Abstract
AIM: To investigate the correlation between the appearance of skin lesions and concentration of interleukin (IL)-17A, IL-23 and interferon-γ (IFN-γ) in Crohn’s disease (CD) patients during anti-tumor necrosis factor-α (TNF-α) therapy
METHODS: A prospective study included 30 adult patients with CD of Caucasian origin (19 men and 11 women; mean age ± SD 32.0 ± 8.6 years) during biological therapy with anti-TNF-α antibodies from January 2012 to March 2013. Eighteen patients were treated with infliximab, seven with adalimumab and five with certolizumab. Inclusion criteria were exacerbation of the underlying disease, Crohn’s Disease Activity Index over 300 and the ineffectiveness of previously used non-biological therapies. Patients with a history of psoriasis, atopic dermatitis and other autoimmune skin lesions were excluded from the study. The control group consisted of 12 healthy subjects. A diagnostic survey was carried out, blood tests and careful skin examination were performed, and the serum levels of IL-17, IL-23 and IFN-γ were measured using an enzyme-linked immunosorbent assays technique. Dermatoses that have developed in the course of biological therapy in patients who had no pre-existing skin lesions of similar character were qualified as skin lesions induced by anti-TNF-α therapy.
RESULTS: Skin manifestations occurred in 18 of CD patients during the anti-TNF-α therapy (60%), in the average time of 10.16 ± 3.42 mo following the beginning of the 52-wk treatment cycle. Skin lesions observed in CD patients during biological therapy included psoriasiform lesions (44.4%), and eczema forms lesions (22.2%). In CD patients with drug induced skin lesions significantly higher levels of hemoglobin (13.3 ± 1.5 g/dL vs 10.8 ± 1.9 g/dL, P = 0.018) and hematocrit (39.9% ± 4.5% vs 34.3% ± 5.4%, P = 0.01), as well as a significantly lower level of platelets (268 ± 62 × 103/μL vs 408 ± 239 × 103/μL, P = 0.046) was observed compared with CD patients without skin manifestations. The concentrations of IL-17A and IL-23 in CD patients with skin lesions developed under anti-TNF-α therapy were significantly higher compared to those in patients without lesions (IL-17A: 39.01 ± 7.03 pg/mL vs 25.71 ± 4.90 pg/mL, P = 0.00004; IL-23: 408.78 ± 94.13 pg/mL vs 312.15 ± 76.24 pg/mL, P = 0.00556).
CONCLUSION: Skin lesions in CD patients during biological therapy may result from significantly increased concentrations of IL-17A and IL-23, which are strongly associated with TNF-α/Th1 immune pathways.
Keywords: Biological therapy, Crohn’s disease, Interleukin 17A, Interleukin 23, Interferon γ, Tumor necrosis factor-α
Core tip: The important and often underestimated problem of skin lesions associated with biological treatment in Crohn’s disease. The authors found the correlation between the appearance of skin lesions and concentrations of interleukin (IL)-17A, IL-23 and interferon-γ in Crohn’s disease patients. This is a new and innovative view on the phenomenon “treat and trigger” in clinical practice. Better understanding the pathway of these disorders can influence the effectiveness of the treatment of skin lesions induced by biological therapy.
INTRODUCTION
Crohn’s disease (CD) belongs to a group of inflammatory bowel diseases (IBD) and is characterized by a chronic, segmental granulomatosus inflammation with periods of exacerbation and remission, which may involve any part of the gastrointestinal tract. The pathogenesis of the intestinal lesions in CD is not entirely identified and understood. Excessive activation of the immune system comes as a result of the interaction of various environmental and infectious factors, genetic predisposition, and the mediation of abnormal intestinal flora. The imbalance between proinflammatory and anti-inflammatory cytokines leads to a disproportionate activation of T helper (Th)1 cells and overproduction of interleukin (IL)-1β, IL-2, IL-10, IL-12, IL-18, transforming growth factor beta (TGF-β) and tumor necrosis factor-α (TNF-α)[1,2]. Clinical manifestations and inflammatory lesions in the intestinal wall are induced by elevated levels of several of these cytokines, mainly TNF-α. Recent studies showed an additional impact of lymphocyte Th17 excessive proinflammatory cytokine secretion in CD[3].
CD symptomatology is non-specific and very diverse. General symptoms, such as fever, weakness, and weight loss are accompanied by symptoms associated with chronic inflammation of the intestinal mucosa, such as abdominal pain and chronic diarrhea. Extraintestinal manifestations occur in 6%-36% of CD patients and include the most common symptoms of arthritis, uveitis, hematologic and hepatobiliary disorders, and skin lesions[4,5]. Non-specific skin lesions can be classified into characteristic for IBD, which include gangrenous dermatitis and erythema nodosum, and uncharacteristic, which include mucositis, aphthous stomatitis, vasculitis, and perianal disease - ulcers, abscesses, and fistulas[1,2,6,7].
