Abstract
AIM: To determine the influence of Adriamycin (ADM) on the changes in Nanog, Oct4, Sox2, as well as, in ARID1 and Wnt5b expression in liver cancer stem cells.
METHODS: The MHCC97-L and HCCLM3 liver cancer cell lines were selected as the cell models in this study, and were routinely cultured. The 50% lethal dose (LD50) in the cell lines was detected by the MTT assay. Expression changes in liver cancer stem cell related genes (Nanog, Oct-4, Sox2, ARID1, and Wnt5b) were detected by western blot following treatment with ADM (LD50).
RESULTS: The LD50 of ADM in MHCC97-L cells was lower than that in HCCLM3 cells (0.4123 ± 0.0236 μmol/L vs 0.5259 ± 0.0125 μmol/L, P < 0.05). Wnt5b and Nanog were expressed in both MHCC97-L and HCCLM3 cells, while only Sox2 was expressed in HCCLM3 cells. However, neither ARID1A nor Oct4 was detected in these two cell lines. Genes, related to the stem cells, showed different expression in liver cancer cells with different metastatic potential following treatment with ADM (LD50). Wnt5b protein increased gradually within 4 h of ADM (LD50) treatment, while Nanog decreased (P < 0.05). After 12 h, Wnt5b decreased gradually, while Nanog increased steadily (P < 0.05). In addition, only Sox2 was expressed in HCCLM3 cells with high metastatic potential following ADM (LD50) treatment. The expression of Sox2 increased gradually with ADM (LD50) in HCCLM3 cells (P < 0.05).
CONCLUSION: ADM increased the death rate of MHCC97-L and HCCLM3 cells, while the growth suppressive effect of ADM was higher in MHCC97-L cells than in HCCLM3 cells.
Keywords: Liver cancer cell, Adriamycin, Stem cell related gene, Western blot, Liver cancer stem cell
Core tip: This study, comprehensively and systematically reports the gene expression of Nanog, Wnt5b, Oct4, and Sox2 and ARID1A, in two hepatoma cell lines. This was performed to explore the molecular mechanism of the recurrence and metastasis of hepatocellular carcinoma, and to detect accurate markers and targets for stem cell-targeted therapeutic interventions. The results showed that Adriamycin (ADM) increased the death rate of MHCC97-L and HCCLM3 cells, while the growth suppressive effect of ADM was higher in MHCC97-L cells than in HCCLM3 cells.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common liver cancers in adults, and is the fifth most common types of cancer in the world. Liver cancer ranks third in all cancer-related deaths[1,2]. The incidence of liver cancer in China accounts for 55% worldwide and mortality due to liver cancer is second to lung cancer[3,4]. Recurrence is the main cause of high mortality in patients with HCC.
Liver cancer stem cells (CSCs) play an important role in cancer development, metastasis, recurrence, and multidrug resistance. However, markers for liver cancer stem cells have yet to be recognized. Thus, research on liver cancer stem cells has mainly focused on identifying markers. Currently, the expression of Oct4, Sox2, and Nanog (the embryonic stem cell specific genes) has been detected in human hepatoma cell lines[5]. These findings have proved the role of stem cells in cancer development. Furthermore, a previous study[6] showed that the expression of target factors, in the Wnt signaling pathways of liver cancer stem cells, led to reduced metastasis and drug resistance. In addition, frequent ARID1A (AT-rich interactive domain-containing protein 1A) mutations were discovered in ovarian cancer, bladder cancer, gastric cancer, and other tumors by a number of researchers[7-10]. Hence, ARID1A may be expressed in hepatoma cells. To date, expression changes in some stem-cell related genes have been detected due to the effect of an anti-cancer drug[11]. However, the numbers of observed genes were limited. In order to study the mechanism of these genes, more genes related to HCC were evaluated in this study in order to understand the full mechanism of HCC. In this research, the hepatoma cell lines MHCC97-L and HCCLM3 were selected as the cell models in this study, as they share the same background, but have different metastatic potential. One of the broad-spectrum anti-cancer drugs, Adriamycin (ADM), was used in this study, and its effects on these cell lines, with different metastatic potential, were observed. Furthermore, the 50% lethal dose (LD50) of ADM in the cell lines was measured. Using the LD50 concentration of ADM, the expression of Nanog, Oct-4, Sox2, ARID1A and Wnt5b were determined at different time intervals (0 h, 2 h, 4 h, 12 h, 24 h, and 72 h) to analyze the molecular mechanism of recurrence and metastasis of HCC, and to identify the accurate markers and targets for stem cell-targeted therapeutic interventions.
