Abstract
AIM: To determine if subclinical abnormal glucose tolerance (SAGT) has influence on survival of non-diabetic patients with liver cirrhosis.
METHODS: In total, 100 patients with compensated liver cirrhosis and normal fasting plasma glucose were included. Fasting plasma insulin (FPI) levels were measured, and oral glucose tolerance test (OGTT) was performed. According to OGTT results two groups of patients were formed: those with normal glucose tolerance (NGT) and those with SAGT. Patients were followed every three months. The mean follow-up was 932 d (range of 180-1925). Survival was analyzed by the Kaplan-Meyer method, and predictive factors of death were analyzed using the Cox proportional hazard regression model.
RESULTS: Of the included patients, 30 showed NGT and 70 SAGT. Groups were significantly different only in age, INR, FPI and HOMA2-IR. Patients with SAGT showed lower 5-year cumulated survival than NGT patients (31.7% vs 71.6%, P = 0.02). Differences in survival were significant only after 3 years of follow-up. SAGT, Child-Pugh B, and high Child-Pugh and Model for End-Stage Liver Disease (MELD) scores were independent predictors of death. The causes of death in 90.3% of cases were due to complications related to liver disease.
CONCLUSION: SAGT was associated with lower survival. SAGT, Child-Pugh B, and high Child-Pugh and MELD scores were independent negative predictors of survival.
Keywords: Diabetes mellitus, Liver cirrhosis, Oral glucose tolerance test, Survival, Glucose metabolism disorders
Core tip: In this controlled prospective long term study we demonstrated that subclinical forms of abnormal glucose tolerance (IGT) or diabetes mellitus (DM) may influence survival of non-diabetic cirrhotic patients. Previously it had been demonstrated that clinically overt DM is associated with low survival of cirrhotic patients. This issue is important since a high proportion of cirrhotic patients (about 70%) without history of DM and with normal fasting plasma glucose have subclinical abnormal glucose tolerance. The use of oral glucose tolerance test for early recognition and treatment of DM may improve prognosis of these patients.
INTRODUCTION
Overt diabetes mellitus (DM) has been reported in 21%-30% of patients with liver cirrhosis (LC)[1]. DM may arise from a progressive disorder of insulin secretion in the presence of liver and muscle resistance to insulin[2,3].
DM is related to LC in two ways: (1) type 2 DM (T2DM) (often associated with metabolic syndrome) causes nonalcoholic fatty liver disease (steatosis, steatohepatitis, LC, and hepatocellular carcinoma)[4-7]; and (2) DM may develop as a complication of LC, which is known as “hepatogenous diabetes” (HD)[8,9]. As liver disease advances, DM becomes clinically evident[10,11]. T2DM and HD are associated with an increased risk of complications of chronic liver diseases and mortality[7,9,12-17].
On the other hand, subclinical abnormal glucose tolerance (SAGT) disorders, such as impaired glucose tolerance or DM (called subclinical since they are detected only by means of an oral glucose tolerance test), may be observed in 45% and 22%, respectively, of patients with LC and no history of DM[18,19]. Therefore, the prevalence of DM and impaired glucose tolerance (IGT) may be underestimated when only fasting plasma glucose (FPG) levels are taken into account. Our group has recently published a study determining the prevalence of T2DM, DH and IGT in patients with LC who underwent oral glucose tolerance test (OGTT). In total, 86% of the cases had either overt or subclinical IGT and DM, with or without insulin resistance (IR)[18].
The negative influence of SAGT on the survival of cirrhotic patients was demonstrated in one study carried out in Japan, which found that patients with IGT or DM diagnosed by OGTT had a significantly lower 5-year survival rate[20]. However, similar findings have not been reported by others elsewhere.
The aims of this study were: (1) to evaluate if SAGT has any influence on survival of patients with compensated LC and normal FPG levels; and (2) to identify mortality predictors.
MATERIALS AND METHODS
Patients
Patients with LC, who were seen in our hospital from August 2007 to August 2012, were prospectively evaluated. Only those with normal FPG (< 100 mg/dL), no history of overt DM, with clinical or histological diagnosis of LC, aged over 18 years old and without clinically evident complications of liver disease (e.g., esophageal or gastric variceal bleeding, hepatic encephalopathy, hepatorenal syndrome, infection, spontaneous bacterial peritonitis, and moderate to severe ascites), were selected for the study (patients with complications were excluded in order to avoid the effect of medications used for treating them, on glucose metabolism of patients). Clinical diagnosis of LC was made by using a combination of clinical symptoms, laboratory tests and imaging studies (abdominal ultrasonography or CT scan)[21,22]. The etiology of cirrhosis was determined as follows: Alcohol-related cirrhosis was determined when daily alcohol consumption was > 80 g in men and > 40 g in women for at least 10 years with negative viral, metabolic and autoimmune markers. Diagnosis of hepatitis C virus and B virus related cirrhosis was determined with specific viral markers (HBsAg or anti-HCV). Autoimmune liver disease was diagnosed with specific autoimmune markers (anti-nuclear antibodies, anti-smooth muscle antibodies or liver-kidney anti-microsomal antibodies). Meanwhile, cryptogenic cirrhosis was established in the absence of any of the causes above described[23].