The aim of the anti-IBD treatment is to induce and maintain remission by taking control of inflammation. To achieve this, 5-aminosalicylic acid, corticosteroids, immunosuppressants and antibiotics are used[2]. More comprehensive studies on the CD pathogenesis lead to the introduction of targeted therapies in the form of biological drugs. Anti-TNF-α monoclonal antibodies act by suppressing the immune pathway, which is dependent on TNF-α. Although very effective in CD treatment, biological drugs interfere with the immune system and can cause a variety of side effects, such as hypersensitivity to the drug, opportunistic infections including reactivation of tuberculosis, autoimmunity with the formation of antibodies, demyelinating disease, an increased risk of lymphoma, and cancer development. A broad, diverse group of the anti-TNF-α side effects are skin lesions. There may be a local hypersensitivity reaction related to drug administration or a generalized reaction based on immunological imbalance.
According to the recent studies, skin lesions induced by biological therapy are the result of an immune imbalance. Similarly to CD, there is an enhanced expression of Th17 cytokines, such as IL-17A in inflammatory skin lesions of patients with psoriasis or atopic dermatitis. It has been speculated that the inhibition of the excessive production of TNF-α and IFN-γ by CD4+ Th1 lymphocytes may stimulate the IL-23-dependent pathway associated with activation of Th17 cytokines[8] and an overproduction of IL-17A, responsible for the development of changes in the dermis. The most common skin lesions, which appear in the course of the treatment with anti-TNF-α are local hypersensitivity reactions, psoriasis and psoriasiform lesions, eczema, lupus-like lesions, vasculitis, bacterial infections of the skin, dermatitis caused by viruses, and skin cancer[9,10].
The aim of this study was to evaluate the concentration of IL-17A, IL-23 and IFN-γ in patients with CD treated with anti-TNF-α drugs and their correlation with appearance of drug induced skin lesion.
MATERIALS AND METHODS
The study was conducted in accordance with the ethical principles of the 1975 Declaration of Helsinki. The study protocol was approved by the Committee of Bioethics of Medical University of Lodz, and all participants gave a written informed consent to participate in the study.
Thirty adult patients with CD of Caucasian origin (19 men and 11 women; mean age ± SD 32.0 ± 8.6 years) during biological therapy with anti-TNF-α antibodies from January 2012 to March 2013 were enrolled in this study. Eighteen patients were treated with infliximab, seven with adalimumab and five with certolizumab. All patients were hospitalized at the Department of Gastroenterology, Medical University of Lodz, Poland. CD was diagnosed and confirmed according to clinical, radiological, endoscopic, and histological criteria developed by the European Crohn’s and Colitis Organisation. The clinical state of each patient was classified according to Crohn’s Disease Activity Index (CDAI). Inclusion criteria for biological therapy were exacerbation of the underlying disease, CDAI over 300 and the ineffectiveness of previously used non-biological therapies. From the study were excluded patients with a history of hypersensitivity, psoriasis, atopic dermatitis and other autoimmune skin lesions.
In the study group there were 18 patients with cutaneous manifestations induced by biological treatment and 12 patients in whom there were no skin lesions observed during the therapy. Dermatoses that have developed in the course of biological therapy in patients who had no pre-existing skin lesions of similar character were qualified as skin lesions induced by anti-TNF-α therapy. Patients with lesions of hypersensitivity underlying character were excluded from this group. In each patient, the skin manifestations were diagnosed by a specialist gastroenterologist and then confirmed by independent, experienced, board-certified dermatologist. The skin lesions were classified based on careful examination and their clinical appearance. Patients with skin lesions induced by anti-TNF-α were not under topical therapy during the study and one-year before the enrolment. Patients in the study group were not taking any other anti-inflammatory drugs that may affect the immunological profile and appearance of skin lesions. After completion of biological treatment, patients were included in the 6-mo follow-up, in which all were retested twice, with carful skin examination. The control group of 12 healthy subjects consisted of students and medical staff and was homogenous to the study group in terms of age and sex.
From all patients, 5 mL venous blood was taken to determine cytokine levels. Serum was quickly frozen at -80 °C and stored until use. In the group of CD patients with skin lesions, the blood was taken at the time of the appearance of first cutaneous manifestations; in CD patients without skin involvement the blood was taken at the end of the course of biologic therapy. Additional laboratory tests included peripheral complete blood count and serum C-reactive protein (CRP). At the same time frames clinical disease characteristics charts, a detailed written questionnaire, and an interview were collected from all patients.
