Abstract
AIM: To assess the effectiveness of pancreatic stents for preventing pancreatitis in high-risk patients after endoscopic retrograde cholangiopancreatography (ERCP).
METHODS: PubMed, Embase, Science Citation Index, and Cochrane Controlled Trials Register were searched to identify relevant trials published in English. Inclusion and exclusion criteria were used to screen for suitable studies. Two reviewers independently judged the study eligibility while screening the citations. The methodological quality of the included trials was assessed using the Jadad scoring system. All results were expressed as OR and 95%CI. Data were analyzed using Stata12.0 software.
RESULTS: Ten eligible randomized controlled trials were selected, including 1176 patients. A fixed-effects model in meta-analysis supported that pancreatic duct stents significantly decreased the incidence of post-ERCP pancreatitis (PEP) in high-risk patients (OR = 0.25; 95%CI: 0.17-0.38; P < 0.001). Pancreatic stents also alleviated the severity of PEP (mild pancreatitis after ERCP: OR = 0.33; 95%CI: 0.21-0.54; P < 0.001; moderate pancreatitis after ERCP: OR = 0.30; 95%CI: 0.13-0.67; P = 0.004). The result of severe pancreatitis after ERCP was handled more rigorously (OR = 0.24; 95%CI: 0.05-1.16; P = 0.077). Serum amylase levels were not different between patients with pancreatic stents and control patients (OR = 1.08; 95%CI: 0.82-1.41; P = 0.586).
CONCLUSION: Placement of prophylactic pancreatic stents may lower the incidence of post-ERCP pancreatitis in high-risk patients and alleviate the severity of this condition.
Keywords: Pancreatic stent, Endoscopic retrograde cholangiopancreatography, Pancreatitis, Hyperamylasemia, Meta-analysis
Core tip: Post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis is the most common and serious complication of ERCP. In the past few decades, a number of clinical randomized controlled trials have shown that pancreatic stents can effectively prevent the occurrence of post-ERCP pancreatitis (PEP). In view of these high quality data, we conducted this meta-analysis to evaluate the effectiveness of pancreatic stent placement in preventing PEP in high-risk patients. Our results supported that pancreatic stent placement is an effective means to prevent PEP and alleviate the severity (mild and moderate) of PEP in high-risk patients. Furthermore, no serious complications were reported in subjects in the stent group.
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) plays a significant role in the diagnosis and treatment of bile duct and pancreatic diseases. Post-ERCP pancreatitis (PEP) is the most common and serious complication of ERCP, and the incidence of PEP ranges from 1% to 30%[1-5]. Independent patient-related and procedure-related risk factors for PEP have been previously reported[3-6], and the risk factors for PEP include female gender, post-ERCP pancreatic history, pancreatic sphincterotomy, sphincter of Oddi dysfunction, sphincter of Oddi manometry, difficult cannulation, pancreatic duct biopsy, and intraductal pancreatic duct ultrasonography. In high-risk patients undergoing ERCP, the incidence of pancreatitis after ERCP ranges from 30% to 40%[7-9]. In the past few decades, a number of clinical randomized controlled trials (RCTs) have shown that pancreatic stents can effectively prevent the occurrence of PEP. Several meta-analyses have also confirmed that placement of pancreatic duct stents significantly decreases the incidence of PEP and alleviates its severity[10-12]. However, all of the included articles in previous meta-analyses were published before 2008. In recent years, several high quality RCTs investigating pancreatic stent placement for the prevention of PEP have been published. In view of these newly added data, we conducted a meta-analysis to evaluate the effectiveness of pancreatic stent placement in preventing PEP in high-risk patients.
MATERIALS AND METHODS
Literature search and study selection
Articles published up to May 2013 were searched in the following databases: PubMed, Embase, Science Citation Index, and the Cochrane Controlled Trials Register. We searched for related RCTs that studied pancreatic stent placement for preventing the incidence of PEP in high-risk patients. The primary search terms were “pancreatic stent”, “ERCP”, and “post-ERCP pancreatitis”, which were limited to the article title and abstract, using human and randomized controlled trials as filters. Reference lists were manually handled to exclude the possibility of omission of eligible studies. This process was repetitively performed until no articles were identified as suitable candidates.
The inclusion criteria were as follows: (1) study type was prospective clinical RCTs, regardless of whether the trial was double-blind; (2) study patients included adults with risk factors and those undergoing ERCP; (3) intervention included pancreatic stent placement before or after ERCP; and (4) the incidence of PEP was required to be the primary outcome, and the severity of PEP and incidence of hyperamylasemia were secondary outcomes. Studies were excluded if any of the required information was not obtainable and if they were retrospective studies or published only in abstract form.
Data extraction and quality assessment
Two investigators (Shi QQ and Ning XY) independently extracted data from the included studies, and a common data form was used during this process. The form contained mainly the following information: first author, publication year, study type, patient characteristics, number of subjects, PEP outcomes, PEP severity, incidence of hyperamylasmia, stent type, and stent size. Any disagreements were resolved through discussion and consensus.