Informed consent was obtained from each patient. The protocol was approved by The Research and Ethics Committee of the Faculty of Medicine of the Autonomous University of Nuevo Leon in Monterrey.
Fasting plasma insulin and OGTT
Fasting plasma insulin (FPI) levels were measured and OGTT was performed according to the World Health Organization criteria[24]. Patients were fasted for at least 12 h, and baseline plasma glucose was measured; then, they were given an oral glucose load (75 g). Additionally, plasma glucose (PG) levels were measured every 30 min for 2 h. The results were interpreted as follows: a) normal glucose tolerance (NGT) if 2-h PG was < 140 mg/dL; IGT if 2-h PG between 140 mg/dL and 200 mg/dL; and DM if 2-h PG > 200 mg/dL. Plasma HbA1c was not used for diagnosing DM due to its low sensitivity reported in cirrhotic patients[19].
FPI was measured using the electrochemiluminescence method (normal values: 5-20 μU/mL). HOMA2-IR index (Homeostasis Model Assessment 2-Insulin Resistance Index) was calculated using the following online software (http://www.dtu.ox.ac.uk/homacalculator/index.php).
Assessments
The following data were recorded in a database specially designed for the study: gender, age, and family history of DM as well as laboratory blood test results (serum hemoglobin, leukocytes, platelets, BUN, creatinine, albumin, bilirubin, aspartate aminotransferase, γ-glutamyltranspeptidase, alkaline phosphatase, cholesterol, triglycerides, and INR). The body mass index (BMI) was calculated and construed as follows: normal if < 24.9, overweight if between 25 and 29.9, and obesity if > 30[25]. Liver function was estimated using Child-Pugh and Model for End-Stage Liver Disease (MELD) classifications[26,27].
Follow-up
All patients were followed every 3 mo in the outpatient department. A full clinical examination was made in each interview. Laboratory blood testing (serum hemoglobin, hematocrit, blood cell count, glucose, creatinine, BUN, liver function tests and coagulation tests) was also performed. Moreover, plasma alpha-fetoprotein and liver ultrasonography were performed every 6 mo. Additionally, hospital admissions were recorded during which clinical course of the patient was determined. Patients or their relatives were contacted by telephone when he or she did not attend to appointments. Callings were done in order to determine the clinical status of the patient, or to inquire whether their death occurred.
During follow-up patients were treated with life style and diet modifications, and in particular cases with oral hypoglycemic agents or insulin administration. No antidiabetic medication was given to those with impaired glucose tolerance.
Statistical analysis
Continuous variables are expressed as means and standard deviations, while non-continuous variables as medians and ranges. Categorical variables are expressed as relative proportions. Intergroup comparisons were made by Student’s t, χ2 and Mann-Whitney tests. Variables were analyzed using the Cox proportional hazard regression model in order to determine independent predictive factors of mortality[28]. Only the variables that were statistically significant in univariate analysis were analyzed in multivariate analysis. The results were expressed as HR with 95%CI, and the P value was calculated.
The cumulative survival was analyzed using the Kaplan-Meier method, and the curves were compared using log-rank test. A P value less than 0.05 was considered statistically significant. All statistical analyses were done using the statistical package SPSS v17.0 (Chicago, Illinois, United States).
RESULTS
Patient population
Of 293 cirrhotic patients evaluated, 124 (42.3%) met the inclusion criteria. However, 24 (8.1%) of them were not finally included due to the following reasons: 13 refused to participate in the study, 8 found difficult to attend consultation because they lived in another state, and 3 did not attend their appointments for performing OGTT (Figure 1).
Figure 1.
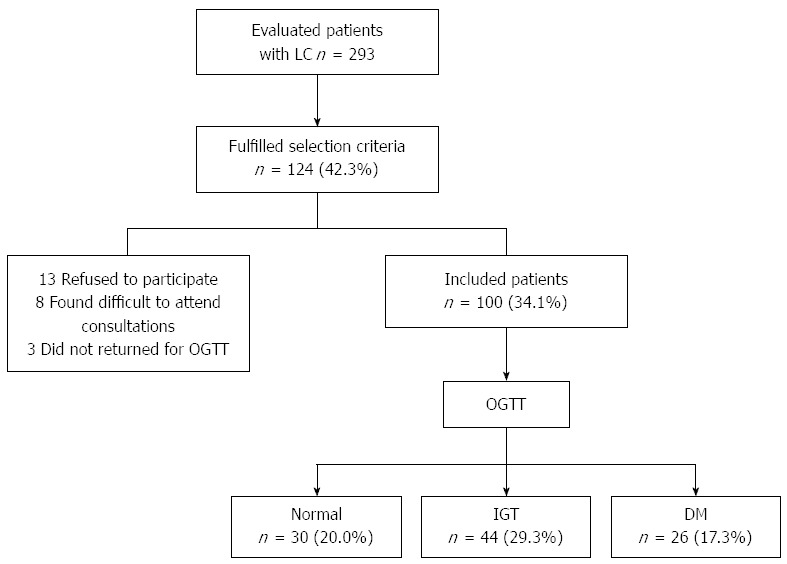
Flowchart describing how selection of patients for the study was done. LC: Liver cirrhosis; OGTT: Oral glucose tolerance test; IGT: Impaired glucose tolerance; DM: Diabetes mellitus.