Cytokine evaluation
The serum concentrations of IL-17A, IL-23 and IFN-γ were determined by the quantitative sandwich enzyme-linked immunosorbent assay (ELISA), using the standard ELISA kits from DIACLONE SAS according to the manufacturer’s instructions. The sensitivity of the methods were < 5.0 pg/mL for IFN-γ, < 2.3 pg/mL for IL-17A, and < 20.0 pg/mL for IL-23. For each detection, calibration blank tests have been taken into account. Each determination was carried out in duplicate in accordance with the principles of laboratory.
Statistical analysis
The data were analyzed using the Statistica 10.0 software (StatSoft, Inc., United States). The results were expressed as means ± SD. Comparison between groups were performed using the Student’s t-test (or nonparametric Mann-Whitney U-test) and Fisher’s exact test (or χ2 test), depending on the distribution of the variables. Correlations were evaluated using the Pearson’s test or Spearman’s rank correlation coefficient test depending on normality of distribution. A value of P < 0.05 was considered statistically significant.
RESULTS
The baseline characteristics of 30 CD patients on biological therapy and 12 health controls are presented in Table 1. Eighteen (60%) of CD patients developed skin lesions during anti-TNF-α therapy, whereas twelve (40%) of CD patients had no skin manifestations. Drug induced skin lesions were observed in twelve patients treated with infliximab (66.7%), four with adalimumab (57.1%), and two with certolizumab (40.0%). Skin lesions in patients with CD occurred in the average time of 10.16 ± 3.42 mo following the beginning of the anti-TNF-α therapy with a 52-wk treatment cycle. Each patient was retested twice in a 6 mo follow-up after the termination of biological therapy. All drug induced skin lesions were reversible and subsided without necessity to use topical or general treatment in the mean time of 2.6 mo after the last dose of the anti-TNF-α agent.
Table 1.
Baseline clinical characteristics and laboratory findings n (%)
| Characteristics |
Crohn disease |
Control group | ||||
| All patients |
Skin lesions |
P value1 | ||||
| + | - | |||||
| Subjects | 30 (100) | 18 (60) | 12 (40) | 12 (100) | ||
| Sex | Women | 11 (36.7) | 5 (27.8) | 6 (50) | 0.215 | 6 (50) |
| Men | 19 (63.3) | 13 (72.2) | 6 (50) | 6 (50) | ||
| Age, yr | 32.0 ± 8.6 | 34.0 ± 8.1 | 29.0 ± 8.7 | 0.119 | 25 ± 5.1 | |
| Smokers | 11 (36.7) | 8 (44.4) | 3 (25) | 0.245 | 4 (33.3) | |
| BMI, kg/m2 | 23.0 ± 3.5 | 23.5 ± 3.5 | 22.2 ± 3.4 | 0.314 | 22.7 ± 2.3 | |
| Duration of disease, yr | 8.2 ± 5.9 | 7.4 ± 4.9 | 9.2 ± 7.3 | 0.452 | NA | |
| Location of lesions | Small intestine | 2 (6.7) | 1 (5.6) | 1 (8.3) | 0.719 | NA |
| Large intestine | 10 (33.3) | 7 (38.9) | 3 (25) | NA | ||
| Small and large intestine | 18 (60) | 10 (55.5) | 8 (66.7) | NA | ||
| Perianal lesions | 6 (20) | 3 (16.7) | 3 (25) | 0.456 | NA | |
| CDAI, points | 177 ± 58 | 177 ± 74 | 177 ± 56 | 0.987 | NA | |
| Disease activity | Low CDAI (150-220) | 11 (36.7) | 7 (38.9) | 4 (33.3) | 0.570 | NA |
| Moderate CDAI (220-450) | 7 (23.3) | 3 (16.7) | 4 (33.3) | NA | ||
| Hemoglobin, g/dL | 12.4 ± 2.1 | 13.3 ± 1.5 | 10.8 ± 1.9 | 0.002 | 13.5 ± 1.2 | |
| Hematocrit | 37.9% ± 5.5% | 39.9%± 4.5% | 34.3% ± 5.4% | 0.010 | 42.8% ± 2.4% | |
| Red blood cell count, × 106/mm3 | 4.35 ± 0.61 | 4.46 ± 0.61 | 4.16 ± 0.59 | 0.252 | 4.91 ± 0.49 | |
| White blood cell count, × 103/μL | 7.49 ± 2.46 | 7.09 ± 2.47 | 8.19 ± 2.39 | 0.293 | 6.7 ± 1.5 | |
| Platelet count, × 103/μL | 315 ± 160 | 268 ± 62 | 400 ± 239 | 0.046 | 279 ± 75 | |
| CRP, mg/L | 19.2 ± 31.0 | 14.9 ± 28.6 | 25.9 ± 35.0 | 0.417 | 4.3 ± 0.8 | |
Statistical significance between Crohn’s disease (CD) patient group with and without skin lesions during anti-tumor necrosis factor-α (TNF-α) therapy. Data are presented as mean ± SD or number (percentage). Baseline clinical characteristics and laboratory findings in Crohn’s disease (CD) patients with and without skin lesions during anti-TNF-α therapy and healthy controls. BMI: Body mass index; CDAI: Crohn’s Disease Activity Index; CRP: C-reactive protein; NA: Not applicable.