The Jadad scale was used to evaluate the quality of the included studies[13]. The scale included scores for 3 items: (1) randomization: suitable (2 points), randomized but unclear (1 point), and improper (0 points); (2) blinding: a suitable blind method was used (2 points), unclear blinding method (1 point), and improper blinding method (0 points); and (3) the dropouts and withdraws were recorded (1 point), not recorded dropouts and withdraws (0 point). This quality scale ranges from 0 to 5 points, and higher scores indicate better quality studies. If an article was given a score of 2 points or less, it was considered to be low quality, whereas articles scoring 3 or more were considered high quality.
Statistical analysis
We performed all statistical analyses using the Stata12.0 software. Categorical data are expressed with OR and 95%CI, and P values less than 0.05 represented statistical significance. The heterogeneity of studies was tested by the χ2 test, and I2 described the percentage of variability attributed to heterogeneity instead of sampling error. I2 values less than 25% indicated no heterogeneity, values between 25% (inclusive) and 50% represented low heterogeneity, values between 50% (inclusive) and 75% represented moderate heterogeneity, and values of 75% or above represented high heterogeneity. Based on these ranking of heterogeneity, a fixed-effects (low heterogeneity) or random-effects model (moderate or high heterogeneity) was selected.
RESULTS
Eligible studies
A comprehensive literature search was performed using PubMed, Embase, Science Citation Index, and the Cochrane Controlled Trials Register databases. The initial search identified a total of 238 articles. Among them, 208 articles were excluded because they were nonrandomized trials, duplicates, reviews, or used inappropriate interventions. Thirty relevant articles were selected and reviewed, but 20 articles were excluded subsequently due to duplication of data, publication in abstract form only, or absence of important outcomes. Eventually, 10 RCTs (including a total of 1176 subjects) met the inclusion criteria and were selected for review and analysis (Figure 1). Among the included studies, two articles had the same first author[14,15]; however, since these studies were independent RCTs, both articles were included in this meta-analysis.
Figure 1.
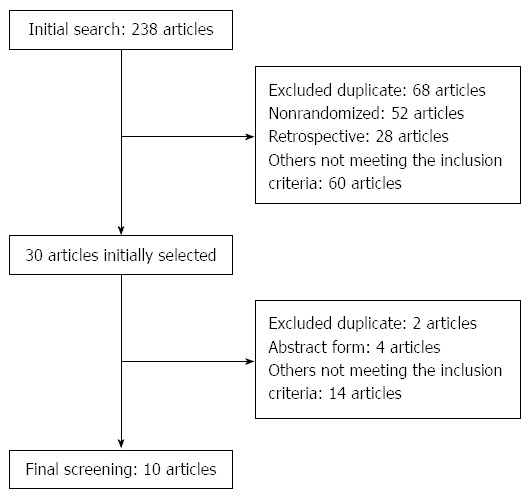
Article search and selection.
Characteristics of included studies
The Jadad scores and main characteristics of the included RCTs are shown in Table 1. The incidence and severity of PEP are presented in Table 2. The definition of post-PEP in all included trials was based on the Cotton’s criteria[9]. The degree of severity of PEP was graded from mild to severe: pancreatitis was considered mild if hospitalization was extended 2 to 3 d after the procedure, moderate if hospitalization was extended 4 to 10 d after the procedure. The incidence of hyperamylasemia after ERCP is described in Table 3. Hyperamylasemia was defined as an amylase level greater than three times the upper limit of normal in the majority of the studies. The incidence of PEP and its severity in patients who experienced failure of pancreatic stent placement are shown in Table 4. The studies were published between 1998 and 2012, and the sample size ranged from 19 to 407 subjects, with a total of 1176 subjects included in the analysis. Eight out of 10 studies reported using 5F stents, with lengths ranging from 2 to 5 cm.
Table 1.
Main characteristics of the included studies
| Ref. | Jadad | Total (n) | Risk factors/procedures | Stent type | Time for spontaneous dislodgement |
| Kawaguch et al[16] | 4 | 120 | History of previous PEP, difficult cannulation, SOD | 5F (3 cm) | 3 d 96.7% (58/60) |
| Lee et al[17] | 5 | 101 | Difficult cannulation | 3F (4, 6, 8 cm) | 7 d 94% (45/48) |
| Pan et al[18] | 2 | 40 | High-risk patients without | 5F | NA |
| Sofuni et al[14] | 5 | 407 | High-risk patients irrespective of the type of the risk factor | 5F (3 cm) | 3 d 95.7% (178/186) |
| Ito et al[19] | 4 | 70 | Difficult cannulation | 5F (4 cm) | NA |
| Sofuni et al[15] | 3 | 201 | High-risk patients irrespective of the type of the risk factor | 5F (3 cm) | 3 d 95.7% (90/94) |
| Tsuchiya et al[20] | 3 | 64 | Bile duct sphincterotomy | 5F (3, 4 cm) | NA |
| Harewood et al[21] | 4 | 19 | Endoscopic ampullectomy | 5F (3, 5 cm) | 1 d 60% (6/10) |
| Fazel et al[22] | 2 | 74 | Difficult cannulation, SOM, billiy ES | 5F (2 cm) | NA |
| Tarnasky et al[23] | 2 | 80 | SOD, SOM | 5F or 7F (2, 2.5 cm) | NA |
PEP: Post-endoscopic retrograde cholangiopancreatography pancreatitis; NA: Not available; ES: Endoscopic sphincterotomy; SOD: Sphincter of Oddi dysfunction; SOM: Sphincter of Oddi manometry.
