Abstract
AIM: To identify factors affecting early local recurrence after transcatheter arterial chemoembolization (TACE) and investigate treatments and outcomes for local recurrence.
METHODS: Early local recurrence and no early local recurrence groups drawn from 134 patients who were initially diagnosed with hepatocellular carcinoma (HCC) and showed a complete response (CR) to TACE treatment between January 1, 2006, and January 31, 2012, were analyzed by univariate and multivariate analyses. Additionally, the subsequent treatment for patients with recurrence was analyzed, and in cases in which TACE had been performed, the cumulative recurrence rates were calculated using the Kaplan-Meier method and compared with those of the primary lesion.
RESULTS: The 1-, 2-, and 3-year survival rates were 92.3%, 60.2%, and 39.8%, respectively, in the early local recurrence group, which were significantly lower than those in both the late local and no local recurrence groups (P < 0.001). On multivariate analyses, non-compact lipiodol uptake, large tumor size, and an alpha-fetoprotein > 20 ng/mL after achieving a CR were significant predictors. When TACE was performed for early and late locally recurrent lesions, a CR was observed in 15 patients (41.7%) and 11 patients (78.6%), and the cumulative recurrence rates at 6, 12, and 24 mo were 17.9%, 43.3%, and 71.2%, respectively, which did not differ significantly from those after the first CR of 20.5%, 44.0%, and 58.6%, respectively (P = 0.639).
CONCLUSION: Closer monitoring and active treatments must be provided to patients with risk factors for early local recurrence of HCC.
Keywords: Chemoembolization, Hepatocellular carcinoma, Recurrence, Survival, Lipiodol
Core tip: We analyzed the risk factors for early local recurrence following transcatheter arterial chemoembolization (TACE) and investigated the treatment method and therapeutic results for local recurrence. This study showed that patients with early local recurrence had significantly lower survival rates and unfavorable treatment responses to repetitive TACE. Therefore, careful follow-up is believed to be necessary in patients exhibiting risk factors for early local recurrence, such as non-compact lipiodol uptake, large tumors, and an alpha-fetoprotein level > 20 ng/mL. Moreover, there was a small chance of a complete response after TACE, and considering other treatments or combining multiple treatments would therefore be advisable.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and has the third highest mortality rate[1]. Curative treatments for HCC include surgical resection and radiofrequency ablation (RFA), but > 50% of cases are not discovered until the disease has reached the incurable stage[2]. The results of randomized controlled trials show that transcatheter arterial chemoembolization (TACE) increases survival rates in patients who are ineligible for curative treatments[3,4]. In some studies, survival rates similar to those of resection were reported when the lipiodol used in the TACE procedure was kept compact, even in cases in which surgical resection was feasible[5].
However, studies have shown that intrahepatic local or distant recurrence commonly occurs after the achievement of complete response (CR). Active treatment for recurrent lesions improves survival rates, but the prognosis is generally unfavorable[6-9]. Therefore, the early detection of recurrences and proper treatment are important for improving survival rates. Local recurrence and intrahepatic distant recurrence exhibit different risk factors and characteristics[10]. Intrahepatic distant recurrence appears to develop in the presence of cirrhosis, whereas local recurrence is known to be influenced by insufficient treatment and tumor characteristics at the time of diagnosis[8,10,11]. Thus, local and intrahepatic distant recurrences should be analyzed separately; however, intrahepatic distant recurrent lesions have been evaluated simultaneously in most studies[7,10], and few cases have demonstrated the treatment effects on local recurrence.
Therefore, this study aimed to assess the risk factors and survival outcomes for early local recurrence after TACE and to investigate the treatment methods for local recurrence and the therapeutic results.
MATERIALS AND METHODS
Subjects
A total of 793 patients were first diagnosed with HCC at Chungnam National University Hospital from January 2007 to January 2012. When curative treatments (liver transplantation, resection, RFA) were difficult to perform due to tumor characteristics, indocyanine green test results, decompensated liver function, jaundice, performance status, or age, or the patients rejected them, even though the patients were satisfied with those conditions, TACE was considered the primary treatment.
Of these 793 patients, 238 subjects received TACE as a primary treatment method in our hospital. Among them, excluding the patients who had other uncontrolled malignancies or who had delayed a procedure by more than 3 mo after the diagnosis of HCC, 148 subjects showed a CR, whereas 14 subjects showed a CR with other methods such as RFA or resection; the latter were thereby excluded from this study because they did not fit the focus of this study. As such, 134 subjects ultimately participated in this study (Figure 1).
Figure 1.
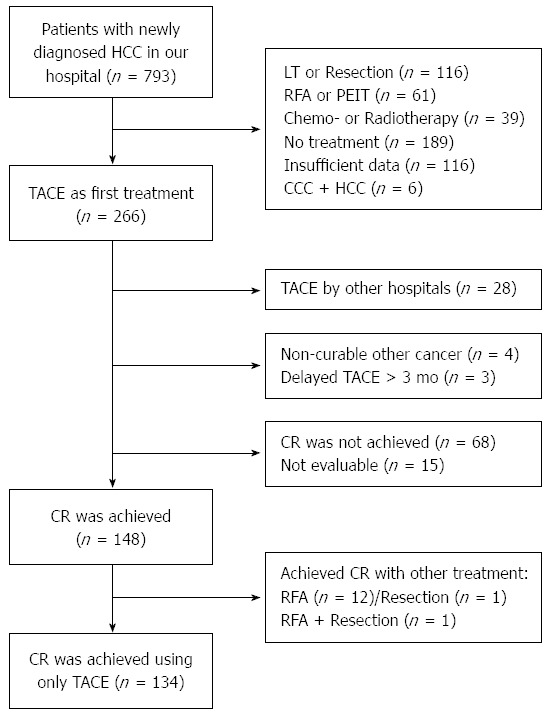
Flow chart summarizing patient outcomes. A: Insufficient data due to transfer to other hospital or loss to follow-up; B: TACE was delayed more than 3 mo from the time of diagnosis. LT: Liver transplantation; PEIT: Percutaneous ethanol injection therapy; CCC: Cholangiocarcinoma; TACE: Transcatheter arterial chemoembolization.