Analysis of patients with and without skin lesions showed no statistically significant differences in terms of the impact of sex, age, body mass index, smoking, disease duration, and location of intestinal inflammatory lesions. The study showed no relationship between the disease activity and the presence of skin lesions. A comparison of laboratory tests revealed that CD patients with drug induced skin lesions had significantly higher levels of hemoglobin (Hb) (13.3 ± 1.5 g/dL vs 10.8 ± 1.9 g/dL, P = 0.018) and hematocrit (Ht) (39.9% ± 4.5% vs 34.3% ± 5.4%, P = 0.01), as well as significantly lower level of platelets (PLT) (268 ± 62 × 103/μL vs 408 ± 239 × 103/μL, P = 0.046) compared with CD patients without skin manifestations. There were no differences between the levels of red blood cell (RBC), white blood cell (WBC) and CRP between CD patients groups with and without skin lesions.
Skin lesions observed in CD patients during biological therapy included psoriasiform lesions (44.4%), eczematiforms lesions (22.2%), erythema (22.2%), excessive skin dryness (22.2%), acne (16.7%) and furunculosis-type lesions (11.1%). In 6 of 18 patients with skin lesions (33.3%), more than one skin manifestation occurred at the same time. Generalized skin lesions were observed in 2 patients (11.1%) in the form of psoriasiform eruptions. Location and frequency of skin lesions are outlined in the Table 2.
Table 2.
Localization of nonspecific skin lesions during anti-tumor necrosis factor-α therapy in Crohn’s disease patients n (%)
| Skin lesions localization | Value |
| Scalp region | 4 (22) |
| Facial region | 8 (44) |
| Presternal region | 3 (17) |
| Intracapsular region | 4 (22) |
| Upper limb | 1 (6) |
| Lower limb | 3 (17) |
| Generalized | 2 (11) |
Analysis of cytokine levels showed a statistically significant increase in serum concentrations of IL-17A, IL-23 and IFN-γ in patients with CD compared to controls. Specifically, substantial differences in the serum levels of IL-17A: 33.69 ± 9.06 pg/mL in CD vs 6.23 ± 4.26 pg/mL in controls (P < 0.000001); IL-23: 370.13 ± 98.58 pg/mL in CD vs 69.58 ± 29.44 pg/mL in controls (P < 0.000001); and IFN-γ: 220.39 ± 65.78 pg/mL in CD vs 44.03 ± 14.30 pg/mL in controls (P < 0.000001) were observed. The statistical analysis of the obtained data showed that there is a significant positive correlation between IL-17A and IL-23 concentrations (r = 0.482, P = 0.007, Figure 1). No correlations were found between the serum levels of IL-17A, IL-23 vs IFN-γ.
Figure 1.
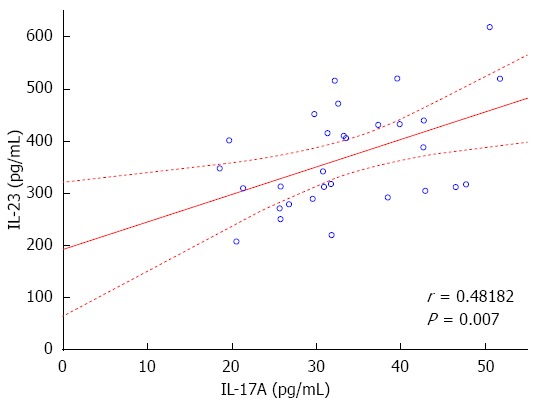
Correlation between the serum concentration. Correlation between the serum concentration of interleukin 17 (IL-17) and IL-23 in Crohn’s disease patients (r = 0.48182, P = 0.007).