Finally, 100 (34.1%) patients were included in the study. Their clinical and biochemical characteristics are shown in Table 1. Although BMI is not the best parameter for assessing nutritional status in cirrhotic patients (particularly in those with ascites), this method showed that most of them (69%) were overweight or obese. The etiology of cirrhosis was predominantly alcoholic and cryptogenic (47% and 26%, respectively). The diagnosis of LC was confirmed by liver biopsy in 44% of cases. In total, 47% and 53% of patients were assigned into the groups A and B of the Child-Pugh classification, respectively. The mean Child-Pugh score was 6.9 ± 1.8 points and the mean MELD score was 8.9 ± 4.8. The mean values of FPI levels and HOMA2-IR index were 15.9 ± 10.2 μU/mL and 2.67 ± 1.6, respectively.
Table 1.
Clinical and biochemical characteristics of cirrhotic patients
| Variable | Total (n = 100) | NGT (n = 30) | SAGT (n = 70) |
| Age, yr | 53.9 ± 11.8 | 49.0 ± 12.0 | 56.0 ± 11.11 |
| BMI, kg/m2 | 26.9 ± 4.2 | 26.4 ± 4.40 | 27.21 ± 4.15 |
| Males | 60 (60) | 18 (60) | 42 (60) |
| Etiology: Alcohol | 47 (47) | 14 (46.6) | 33 (47.1) |
| HBV | 4 (4) | 1 (3.3) | 3 (4.2) |
| HCV | 13 (13) | 2 (6.6) | 11 (15.7) |
| Autoimmunity | 10 (10) | 3 (10) | 7 (10) |
| Cryptogenic | 26 (26) | 10 (33.3) | 16 (22.8) |
| Liver biopsy | 44 (44) | 13 (43.3) | 31 (44.2) |
| Length of LC, mo | 21.6 + 27.6 | 21.4 ± 30.6 | 21.7 ± 33.2 |
| Hemoglobin, g/dL | 11.8 ± 2.3 | 11.6 ± 2.1 | 11.9 ± 2.3 |
| Platelets, × mm3 | 128140 ± 89501 | 159186 ± 112108 | 114834 ± 73269 |
| INR | 1.3 ± 0.3 | 1.2 ± 0.3 | 1.36 ± 0.32 |
| Creatinine, mg/dL | 0.8 ± 0.2 | 0.90 ± 0.33 | 0.83 ± 0.2 |
| Albumin, g/dL | 3.2 ± 0.73 | 3.33 ± 0.71 | 3.21 ± 0.74 |
| ALT, UI/L | 46.5 ± 40.8 | 48.9 ± 53.9 | 45.4 ± 34.0 |
| Total bilirubin, mg/dL | 1.71 ± 0.90 | 1.55 ± 0.88 | 1.78 ± 0.91 |
| Fasting plasma insulin, μU/mL | 15.9 ± 10.2 | 11.9 ± 7.6 | 18.2 ± 113 |
| HOMA2 score | 2.67 ± 1.6 | 2.36 ± 1.46 | 4.6 ± 3.34 |
| Child-Pugh A | 47 (47) | 15 (50) | 32 (45.7) |
| B | 53 (53) | 15 (50) | 38 (54.2) |
| Child Pugh score | 6.8 ± 1.8 | 6.5 ± 1.61 | 6.82 ± 1.67 |
| MELD score | 8.94 ± 4.8 | 8.3 ± 5.2 | 9.21 ± 4.63 |
| Survival, d | 1237 | 1448 | 1116 |
| (990-1925) | (1256-1925) | (990-1790)5 |
Data are expressed as absolute numbers (percentage) or mean ± SD.
P = 0.003;
P = 0.025;
P = 0.03;
P = 0.009;
P = 0.045. NGT: Normal glucose tolerance; SAGT: Subclinical abnormal glucose tolerance; HOMA: Homeostatic model assessment; LC: Liver cirrhosis. BMI: Body mass index; MELD: Model for End-Stage Liver Disease; HBV: Hepatitis B virus; HCV: Hepatitis C virus; INR: International normalized ratio; ALT: Alanine transaminase.
Clinical characteristics and survival rate of patients
Two groups were formed according to the results of OGTT: those with NGT (30 patients) and those with SAGT (70 patients, 44 with IGT and 26 with DM). Clinical and biochemical characteristics are shown in Table 1. SAGT patients were significantly older and had higher values of INR, FPI and HOMA2-IR. Although Child-Pugh and MELD scores were mildly higher in patients with SAGT, these differences were not statistically significant.
