Abstract
Spontaneous aneurysmal thrombosis as a cause for acute ischemic stroke is a rarely described complication of small unruptured intracranial aneurysms. We present an interesting case of a stroke in a woman with an occult acutely thrombosed middle cerebral artery bifurcation aneurysm that was found during successful thrombus aspiration of the occluded parent vessel. Although rare, small aneurysms have to be considered as a possible cause for a thrombotic vessel occlusion. Catheters and thrombectomy devices have to be used carefully while performing mechanical revascularization.
Keywords: aneurysm, mechanical recanalization, Mortality, thrombosis, stroke
Introduction
Around 1%–5% of the adult population harbor an intracranial aneurysm1 and according to the International Study of Unruptured Intracranial Aneurysms 30% of these are middle cerebral artery (MCA) aneurysms1,2. The major complication associated with intracranial aneurysms is aneurysm rupture resulting in subarachnoid hemorrhage. Complete aneurysm thrombosis is a rare event and is mostly seen in giant cerebral aneurysms.3 Spontaneous occlusion of small aneurysms is rarely described in the literature and the mechanism is not well understood. Structural characteristics of the aneurysm (i.e., neck configuration, size, and location) have been discussed to facilitate aneurysmal thrombosis.4–6 In addition, changes in blood flow within the aneurysm sac may promote intraluminal thrombus formation. We report the case of an AIS in a woman due to thrombus formation in a small occult MCA aneurysm with extension into the parent vessel.
Case report
A 62-year-old, left-handed woman presented to the Emergency Department with increased somnolence, dysarthria, facial droop, and left-sided hemiplegia; all other vital signs were normal. The initial obtained NIHSS was 18. Electrocardiogram showed no evidence of atrial fibrillation. The patient’s past medical history was unremarkable except for a mild hyperlipidemia. Laboratory results showed a mild normocytic anemia and an elevated serum glucose level. A noncontrast head computed tomography (CT) showed a hyperdense right MCA sign and the CT-angiography (CTA) showed a proximally occluded right M1 segment. Because of large penumbra and symptom onset more than 4.5 h ago, the patient did not qualify for IV t-PA. Thus, she was immediately transferred to the neuroangiography suite for mechanical revascularization. A Penumbra Reperfusion Catheter 054 (Penumbra, Inc., Alameda, CA, USA) was used to successfully recanalize the occluded vessel. During one of several passes through the thrombus, it was felt that the separator extended beyond the boundaries of the MCA bifurcation at the opercular turn of the MCA and into the Sylvian fissure. An outpouching at the MCA bifurcation potentially consistent with a vessel rupture and/or pseudoaneurysm formation was visualized ( Figure 1B). As the patient had received intra-arterial t-PA, the neurosurgeons were alerted about potential need for surgical intervention.
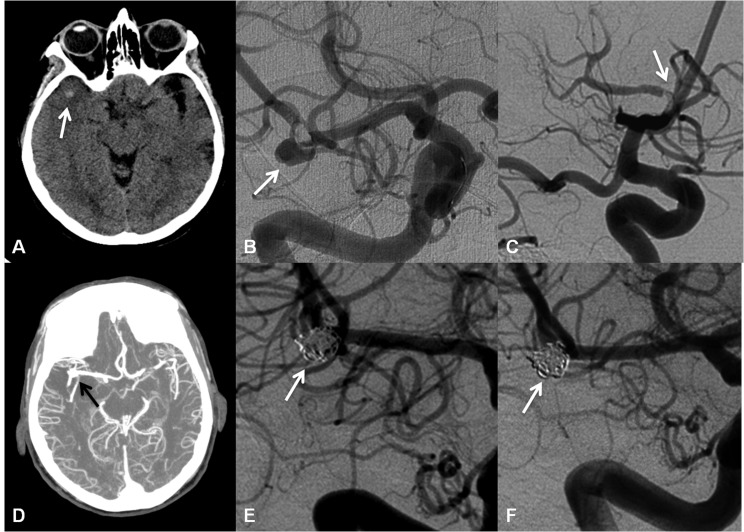
Figure 1. Head computed tomography on admission of the patient shows a small round hyperdensity adjacent to the course of the thrombosed middle cerebral artery within the Sylvian fissure (arrow). In retrospect, this represents the occluded aneurysm (A). Right internal carotid artery angiogram in frontal view during the thrombo-aspiration process shows a hidden M1—M2 bifurcation aneurysm (arrow) with an intraluminal thrombus (B). The final control angiography after completion of mechanical recanalization shows complete reperfusion of the M1 segment and partial recanalization of the middle cerebral artery bifurcation (arrow) (C). Computed tomography angiography post endovascular treatment now shows a reperfused right middle cerebral artery with the M1—M2 junction aneurysm (arrow) (D). The elective coiling procedure successfully excluded the aneurysm from the blood circulation (arrow) without any residual flow into the aneurysm sac (E). Α 6-month followup angiography shows that the aneurysm remains entirely occluded (arrow). No aneurysm reperfusion can be detected (F).