The statistical analysis revealed a significant increase in IL-17A and IL-23 serum concentrations in CD patients with skin lesions during anti-TNF-α therapy compared with CD patients without skin manifestations (IL-17A: 39.01 ± 7.03 pg/mL vs 25.71 ± 4.90 pg/mL, P = 0.00004; IL-23: 408.78 ± 94.13 pg/mL vs 312.15 ± 76.24 pg/mL, P = 0.00556, respectively). There were no statistically significant differences in serum level of IFN-γ between patients with and without skin manifestation (IFN-γ: 223.12 ± 66.52 pg/mL vs 216.29 ± 67.38 pg/mL, P = 0.96622, Figure 2). The analysis showed no significant difference between IL-17A, IL-23 and IFN-γ serum levels in CD patients with skin lesions depending on the type and number of skin lesions. There was also no statistically significant relationship between IL-17A, IL-23 and IFN-γ serum levels in CD patients and age, sex, duration of disease, location of intestinal lesions and the length of anti-TNF-α therapy. The analysis showed that CDAI did not correlate with cytokine serum levels.
Figure 2.
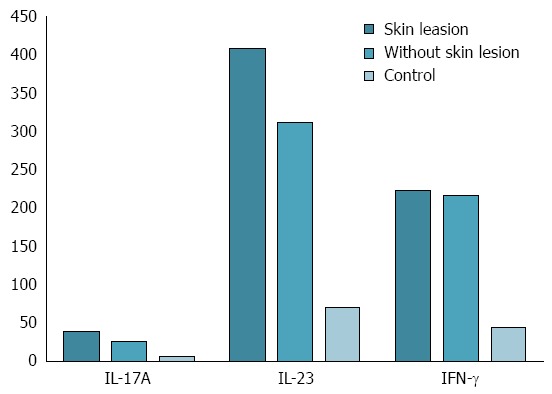
Interleukin 17A, interleukin 23 and interferon gamma serum concentration in controls and Crohn’s disease patients with and without skin adverse side effects of anti-tumor necrosis factor-α therapy. Interleukin 17A (IL-17A): With skin lesions vs without skin lesions: P = 0.000044; with skin lesions vs control: P = 0.000005; without skin lesions vs control: P = 0.000037; IL-23: With skin lesions vs without skin lesions: P = 0.005557; with skin lesions vs control: P = 0.000005; without skin lesions vs control: P = 0.000037; interferon gamma (IFN-γ): With skin lesions vs without skin lesions: P = 0.966233; with skin lesions vs control: P = 0.000005; without skin lesions vs control: P = 0.000037.
The study demonstrated statistically significant positive correlation between IL-17A serum concentration and Hb (r = 0.505, P = 0.01, Figure 3) and Ht (r = 0.474, P = 0.022) levels in all CD patients. On the other hand, a negative correlation between IL-17A serum concentration and WBC and PLT was observed in CD patients, but without statistical significance. There were no significant correlations between the serum concentrations of IL-23 and IFN-γ and the levels of Hb, Ht, PLT, and WBC. Cytokine serum concentrations did not correlate with RBC and CRP.
Figure 3.
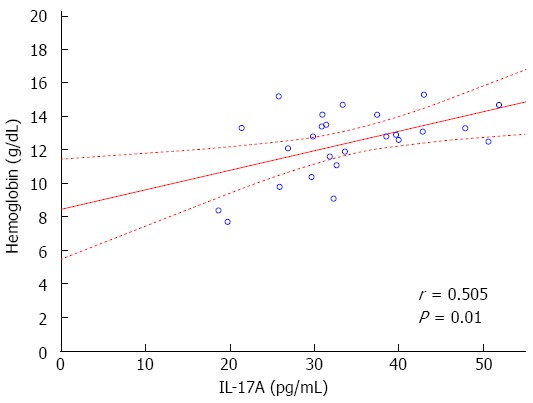
Scatter plot. Scatter plot showing correlation between hemoglobin rate and interleukin 17A (IL-17A) serum levels in Crohn’s disease patients (r = 0.505, P = 0.01).
In CD patients with skin lesions, a statistically significant positive correlation between the IL-17A serum concentration and Hb level was shown (r = 0.521, P = 0.047). In these patients correlations between IL-17A and Ht and PLT were observed, but without statistical significance (Hct: r = 0.4703 P = 0.077; PLT: r = -0.4808, P = 0.07). In CD patients with skin lesions there were no statistically significant correlations between cytokine serum concentrations and RBC, WBC, CRP levels or CDAI score.