HCC is diagnosed on the basis of histopathological findings or blood alpha-fetoprotein (AFP) levels and radiologic evaluations satisfying the diagnostic criteria of the American Association for the Study of Liver Diseases[12]. This study was approved by the Institutional Review Board at Chungnam National University Hospital, Daejeon, South Korea.
Procedure of TACE
TACE was performed by two experienced interventional radiologists. First, portal circulation was evaluated using superior mesenteric angiography with 5-French RH (COOK, Bloomington, IN, United States) or Yashiro (Terumo, Tokyo, Japan) angiocatheters. Hepatic angiography was then implemented, utilizing a coaxial microcatheter (Microferret; COOK; or Progreat; Terumo, Tokyo, Japan or Renegade; Boston Scientific, Watertown, MA, United States). The feeding artery of the hepatic tumors was found, and the catheter was advanced to the distal area. Doxorubicin hydrochloride emulsion (Adriamycin-RDF; Ildong Pharm. Co. Ltd., Seoul, Korea) and lipiodol (Lipiodol Ultra-Fluide; Guerbet, Paris, France) were injected, and embolization was performed using gelatin sponge particles (Cutanplast; Mascia Bruneli Spa, Milano, Italy) or polyvinyl alcohol (Contour; Boston Scientific Corp., Natick, MA, United States).
Definition of CR
Contrast-enhanced dynamic computed tomography (CT) or magnetic resonance imaging was performed 1-3 mo after TACE. In the hypervascular tumors, the absence of contrast enhancement of all intra-hepatic lesions in the arterial phase was defined as a CR. For the hypovascular tumors, however, compact lipiodol uptake without any defect observed was defined as a CR[13]. If contrast enhancements remained after TACE or hypovascular tumors without compact lipiodol uptake remained, TACE was performed repeatedly until a CR was achieved. Local recurrence was observed only in patients who achieved a CR. Portal vein thrombosis (PVT) and extra-hepatic lesions rarely disappeared after TACE and were excluded from the criteria of CR evaluation to assess its effects on local recurrence.
In particular, the presence of lipiodol intake > 75% with 200 Hounsfield units on pre-contrast CT images was defined as compact uptake[8,14].
Treatment responses and follow-up
Patients identified as achieving a CR underwent examinations using the same methods every 3 mo. With regard to lesions in which a CR was noted, the new detection of contrast-enhanced lesions in the arterial phase, contrast-lost lesions in the portal and venous phase at the tumor margin, or an increase in lesion size after CR was achieved were considered local recurrences[7,8,15-17]. According to the duration from the first CR to recurrence, local recurrence within 1 year and local recurrence after 1 year were defined as early local recurrence and late local recurrence, respectively[10,18].
In cases of local recurrence, treatment methods were analyzed after dividing the patients into early and late local recurrence groups. After 1-3 mo, cases in which the contrast-enhanced lesions in the arterial phase in the recurrence area disappeared on radiological evaluation were considered to have achieved a second CR. This study aimed to analyze the effects of locally recurrent lesions, and the treatment outcomes of distant intra-hepatic recurrence observed in the follow-up after the CR were therefore excluded from the criteria of a second CR. However, the frequency of distant intra-hepatic recurrence is described separately.
Patients lost to follow-up within 1 year and those who died were excluded from the analyses of survival rates and risk factors in the early and late recurrence groups, as the presence of early local recurrence could not be determined. The reasons for this are reported.
Complications
We retrospectively reviewed medical records and follow-up radiological findings for evidence of acute TACE-related complications for 238 patients (for a total of 523 TACE procedures), including non-CR patients.
Post-embolization syndrome was defined as the presence of fever (≥ 38.0 °C), nausea, vomiting, the need for the administration of analgesics, or the occurrence of abdominal pain with a visual analogue scale (VAS) score ≥ 4 points. We used the VAS to evaluate abdominal pain by indicating a position along a continuous line between two end points (0 = no pain, 10 = maximum pain).
Hepatic failure after TACE was identified when at least one of the following conditions was met within 2 wk after TACE: hepatic encephalopathy or ascites was newly found; ascites was significantly increased; the Child-Pugh score was increased by more than 2 points; the overall serum bilirubin level was elevated by more than twice the normal level if the pre-TACE level was normal or the baseline level if the pre-TACE level was abnormal; or the prothrombin time was increased by > 3 s compared with the baseline value[19-22].
Statistical analysis
The patient clinical characteristics are expressed as the mean and median. To compare the early and no early local recurrence groups, significant factors were evaluated by univariate and multivariate analyses through binary logistic regression. The Kaplan-Meier method was used in the analyses of cumulative survival rates and cumulative recurrence rates between groups, and the differences between them were compared using a log-rank test. To compare the TACE treatment results between cases with a first CR and cases with a second CR after additional TACE was performed because of the first local recurrence, the cumulative recurrence rate was calculated, and differences between groups were analyzed using the log-rank test. Statistical analyses were performed using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, United States). P values < 0.05 were considered statistically significant.
RESULTS
Patient clinical characteristics
The mean patient age was 63.1 years, and 89 (66.4%) of the patients were men. Viral hepatitis B infection was the most frequent cause of HCC (73 subjects, 54.5%), followed by viral hepatitis C infection (24 subjects, 17.9%) and alcohol consumption (22 subjects, 16.4%). According to the Child-Pugh classification, hepatic function was classified as class A in 93 patients (69.4%), as class B in 39 subjects (29.1%), and as class C in two subjects (1.5%). Regarding the tumor characteristics, 88 (65.7%) and 46 (34.3%) patients had solitary and multiple lesions, respectively, and the median tumor diameter was 2.6 cm (range, 1.0-12.7 cm). Nine patients (6.7%) had concomitant PVT.