MATERIALS AND METHODS
Materials and reagents
The human hepatoma cell lines MHCC97-L and HCCLM3 (from BIOVOL Biological Technology Co., Ltd.), antibodies of Nanog, Sox2, Oct4, ARID1A and Wnt5b (from Cell Signaling Technology Company), ADM and other reagents were purchased in China. DMEM medium, fetal bovine serum (FBS) and the MTT kit were purchased from Invitrogen Company, United States. The equipment used included a laser scanning confocal microscope (Olympus, Japan), inverted microscope (CKX41, Olympus, Japan), clean benches (SW-CJ-2FD, Suzhou Purification Equipment Co., Ltd.), CO2 incubator thermostat (Model 310, Thermo Company, United States), and an ultra-low temperature freezer (MDF-382E, SANYO Company, Japan).
Cell culture
The human hepatoma cell lines MHCC97-L and HCCLM3 were incubated with DMEM medium + 10% FBS + 1% penicillin at 37 °C and 5% CO2. A small amount of cell suspension was drawn to adjust the concentration to 103-104 cells/well, and each well contained 100 μL. ADM, at different concentrations, was added to the bottom of the well (96-well plate) containing the cells. Then, 16-48 h later, the cells were observed under an inverted microscope, and 20 μL of MTT solution (5 mg/mL, 0.5% MTT) was added. After 4 h incubation, the culture liquid was discarded, and 150 μL of dimethyl sulfoxide was added. The plate was then placed on a shaker for 10 min to fully dissolve the crystals. Finally, absorption of the cells, in each well, was evaluated using an ELISA meter at OD 490 nm. A null-balanced well (medium, MTT, methyl sulfoxide) and a control well (cells, the same concentration of the drug dissolution medium, medium, MTT, methyl sulfoxide) were included.
Analysis of PAGE-SDS electrophoresis and Western blot
First, the extracted protein was denatured at 100 °C for 8 min and then centrifuged at 12000 r/min for 5 min. Subsequently, the denatured protein was placed in the prepared polyacrylamide gel and electrophoresed with a voltage of 80 V for 2 h. When the indicator, bromophenol blue, reached the bottom, the electrophoresis was terminated. The gel was then cut to an appropriate size and processed with a nitrocellulose filter membrane film. Subsequently, the transformation was performed at a current density of 1 mA/cm2 for 1.5 h. The film strip was then processed by immune blot and the standard protein band was stained as a control. Antibody I of Nanog was a Nanog (D73G4) XP®sRabbit mAb, which was incubated at 4 °C overnight, and then, II anti DyLight 649 labeled Goat anti rabbit IgG (H + L) was incubated for 2 h at room temperature. The colored liquid was then added and the band appeared. To terminate the reaction, double distilled water was added. To detect the expression of Oct4, Sox2, ARID1A, and Wnt5b, corresponding antibodies were added, according to the method mentioned above.
Data processing
SPSS16.0 software was used for statistical analysis. The LD50 of ADM was calculated using the regression model. All results were expressed as mean ± SD. ANOVA was performed to compare multiple groups, while the SNK (Student-Newman-Keuls) test was performed for pair wise comparisons to determine if the data were normally distributed and the homogeneity of variance. P < 0.05 was considered statistically significant.
RESULTS
ADM inhibition of hepatocellular carcinoma cells
Growth inhibition of the human hepatoma cell lines MHCC97-L and HCCLM3 by ADM was detected by MTT assay. The results showed that ADM inhibited both human hepatoma cell lines to different degrees. At higher concentrations, ADM showed a stronger effect (Figure 1). The calculated ADM LD50 for MHCC97-L cells (0.4123 ± 0.0236 μmol/L) and HCCLM3 cells (0.5259 ± 0.0125 μmol/L) (P < 0.05) was based on the inhibition rate and the corresponding ADM concentration, using a linear regression method.