Table 2.
Incidence and severity of post-endoscopic retrograde cholangiopancreatography pancreatitis
| Ref. |
Stent group (n) |
Non-stent group (n) |
P value | ||||||||
| S-sten | Mild PEP | Moderate PEP | Serve PEP | Total PEP | n | Mild PEP | Moderate PEP | Serve PEP | Total PEP | ||
| Kawaguch et al[16] | 60 | 1 | 0 | 0 | 1 | 60 | 8 | 0 | 0 | 8 | 0.032 |
| Lee et al[17] | 48 | 5 | 1 | 0 | 6 | 51 | 12 | 2 | 1 | 15 | 0.031 |
| Pan et al[18] | 20 | NA | 4 | 20 | NA | 14 | < 0.01 | ||||
| Sofuni et al[14] | 186 | 12 | 4 | 0 | 16 | 204 | 22 | 8 | 1 | 31 | 0.021 |
| Ito et al[19] | 32 | 0 | 0 | 0 | 0 | 35 | 8 | 0 | 0 | 8 | NA |
| Sofuni et al[15] | 94 | 2 | 1 | 0 | 3 | 103 | 8 | 6 | 0 | 14 | 0.019 |
| Tsuchiya et al[20] | 32 | 1 | 0 | 0 | 1 | 32 | 2 | 1 | 1 | 4 | NS |
| Harewood et al[21] | 10 | 0 | 0 | 0 | 0 | 8 | NA | 0 | 3 | 0.020 | |
| Fazel et al[22] | 38 | 2 | 0 | 0 | 2 | 32 | 5 | 2 | 3 | 10 | < 0.05 |
| Tarnasky et al[23] | 41 | 1 | 0 | 0 | 1 | 39 | 5 | 5 | 0 | 10 | 0.003 |
PEP: Post-endoscopic retrograde cholangiopancreatography pancreatitis; S-stent: Successful stent; NA: Not available; NS: Not significant.
Table 3.
Incidence of hyperamylasemia
| Ref. | Year |
Hyperamylasemia |
||
| Stent (n) | Control (n) | P value | ||
| Kawaguchi et al[16] | 2012 | 18 | 23 | 0.862 |
| Lee et al[17] | 2012 | 8 | 6 | 0.538 |
| Sofuni et al[14] | 2011 | 86 | 68 | 0.200 |
| Ito et al[19] | 2010 | 21 | 26 | NA |
| Sofuni et al[15] | 2007 | 31 | 34 | 0.996 |
| Tsuchiya et al[23] | 2007 | 11 | 16 | NS |
NA: Not available; NS : Not significant.
Table 4.
Incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis in subjects with unsuccessful stent placement
| Ref. | Candidate for stent (n) | Successful placement (n) | Unsuccessful placement (n) | PEP (n) |
| Lee et al[17] | 50 | 48 | 2 | 1 |
| Sofuni et al[14] | 203 | 186 | 17 | 3 |
| Ito et al[19] | 35 | 32 | 3 | 1 |
| Sofuni et al[15] | 98 | 94 | 4 | 0 |
PEP: Post-endoscopic retrograde cholangiopancreatography pancreatitis.
Quality assessment
The studies included in this meta-analysis were reasonably well designed and performed, with an average Jadad score of 3.4 points (Table 1). All of the trials were RCTs and had detailed records of dropouts and withdraws. Three trials described randomization methods, 5 studies used appropriate blinding, and all studies defined PEP according to the cotton index[9] as the primary outcome. Only two studies did not have detailed records of the severity of PEP[18,21].
Incidence of PEP
The incidence of PEP is shown in Table 3. All of the included RCTs reported the incidence of PEP in both the stent group and the non-stent group. A total of 1176 patients were included; of these, 156 patients developed PEP, including 34 patients in the stent group, 117 patients in the non-stent group, and 5 patients who underwent unsuccessful stent placement. The incidence of PEP was 13.27% (156/1176). Due to the low heterogeneity among the included studies (I2 = 17.0%, P = 0.286), a fixed-effects model was used to calculate ORs. The incidence of PEP was significantly different between subjects in the stent group and those in the non-stent group (OR = 0.25; 95%CI: 0.17-0.38; P < 0.001; Figure 2A).
