. HIV-1 regulator of virion expression (Rev) protein binds to an RNA stem-loop structure located within the Rev response element region. Cell. 1990 Feb 23;60(4):685–693. doi: 10.1016/0092-8674(90)90671-z. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Krutzsch H. C., Oroszlan S. Myristyl amino-terminal acylation of murine retrovirus proteins: an unusual post-translational proteins modification. Proc Natl Acad Sci U S A. 1983 Jan;80(2):339–343. doi: 10.1073/pnas.80.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S. M., Chavez M., Gerstberger S., Venkatesan S. A specific sequence with a bulged guanosine residue(s) in a stem-bulge-stem structure of Rev-responsive element RNA is required for trans activation by human immunodeficiency virus type 1 Rev. J Virol. 1992 Jun;66(6):3699–3706. doi: 10.1128/jvi.66.6.3699-3706.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Petropoulos C. J., Federspiel M. J., Sutrave P., Forry-Schaudies S., Bradac J. A. Vectors and genes for improvement of animal strains. J Reprod Fertil Suppl. 1990;41:39–49. [PubMed] [Google Scholar]
- Lawrence J. B., Cochrane A. W., Johnson C. V., Perkins A., Rosen C. A. The HIV-1 Rev protein: a model system for coupled RNA transport and translation. New Biol. 1991 Dec;3(12):1220–1232. [PubMed] [Google Scholar]
- Lu X. B., Heimer J., Rekosh D., Hammarskjöld M. L. U1 small nuclear RNA plays a direct role in the formation of a rev-regulated human immunodeficiency virus env mRNA that remains unspliced. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7598–7602. doi: 10.1073/pnas.87.19.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machala O., Donner L., Svoboda J. A full expression of the genome of Rous sarcoma virus in heterokaryons formed after fusion of virogenic mammalian cells and chicken fibroblasts. J Gen Virol. 1970 Sep;8(3):219–229. doi: 10.1099/0022-1317-8-3-219. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Le S. Y., Maizel J. V., Cullen B. R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989 Mar 16;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- McKeown M. Alternative mRNA splicing. Annu Rev Cell Biol. 1992;8:133–155. doi: 10.1146/annurev.cb.08.110192.001025. [DOI] [PubMed] [Google Scholar]
- Mermer B., Felber B. K., Campbell M., Pavlakis G. N. Identification of trans-dominant HIV-1 rev protein mutants by direct transfer of bacterially produced proteins into human cells. Nucleic Acids Res. 1990 Apr 25;18(8):2037–2044. doi: 10.1093/nar/18.8.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Vogt V. M., Ripley S., Eisenman R. N. The NH2-terminal sequence of the avian oncovirus gag precursor polyprotein (Pr76gag). Virology. 1978 Dec;91(2):423–433. doi: 10.1016/0042-6822(78)90388-4. [DOI] [PubMed] [Google Scholar]
- Quintrell N., Hughes S. H., Varmus H. E., Bishop J. M. Structure of viral DNA and RNA in mammalian cells infected with avian sarcoma virus. J Mol Biol. 1980 Nov 15;143(4):363–393. doi: 10.1016/0022-2836(80)90218-1. [DOI] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Oroszlan S. In vivo modification of retroviral gag gene-encoded polyproteins by myristic acid. J Virol. 1983 May;46(2):355–361. doi: 10.1128/jvi.46.2.355-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Fenyö E. M., Pavlakis G. N. Env and Vpu proteins of human immunodeficiency virus type 1 are produced from multiple bicistronic mRNAs. J Virol. 1990 Nov;64(11):5448–5456. doi: 10.1128/jvi.64.11.5448-5456.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Pavlakis G. N. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J Virol. 1992 Jan;66(1):150–159. doi: 10.1128/jvi.66.1.150-159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomin L., Felber B. K., Pavlakis G. N. Different sites of interaction for Rev, Tev, and Rex proteins within the Rev-responsive element of human immunodeficiency virus type 1. J Virol. 1990 Dec;64(12):6010–6017. doi: 10.1128/jvi.64.12.6010-6017.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer K. S., Boettiger D. Complementation rescue of Rous sarcoma virus from transformed mammalian cells by polyethylene glycol-mediated cell fusion. J Virol. 1977 Jul;23(1):133–141. doi: 10.1128/jvi.23.1.133-141.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Chang L. J., Cripe T. P., Turek L. P. Efficient transformation by Prague A Rous sarcoma virus plasmid DNA requires the presence of cis-acting regions within the gag gene. J Virol. 1987 Nov;61(11):3401–3409. doi: 10.1128/jvi.61.11.3401-3409.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M. Synthesis and processing of avian sarcoma retrovirus RNA. Adv Virus Res. 1988;35:1–38. doi: 10.1016/s0065-3527(08)60707-1. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Machala O., Donner L., Sovovã Comparative study of RSV rescue from RSV-transformed mammalian cells. Int J Cancer. 1971 Nov 15;8(3):391–400. doi: 10.1002/ijc.2910080306. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Vogt P. K. Appearance of virus-specific DNA in mammalian cells following transformation by Rous sarcoma virus. J Mol Biol. 1973 Mar 15;74(4):613–626. doi: 10.1016/0022-2836(73)90052-1. [DOI] [PubMed] [Google Scholar]
- Viglianti G. A., Sharma P. L., Mullins J. I. Simian immunodeficiency virus displays complex patterns of RNA splicing. J Virol. 1990 Sep;64(9):4207–4216. doi: 10.1128/jvi.64.9.4207-4216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Bruckenstein D. A., Bell A. P. Avian sarcoma virus gag precursor polypeptide is not processed in mammalian cells. J Virol. 1982 Nov;44(2):725–730. doi: 10.1128/jvi.44.2.725-730.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb J. M., Ortiz-Conde B. A., Hughes S. H. Replication of avian leukosis viruses with mutations at the primer binding site: use of alternative tRNAs as primers. J Virol. 1995 Oct;69(10):6228–6238. doi: 10.1128/jvi.69.10.6228-6238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Achacoso J. A. Creation and expression of myristylated forms of Rous sarcoma virus gag protein in mammalian cells. J Virol. 1989 Oct;63(10):4331–4343. doi: 10.1128/jvi.63.10.4331-4343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Weldon R. A., Jr, Nelle T. D., Erdie C. R. Suppression of retroviral MA deletions by the amino-terminal membrane-binding domain of p60src. J Virol. 1991 Jul;65(7):3804–3812. doi: 10.1128/jvi.65.7.3804-3812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow B. J., Trono D. The blocks to human immunodeficiency virus type 1 Tat and Rev functions in mouse cell lines are independent. J Virol. 1993 Apr;67(4):2349–2354. doi: 10.1128/jvi.67.4.2349-2354.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin A. S., Harford J. B., Felber B. K. Rev of human immunodeficiency virus and Rex of the human T-cell leukemia virus type I can counteract an mRNA downregulatory element of the transferrin receptor mRNA. Nucleic Acids Res. 1994 Nov 11;22(22):4725–4732. doi: 10.1093/nar/22.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin A. S., Valentin A., Pavlakis G. N., Felber B. K. Continuous propagation of RRE(-) and Rev(-)RRE(-) human immunodeficiency virus type 1 molecular clones containing a cis-acting element of simian retrovirus type 1 in human peripheral blood lymphocytes. J Virol. 1994 Dec;68(12):7944–7952. doi: 10.1128/jvi.68.12.7944-7952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]