A total of 107 (79.9%), 19 (14.2%), 5 (3.7%), and 3 (2.2%) cases achieved a CR after one, two, three, and four rounds of TACE, respectively (Table 1).
Table 1.
Baseline patient characteristics n (%)
| Characteristic | Total patients, n = 134 |
| Male sex | 89 (66.4) |
| Age, yr, mean ± SD | 63.1 ± 10.4 |
| Etiology of primary hepatic tumor | |
| Viral hepatitis B infection | 73 (54.5) |
| Viral hepatitis C infection | 24 (17.9) |
| Alcohol consumption | 22 (16.4) |
| Others | 9 (6.7) |
| Viral infection + alcohol consumption | 6 (4.5) |
| Decompensated cirrhosis | 51 (38.1) |
| Child-Pugh class (A/B/C) | 93 (69.4)/39 (29.1)/2 (1.5) |
| Portal hypertension (present) | 93 (69.4) |
| Tumor related characteristics | |
| Tumor maximal diameter, median (range) | 2.6 (1.0-12.7) |
| Tumor number (single/multiple) | 88 (65.7)/46 (34.3) |
| Portal vein thrombosis (present) | 9 (6.7) |
| Well defined/infiltrating | 131 (97.8)/3 (2.2) |
| Ruptured HCC | 4 (3.0) |
| Tumor staging (AJCC 7th edition) | |
| Stage I | 84 (62.7) |
| Stage II | 36 (26.9) |
| Stage IIIA/IIIB/IIIC | 7 (5.2)/5 (3.7)/- |
| Stage IVA/IVB | -/2 (1.5) |
| Laboratory finding | |
| Total bilirubin, mg/dL, median (range) | 0.90 (0.20-5.20) |
| Prothrombin time, s, mean ± SD | 13.41 ± 2.10 |
| Albumin, mg/dL, mean ± SD | 3.61 ± 0.53 |
| TACE no. to CR (1/2/3/≥ 4) | 107/19/5/3 (79.9/14.2/3.7/2.2) |
AJCC: American Joint Committee on Cancer; HCC: Hepatocellular carcinoma; SD: Standard deviation; TACE: Transcatheter arterial chemoembolization; CR: Complete response.
Local recurrence and treatment methods after the first CR
A CR was noted in a total of 134 subjects, and the mean follow-up duration was 26.4 months (range, 2.3-66.0 mo). Among these subjects, early local recurrence was found in 52 subjects, late local recurrence was observed in 19 subjects, and no detectable local recurrence by the last examination was noted in 63 subjects. Among the 52 patients with early local recurrence, TACE was performed on 36 (69.2%); of those, a CR was achieved a second time in 15 subjects (41.7%). Among the 19 patients with late local recurrence, TACE was performed on 14 subjects (73.7%); of them, a CR was noted in 11 subjects (78.6%) (Figure 2).
Figure 2.
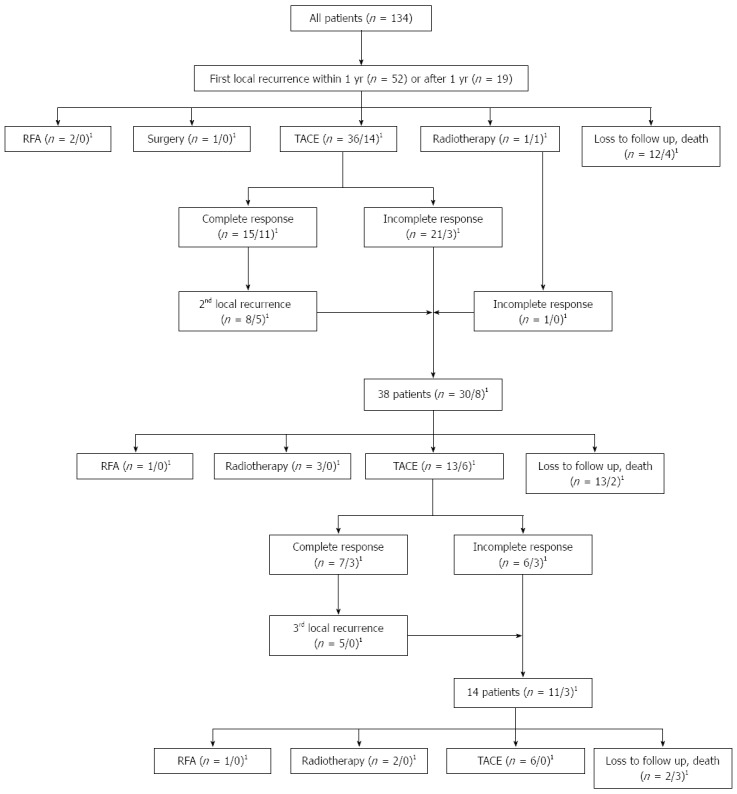
Flow chart shows the first, second, and third local recurrences and the following treatments. 1n = patients with local recurrence within 1 year/local recurrence after 1 year.
RFA, resection, and radiotherapy were employed in two, one, and two patients with early local recurrence, respectively. Among the patients who received RFA, one had 1.7-cm and 1.4-cm hypervascular tumors, and the other had a 3.1-cm hypovascular tumor and a 1.0-cm hypervascular tumor at the time of diagnosis. RFA was performed for the 1.4-cm and 3.1-cm locally recurrent tumors. Recurrence has not been observed since that time.
The patient who underwent resection showed decompensated cirrhosis with a 1.2-cm hypervascular tumor at the time of diagnosis, and TACE was performed because the lesions were not clearly visible on ultrasonography. Wedge resection was then performed for the treatment of local recurrence, and no recurrence has been noted since.
Radiotherapy was employed in two patients. The first patient’s condition has been stable for 1.5 years. However, the disease progressed in the other patient and resulted in an incomplete response after additional TACE. Since then, that patient has received conservative treatment after chemotherapy.