DISCUSSION
Skin lesions induced by anti-TNF-α therapy in patients with CD consist of a heterogenic group of dermatopathologies. In our study, skin lesions occurred in 60% of CD patients during biological therapy and approximately 33% were of a polymorphic nature. A wide variety of skin lesions occurring in patients during the anti-TNF-α therapy was also observed. The most common types of dermatoses were psoriasiform eruptions or eczematiforms lesions. The face was the most commonly observed location; other privileged locations were scalp and intrascapular or presternal region.
Observations of skin lesions obtained in our study can be compared with a large comparative meta-analysis by Moustou et al[9], which evaluated skin complications during biological treatment regardless of the underlying disease. The occurrence of anti-TNF-α induced skin lesions was described as an adverse reaction of medium or high probability. The highest correlation concerned appearance of psoriasis or psoriasiform lesions. It was suggested that the skin manifestations may be the result of the participation of the same cytokines or combining activation of various immune pathways in the course of biological treatment in CD patients[9,11].
In this study we aimed at further characterizing the IL-17A and IL-23-related pathways in relation to anti-TNF-α therapy in patients with CD. The pathogenesis of autoimmune diseases is not fully understood. Although a similar cytokine activation profile is observed in IBD, psoriasis, rheumatoid arthritis and systemic lupus erythematosus, causing generalized immune imbalance, clinical symptoms are different and may involve various organs. Available data suggest that autoimmune diseases, including IBD, are probably connected with a genetic predisposition to specific tissue responses to elevated levels of proinflammatory cytokines[12].
The main immune pathway in CD is associated with disproportionate activation of Th1 cells. Th1 cells are responsible for the secretion of several proinflammatory cytokines, such as IL-2, IL-3, IL-12, IL-18, TGF-β, IFN-γ and TNF-α, which is the main proinflammatory cytokine in the persistent inflammation of the intestinal mucosa[1,2]. Recent studies point at other pathways in the pathogenesis of CD and suggest a strong link between IL-23, IL-17A, TNF-α and IFN-γ. In a study by Hovhannisyan et al[3] the elevated levels of IL-17A were observed in the mucosa and serum of CD patients. This may suggest that in some cases IBD development may be due to an excessive activation of TNF-α pathway and simultaneous Th17 lymphocyte activation dependent on IL-23. Another study demonstrated that CD4+ Th17 lymphocytes are responsible for the induced skin and gut inflammation[13].
Biological therapy interferes with the immune system and inhibits the Th1 lymphocytes pathway linked with TNF-α. As a result of the TNF-α dependent pathway inhibition, the precursor cells T cell CD4+ under the influence of TGF-β and IL-6 can enter the pathway dependent on IL-23. Increasing the level of IL-23 stimulates Th17 lymphocytes, which secrete mainly IL-17A, initiating the inflammatory response upon binding with specific receptors[8]. The location of these receptors, as they may be skin or intestinal mucosa, is responsible for clinical symptoms manifested by the CD patients[14,15].
Our study showed an increased level of IL-23 in all CD patients compared to a control group and was the highest in the group with skin manifestations. IL-23 is an important cytokine involved in the development of skin lesions during biological therapy in CD patients. Its role in lymphocyte differentiation is crucial in the formation of psoriasiform eruptions. Also, an increase in the number of lymphocytes and dendritic cells with abnormal expression of IL-23R on the surface can also play an important role in this process[16]. Several studies have shown that IL-23 is responsible for the final redirection of the immune system on the Th-17 pathway. Various cytokines, such as IL-12, IL-1β and IL-6 are responsible for the initial differentiation of immune activation, while IL-23 directly affects the proliferation of Th-17 lymphocytes[12,14,17].
In our study an increased level of IL-17A was detected in CD patients. IL-17 participate in the development of autoimmune disorders and include six polymorphic cytokines (IL-17A to IL-17F). In the pathogenesis of skin lesions, mainly psoriasiforms, the highest concentration of IL-17 variant A or F, or both of these cytokines were recorded. Lesions induced by an excess of IL-17A are characterized by hyperproliferation of keratinocytes and traceability of activated T-cells in the dermis[18,19]. Activated Th17 lymphocytes in the dermis secrete IL-17A and IL-22, which are probably the ultimate causative factor of skin lesions, based on the increased expression of proinflammatory genes in kerationcytes[20]. This report is confirmed by Ariza et al[8] in a mouse psoriasis model.