Figure 2.
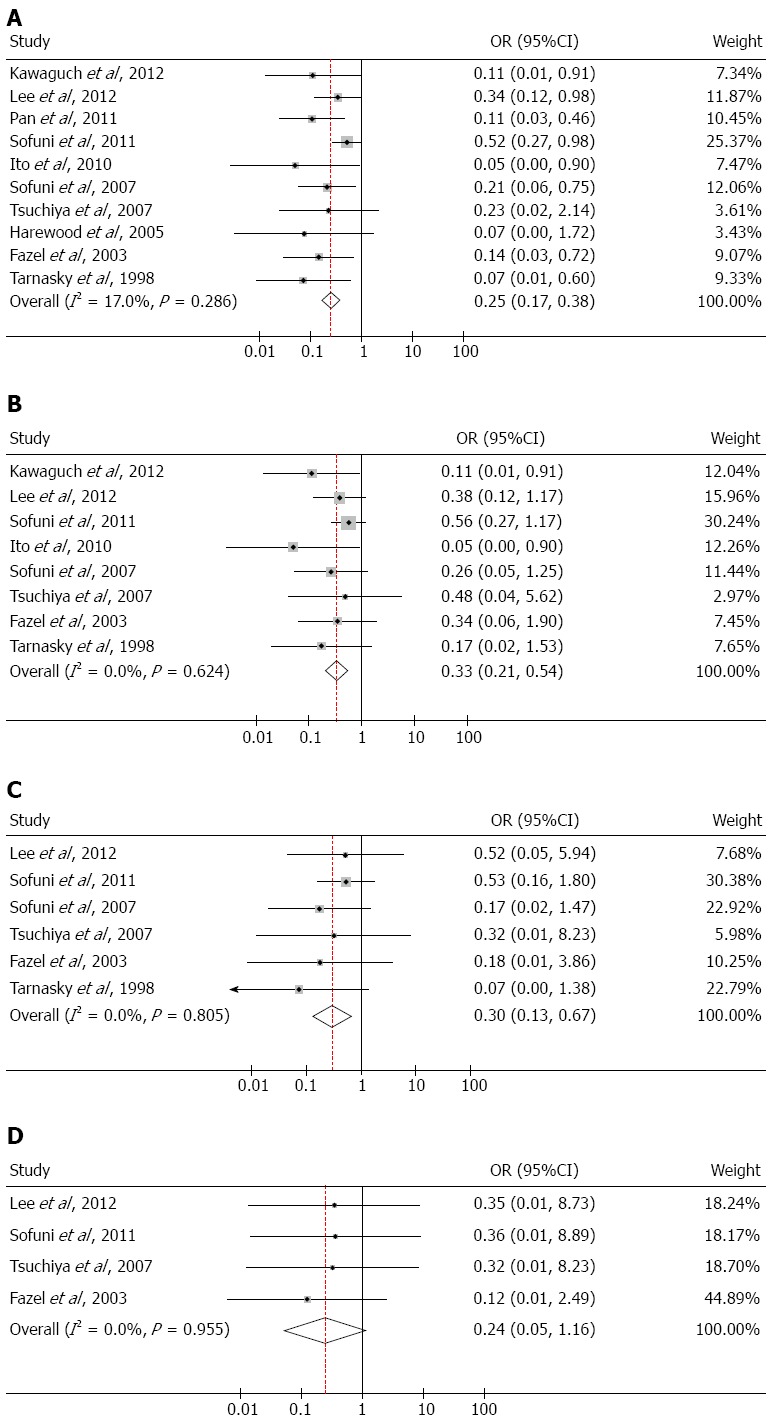
Forest plots. A: Forest plot showing the effects of pancreatic stents to prevent patients after post-endoscopic retrograde cholangiopancreatography (PEP); B: Forest plot showing the effects of pancreatic stent placement in the prevention of mild PEP; C: Forest plot showing the effects of pancreatic stents in the prevention of moderate PEP; D: Forest plot showing the effects of pancreatic stents in the prevention of severe PEP.
Sensitivity analysis
Subsequently, we performed sensitivity analyses to explore the stability of the pooled results. Exclusion of three low-quality studies (Jadad score ≤ 2) only slightly changed the overall estimate (OR = 0.31; 95%CI: 0.20-0.49; P < 0.001) and still resulted in low heterogeneity I2 = 3.5%, P = 0.399). Exclusion of one study with the largest sample size (N = 407) also only slightly changed the overall estimate (OR = 0.16; 95%CI: 0.09-0.28, P < 0.001), and no evidence of heterogeneity was observed among the remaining studies (I2 = 0.9%, P = 0.847). Further exclusion of any single trial also did not significantly alter the overall combined OR (data not shown).