Follow-up
The mean follow-up was 932 d (range 180-1925). At the end of follow-up, 19 (19%) patients (5 NGT patients and 14 SAGT patients) developed overt IGT (7 cases) or overt DM (12 cases). All patients with SAGT were prescribed dietary and life style modifications. At different times of follow-up, oral antidiabetic medications were prescribed in 6, and subcutaneous insulin was prescribed in 2 out of 12 patients with overt DM.
In total, 49% of alcoholic patients consistently stopped alcohol consumption, 47% of patients with hepatitis C virus infection were treated with antiviral therapy, 50% and 90% of patients with hepatitis B virus infection or autoimmune liver disease were treated. There were no dropouts.
Cumulative survival
The mean survival time was 1448 d (range: 1256-1925) in the patients with NGT, while it was 1116 d (range 990-1790) in the patients with SAGT (P = 0.045). Cumulative survival curve of patients with NGT and SAGT is shown in Figure 2. SAGT patients had significantly lower survival (P = 0.02).
Figure 2.
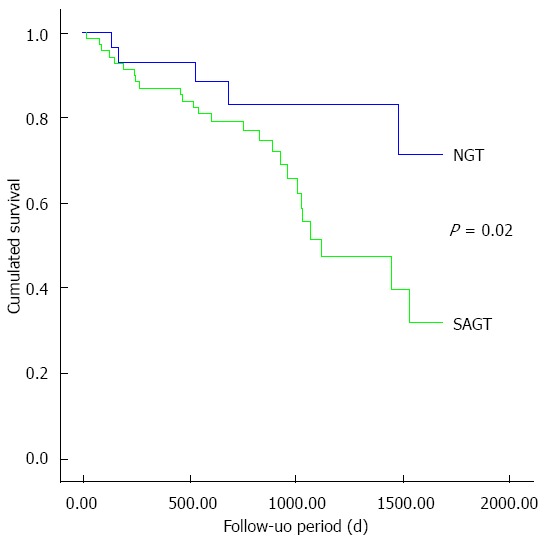
Cumulated survival of cirrhotic patients with normal glucose tolerance or subclinical abnormal glucose tolerance. Note that significant differences between both groups are evident after 3 years of follow-up. NGT: Normal glucose tolerance; SAGT: Subclinical abnormal glucose tolerance.
One to 5-year survival rates are shown in Table 2. Statistically significant differences were observed only after three years of follow-up. Increasing differences were observed over time until the end of follow-up.
Table 2.
Annual cumulated survival of cirrhotic patients n (%)
| Year(s) | NGT (n = 30) | SAGT (n = 70) | P value (χ2 test) |
| 1 | 2 (93) | 9 (87) | 0.36 |
| 2 | 4 (83.2) | 14 (79) | 0.34 |
| 3 | 4 (83.2) | 23 (51.4) | 0.04 |
| 4 | 4 (83.2) | 24 (47.5) | 0.03 |
| 5 | 5 (71.6) | 26 (31.7) | 0.02 |
NGT: Normal glucose tolerance; SAGT: Subclinical abnormal glucose tolerance.
Causes of death
In total 31 patients died (5 from the NGT group and 26 from the SAGT group). The causes of death are shown in Table 3. Twenty eight (90.3%) patients died due to the following complications related to liver disease: 8 due to esophageal variceal bleeding (25.8%), 6 due to chronic liver failure (19.3%), 5 due to spontaneous bacterial peritonitis and other infections (16.2%), 5 due to hepatorenal syndrome (16.2%) and 4 due to hepatocellular carcinoma (12.9%). Additionally, 2 of the remaining patients died due to renal impairment (6.4%), and 1 due to cardiovascular disease (3.2%). There were no significant differences in the causes of death among patients with NGT vs those with SAGT.
Table 3.
Causes of death n (%)
| Cause | Total (n = 31) |
| Variceal bleeding | 8 (25.8) |
| Liver failure | 6 (19.3) |
| SBP e infection | 5 (16.2) |
| Hepatorenal syndrome | 5 (16.2) |
| HCC | 4 (12.9) |
| Renal impairment | 2 (6.4) |
| Cardiovascular | 1 (3.2) |
SBP: Spontaneous bacterial peritonitis; HCC: Hepatocellular carcinoma.
Predictors of death
Serum creatinine, albumin, INR, Child-Pugh B, Child-Pugh and MELD scores, SAGT as well as FPI were statistically significant according to univariate analysis. However, only Child-Pugh B (HR = 8.2, 95%CI: 2.0-28.5, P = 0.02), Child-Pugh score (HR = 10.2, 95%CI: 2.4-21.9, P = 0.008), MELD score (HR = 12.3, 95%CI: 6.4-33.6, P = 0.001) and SAGT (HR = 3.2, 95%CI: 2.8-54.2, P = 0.01) were independent predictors of survival according to multivariate analysis (Table 4).
Table 4.