Figure 1.
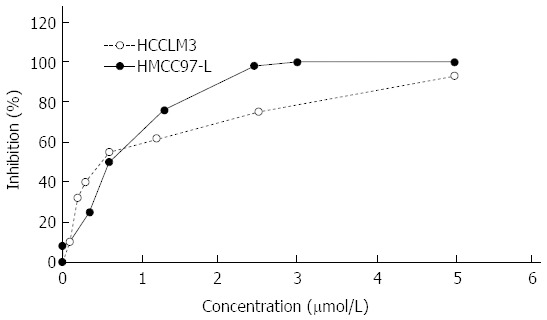
Adriamycin growth inhibition curve for MHCC97-L and HCCLM3 cells. Adriamycin (ADM) (at concentrations of 0, 0.005, 0.1, 0.35, 0.6, 1.3, 2.45, 5 μmol/L) was added to MHCC97-L and HCCLM3 cells, and the cell death rate was calculated accordingly. The concentration and growth inhibition rates were taken as the X-axis and Y-axis, respectively. ADM LD50 for MHCC97-L cells was significantly lower than that for HCCLM3 cells (0.4123 ± 0.0236 μmol/L vs 0.5259 ± 0.0125 μmol/L, P < 0.05).
Influence of ADM on stem cell-related gene expression in hepatoma cell lines with different metastatic potential
Influence of ADM on the expression of Nanog, Wnt5b, Oct4, Sox2, and ARID1A in the hepatoma cell line MHCC97-L with low metastatic potential: In the low-metastatic human hepatoma cell line, MHCC97-L, Wnt5b, and Nanog proteins were expressed and the changes were both time- and ADM concentration-dependent. Within a short period (≤ 4 h), the longer the ADM (LD50) inhibition time, the higher the protein level of Wnt5. However, 12 h later, Wnt5b protein levels gradually decreased (Figure 2A). The expression of Nanog was the opposite to that of Wnt5 (Figure 2B), and its expression curve was a parabola.
Figure 2.

Wnt5b and Nanog expression in cell line MHCC97-L treated with Adriamycin. A: Wnt5b expression in cell line MHCC97-L treated with Adriamycin (ADM). The expression of Wnt5b was evaluated at different time intervals (0 h, 2 h, 4 h, 12 h, 24 h, and 72 h) by western blot. The band gray value was highest at 12 h. The relative gene expression was indicated by the Wnt5b protein band gray value or β-actin band gray value calculated using western blot. The relative expression curve was a normal distribution line; B: Nanog expression in cell line MHCC97-L treated with ADM. The expression of Nanog was measured at different time intervals (0 h, 2 h, 4 h, 12 h, 24 h, and 72 h) by western blot. The band gray value was lowest at 12 h, and highest at 72 h. The relative expression of the gene was indicated by the Nanog protein band gray value or β-actin band gray value calculated using western blot. The relative expression curve was a parabola.
There was a statistically significant difference between the expression changes of Wnt5b and Nanog in MHCC97-L cells (P < 0.05) (Table 1). However, Sox2, ARID1A, and Oct4 were not expressed in these cells.
Table 1.
Comparison of expression changes in stem-cell genes in the hepatoma cell lines with different metastatic potential treated with Adriamycin (mean ± SD)
| Group | n | Wnt5b | Nanog | Sox2 |
| MHCC97-L | 3 | 31.5 ± 1.2↑ | 30.8 ± 0.2↓ | - |
| HCCLM3 | 3 | 36.7 ± 2.1↑ | 90.4 ± 0.8↓ | 6.0 ± 2.3↑ |
| t value | 6.03 | 45.40 | - | |
| P value | 0.04 | 0.00 | - |
Influence of ADM on the expression of Nanog, Wnt5b, Oct4, Sox2, and ARID1A in the hepatoma cell line HCCLM 3 with high metastatic potential: In the high-metastatic human hepatoma cell line HCCLM3, Wnt5b and Nanog proteins were significantly expressed and were associated with prolonged ADM inhibition of these cells. Both, the gene expression curve patterns, and the time-dependent acceleration and deceleration, were similar in cell line HCCLM 3 and MHCC97-L (Figure 3A). Nanog expression level gradually decreased within the first 4 h and reversed after 12 h (Figure 3B). However, the Sox2 protein expression level in cell line HCCLM3 increased in a time-dependent manner (Figure 3C).