Comparison of early and late locally recurrent lesions in patients treated with TACE when local recurrence was detected
Comparing the factors that could possibly affect the TACE therapeutic result, no significant differences were observed in the number of lesions, median maximal tumor diameter by procedure, Child-Pugh class, age, sex, PVT incidence, lipiodol uptake, post-CR AFP, or the presence of decompensated liver cirrhosis at the time of treatment. A total of 19 of 36 patients in the early recurrence group had a maximum recurrent lesion diameter > 2 cm, a proportion that was significantly larger than that in the late recurrence group (3/14) (P = 0.045, Pearson’s χ2 test).
Comparison of cumulative survival rates in the early local recurrence group
The overall survival rates of the 134 patients were 93.0%, 77.0%, 62.3%, and 32.2% at 1, 2, 3, and 5 years, respectively. A total of 117 subjects, except for 17 patients who died or were lost to follow-up within 1 year, were analyzed following their classification into the early local recurrence, late local recurrence, and no local recurrence groups. Accordingly, the early local recurrence group showed 1-, 2-, 3-, and 5-year survival rates of 92.3%, 60.2%, 39.8%, and 26.6%, respectively; the late local recurrence group showed rates of 100.0%, 94.4%, 94.4%, and 41.3%, respectively; and the no local recurrence group had rates of 100.0%, 95.4%, 77.8%, and 41.3%, respectively (Figure 3).
Figure 3.
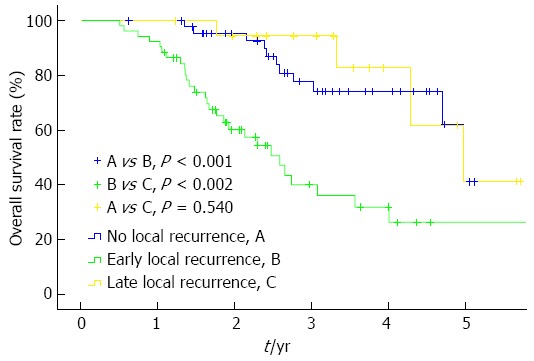
Overall survival rates according to the timing of local recurrence. The 1-, 2-, 3-, and 5-year survival rates were 92.3%, 60.2%, 39.8%, and 26.6%, respectively, which were significantly lower than those following both the late local recurrence and no local recurrence (Kaplan-Meier method, log-rank test, overall P < 0.001).
Nine patients died within 1 year. Among them, five died due to hepatic dysfunction and progression of the hepatic tumor (hepatic failure, two; hepatorenal syndrome, one; hepatic tumor, one; unknown, one), whereas four patients died of other conditions (cerebral hemorrhage, one; tension pneumothorax, one; pneumonia, two). Five patients were lost to follow-up. The other three patients underwent resection or liver transplantation within 1 year, and the embolized lesions were removed; because the presence of recurrence could not be confirmed in these patients, they were excluded from the analysis.
Analyses of risk factors involved in early local recurrence
Univariate analysis of the 12 factors showed that sex (P = 0.042), multiple tumors (P = 0.015), non-compact lipiodol uptake (P < 0.001), tumor size 2-5 and > 5 cm (P = 0.010 and 0.001, respectively), an AFP level > 20 ng/mL after a CR was achieved (P = 0.044), and more than 2 rounds of TACE before the achievement of a CR (P = 0.030) were statistically significant for early local recurrence.
When factors significant in the univariate analysis were entered into multivariate analyses, non-compact lipiodol uptake (OR = 9.092; P = 0.001), tumor sizes of 2-5 and > 5 cm (OR = 3.911 and 5.218; P = 0.012 and 0.043, respectively), and an AFP level > 20 ng/mL after CR achievement (OR = 3.464, P = 0.016) showed a significant independent relationship with early local recurrence (Table 2). All eight patients with PVT manifested early local recurrence, indicating a statistically significant difference; however, multivariate analysis and OR analysis were not performed (P = 0.001, Fisher’s exact test).
Table 2.
Univariate and multivariate analysis of factors significantly predictive of early local recurrence after achieving a complete response
| Variable |
Univariate analysis |
Multivariate analysis |
||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age (≥ 65 yr) | 0.489 | 0.233-1.026 | 0.058 | - | - | - |
| Sex (female) | 2.239 | 1.028-4.876 | 0.042 | 1.969 | 0.704-5.507 | 0.197 |
| Tumor number, multiple | 2.614 | 1.203-5.680 | 0.015 | 2.171 | 0.784-6.012 | 0.136 |
| Tumor size (cm) | ||||||
| 2-5 | 3.107 | 1.318-7.324 | 0.010 | 3.911 | 1.344-11.383 | 0.012 |
| > 5 | 9.000 | 2.401-33.738 | 0.001 | 5.218 | 1.056-25.767 | 0.043 |
| Child-Pugh score (≥ 8) | 2.773 | 0.785-9.788 | 0.113 | - | - | - |
| Decompensated LC | 0.830 | 0.385-1.788 | 0.634 | - | - | - |
| Gelfoam or PVA use | 1.487 | 0.662-3.340 | 0.336 | - | - | - |
| Bilirubin (> 2.0 mg/dL) | 3.351 | 0.623-18.030 | 0.159 | - | - | - |
| Albumin (< 3.5 mg/dL) | 1.550 | 0.732-3.284 | 0.252 | - | - | - |
| Post-CR AFP (> 20 ng/mL) | 2.362 | 1.023-5.456 | 0.044 | 3.464 | 1.262-9.507 | 0.016 |
| Lipiodol uptake (non-compact) | 9.342 | 2.916-29.929 | < 0.001 | 9.092 | 2.537-32.508 | 0.001 |
| TACE number to CR (≥ 2) | 2.765 | 1.105-6.924 | 0.030 | 2.029 | 0.715-5.756 | 0.715 |
AFP: α-fetoprotein; CR: Complete response; OR: Odds ratio; LC: Liver cirrhosis; PVA: Polyvinyl alcohol; TACE: Transcatheter arterial chemoembolization; PVT: Portal vein thrombosis.