Univariate and multivariate analyses: Independent predictors of death
| Variable | HR | 95%CI | P value | HR | 95%CI | P value |
| Creatinine | 1.6 | 1.2-40.2 | 0.030 | 2.62 | 0.5-3.46 | 0.650 |
| Albumin | 3.4 | 1.8-22.3 | 0.015 | 6.3 | 0.4-32.1 | 0.180 |
| INR | 2.2 | 1.6-32.4 | 0.050 | 3.5 | 0.3-23.6 | 0.230 |
| Child-Pugh B | 7.2 | 3.4-33.0 | 0.0003 | 8.2 | 2.0-28.5 | 0.020 |
| Child Pugh score | 5.2 | 2.9-25.8 | 0.008 | 10.2 | 2.4-21.9 | 0.008 |
| MELD score | 8.1 | 4.2-18.7 | 0.001 | 12.3 | 6.4-33.6 | 0.001 |
| SAGT | 4.5 | 2.0-15.2 | 0.042 | 3.2 | 2.8-54.2 | 0.010 |
| Fasting serum insulin | 1.2 | 1.0-33.5 | 0.040 | 3.2 | 0.1-27.3 | 0.320 |
MELD: Model for end stage liver disease; INR: International normalized ratio; SAGT: Subclinical abnormal glucose tolerance.
DISCUSSION
Our study suggests that SAGT may have negative impact on the survival of patients with LC, as those who showed this abnormality had lower 5-year survival than those who had NGT. Significant differences in survival were observed after 3 years of follow-up (Table 2). These results are consistent with another study carried out in Japan, with 58 cirrhotic patients without overt DM who underwent OGTT[20]. In that study, 5-year survival rate was lower in patients with IGT and DM compared to those with NGT. Albumin and abnormal OGTT were independent negative predictors of survival[20]. Despite the similarities in results of both studies, ours differs in the following points: (1) 74% of our patients had alcoholic and cryptogenic cirrhosis, while 78.5% of patients in the Japanese study had viral etiology (HBV and HCV). The causes of chronic liver disease in our patients were identical to those observed in most Western countries, where alcohol and metabolic syndrome are the predominant causes (especially nowadays, the frequency of obesity and nonalcoholic fatty liver disease have dramatically increased), while viral etiology is predominantly observed in Asian countries[29-31]; (2) At time of inclusion, our patients did not have liver-related complications, whereas in the Japanese study, up to 30% had ascites and hepatic encephalopathy; and (3) The number of patients in our study was almost twice.
The mechanism by which SAGT may be involved in the occurrence of death of cirrhotic patients is difficult to explain. DM may induce progression of liver fibrosis, inflammation and liver failure, or may increase cardiovascular complications and atherosclerosis. In a study with virus C-infected patients, IR and DM were significantly associated with liver fibrosis[32]. Furthermore, in other reports, it has been suggested that DM can increase fibrosis, incidence of hepatocellular carcinoma, and resistance to antiviral therapy in patients with hepatitis C[33]. DM may be involved in the progression of liver fibrosis and inflammation through diverse mechanisms: it is likely that adipokine production (such as leptin and Tumor necrosis factor-alpha, which activate inflammatory pathways exacerbating liver injury) is increased by insulin resistance[34-36]. Leptin and oxidative stress associated with liver inflammation may activate transforming growth factor beta 1 (TGF-β1), which is one of the most potent profibrogenic cytokines produced in the liver. TGF-β1 activates hepatic stellate cells which are the major source of collagen I and III and extracellular matrix proteins[37-39]. In our study, SAGT as well as high Child-Pugh and MELD scores were independent predictors of death. This suggests that SAGT combined with other etiologic agents of liver cirrhosis might have induced liver failure and death.
On the other hand, as it has been pointed out in other publications, most of our patients with SAGT died from liver disease related complications[9,20,40] (Table 3). DM increases the incidence of severe infections by inducing immunosuppression. Cirrhotic patients with DM have a higher prevalence of infections compared to non-diabetic ones. In a recent study, spontaneous bacterial peritonitis was more frequent in patients with cryptogenic cirrhosis (which is associated with DM) compared to those with cirrhosis of other causes[41]. These infected patients had higher in-hospital mortality due to sepsis, liver failure, and hepatorenal syndrome[42]. In addition, DM may increase the risk of variceal bleeding, as postprandial hyperglycemia that occurs in diabetic patients produces splanchnic vasodilatation and increases the flow and pressure of the porto systemic venous system[43]. Also, esophageal variceal bleeding increases the risk of infection and death by inducing bacterial intestinal translocation[44]. DM has been also associated with an increased risk of hepatic encephalopathy (HE). A significant association between malnutrition and diabetes with HE was observed in a study with cirrhotic patients[45]. In multivariate analysis, Child-Pugh classification, malnutrition and DM showed independent correlations with HE. Plasma ammonium ion levels were related to insulin resistance and muscle mass. Authors concluded that nutritional status and insulin resistance might be implicated in the pathogenesis of HE[45].
On the other hand, some authors reported low frequency of diabetic complications in cirrhotic patients[46]. However, these observations have been recently challenged: in two published studies, patients with chronic hepatitis C compared to controls, showed significant higher carotid intima media thickness, greater number of carotid plaques and carotid atherosclerosis affection[47,48]. In one of these studies[47], older age and severe hepatic fibrosis were independently associated with early carotid atherosclerosis. In the other one[48], diabetes and metabolic syndrome were associated with carotid plaques. The precise prevalence of cardiovascular disease or atherosclerosis and their role in the induction of death in cirrhotic patients with DM need to be defined in future well conducted studies.