Incidence of mild PEP
The incidence of mild PEP is shown in Table 3. Eight out of 10 RCTs reported the incidence of mild PEP. No heterogeneity was found between these studies (I2 = 0.0%, P = 0.624), and a fixed-effects model was used to incorporate ORs. The outcome of statistical analysis demonstrated that pancreatic stents could effectively prevent the occurrence of mild PEP (OR = 0.33; 95%CI: 0.21-0.54; P < 0.001; Figure 2B).
Incidence of moderate PEP
As shown in Table 3, among the 8 RCTs that reported the severity of PEP, two studies reported no moderate PEP in either the stent group or the non-stent group. There was no significant change with these studies incorporated into the analysis, and thus, they were excluded. No heterogeneity was found in the remaining studies (I2 = 0.0%, P = 0.805), and a fixed-effects model was used to incorporate ORs. The results indicated that pancreatic stents were also effective in the prevention of moderate PEP (OR = 0.30; 95%CI: 0.13-0.67; P = 0.004; Figure 2C).
Incidence of severe PEP
As shown in Table 3, nine studies reported that no subject in the stent group experienced severe PEP. Among these studies, four reported the occurrence of severe PEP in six patients in the non-stent group. The other five trials were excluded from this analysis since no severe PEP was reported. No heterogeneity was found in the four included studies (I2 = 0.0%, P = 0.955), and a fixed-effects model was used to incorporate ORs. The incidence of severe PEP was not significantly different between the stent group and the non-stent group (OR = 0.24; 95%CI: 0.05-1.16; P = 0.077; Figure 2D).
Incidence of hyperamylasemia
Six studies reported the incidence of hyperamylasemia after ERCP, and low heterogeneity was found among these studies (I2 = 26.5%, P = 0.236); therefore, a fixed-effects model was used to analyze these data. The results demonstrated that pancreatic stent placement did not increase the incidence of hyperamylasemia (OR = 1.08; 95%CI: 0.82-1.41; P = 0.586; Figure 3).
Figure 3.
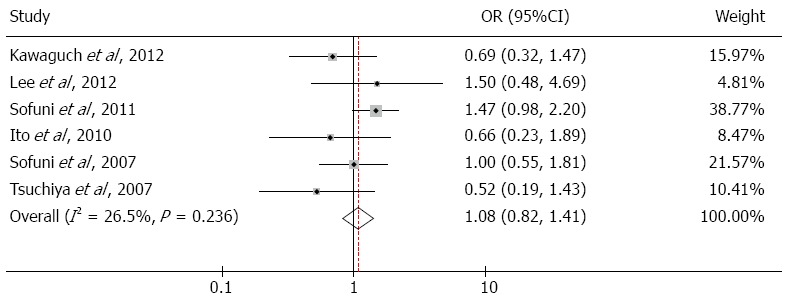
Forest plot of hyperamylasemia.
Publication bias
Assessment of publication bias using Begg’s tests showed that there was no potential publication bias among the included trials (Begg’s test, P = 0.210; Figure 4).
Figure 4.
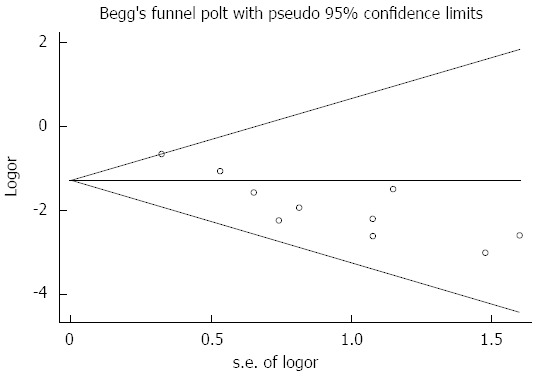
Tests for publication bias for odds ratio of the incidence of post-endoscopic retrograde cholangiopancreatography.
DISCUSSION
PEP remains the most common and serious complication of ERCP. Some researchers have tried to solve this problem by means of drug research or related interventions[24-26]. However, more researches have investigated the effectiveness of pancreatic stents in prevention of PEP[14-23]. Three previous meta-analyses have reported that pancreatic stent placement is an effective method to prevent PEP[10-12]. However, some of these meta-analyses included analysis of abstracts rather than full articles, and no high-quality analysis concerning the incidence of PEP following stent placement in high-risk patients had been performed. Therefore, we conducted the current meta-analysis to provide better evidence for the effectiveness of pancreatic stent placement in preventing PEP.