IL-17A and IL-23 play crucial role in the formation of skin lesions, mainly manifested as psoriasis and psoriasiform eruptions. Lesions are caused by many coexisting factors, such as immune imbalance caused by the CD and therapy with anti-TNF-α agents, abnormal infiltration of inflammatory cells in dermis, immune disorders secondary to malnutrition and anti-TNF-α agents, delamination of the skin layers, and secondary bacterial infections of skin lesions. In clinical practice most skin infections, such as furuncles and furunculosis were observed in the end of the cycle of therapy with anti-TNF-α agents, apparently due to the highest degree of suppression of TNF-α pathway and activation of pathways dependent on IL-23 and IL-17A.
In our study we observed a positive correlation between increased levels of IL-23 and IL-17A in all CD patients. A similar relationship in CD patients with skin lesions induced by anti-TNF-α therapy was found, which is in good agreement with previously published data[21,22].
We also aimed to evaluate the relationship between the occurrence of the skin lesions and serum concentrations of IL-17A, IL-23 and INF-γ in CD patients during the treatment with anti-TNF-α monoclonal antibodies. The study showed significant increase of the analyzed cytokine levels in the relation to the control group, which consisted of healthy subjects. Moreover, an association between IL-23 and IL-17A and the appearance of skin lesions in CD patients during the biological treatment was observed. Therefore we suggest that elevated levels of IL-23 and IL-17A may be responsible for the formation of skin lesions. Furthermore, since all tested cytokines were elevated in CD patients compared to the control group, the participation of these molecules in the inflammatory pathway of CD may also be suggested.
Finally, we observed a statistically significant relationship between Hb, Ht and PLT, the appearance of induced skin lesions and cytokine levels during the treatment with anti-TNF-α drugs. The levels of Hb, Ht and PLT may reflect an appropriate gut response to anti-TNF-α therapy and the reduction of intestinal inflammatory process. In our study a positive correlation between Hb and Ht with IL-17A were recorded. Lower level of PLT and higher levels of Hb and Ht suggested more significant suppression of TNF-α related gut inflammatory pathway and more excessive activation Th17 lymphocytes in the dermis in CD patients with skin lesions. This statement is also indicated by the occurrence of drug induced skin lesions in the late phase of anti-TNF-α therapy.
In conclusion, the use of targeted therapy and modulation of the immune response of the patient, mainly through anti-TNF-α drugs has become a growing trend in IBD treatment. Therefore, a clear understanding of IL-23/IL-17A and TNF-α immune pathways involved in intestinal inflammation in the course of CD, as well as side effects of anti-TNF-α drugs, including those concerning skin, will allow clinicians to select the appropriate therapy based on the individual patient cytokine profile.
Our study clearly showed that skin lesions in CD patients after biological therapy may result from significantly increased concentrations of IL-17A and IL-23, which are strongly associated with TNF-α/Th1 immune pathways. Our observations lead to a better understanding of the mechanisms underlying IBD and may be crucial for the development of new biological drugs, which could provide more effective induction and maintenance of the intestinal remission in IBD, with fewer side effects.
Further studies to confirm our observations in a larger cohort and to verify whether there are differences between the anti-TNF-α drugs used in terms of skin manifestations during biological therapy are thus warranted.
COMMENTS
Background
Skin lesions induced during anti-tumor necrosis factor (TNF)-therapy in patients with Crohn’s disease (CD) are important and often underestimated problem in clinical practice. According to recent studies, this broad and diverse group of side effects may be the result of an immune imbalance.
Research frontiers
The aim of this study was to investigate the correlation between the appearance of drug induced skin lesions and the concentration of interleukin (IL)-17A, IL-23 and interferon (IFN)-γ in Crohn’s disease patients during anti-TNF-α therapy.
Innovations and breakthroughs
Earlier studies reported drug induced skin lesion under biological therapy with anti-TNF-α agents. This study clearly showed that skin lesions in CD patients after biological therapy may result from significantly increased concentrations of IL-17A and IL-23, which are strongly associated with TNF-α/Th1 immune pathways.
Applications
The observations lead to a better understanding of the mechanisms underlying inflammatory bowel diseases (IBD) and may be crucial for the development of new biological drugs, which could provide more effective induction and maintenance of the intestinal remission in IBD, with fewer side effects.
Terminology
CD belongs to a group of IBD and is characterized by a chronic, segmental granulomatosus inflammation with periods of exacerbation and remission, which may involve any part of the gastrointestinal tract. Biological therapy with anti-TNF-α is a targeted therapy in CD patients, directed against the main proinflammatory cytokine. As skin lesions induced by anti-TNF-α therapy, dermatoses that have developed in the course of biological therapy in patients who had no pre-existing skin lesions of similar character were qualified.