In our study, we did not observe significant differences in the causes of death among patients with NGT compared to those with SAGT (Table 3). This may be due to the small number of individuals in each group. Some differences might be clearly evident using a larger sample of patients.
Although the negative impact of DM on survival of cirrhotic patients is well known a long time ago, the beneficial effects of early detection and treatment of DM and IGT for reducing complications and mortality rates have not been clearly determined. In our study, analysis of this issue was not performed due to the low number of patients. This important point has to be determined in future studies.
In conclusion, SAGT disorders were associated with reduced long term survival in patients with compensated LC and normal FPG. Additionally, SAGT, Child-Pugh B, and high Child-Pugh and MELD scores were independent negative predictors of survival. These findings suggest that SAGT may give rise to morbid conditions that increase mortality of patients. According to this, OGTT is useful for accurate assessment of mortality in non-diabetic cirrhotic patients.
COMMENTS
Background
Overt diabetes mellitus (DM) may be found in 30% of patients with liver cirrhosis of diverse etiology. Since a long time ago, it has been known that overt DM is linked to a reduction of survival of patients with liver cirrhosis. Causes of death of these patients are mostly due to liver disease complications. Nonetheless, 60% of non-diabetic patients with liver cirrhosis may have abnormal glucose tolerance disorders, either impaired glucose tolerance (IGT) or DM, after an oral glucose tolerance test.
Research frontiers
The impact of subclinical forms of IGT and DM on survival of cirrhotic patients has not been extensively described. In this paper, the authors demonstrate that patients with these forms of abnormal glucose tolerance have reduced long term survival than those with normal glucose tolerance. Nevertheless, this study does not allow demonstrating the mechanisms by which abnormal glucose tolerance may reduce survival of patients
Related publications
The impact of subclinical forms of abnormal glucose tolerance on survival of cirrhotic patients has been described in only one study performed in Japan. In this study, patients with IGT and DM detected by oral glucose tolerance test (OGTT) had reduced 5-year survival than those with normal glucose tolerance.
Innovations and breakthroughs
Most of the patients in the previously published study had cirrhosis of viral etiology and 30% had liver complications, while in this study, patients were clinically compensated and most of them had alcoholic and cryptogenic etiology. In addition the sample size of this study was double.
Applications
The practical applications of the findings of this study are clear: early detection of abnormal glucose tolerance conditions through OGTT allows early treatment of DM and consequently a reduction of complications and mortality of patients. The use of OGTT is suggested in all patients with liver cirrhosis without previous DM, in order to assess prognosis of survival. Those with DM may be included in a close monitoring and an early treatment program. Future studies concerning the mechanisms by which DM induces death of cirrhotic patients may allow focusing prevention and therapeutic measures.
Terminology
Subclinical abnormal glucose tolerance refers to impaired glucose tolerance and diabetes mellitus detected by an oral glucose tolerance test.
Peer review
The investigators correlated the subclinical abnormal glucose tolerance and reduced survival in cirrhosis patients, and found that the subclinical abnormal glucose tolerance was associated with lower survival.
Footnotes
Supported by Gastroenterology and Endocrinology Services of the University Hospital, Universidad Autónoma de Nuevo León, Monterrey, México
P- Reviewers: Hee Y, Lonardo A, Perazzo H S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Hickman IJ, Macdonald GA. Impact of diabetes on the severity of liver disease. Am J Med. 2007;120:829–834. doi: 10.1016/j.amjmed.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Petrides AS, Vogt C, Schulze-Berge D, Matthews D, Strohmeyer G. Pathogenesis of glucose intolerance and diabetes mellitus in cirrhosis. Hepatology. 1994;19:616–627. doi: 10.1002/hep.1840190312. [DOI] [PubMed] [Google Scholar]
- 3.Petrides AS, Stanley T, Matthews DE, Vogt C, Bush AJ, Lambeth H. Insulin resistance in cirrhosis: prolonged reduction of hyperinsulinemia normalizes insulin sensitivity. Hepatology. 1998;28:141–149. doi: 10.1002/hep.510280119. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560–578. doi: 10.1007/s10620-009-1081-0. [DOI] [PubMed] [Google Scholar]