Our analysis demonstrated that prophylactic pancreatic stent placement before or after ERCP significantly decreased the odds of PEP occurrence in high-risk patients. Additionally, our analysis demonstrated that stent placement also significantly prevented both mild and moderate PEP. These data were similar to those of previously published meta-analyses[10,11]. However, we also found that prophylactic pancreatic stent placement did not reduce the incidence of severe PEP, and further careful analyses must be performed to better understand this finding. As shown in Table 2, among all the included trials, no occurrence of severe PEP was found in the stent group, while six patients suffered from severe PEP in the non-stent group, yielding an incidence of 0.51% (6/1176); this was too low to obtain significant results with such a small sample size. A domestic meta-analysis[10] demonstrated statistically significant results using the same type of analysis; however, this study included studies published before 2008 only and included three abstracts, which makes its reliability hard to assess. Therefore, additional prospective and high-quality studies are needed to evaluate whether pancreatic stents can prevent severe PEP.
An analysis of the incidence of post-ERCP hyperamylasemia was also performed. Importantly, pancreatic stent placement did not increase the incidence of post-ERCP hyperamylasemia. However, this result was inconsistent with a previous meta-analysis[11], which suggested that stents could decrease the occurrence of hyperamylasemia. Since our study used a different index to define hyperamylasemia, it is difficult to determine which study is more accurate. Five out of 10 RCTs reported the occurrence of abdominal pain[14,15,18,20,21]; however, various definitions were used in the studies, and it was impossible to synthesize the data in this regard. Other relevant complications after pancreatic stent placement were recorded in some studies. For example, 4 studies[14-16,22] reported no occurrence of hemorrhage, perforation, infection (cholangitis or cholecytitis), or other complications in the stent group. One study[19] reported mild cholangitis in the stent group, and another study[23] reported mild cholangitis and guidewire perforation during stent placement. The remaining four articles did not give details on complications during or after stent placement. Analysis based on the above-mentioned six studies (including 475 subjects) revealed that two (0.42%) subjects experienced cholangitis and one (0.21%) subject had a perforation. Bases on these low incidences of complications, we concluded that pancreatic stent placement is a very safe procedure, consistent with the report by Ding[27], who demonstrated that placement of pancreatic stents was safe during emergency ERCP for acute biliary pancreatitis and difficult sphincterotomy. However, further prospective trials are needed to confirm this viewpoint.
Four RCTs[14,15,17,19] reported the incidence of unsuccessful stent placement (Table 4), and no placement failure occurred in the other six RCTs. Of these studies, a total of 587 patients were allocated to the stent group, and failure occurred in 26 subjects (4.43%). Importantly, of these 26 subjects, five acquired mild pancreatitis (19.23%), while no moderate or severe pancreatitis occurred. This percentage was higher than that in subjects with successful stent placement (6.06%, 34/561), but the incidence of PEP among these high-risk patients reached 30%-40%[7-9]. Therefore, it is hard to conclude whether the failure of stent placement results in a higher incidence of PEP.
Five studies reported the rate of spontaneous dislodgment of stents, as shown in Table 1. Various endpoints of time were used. No guidelines are available describing how long the stent must remain correctly placed to obtain the greatest effectiveness for preventing PEP. Conigliaro et al[28] and Cha et al[29] conducted an RCT to address this question. They randomly allocated patients with successfully placed stents to two groups: one underwent immediate removal of the stent after the procedure, and the other group did not have the stent removed for a period of 7-10 d[28] or 96 h[29]. Both studies demonstrated that the incidence of PEP was higher in subjects who underwent immediate removal; therefore, leaving the stent in place for a period of 4-10 d is an excellent choice to prevent PEP. However, further studies are required to determine the optimal stent placement time.
Among the 10 RCTs, eight studies selected 5F stents (with lengths ranging from 2 to 5 cm), and the other two studies used 3F or 5F stents. Chahal et al[30] compared the stability of 5F (3 cm) and 3F (8 cm or longer) stents and found that 5F stents exhibited a higher frequency of spontaneous dislodgement (P < 0.0001). Additionally, the incidence of PEP was lower (although no statistical significance was found: P = 0.3), and a higher rate of successful stent placement was observed (P = 0.0003). Zolotarevsky et al[31] also proved that 5F stents were more effective than 3F stents in the prevention of PEP. However, these results were contradictory with a retrospective study[32]. Therefore, more prospective RCTs are needed to confirm which stent type is optimal for prevention of PEP.
Our study has some limitations. First, we only searched relevant trials published in English in four databases, and this conduct may result in sample bias and geographical bias. Second, various lengths of stents were used, which may result in evaluation bias. Finally, included studies included in this analysis are not sufficient to confirm the relationship between pancreatic stents and severe PEP.
In conclusion, this meta-analysis supported that pancreatic stent placement is an effective means to prevent PEP and alleviate the severity (mild and moderate) of PEP in high-risk patients. Furthermore, no serious complications were reported in subjects in the stent group. However, more high-quality RCTs are needed to further confirm whether pancreatic stent placement can prevent severe PEP as well. Additionally, more studies are needed to determine the optimal time for stent placement and whether 3F or 5F stents are most appropriate. Thus, more prospective RCTs are needed to resolve these disagreements.