At this point the noncontrast head CT was carefully reviewed and in addition to the hyperdense MCA sign, there was a hyperdense nodular structure at the right MCA bifurcation consistent with a thrombosed aneurysm ( Figure 1A). The revascularization was carefully continued. Initial followup angiogram showed a MCA bifurcation aneurysm measuring 5.2 mm in transverse × 4.8 mm craniocaudal in size with a neck measuring 3.7 mm in width ( Figure 1B). There was persistent filling defect around the proximal neck, consistent with thrombus. Penumbra device aspiration was continued. Final followup angiogram showed small amounts of clot within a proximal M2 branch while the M1 segment was entirely patent ( Figure 1C). Followup noncontrast head CT post-intervention showed a small area of hypodensity within the lateral aspect of the right temporal lobe that may reflect infarct. Post-treatment, CTA visualized the patent right MCA with a bifurcation aneurysm ( Figures 1D). Four days after the thrombectomy procedure, the patient underwent successful and complete coil embolization of the aneurysm ( Figure 1E). After the procedure the patient was transferred to the neurocritical care unit. Two days later she was transferred to the stroke service on a regular medical floor. A transesophageal echocardiogram (TEE) revealed normal left-ventricular ejection fraction and a small patent foramen ovale with slight mitral valve prolapse. The patient did not require surgical treatment for these findings. The patient was continued on medical therapy consisting of aspirin 81 mg p.o./day, atorvastatin 80 mg p.o./day, and lisinopril 2.5 mg p.o./day. A prothrombotic panel was negative. The patient was then sent to a facility for a several-week extensive rehabilitation. A followup angiography was performed 6 months after the coiling procedure, and showed complete aneurysm obliteration ( Figure 1F). NIHSS was 2.
Discussion
Cerebral infarction caused by spontaneous thrombosis of a small unruptured cerebral aneurysm is a rarely described event.7 Current literature only provides limited information on management and treatment recommendations of such cases.7 Complete aneurysm thrombosis is well known to occur after subarachnoid hemorrhage and is observed in around 50% of giant saccular and fusiform aneurysms.6 Only a few cases that describe clinical ischemic events related to small partially occluded aneurysms can be found.7 Although intra-aneurysmal thrombosis in small intracranial aneurysms is infrequently reported, our case shows that the possibility of a hidden aneurysm has to be considered when mechanical revascularization of an occluded vessel is performed and that small aneurysms indeed can serve as a source of thrombus formation and emboli. In our patient, the intra-aneurysmal thrombus completely occluded the entire aneurysm sac and extended distally and proximally into the parent artery causing complete vessel occlusion. Arterial branches originating near the aneurysm were patent and thus suggesting of thrombus formation within the aneurysm and subsequent extension into the MCA rather than being embolic in nature.