- 5.Blendea MC, Thompson MJ, Malkani S. Diabetes and Chronic liver disease: Etiology and pitfalls in monitoring. Clin Diabetes. 2010;28:139–144. [Google Scholar]
- 6.Porepa L, Ray JG, Sanchez-Romeu P, Booth GL. Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. CMAJ. 2010;182:E526–E531. doi: 10.1503/cmaj.092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loria P, Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatol Res. 2013;43:51–64. doi: 10.1111/j.1872-034X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Compean D, Jaquez-Quintana JO, Maldonado-Garza H. Hepatogenous diabetes. Current views of an ancient problem. Ann Hepatol. 2009;8:13–20. [PubMed] [Google Scholar]
- 9.Holstein A, Hinze S, Thiessen E, Plaschke A, Egberts EH. Clinical implications of hepatogenous diabetes in liver cirrhosis. J Gastroenterol Hepatol. 2002;17:677–681. doi: 10.1046/j.1440-1746.2002.02755.x. [DOI] [PubMed] [Google Scholar]
- 10.Del Vecchio Blanco C, Gentile S, Marmo R, Carbone L, Coltorti M. Alterations of glucose metabolism in chronic liver disease. Diabetes Res Clin Pract. 1990;8:29–36. doi: 10.1016/0168-8227(90)90093-9. [DOI] [PubMed] [Google Scholar]
- 11.Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734–743. doi: 10.2337/dc06-1539. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi G, Marchesini G, Zoli M, Bugianesi E, Fabbri A, Pisi E. Prognostic significance of diabetes in patients with cirrhosis. Hepatology. 1994;20:119–125. doi: 10.1016/0270-9139(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 13.Lonardo A, Carulli N, Loria P. HCV and diabetes. A two-question-based reappraisal. Dig Liver Dis. 2007;39:753–761. doi: 10.1016/j.dld.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Quintana JO, García-Compean D, González JA, Pérez JZ, González FJ, Espinosa LE, Hernández PL, Cabello ER, Villarreal ER, Rendón RF, et al. The impact of diabetes mellitus in mortality of patients with compensated liver cirrhosis-a prospective study. Ann Hepatol. 2011;10:56–62. [PubMed] [Google Scholar]
- 15.Harrison SA. Liver disease in patients with diabetes mellitus. J Clin Gastroenterol. 2006;40:68–76. doi: 10.1097/01.mcg.0000190774.91875.d2. [DOI] [PubMed] [Google Scholar]
- 16.de Marco R, Locatelli F, Zoppini G, Verlato G, Bonora E, Muggeo M. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care. 1999;22:756–761. doi: 10.2337/diacare.22.5.756. [DOI] [PubMed] [Google Scholar]
- 17.El-Serag HB, Everhart JE. Diabetes increases the risk of acute hepatic failure. Gastroenterology. 2002;122:1822–1828. doi: 10.1053/gast.2002.33650. [DOI] [PubMed] [Google Scholar]
- 18.García-Compeán D, Jáquez-Quintana JO, Lavalle-González FJ, Reyes-Cabello E, González-González JA, Muñoz-Espinosa LE, Vázquez-Elizondo G, Villarreal-Pérez JZ, Maldonado-Garza HJ. The prevalence and clinical characteristics of glucose metabolism disorders in patients with liver cirrhosis. A prospective study. Ann Hepatol. 2012;11:240–248. [PubMed] [Google Scholar]
- 19.Cacciatore L, Cozzolino G, Giardina MG, De Marco F, Sacca L, Esposito P, Francica G, Lonardo A, Matarazzo M, Varriale A. Abnormalities of glucose metabolism induced by liver cirrhosis and glycosylated hemoglobin levels in chronic liver disease. Diabetes Res. 1988;7:185–188. [PubMed] [Google Scholar]
- 20.Nishida T, Tsuji S, Tsujii M, Arimitsu S, Haruna Y, Imano E, Suzuki M, Kanda T, Kawano S, Hiramatsu N, et al. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006;101:70–75. doi: 10.1111/j.1572-0241.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 21.Cozzolino G, Lonardo A, Francica G, Amendola F, Cacciatore L. Differential diagnosis between hepatic cirrhosis and chronic active hepatitis: specificity and sensitivity of physical and laboratory findings in a series from the Mediterranean area. Am J Gastroenterol. 1983;78:442–445. [PubMed] [Google Scholar]
- 22.Udell JA, Wang CS, Tinmouth J, FitzGerald JM, Ayas NT, Simel DL, Schulzer M, Mak E, Yoshida EM. Does this patient with liver disease have cirrhosis? JAMA. 2012;307:832–842. doi: 10.1001/jama.2012.186. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 24.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen DM, El-Serag HB. The epidemiology of obesity. Gastroenterol Clin North Am. 2010;39:1–7. doi: 10.1016/j.gtc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 27.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 28.Cox D. Regression models and life tables. J Roy Statist Soc. 1972;34:187–220. [Google Scholar]
- 29.Petrasch S, Stein H, Kosco MH, Brittinger G. Follicular dendritic cells in non-Hodgkin lymphomas: localisation, characterisation and pathophysiological aspects. Eur J Cancer. 1991;27:1052–1056. doi: 10.1016/0277-5379(91)90280-q. [DOI] [PubMed] [Google Scholar]