COMMENTS
Background
Post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) is the most common and serious complication of ERCP, and the incidence of PEP ranges from 30% to 40% in high-risk patients. A number of clinical randomized control trials have shown that pancreatic stents can effectively prevent the occurrence of PEP, however, whether pancreatic stent placement contributes to prevent PEP remains uncertain.
Research frontiers
In recent years, several meta-analyses have confirmed that placement of pancreatic duct stents significantly reduces the incidence of PEP and alleviates its severity. However, all of the included articles in previous meta-analyses were published before 2008.
Innovations and breakthroughs
Based on this meta-analysis, pancreatic stent placement is an effective means to prevent PEP and alleviate the severity (mild and moderate) of PEP in high-risk patients. Different from the previous studies, our conclusions was based on high quality articles. Furthermore, the authors also pay attention to the complications in the stent group.
Applications
Evidence suggested that pancreatic stents can effectively prevent the occurrence of PEP (mild and moderate), and these results will provide valuable information to the doctors to prevent post-ERCP pancreatitis.
Peer review
PEP is the most common and serious complication of ERCP. The authors performed a good analysis to deal with this important issue and found that pancreatic stent placement is an effective means to prevent PEP and alleviate its severity.
Footnotes
Supported by The Natural Science Foundation of Guangxi Zhuang Autonomous Region, China, No. 2012GXNSFAA053143 and No. 1355005-3-2
P- Reviewers: Abu-Zidan FM, Hardt PD, Jokic R S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Abdel Aziz AM, Lehman GA. Pancreatitis after endoscopic retrograde cholangio-pancreatography. World J Gastroenterol. 2007;13:2655–2668. doi: 10.3748/wjg.v13.i19.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 3.Cheng CL, Sherman S, Watkins JL, Barnett J, Freeman M, Geenen J, Ryan M, Parker H, Frakes JT, Fogel EL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol. 2006;101:139–147. doi: 10.1111/j.1572-0241.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 4.Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425–434. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
- 5.Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781–1788. doi: 10.1111/j.1572-0241.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 6.Moffatt DC, Coté GA, Avula H, Watkins JL, McHenry L, Sherman S, Lehman GA, Fogel EL. Risk factors for ERCP-related complications in patients with pancreas divisum: a retrospective study. Gastrointest Endosc. 2011;73:963–970. doi: 10.1016/j.gie.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb K, Sherman S. ERCP and biliary endoscopic sphincterotomy-induced pancreatitis. Gastrointest Endosc Clin N Am. 1998;8:87–114. [PubMed] [Google Scholar]
- 8.Freeman ML. Adverse outcomes of ERCP. Gastrointest Endosc. 2002;56:S273–S282. doi: 10.1067/mge.2002.129028. [DOI] [PubMed] [Google Scholar]
- 9.Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 10.He LX, Gong P, Bie LK. The pancreatic stents to prevent the high risk patient s from post-ERCP pancreatitis: a meta-analysis. Zhonghua Xiaohua Neijing Zazhi. 2010;27:94–98. [Google Scholar]
- 11.Choudhary A, Bechtold ML, Arif M, Szary NM, Puli SR, Othman MO, Pais WP, Antillon MR, Roy PK. Pancreatic stents for prophylaxis against post-ERCP pancreatitis: a meta-analysis and systematic review. Gastrointest Endosc. 2011;73:275–282. doi: 10.1016/j.gie.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 12.Mazaki T, Masuda H, Takayama T. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2010;42:842–853. doi: 10.1055/s-0030-1255781. [DOI] [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Sofuni A, Maguchi H, Mukai T, Kawakami H, Irisawa A, Kubota K, Okaniwa S, Kikuyama M, Kutsumi H, Hanada K, et al. Endoscopic pancreatic duct stents reduce the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients. Clin Gastroenterol Hepatol. 2011;9:851–88; quiz e110. doi: 10.1016/j.cgh.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Sofuni A, Maguchi H, Itoi T, Katanuma A, Hisai H, Niido T, Toyota M, Fujii T, Harada Y, Takada T. Prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis by an endoscopic pancreatic spontaneous dislodgement stent. Clin Gastroenterol Hepatol. 2007;5:1339–1346. doi: 10.1016/j.cgh.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi Y, Ogawa M, Omata F, Ito H, Shimosegawa T, Mine T. Randomized controlled trial of pancreatic stenting to prevent pancreatitis after endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2012;18:1635–1641. doi: 10.3748/wjg.v18.i14.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee TH, Moon JH, Choi HJ, Han SH, Cheon YK, Cho YD, Park SH, Kim SJ. Prophylactic temporary 3F pancreatic duct stent to prevent post-ERCP pancreatitis in patients with a difficult biliary cannulation: a multicenter, prospective, randomized study. Gastrointest Endosc. 2012;76:578–585. doi: 10.1016/j.gie.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Pan XP, Dang T, Meng XM, Xue KC, Chang ZH, Zhang YP. Clinical study on the prevention of post-ERCP pancreatitis by pancreatic duct stenting. Cell Biochem Biophys. 2011;61:473–479. doi: 10.1007/s12013-011-9230-4. [DOI] [PubMed] [Google Scholar]