Peer review
This is a nice small study correlating plasma levels of ILs and IFN with that of the appearance of skin lesions with biological treatment in CD patients. What is shown in highest expression levels of IL-17 and IL-23 in CD patients with anti-TNF-α drug induced skin lesions. The results are interesting and may represent an immunological mechanism of skin lesions induced by biological therapy in CD patients.
Footnotes
Supported by The student’s grant from the Foundation for Medical University of Lodz
P- Reviewers: Goral V, Martin-Villa JM, Scharl M, Steele SR S- Editor: Gou SX L- Editor: A E- Editor: Ma S
References
- 1.Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957–965. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakatos L, Pandur T, David G, Balogh Z, Kuronya P, Tollas A, Lakatos PL. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9:2300–2307. doi: 10.3748/wjg.v9.i10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ardizzone S, Puttini PS, Cassinotti A, Porro GB. Extraintestinal manifestations of inflammatory bowel disease. Dig Liver Dis. 2008;40 Suppl 2:S253–S259. doi: 10.1016/S1590-8658(08)60534-4. [DOI] [PubMed] [Google Scholar]
- 6.Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, Li S, Dooley LT, Arnold C, Gottlieb AB. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56:31.e1–31.15. doi: 10.1016/j.jaad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Hyams JS. Inflammatory bowel disease. Pediatr Rev. 2005;26:314–320. doi: 10.1542/pir.26-9-314. [DOI] [PubMed] [Google Scholar]
- 8.Ariza ME, Williams MV, Wong HK. Targeting IL-17 in psoriasis: from cutaneous immunobiology to clinical application. Clin Immunol. 2013;146:131–139. doi: 10.1016/j.clim.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Moustou AE, Matekovits A, Dessinioti C, Antoniou C, Sfikakis PP, Stratigos AJ. Cutaneous side effects of anti-tumor necrosis factor biologic therapy: a clinical review. J Am Acad Dermatol. 2009;61:486–504. doi: 10.1016/j.jaad.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 10.Wollina U, Hansel G, Koch A, Schönlebe J, Köstler E, Haroske G. Tumor necrosis factor-alpha inhibitor-induced psoriasis or psoriasiform exanthemata: first 120 cases from the literature including a series of six new patients. Am J Clin Dermatol. 2008;9:1–14. doi: 10.2165/00128071-200809010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Ramos-Casals M, Brito-Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, Cuadrado MJ, Khamashta MA. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007;86:242–251. doi: 10.1097/MD.0b013e3181441a68. [DOI] [PubMed] [Google Scholar]
- 12.Safrany E, Szabo M, Szell M, Kemeny L, Sumegi K, Melegh BI, Magyari L, Matyas P, Figler M, Weber A, et al. Difference of interleukin-23 receptor gene haplotype variants in ulcerative colitis compared to Crohn’s disease and psoriasis. Inflamm Res. 2013;62:195–200. doi: 10.1007/s00011-012-0566-z. [DOI] [PubMed] [Google Scholar]
- 13.Tillack C, Ehmann LM, Friedrich M, Laubender RP, Papay P, Vogelsang H, Stallhofer J, Beigel F, Bedynek A, Wetzke M, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γ-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2014;63:567–577. doi: 10.1136/gutjnl-2012-302853. [DOI] [PubMed] [Google Scholar]
- 14.Cayatte C, Joyce-Shaikh B, Vega F, Boniface K, Grein J, Murphy E, Blumenschein WM, Chen S, Malinao MC, Basham B, et al. Biomarkers of Therapeutic Response in the IL-23 Pathway in Inflammatory Bowel Disease. Clin Transl Gastroenterol. 2012;3:e10. doi: 10.1038/ctg.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319–324. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- 16.Thareja S, Paghdal K, Lien MH, Fenske NA. Reticular erythematous mucinosis--a review. Int J Dermatol. 2012;51:903–909. doi: 10.1111/j.1365-4632.2011.05292.x. [DOI] [PubMed] [Google Scholar]
- 17.Geremia A, Arancibia-Cárcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385–422. doi: 10.1146/annurev-pathol-011811-132448. [DOI] [PubMed] [Google Scholar]
- 19.Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535–546. doi: 10.1016/j.clindermatol.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 21.Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172–176. doi: 10.1590/s0004-28032012000200014. [DOI] [PubMed] [Google Scholar]
- 22.Furuya Y, Ozeki T, Takayanagi R, Yokoyama H, Okuyama K, Yamada Y. Theory based analysis of anti-inflammatory effect of infliximab on Crohn’s disease. Drug Metab Pharmacokinet. 2007;22:20–25. doi: 10.2133/dmpk.22.20. [DOI] [PubMed] [Google Scholar]