Previous reports have described aneurysms that were found after endovascular recanalization of an ICA occlusion and two MCA occlusions (Saito et al8 and Hayashi et al9, respectively). However, both authors proposed based on the presence of atrial fibrillation and displacement of the clot during endovascular treatment while the discovered aneurysm neck appeared to be smooth that the etiology for the parent vessel thrombosis was more likely due to a cardioembolic event. In our patient, a cardioembolic event seems unlikely as there was no history of an atrial fibrillation (AF) prior to admission and no AF was detected at any time during hospitalization. Also, TEE did not reveal a mural thrombus or cardiac vegetations as potential source of emboli. Saito et al8 discussed that during the mechanical revascularization the aneurysm was at high risk of rupture. We agree and conclude that the Penumbra System used in our case seems to be a safe device if used carefully.In the current literature, most if not all of the thrombosed aneurysms detected with angiography, CT, MRI, or incidentally found after mechanical recanalization of the occluded vessel, were treated with medical therapy or surgical clipping or just controlled on followup examinations3,6–9. The MCA bifurcation aneurysm in our patient was coiled using two Codman Trufill DCS Orbit detachable coils (Codman Neurovascular, Johnson & Johnson Medical, Raynham, MA, USA) and one Deltapaq 10 Micrus Cerecyte detachable coil (Micrus Endovascular Corporation Industry, San Jose, CA, USA, now part of Codman & Shurtleff, Johnson & Johnson Medical, Raynham, MA, USA). The coil embolization resulted in complete aneurysm occlusion without residual filling. Also at 6-month followup, the aneurysm remained entirely occluded and without significant residual.
Conclusion
Although small aneurysms are considered to show less thrombus formation than bigger aneurysms and the occurrence of thrombotic parent vessel occlusion may be rare, it still presents an important complication. In any case, catheters and devices have to be handled very carefully to minimize the risk of vessel rupture. As no guidelines regarding the optimal treatment of such incidentally found thrombosed aneurysms exist, our findings suggest coiling to be a safe and effective treatment option in aneurysms suitable for this endovascular approach.
- AF
Atrial fibrillation
- AIS
Acute ischemic stroke
- CT
Computed tomography
- CTA
Computed tomography angiography
- MCA
Middle cerebral artery
- TEE
Transesophageal echocardiogram
List of Commercial Products
-
-
Penumbra Inc., Alameda, CA, USA
-
-
Codman Neurovascular, Johnson&Johnson Medical, Raynham, MA, USA
-
-
Micrus Endovascular Corporation Industry, San Jose, CA, USA, now part of Codman &
-
-
Shurtleff, Johnson&Johnson Medical, Raynham, MA, USA
Footnotes
Funding: none
Conflict of Interest: no conflict of interest exists.
References
- 1.Valencia C, Villa-Uriol MC, Pozo JM, et al. 2010Morphological descriptors as rupture indicators in middle cerebral artery aneurysms Conf Proc IEEE Eng Med Biol Soc;20106046–9. [DOI] [PubMed] [Google Scholar]
- 2.Wiebers DO, Whisnant JP, Huston J., 3rd2003;Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment Lancet 362103–10. [DOI] [PubMed] [Google Scholar]
- 3.Brownlee RD, Tranmer BI, Sevick RJ, et al. Spontaneous thrombosis of an unruptured anterior communicating artery aneurysm. An unusual cause of ischemic stroke. Stroke. 1995;26:1945–9. doi: 10.1161/01.str.26.10.1945. [DOI] [PubMed] [Google Scholar]
- 4.Whittle IR, Dorsch NW, Besser M. Spontaneous thrombosis in giant intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1982;45:1040–7. doi: 10.1136/jnnp.45.11.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen JE, Rajz G, Umansky F, et al. Thrombosis and recanalization of symptomatic nongiant saccular aneurysm. Neurol Res. 2003;25:857–9. doi: 10.1179/016164103771953961. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JE, Itshayek E, Gomori JM, et al. Spontaneous thrombosis of cerebral aneurysms presenting with ischemic stroke. J Neurol Sci. 2007;254:95–8. doi: 10.1016/j.jns.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Mokin M, Darkhabani Z, Binning MJ, Levy EI, Siddiqui AH. Small unruptured partially thrombosed aneurysms and stroke: report of three cases and review of the literature. J NeuroIntervent Surg. 2012;4:e6. doi: 10.1136/neurintsurg-2011-010026. [DOI] [PubMed] [Google Scholar]
- 8.Saito N, Hayashi N, Okubo T, et al. Internal carotid artery aneurysm visualized during successful endovascular treatment of carotid embolism. AJNR Am J Neuroradiol. 2000;21:546–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi K, Takahashi N, Furuichi S, et al. Two cases of cerebral aneurysm detected after recanalization of the middle cerebral artery. No Shinkei Geka. 1998;26:1103–7. [PubMed] [Google Scholar]