Figure 3.

Wnt5b, Nanog and Sox2 expression in cell line HCCLM3 treated with Adriamycin. A: Wnt5b expression in cell line HCCLM3 treated with Adriamycin (ADM). The expression of Wnt5b was evaluated at different time intervals (0 h, 2 h, 4 h, 12 h, 24 h, and 72 h) by western blot. The band gray value was highest at 4 h, and lower at both ends. The relative gene expression was indicated by the Wnt5b protein band gray value or β-actin band gray value calculated using western blot. The relative expression curve was a normal distribution line; B: Nanog expression in cell line HCCLM3 treated with ADM. The expression of Nanog was measured at different time intervals (0 h, 2 h, 4 h, 12 h, 24 h, and 72 h) by western blot. The band gray value was lowest at 4 h and highest at 72 h. The relative gene expression was indicated by the Nanog protein band gray value or β-actin band gray value calculated using western blot. The relative expression curve was a parabola, but was more concave than that in MHCC97-L cells; C: Sox2 expression in cell line HCCLM3 treated with ADM. The expression of Nanog was measured at different time intervals (0 h, 2 h, 4 h, 12 h, 24 h, and 72 h) by western blot. The band gray value increased with time and was highest at 72 h. The relative gene expression was indicated by the Nanog protein band gray value or β-actin band gray value calculated using western blot. The relative gene expression correlated positively with time.
Relative expressions were calculated as mentioned above. There was a statistically significant difference between the expression changes of Sox2, Wnt5b, and Nanog, caused by ADM in HCCLM3 cells (P < 0.05). However, ARID1A and Oct4 were not expressed in these cells.
Difference in expression changes of stem-cell genes in the hepatoma cell lines with different metastatic potential following treatment with ADM: There was a difference between the expression changes of stem-cell genes in the hepatoma cell lines with different metastatic potential within 4 h following treatment with ADM (LD50). Interestingly, the expression changes of Wnt5 and Nanog in cell line MHCC97-L were smaller than those in cell line HCCLM 3, and the difference was statistically significant (P < 0.05).
DISCUSSION
To our knowledge, this may be the first study to report the gene expression of Nanog, Wnt5b, Oct4 and Sox2, and ARID1A comprehensively and systematically in two hepatoma cell lines. This was carried out to explore the molecular mechanism of the recurrence and metastasis of HCC, and to detect the accurate markers and targets for stem cell-targeted therapeutic interventions.
Study background
The concept of CSCs was put forward by Reya in 2001. Due to their characteristics, they have a natural resistance to drugs, consequently, leading to tumor recurrence and metastasis after chemotherapy[12]. As a broad-spectrum anticancer drug, ADM, can induce apoptosis of tumor cells by interfering with the transcription process[13]. However, most of the traditional chemotherapy agents, including ADM, can only kill cells in the proliferating period. Therefore, it reduces the tumor volume or can cause complete remission, but cannot eradicate CSCs. This can lead to a series of stress protective reactions thus the cells become resistant to drugs. This study evaluated the relationship between HCC recurrence, multidrug resistance, and CSCs by evaluating the influence of ADM on CSCs-related gene expression in hepatoma cell lines with different metastatic potential.