- 30.Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;25:883–889. doi: 10.1111/j.1365-2036.2007.03246.x. [DOI] [PubMed] [Google Scholar]
- 31.Tellez-Avila FI, Sanchez-Avila F, García-Saenz-de-Sicilia M, Chavez-Tapia NC, Franco-Guzman AM, Lopez-Arce G, Cerda-Contreras E, Uribe M. Prevalence of metabolic syndrome, obesity and diabetes type 2 in cryptogenic cirrhosis. World J Gastroenterol. 2008;14:4771–4775. doi: 10.3748/wjg.14.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taura N, Ichikawa T, Hamasaki K, Nakao K, Nishimura D, Goto T, Fukuta M, Kawashimo H, Fujimoto M, Kusumoto K, et al. Association between liver fibrosis and insulin sensitivity in chronic hepatitis C patients. Am J Gastroenterol. 2006;101:2752–2759. doi: 10.1111/j.1572-0241.2006.00835.x. [DOI] [PubMed] [Google Scholar]
- 33.Pattullo V, Heathcote J. Hepatitis C and diabetes: one treatment for two diseases? Liver Int. 2010;30:356–364. doi: 10.1111/j.1478-3231.2009.02185.x. [DOI] [PubMed] [Google Scholar]
- 34.Roden M. Mechanisms of Disease: hepatic steatosis in type 2 diabetes--pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab. 2006;2:335–348. doi: 10.1038/ncpendmet0190. [DOI] [PubMed] [Google Scholar]
- 35.Flisiak R, Pytel-Krolczuk B, Prokopowicz D. Circulating transforming growth factor beta(1) as an indicator of hepatic function impairment in liver cirrhosis. Cytokine. 2000;12:677–681. doi: 10.1006/cyto.1999.0660. [DOI] [PubMed] [Google Scholar]
- 36.Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin--a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 37.Bertolani C, Marra F. The role of adipokines in liver fibrosis. Pathophysiology. 2008;15:91–101. doi: 10.1016/j.pathophys.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Jonsson JR, Moschen AR, Hickman IJ, Richardson MM, Kaser S, Clouston AD, Powell EE, Tilg H. Adiponectin and its receptors in patients with chronic hepatitis C. J Hepatol. 2005;43:929–936. doi: 10.1016/j.jhep.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 39.Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, Orlandoni P, Macarri G, Perego L, Benedetti A, et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743–1751. doi: 10.1002/hep.510290632. [DOI] [PubMed] [Google Scholar]
- 40.Hagel S, Bruns T, Herrmann A, Stallmach A, Schmidt C. Abnormal glucose tolerance: a predictor of 30-day mortality in patients with decompensated liver cirrhosis. Z Gastroenterol. 2011;49:331–334. doi: 10.1055/s-0029-1245933. [DOI] [PubMed] [Google Scholar]
- 41.Sorrentino P, Tarantino G, Conca P, Perrella A, Perrella O. Clinical presentation and prevalence of spontaneous bacterial peritonitis in patients with cryptogenic cirrhosis and features of metabolic syndrome. Can J Gastroenterol. 2004;18:381–386. doi: 10.1155/2004/739509. [DOI] [PubMed] [Google Scholar]
- 42.Cheruvattath R, Balan V. Infections in Patients With End-stage Liver Disease. J Clin Gastroenterol. 2007;41:403–411. doi: 10.1097/01.mcg.0000248018.08515.f9. [DOI] [PubMed] [Google Scholar]
- 43.Moreau R, Chagneau C, Heller J, Chevenne D, Langlet P, Deltenre P, Hillaire S, Lefilliatre P, Pateron D, Sogni P, et al. Hemodynamic, metabolic and hormonal responses to oral glibenclamide in patients with cirrhosis receiving glucose. Scand J Gastroenterol. 2001;36:303–308. doi: 10.1080/003655201750074654. [DOI] [PubMed] [Google Scholar]
- 44.Thalheimer U, Triantos CK, Samonakis DN, Patch D, Burroughs AK. Infection, coagulation, and variceal bleeding in cirrhosis. Gut. 2005;54:556–563. doi: 10.1136/gut.2004.048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalaitzakis E, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, Jalan R, Björnsson E. Malnutrition and diabetes mellitus are related to hepatic encephalopathy in patients with liver cirrhosis. Liver Int. 2007;27:1194–1201. doi: 10.1111/j.1478-3231.2007.01562.x. [DOI] [PubMed] [Google Scholar]
- 46.Marchesini G, Ronchi M, Forlani G, Bugianesi E, Bianchi G, Fabbri A, Zoli M, Melchionda N. Cardiovascular disease in cirrhosis--a point-prevalence study in relation to glucose tolerance. Am J Gastroenterol. 1999;94:655–662. doi: 10.1111/j.1572-0241.1999.00931.x. [DOI] [PubMed] [Google Scholar]
- 47.Petta S, Torres D, Fazio G, Cammà C, Cabibi D, Di Marco V, Licata A, Marchesini G, Mazzola A, Parrinello G, et al. Carotid atherosclerosis and chronic hepatitis C: a prospective study of risk associations. Hepatology. 2012;55:1317–1323. doi: 10.1002/hep.25508. [DOI] [PubMed] [Google Scholar]
- 48.Adinolfi LE, Restivo L, Zampino R, Guerrera B, Lonardo A, Ruggiero L, Riello F, Loria P, Florio A. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496–502. doi: 10.1016/j.atherosclerosis.2012.01.051. [DOI] [PubMed] [Google Scholar]