Effects of TACE on local recurrence area
The cumulative local recurrence rates after the first CR were 20.5%, 44.0%, 58.6% and 64.3% at 6, 12, 24 and 36 mo, respectively. When TACE was performed again in the local recurrence area, the cumulative local recurrence rates after the second CR at 6, 12, 24 and 36 mo were 17.9%, 43.3%, 71.2% and 71.2%, respectively. The local recurrence rate tended to be similar until 12 mo. After the second CR, however, late local recurrence was observed more frequently than the first CR over time, but the difference was not statistically significant (Figure 4).
Figure 4.
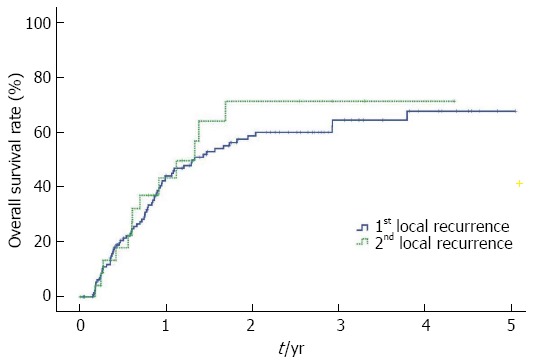
Cumulative local recurrence rates were not significantly different between the first and second complete responses (Kaplan-Meier method, log-rank test, P = 0.639).
Frequency of distant intra-hepatic recurrence
The cumulative distant intra-hepatic recurrence rates of the 134 patients were 15.4%, 38.4%, 59.4%, and 65.3% at 6, 12, 24 and 36 mo, respectively. The early local recurrence group showed 6-, 12-, 24- and 36-mo cumulative distant intra-hepatic recurrence rates of 21.2%, 52.7%, 69.7% and 75.8%, respectively; the late local recurrence group showed rates of 15.8%, 26.3%, 59.1% and 65.9%, respectively; and the no local recurrence group had rates of 8.7%, 28.3%, 48.2% and 54.2%, respectively (Figure 5).
Figure 5.
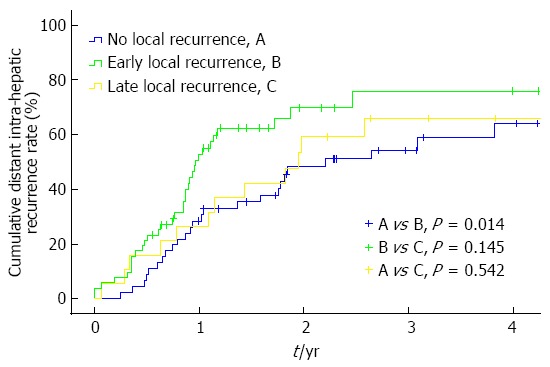
Cumulative distant intra-hepatic recurrence rates showed a significantly high frequency in the early local recurrence group compared with the no local recurrence group. However, no statistically significant difference was observed compared with the late local recurrence group (Kaplan-Meier method, log-rank test, P = 0.034 overall).
Complications
Complications, including lack of a CR, were assessed for all 523 TACEs (in the 238 patients) performed during the corresponding period (Table 3). Post-embolization syndrome occurred in 434 cases (83.0%). Acute hepatic failure was concomitant in 110 cases (21.0%); of these, recovery was not noted until the next TACE in 19 cases (3.6%). Biloma occurred in six cases (1.1%); an infected biloma was suspected in 3 cases and improved after percutaneous catheter drainage and antibiotic treatments. A liver abscess was noted in five cases (1.0%). All five of those patients received antibiotic treatments for 4-6 wk, and percutaneous catheter drainage was performed in one case. One patient who was incapable of undergoing percutaneous catheter drainage due to a low platelet count died of sepsis. There were 11 cases (2.1%) of upper gastrointestinal bleeding with considerable ulcer bleeding and slightly high varix bleeding.
Table 3.
Complications from the chemoembolization procedure n (%)
| Complication | Frequency per 523 procedures |
| Postembolization syndrome | 434 (83.0) |
| Fever | 305 (58.3) |
| Abdominal pain | 326 (62.3) |
| Nausea/vomiting | 135 (25.8)/26 (5.0) |
| Acute hepatic failure | 110 (21.0) |
| Reversible/Irreversible | 91 (17.4)/19 (3.6) |
| Biloma | 6 (1.1) |
| Liver abscess | 5 (1.0) |
| Septicemia | 6 (1.1) |
| Acalculous cholecystitis | 2 (0.4) |
| Diarrhea, simple/infectious (including PMC) | 22 (4.2)/3 (0.6) |
| Headache | 83 (15.9) |
| Dizziness | 15 (2.9) |
| Itching sense | 24 (4.6) |
| Acute urticaria, drug eruption, skin rash | 6 (1.1) |
| Local bleeding (including rebleeding, hematoma) | 5 (1.0) |
| Upper GI bleeding, total | 11 (2.1) |
| Esophageal varix bleeding | 4 (0.8) |
| Gastric varix bleeding | 2 (0.4) |
| Ulcer bleeding | 4 (0.8) |
| Mallory-Weiss tear | 1 (0.2) |
| Others | |
| Splenic infarction | 1 (0.2) |
| Stress-induced cardiomyopathy | 1 (0.2) |
| Paralytic ileus | 1 (0.2) |
| Skin necrosis | 1 (0.2) |
PMC: Pseudomembranous colitis; GI: Gastrointestinal.
DISCUSSION
Studies have reported that patients who achieved a CR that occurred after TACE had 1- and 3-year survival rates of 96%-99% and 60%-64%, respectively[6,9]. Studies have also shown that the 3- and 5-year survival rates, except in patients with Child-Pugh C class or PVT, were 92% and 62%, respectively[10,18]. In the present study, the 1-, 3-, and 5-year survival rates of patients who achieved a CR were 93.0%, 62.3%, and 32.2%, respectively, which were not remarkably different from the rates reported by other studies.