Study findings
ADM significantly inhibited the growth of hepatoma cells with different metastatic potential. Moreover, the LD50 of ADM in the hepatoma cell line with high metastatic potential was higher than that in the cell line with low metastatic potential. According to clinical findings, when recurrence and metastasis occur at an early stage, HCC patients tend to show higher resistance to drugs. ADM at < 0.625 μmol/L showed stronger inhibition of the HCCLM3 cell line than the MHCC97-L cell line, without an apparent killing effect; and with increased concentration (> 1.25 μmol/L), the inhibitory action of ADM increased. Consistently, the HCCLM3 cell line with higher metastatic potential showed stronger resistance to drugs than the MHCC97-L cell line. While ADM inhibited cancer cell growth, it led to stimulation of CSCs. Thus, the degree of drug resistance increased. As demonstrated by Ma et al[14], cultured hepatoma cells treated with ADM and fluorouracil showed enhanced expression of survival proteins in stem-cell related pathways; suggesting the possible correlation of CSCs with drug resistance.
This study determined the expression of Wnt5b, Nanog, Sox2, ARID1A and Oct4 at different time intervals in two cell line models treated with ADM. Wnt5 was expressed in HCC cell lines MHCC97-L and HCCLM3. Wnt5 plays a critical role in embryonic development by regulating cell growth, differentiation, and development[15]. Abnormal expression of the Wnt signal was detected in more than 30% of HCCs, which highlighted its role in carcinogenesis. Mutations in the Wnt signaling pathways can result in appropriate activation, which may be related to tumor occurrence and development[16]. Research has shown that the expression of Wnt5b in normal liver tissues was higher than that in adjacent cancer tissues and HCC tissue; and the expression in adjacent cancer tissues was highest[17]. In this study, the expression of Wnt5 initially increased, then decreased in both cell lines following treatment with ADM (LD50). Also, the Wnt5 expression level in cell line HCCLM3 was lower than that in cell line MHCC97-L. Yuan et al[18] also observed similar findings. Thus, it can be speculated that Wnt5 was negatively related with the malignancy of liver cancer cells.
Nanog was discovered in May, 2003. It plays a key role in stem-cell totipotency in the embryo. In addition, it is expressed in embryonic tumor cells and ESCs[19-22], but not in differentiated ESCs[23]. There are no reports on the expression of Nanog in differentiated cells. Therefore, there should be at least some undifferentiated cells in HCC, as the expression of Nanog was detected in both cell lines. In addition, the expression is thought to be related to the transformation of somatic cells to cancer cells, as found by Lin[24] and Piestun[25]. In the present study of two hepatoma cell models, Nanog expression decreased gradually in a short time period (T < 4 h) due to ADM (LD50), and later increased (T > 4 h). Based on this phenomenon, Nanog is thought to be an important factor that could set the cell state during turbulent periods. Moreover, ADM can induce the transformation of hepatic cancer cells from differentiated to undifferentiated states. By keeping these undifferentiated cells in the stationary phase, ADM promoted tumor cells to avoid the killing effect of chemotherapeutic drugs. In conclusion, the expression of Nanog was related to tumor recurrence or distant metastasis.
The expression patterns of Sox2, ARID1A, and Oct4 were also studied. Sox2 expression was found only in cell line HCCLM3 which has high metastatic potential, and both ARID1A and Oct4 were not expressed in these two cell models. Sox2 is a member of the SOX family, which may be involved in a variety of solid tumors[26-29]. The abnormal expression of Sox2 in breast cancer[30], hypopharyngeal squamous cell carcinoma[30], lung adenocarcinoma[31], ovarian cancer[32] and other diseases, has been detected in recent years. In this study, Sox2 expression was only detected in the HCC cell line HCCLM3 with high metastatic potential, and the level of expression was time-dependent. Other studies have reported similar results[32-35]. It is suggested that the higher the expression of Sox2, the higher the tumor malignancy; and high expression of Sox2 may involve liver cell carcinogenesis. Sox2 expression level, and the invasion and metastasis of HCC may be interrelated.
As an embryonic stem cell specific gene, Oct4 is mainly expressed in embryo and germ cell tumors. Although, some researchers have detected its expression in the HCC cell line, Mahlava, it was not observed in this study. Further study is required to determine the relationship between Oct4 and the occurrence and development of HCC.
Problems and prospects of the study
The correlation between Nanog and Wnt5b should be explored as both were expressed in the HCC cell lines, MHCC97-L and HCCLM3, at the same time, but their expression was negatively correlated. However, research on the interaction between these two factors would be very costly, both in time and funding. Further study will be initiated, when conditions permit.