- 19.Ito K, Fujita N, Noda Y, Kobayashi G, Obana T, Horaguchi J, Takasawa O, Koshita S, Kanno Y, Ogawa T. Can pancreatic duct stenting prevent post-ERCP pancreatitis in patients who undergo pancreatic duct guidewire placement for achieving selective biliary cannulation? A prospective randomized controlled trial. J Gastroenterol. 2010;45:1183–1191. doi: 10.1007/s00535-010-0268-7. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchiya T, Itoi T, Sofuni A, Itokawa F, Kurihara T, Ishii K, Tsuji S, Kawai T, Moriyasu F. Temporary pancreatic stent to prevent post endoscopic retrograde cholangiopancreatography pancreatitis: a preliminary, single-center, randomized controlled trial. J Hepatobiliary Pancreat Surg. 2007;14:302–307. doi: 10.1007/s00534-006-1147-8. [DOI] [PubMed] [Google Scholar]
- 21.Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367–370. doi: 10.1016/j.gie.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Fazel A, Quadri A, Catalano MF, Meyerson SM, Geenen JE. Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study. Gastrointest Endosc. 2003;57:291–294. doi: 10.1067/mge.2003.124. [DOI] [PubMed] [Google Scholar]
- 23.Tarnasky PR, Palesch YY, Cunningham JT, Mauldin PD, Cotton PB, Hawes RH. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology. 1998;115:1518–1524. doi: 10.1016/s0016-5085(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 24.Shah TU, Liddle R, Branch MS, Jowell P, Obando J, Poleski M. Pilot study of aprepitant for prevention of post-ERCP pancreatitis in high risk patients: a phase II randomized, double-blind placebo controlled trial. JOP. 2012;13:514–518. doi: 10.6092/1590-8577/855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otsuka T, Kawazoe S, Nakashita S, Kamachi S, Oeda S, Sumida C, Akiyama T, Ario K, Fujimoto M, Tabuchi M, et al. Low-dose rectal diclofenac for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a randomized controlled trial. J Gastroenterol. 2012;47:912–917. doi: 10.1007/s00535-012-0554-7. [DOI] [PubMed] [Google Scholar]
- 26.Ding X, Chen M, Huang S, Zhang S, Zou X. Nonsteroidal anti-inflammatory drugs for prevention of post-ERCP pancreatitis: a meta-analysis. Gastrointest Endosc. 2012;76:1152–1159. doi: 10.1016/j.gie.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Ding G, Qin M, Cai W, Zou F, Zhao H. The safety and utility of pancreatic duct stents in the emergency ERCP of acute biliary pancreatitis but difficult sphincterotomy. Hepatogastroenterology. 2012;59:2374–2376. doi: 10.5754/hge12363. [DOI] [PubMed] [Google Scholar]
- 28.Conigliaro R, Manta R, Bertani H, Manno M, Barbera C, Caruso A, Olivetti G, Melotti G, Frazzoni M. Pancreatic duct stenting for the duration of ERCP only does not prevent pancreatitis after accidental pancreatic duct cannulation: a prospective randomized trial. Surg Endosc. 2013;27:569–574. doi: 10.1007/s00464-012-2487-x. [DOI] [PubMed] [Google Scholar]
- 29.Cha SW, Leung WD, Lehman GA, Watkins JL, McHenry L, Fogel EL, Sherman S. Does leaving a main pancreatic duct stent in place reduce the incidence of precut biliary sphincterotomy-associated pancreatitis? A randomized, prospective study. Gastrointest Endosc. 2013;77:209–216. doi: 10.1016/j.gie.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Chahal P, Tarnasky PR, Petersen BT, Topazian MD, Levy MJ, Gostout CJ, Baron TH. Short 5Fr vs long 3Fr pancreatic stents in patients at risk for post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2009;7:834–839. doi: 10.1016/j.cgh.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Zolotarevsky E, Fehmi SM, Anderson MA, Schoenfeld PS, Elmunzer BJ, Kwon RS, Piraka CR, Wamsteker EJ, Scheiman JM, Korsnes SJ, et al. Prophylactic 5-Fr pancreatic duct stents are superior to 3-Fr stents: a randomized controlled trial. Endoscopy. 2011;43:325–330. doi: 10.1055/s-0030-1256305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rashdan A, Fogel EL, McHenry L, Sherman S, Temkit M, Lehman GA. Improved stent characteristics for prophylaxis of post-ERCP pancreatitis. Clin Gastroenterol Hepatol. 2004;2:322–329. doi: 10.1016/s1542-3565(04)00062-x. [DOI] [PubMed] [Google Scholar]