However, the survival rate was lower than that of patients without recurrence and that of patients in need of repetitive treatments due to frequent recurrence after the procedure[9,10].
In the present study, we focused on local recurrence and found that the early local recurrence group had a significantly lower survival rate. The risk factors for local recurrence are known to be the levels of proteins induced by vitamin K absence, lipiodol uptake, and multiple tumors[8,10,18]. In this study, the risk factors of early local recurrence were non-compact lipiodol uptake, large tumor size, and an AFP level > 20 mg/mL after a CR was achieved. A few studies have reported that compact lipiodol uptake later results in complete necrosis in the resected tissues[23-26] and is known to influence cell differentiation, blood vessel distribution, and tumor size[14,26-30]. In addition, a study was published indicating that even in the presence of complete necrosis, cancer cells remaining around the fibrous capsules surrounding tumors were found in the tissues that were surgically removed[24]. The increased frequency of early local recurrence for non-compact lipiodol intake indicated the possibility of incomplete necrosis in the presence of non-compact lipiodol intake despite the observation of a CR upon radiologic evaluation.
Insufficient embolization or undetected residual lesions were assumed to be the cause of early recurrence, and undetected lesions on CT that were detected during TACE were reported to occur in 45% of cases[9,10]. In our hospital, areas of local recurrence that were invisible on CT were detected in many cases when performing TACE for other lesions. Therefore, in cases of large multiple tumors, repeating the procedures until the AFP level decrease to < 20 ng/mL after a CR is achieved is considered[10], but this is believed to require a careful approach because the AFP level may not drop immediately after the procedures and may remain elevated for other reasons.
In our study, early locally recurrent lesions had a different CR rate for subsequent TACE treatments than did late locally recurrent lesions. Comparison of the groups revealed no specific differences except for a slightly high ratio of the maximum diameter of the recurrent lesions (> 2 cm) in the early local recurrence group. Tumor size is also known to be predictive of CR after TACE[9]. However, because of the low median maximum diameter of the early recurrent lesions (2.1 cm), it is difficult to explain the result of the low CR frequency after re-treatment, and the treatment response of the early locally recurrent lesion itself may not be good. This may be because HCC consists of different cell types, both well-differentiated hepatic tumor cells that are vulnerable to hypoxia induced by TACE and undifferentiated hepatic tumor cells that are relatively resistant to hypoxia. Therefore, following TACE, the undifferentiated cells are retained, making HCC more aggressive or decreasing the treatment response to TACE[31,32]. Additionally, studies have reported that the doubling time of recurrent lesions is shorter than that of the first diagnosed HCC and that the prognosis was not good in cases with a short doubling time[17]. Therefore, it would be necessary to examine the contrast-enhancement patterns of the tumor as well as its doubling time and the differentiation of locally recurrent lesions. In cases of early recurrence, other treatments such as RFA, resection, or a combination with other treatments can be considered.
TACE was performed most commonly to re-treat recurrent lesions (65%-84%), followed by RFA (12%-19%)[10,33], likely because TACE was implemented when RFA was not feasible at the time of the procedure and because multiple hepatic tumors were detected at the same time as the local recurrence. Tezuka et al[7] performed embolization only in patients in whom local recurrence occurred after a CR was achieved and determined the recurrence-free rate; they showed that this rate was not different from the previously published recurrence-free rate after the first embolization, thereby suggesting that TACE was still effective against the recurrent lesions. Although further studies are required due to the small number of subjects in the present study, the cumulative recurrence rate between the first lesions and recurrent lesions did not differ when a CR was achieved after TACE. However, the frequency of achieving a CR was low in patients with early local recurrence, and care must therefore be taken with regard to repeated TACE for early locally recurrent lesions. In addition, because recurrence after RFA was not observed in two patients with early local recurrence, the selected patients had a small chance of recurrence due to compact lipiodol uptake by other lesions treated in the past, even in cases of small and multiple tumors.
This study has a few limitations. First, it was retrospective, and most of the recurrent lesions were cured by TACE, so other treatment methods were not usually included. Second, our findings appear to support the role of PVT as a possible risk factor of intrahepatic recurrence[9,34]; however, as early local recurrence occurred in all eight patients with PVT in this study, OR and multivariate analyses could not be performed because none of the patients with PVT at diagnosis exhibited no local recurrence after achieving a CR. Significant results may be obtained in larger studies. Third, patients who underwent lesion removal or were lost to follow-up within 1 year and those who died were excluded because the presence of early local recurrence could not be confirmed, which may have led to selection bias. However, even when the five dead patients were included in the no early local recurrence group, the survival rate was statistically higher in this group than in the early local recurrence group. Finally, because the cumulative recurrence rate of patients with a second CR after local recurrence was compared with that of all patients with the first CR, only a small number of patients were included.
In conclusion, this study showed that the early local recurrence group had a significantly lower survival rate when compared with the late local recurrence group and the no local recurrence group. Responses to TACE were examined, and risk factor analyses were performed; we found that the lesions recurring within 1 year and those recurring after 1 year were different. Therefore, it is necessary to consider the use of active follow-up and treatments in such patients and other treatment methods for recurrent lesions.
COMMENTS
Background
Transcatheter arterial chemoembolization (TACE) is the mainstay of treatment for patients with unresectable hepatocellular carcinoma and can be performed in patients in the early disease stage for which curative treatment is not feasible. Recent randomized controlled trials and a meta-analysis have shown significant beneficial effects on patient survival.
Research frontiers
Frequent tumor recurrence after complete response (CR) is associated with a worse prognosis. TACE has a higher local recurrence frequency than all other treatment methods. Therefore, the early diagnosis of recurrence and selection of the appropriate therapy are very important factors that increase patient survival rates. However, few studies to date have examined local recurrence or the efficacy of the post-recurrence treatment.