In conclusion, ADM increased the death rate of MHCC97-L and HCCLM3 cells, while the suppressive effect of ADM was higher in MHCC97-L cells than in HCCLM3 cells. In addition, a negative correlation between Wnt5b and the malignancy rate of liver cancer stem cells was found. Also, Sox2 was expressed only in the cell line with high metastatic potential, and was combined with Nanog. Sox2 expression increased gradually, which indicates a potential relationship between the metastatic capability of liver cancer and drug resistance.
COMMENTS
Background
There are no recognized markers for liver cancer stem cells. However, liver cancer stem cells play an important role in cancer development, metastasis, recurrence, and multidrug resistance. Therefore, research on liver cancer stem cells are mainly focused on identifying markers.
Research frontiers
To date, the expression changes of some stem-cell related genes have been detected as the effect of an anti-cancer drug. However, the numbers of observed genes were limited. In order to study the mechanism of the genes related to hepatocellular carcinoma (HCC), more genes should be studied to understand the full mechanism of HCC.
Innovations and breakthroughs
This study reported the gene expression of Nanog, Wnt5b, Oct4, Sox2, and ARID1A comprehensively and systematically in two hepatoma cell lines. This was performed to explore the molecular mechanism of recurrence and metastasis of HCC and detect the accurate markers and targets of stem cell-targeted therapeutic interventions.
Applications
It is of benefit to identify the accurate markers and targets for stem cell-targeted therapeutic intervention.
Peer review
The manuscript untitled “Adriamycin influence on changes of Nanog, Oct-4, Sox2, ARID1 and Wnt5b expression in cancer stem cell” is very well written. In this study, the authors reported genes expression of Nanog, Wnt5b, Oct4 and Sox2 and ARID1A in two hepatoma cell lines comprehensively and systematically in order to explore the molecular mechanism of recurrence and metastasis of hepatocellular carcinoma.
Footnotes
Supported by National Natural Science Foundation, No. 81372317
P- Reviewer: Cao L S- Editor: Qi Y L- Editor: Webster JR E- Editor: Liu XM
References
- 1.Tsim NC, Frampton AE, Habib NA, Jiao LR. Surgical treatment for liver cancer. World J Gastroenterol. 2010;16:927–933. doi: 10.3748/wjg.v16.i8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene CM, Varley RB, Lawless MW. MicroRNAs and liver cancer associated with iron overload: therapeutic targets unravelled. World J Gastroenterol. 2013;19:5212–5226. doi: 10.3748/wjg.v19.i32.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadri S, Soleimani M, Kiani J, Atashi A, Izadpanah R. Multipotent mesenchymal stem cells from adult human eye conjunctiva stromal cells. Differentiation. 2008;76:223–231. doi: 10.1111/j.1432-0436.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samartzis EP, Noske A, Dedes KJ, Fink D, Imesch P. ARID1A mutations and PI3K/AKT pathway alterations in endometriosis and endometriosis-associated ovarian carcinomas. Int J Mol Sci. 2013;14:18824–18849. doi: 10.3390/ijms140918824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones S, Wang TL, Shih IeM, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA, Vogelstein B, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, Wu R, Chen C, Li X, Zhou L, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet. 2011;43:875–878. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, Chan TL, Kan Z, Chan AS, Tsui WY, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43:1219–1223. doi: 10.1038/ng.982. [DOI] [PubMed] [Google Scholar]