Innovations and breakthroughs
The risk of early local recurrence was high in cases of non-compact lipiodol uptake, large tumor size, and an AFP level > 20 mg/mL. The presence of an early local recurrence led to a significantly lower survival rate compared with the no early local recurrence group. In addition, lesions with early local recurrence were associated with a low likelihood of achieving a CR, even when TACE was repeated. This is the first study to mention the importance of early local recurrence.
Applications
Based on the results of this study, more active follow-up is needed for patients at high risk of local recurrence. In addition, considering other treatment methods or combining current treatments with those for early local recurrence would be advisable.
Terminology
Recurrent lesions are classified by the timing of recurrence and the distance from primary lesions. Recurrence within 1 year after a CR is achieved and that after 1 year are categorized as early recurrence and late recurrence, respectively. Depending on the distance from the lesion, recurrent lesions are classified as local recurrence and intrahepatic distant recurrence. The definitions differ slightly among studies, but local recurrence is most often defined as either a recurrence bordering the initial lesion site or within 2 cm of the initial lesion.
Peer review
The paper, “Risk factors and therapeutic results of early local recurrence after TACE” by Rou WS et al suggests that an early local recurrence after TACE is associated with a worse outcome in patients with HCC and multinodularity, the tumor size and a higher AFP level are independent factors that can predict early local recurrence.
Footnotes
P- Reviewers: Akarsu K, Kinoshita A, Kapoor S, Wei HS S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Pinter M, Hucke F, Graziadei I, Vogel W, Maieron A, Königsberg R, Stauber R, Grünberger B, Müller C, Kölblinger C, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590–599. doi: 10.1148/radiol.12111550. [DOI] [PubMed] [Google Scholar]
- 3.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 5.Lee HS, Kim KM, Yoon JH, Lee TR, Suh KS, Lee KU, Chung JW, Park JH, Kim CY. Therapeutic efficacy of transcatheter arterial chemoembolization as compared with hepatic resection in hepatocellular carcinoma patients with compensated liver function in a hepatitis B virus-endemic area: a prospective cohort study. J Clin Oncol. 2002;20:4459–4465. doi: 10.1200/JCO.2002.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Yang MJ, An SY, Moon EJ, Lee MS, Hwang JA, Cheong JY, Won JH, Kim JK, Wang HJ, Cho SW. [Comparison of radiofrequency ablation and transarterial chemoembolization for the treatment of a single hepatocellular carcinoma smaller than 4 cm] Korean J Hepatol. 2009;15:474–485. doi: 10.3350/kjhep.2009.15.4.474. [DOI] [PubMed] [Google Scholar]
- 7.Tezuka M, Hayashi K, Okada Y, Irie T, Ina H. Therapeutic results of computed-tomography-guided transcatheter arterial chemoembolization for local recurrence of hepatocellular carcinoma after initial transcatheter arterial chemoembolization: the results of 85 recurrent tumors in 35 patients. Dig Dis Sci. 2009;54:661–669. doi: 10.1007/s10620-008-0388-6. [DOI] [PubMed] [Google Scholar]
- 8.Kinugasa H, Nouso K, Takeuchi Y, Yasunaka T, Onishi H, Nakamura S, Shiraha H, Kuwaki K, Hagihara H, Ikeda F, et al. Risk factors for recurrence after transarterial chemoembolization for early-stage hepatocellular carcinoma. J Gastroenterol. 2012;47:421–426. doi: 10.1007/s00535-011-0492-9. [DOI] [PubMed] [Google Scholar]
- 9.Lee JK, Chung YH, Song BC, Shin JW, Choi WB, Yang SH, Yoon HK, Sung KB, Lee YS, Suh DJ. Recurrences of hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2002;17:52–58. doi: 10.1046/j.1440-1746.2002.02664.x. [DOI] [PubMed] [Google Scholar]
- 10.Jin YJ, Chung YH, Kim JA, Park W, Lee D, Shim JH, Lee D, Kim KM, Lim YS, Lee HC, et al. Predisposing factors of hepatocellular carcinoma recurrence following complete remission in response to transarterial chemoembolization. Dig Dis Sci. 2013;58:1758–1765. doi: 10.1007/s10620-013-2562-8. [DOI] [PubMed] [Google Scholar]
- 11.Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, Sone Y, Toyoda H, Shimada S, Takahashi M, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87–92. doi: 10.1053/jhep.1997.v25.pm0008985270. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 13.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 14.Stefanini GF, Amorati P, Biselli M, Mucci F, Celi A, Arienti V, Roversi R, Rossi C, Re G, Gasbarrini G. Efficacy of transarterial targeted treatments on survival of patients with hepatocellular carcinoma. An Italian experience. Cancer. 1995;75:2427–2434. doi: 10.1002/1097-0142(19950515)75:10<2427::aid-cncr2820751007>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Murakami T, Ishimaru H, Sakamoto I, Uetani M, Matsuoka Y, Daikoku M, Honda S, Koshiishi T, Fujimoto T. Percutaneous radiofrequency ablation and transcatheter arterial chemoembolization for hypervascular hepatocellular carcinoma: rate and risk factors for local recurrence. Cardiovasc Intervent Radiol. 2007;30:696–704. doi: 10.1007/s00270-007-9003-z. [DOI] [PubMed] [Google Scholar]
- 16.Ishii H, Okada S, Nose H, Okusaka T, Yoshimori M, Takayama T, Kosuge T, Yamasaki S, Sakamoto M, Hirohashi S. Local recurrence of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1996;77:1792–1796. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1792::AID-CNCR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 17.Tezuka M, Hayashi K, Kubota K, Sekine S, Okada Y, Ina H, Irie T. Growth rate of locally recurrent hepatocellular carcinoma after transcatheter arterial chemoembolization: comparing the growth rate of locally recurrent tumor with that of primary hepatocellular carcinoma. Dig Dis Sci. 2007;52:783–788. doi: 10.1007/s10620-006-9537-y. [DOI] [PubMed] [Google Scholar]
- 18.Park W, Chung YH, Kim JA, Jin YJ, Lee D, Shim JH, Lee D, Kim KM, Lim YS, Lee HC, et al. Recurrences of hepatocellular carcinoma following complete remission by transarterial chemoembolization or radiofrequency therapy: Focused on the recurrence patterns. Hepatol Res. 2013;43:1304–1312. doi: 10.1111/hepr.12083. [DOI] [PubMed] [Google Scholar]
- 19.Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747–1752. doi: 10.1002/cncr.10407. [DOI] [PubMed] [Google Scholar]
- 20.Jeon SH, Park KS, Kim YH, Shin YS, Kang MK, Jang BK, Chung WJ, Cho KB, Hwang JS. [Incidence and risk factors of acute hepatic failure after transcatheter arterial chemoembolization for hepatocellular carcinoma] Korean J Gastroenterol. 2007;50:176–182. [PubMed] [Google Scholar]
- 21.Hsin IF, Hsu CY, Huang HC, Huang YH, Lin HC, Lee RC, Chiang JH, Lee FY, Huo TI, Lee SD. Liver failure after transarterial chemoembolization for patients with hepatocellular carcinoma and ascites: incidence, risk factors, and prognostic prediction. J Clin Gastroenterol. 2011;45:556–562. doi: 10.1097/MCG.0b013e318210ff17. [DOI] [PubMed] [Google Scholar]
- 22.Huang YS, Chiang JH, Wu JC, Chang FY, Lee SD. Risk of hepatic failure after transcatheter arterial chemoembolization for hepatocellular carcinoma: predictive value of the monoethylglycinexylidide test. Am J Gastroenterol. 2002;97:1223–1227. doi: 10.1111/j.1572-0241.2002.05709.x. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo N, Uchida H, Soda S, Ohshima M, Nakano H, Ohishi H, Nagano N, Kitanura I, Nishimura Y, Nishimine K, et al. Histopathological study of the resected specimens after Segmental Lp-TAE using Lipiodol mixed with anticancer agent for hepatocellular carcinoma. Anti-tumor effect and influence on non-tumor area. Acta Hepatol Jpn. 1990;31:1084–1093. [Google Scholar]
- 24.Raby N, Karani J, Michell M, Gimson A, Nunnerley H, Williams R. Lipiodol enhanced CT scanning in assessment of hepatocellular carcinoma. Clin Radiol. 1989;40:480–485. doi: 10.1016/s0009-9260(89)80254-5. [DOI] [PubMed] [Google Scholar]
- 25.Matsuo N, Uchida H, Sakaguchi H, Nishimine K, Nishimura Y, Hirohashi S, Ohishi H. Optimal lipiodol volume in transcatheter arterial chemoembolotherapy for hepatocellular carcinoma: study based on lipiodol accumulation patterns and histopathologic findings. Semin Oncol. 1997;24:S6–61-S6-S6-61-70. [PubMed] [Google Scholar]
- 26.Kwan SW, Fidelman N, Ma E, Kerlan RK, Yao FY. Imaging predictors of the response to transarterial chemoembolization in patients with hepatocellular carcinoma: a radiological-pathological correlation. Liver Transpl. 2012;18:727–736. doi: 10.1002/lt.23413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki Y, Imaoka S, Kasugai H, Fujita M, Kawamoto S, Ishiguro S, Kojima J, Ishikawa O, Ohigashi H, Furukawa H. A new approach to chemoembolization therapy for hepatoma using ethiodized oil, cisplatin, and gelatin sponge. Cancer. 1987;60:1194–1203. doi: 10.1002/1097-0142(19870915)60:6<1194::aid-cncr2820600607>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 28.Kanematsu T, Furuta T, Takenaka K, Matsumata T, Yoshida Y, Nishizaki T, Hasuo K, Sugimachi K. A 5-year experience of lipiodolization: selective regional chemotherapy for 200 patients with hepatocellular carcinoma. Hepatology. 1989;10:98–102. doi: 10.1002/hep.1840100119. [DOI] [PubMed] [Google Scholar]
- 29.Takayasu K, Shima Y, Muramatsu Y, Moriyama N, Yamada T, Makuuchi M, Hasegawa H, Hirohashi S. Hepatocellular carcinoma: treatment with intraarterial iodized oil with and without chemotherapeutic agents. Radiology. 1987;163:345–351. doi: 10.1148/radiology.163.2.3031724. [DOI] [PubMed] [Google Scholar]
- 30.Nishimine K, Uchida H, Matsuo N, Sakaguchi H, Hirohashi S, Nishimura Y, Guo Q, Ohishi H, Nagano N, Yoshioka T. Segmental transarterial chemoembolization with Lipiodol mixed with anticancer drugs for nonresectable hepatocellular carcinoma: follow-up CT and therapeutic results. Cancer Chemother Pharmacol. 1994;33 Suppl:S60–S68. doi: 10.1007/BF00686670. [DOI] [PubMed] [Google Scholar]
- 31.Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41:864–880. doi: 10.1016/j.jhep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Arora AS, de Groen PC, Croall DE, Emori Y, Gores GJ. Hepatocellular carcinoma cells resist necrosis during anoxia by preventing phospholipase-mediated calpain activation. J Cell Physiol. 1996;167:434–442. doi: 10.1002/(SICI)1097-4652(199606)167:3<434::AID-JCP7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 33.Terzi E, Golfieri R, Piscaglia F, Galassi M, Dazzi A, Leoni S, Giampalma E, Renzulli M, Bolondi L. Response rate and clinical outcome of HCC after first and repeated cTACE performed “on demand”. J Hepatol. 2012;57:1258–1267. doi: 10.1016/j.jhep.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 34.Shirabe K, Kajiyama K, Harimoto N, Masumoto H, Fukuya T, Ooya M, Maehara Y. Prognosis of hepatocellular carcinoma accompanied by microscopic portal vein invasion. World J Gastroenterol. 2009;15:2632–2637. doi: 10.3748/wjg.15.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]