- 11.Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, et al. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433–10444. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- 12.Zhou WL, Medine CN, Zhu L, Hay DC. Stem cell differentiation and human liver disease. World J Gastroenterol. 2012;18:2018–2025. doi: 10.3748/wjg.v18.i17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bessone F. Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage? World J Gastroenterol. 2010;16:5651–5661. doi: 10.3748/wjg.v16.i45.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncoqene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 15.Boyault S, Rickman DS, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, Hérault A, Saric J, Belghiti J, Franco D, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 16.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 17.Yuan F, Zhou W, Zou C, Zhang Z, Hu H, Dai Z, Zhang Y. Expression of Oct4 in HCC and modulation to wnt/β-catenin and TGF-β signal pathways. Mol Cell Biochem. 2010;343:155–162. doi: 10.1007/s11010-010-0509-3. [DOI] [PubMed] [Google Scholar]
- 18.Yuan F, Zhou W, Zou C, Zhang Z, Hu H, Dai Z, Zhang Y. Expression of Oct4 in HCC and modulation to wnt/β-catenin and TGF-β signal pathways. Mol Cell Biochem. 2010;343:155–162. doi: 10.1007/s11010-010-0509-3. [DOI] [PubMed] [Google Scholar]
- 19.Hart AH, Hartley L, Parker K, Ibrahim M, Looijenga LH, Pauchnik M, Chow CW, Robb L. The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer. 2005;104:2092–2098. doi: 10.1002/cncr.21435. [DOI] [PubMed] [Google Scholar]
- 20.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 21.Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14:4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 22.Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 23.Wu da Y, Yao Z. Isolation and characterization of the murine Nanog gene promoter. Cell Res. 2005;15:317–324. doi: 10.1038/sj.cr.7290300. [DOI] [PubMed] [Google Scholar]
- 24.Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- 25.Piestun D, Kochupurakkal BS, Jacob-Hirsch J, Zeligson S, Koudritsky M, Domany E, Amariglio N, Rechavi G, Givol D. Nanog transforms NIH3T3 cells and targets cell-type restricted genes. Biochem Biophys Res Commun. 2006;343:279–285. doi: 10.1016/j.bbrc.2006.02.152. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y, Sun G, Zhao C, Stewart R. Neural stem cell self-renewal. Crit Rev Oncol Hematol. 2008;65:43–53. doi: 10.1016/j.critrevonc.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, Miki C, Kusunoki M. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009;16:3488–3498. doi: 10.1245/s10434-009-0617-z. [DOI] [PubMed] [Google Scholar]
- 29.Otsubo T, Akiyama Y, Yanagihara K, Yuasa Y. SOX2 is frequently downregulated in gastric cancers and inhibits cell growth through cell-cycle arrest and apoptosis. Br J Cancer. 2008;98:824–831. doi: 10.1038/sj.bjc.6604193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lengerke C, Fehm T, Kurth R, Neubauer H, Scheble V, Müller F, Schneider F, Petersen K, Wallwiener D, Kanz L, et al. Expression of the embryonic stem cell marker SOX2 in early-stage breast carcinoma. BMC Cancer. 2011;11:42. doi: 10.1186/1471-2407-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge N, Lin HX, Xiao XS, Guo L, Xu HM, Wang X, Jin T, Cai XY, Liang Y, Hu WH, et al. Prognostic significance of Oct4 and Sox2 expression in hypopharyngeal squamous cell carcinoma. J Transl Med. 2010;8:94. doi: 10.1186/1479-5876-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sholl LM, Barletta JA, Yeap BY, Chirieac LR, Hornick JL. Sox2 protein expression is an independent poor prognostic indicator in stage I lung adenocarcinoma. Am J Surg Pathol. 2010;34:1193–1198. doi: 10.1097/PAS.0b013e3181e5e024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye F, Li Y, Hu Y, Zhou C, Hu Y, Chen H. Expression of Sox2 in human ovarian epithelial carcinoma. J Cancer Res Clin Oncol. 2011;137:131–137. doi: 10.1007/s00432-010-0867-y. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Shi L, Zhang L, Li R, Liang J, Yu W, Sun L, Yang X, Wang Y, Zhang Y, et al. The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J Biol Chem. 2008;283:17969–17978. doi: 10.1074/jbc.M802917200. [DOI] [PubMed] [Google Scholar]
- 35.Park ET, Gum JR, Kakar S, Kwon SW, Deng G, Kim YS. Aberrant expression of SOX2 upregulates MUC5AC gastric foveolar mucin in mucinous cancers of the colorectum and related lesions. Int J Cancer. 2008;122:1253–1260. doi: 10.1002/ijc.23225. [DOI] [PubMed] [Google Scholar]


