Abstract
Mucoepidermoid carcinoma (MEC) is a rare primary esophageal malignancy. It is characterized by poor clinical recognition, pre-operative diagnostic challenges and a lack of standardized therapeutic guidelines. We report the clinicopathological features of a hitherto unreported variant of esophageal MEC, sclerosing MEC with “tissue eosinophilia”, in a mid-esophageal location in a 51-year-old female. The diagnosis of the initial biopsy was challenging, because of the small size, poor orientation and inadequate representation of the MEC components. Recognition of the resectability of the tumor prompted surgical resection and enabled a demonstration of the low grade foci containing intermediate cells, mucin pools and the hitherto undescribed presence of stromal sclerosis and tissue eosinophils in esophageal MEC. Heightened clinicopathological awareness of esophageal MEC facilitated a definitive diagnosis and patient management. Increased recognition and global documentation of esophageal sclerosing MEC with “tissue eosinophilia” is necessary to improve the understanding and diagnosis of this malignancy in this location and to improve management guidelines.
Keywords: Mucoepidermoid, Carcinoma, Sclerosing, Tissue eosinophilia, Esophagus
Core tip: Primary mucoepidermoid cancer (MEC) of the esophagus, including the sclerosing variant, is rarely reported, and sclerosing MEC with “tissue eosinophilia” has never been reported in this location. The rarity of this latter condition has precluded a thorough understanding of esophageal MEC. Heightened recognition of MEC in this location is also necessary to distinguish MEC from squamous carcinoma, adenocarcinoma or adenosquamous carcinoma. This distinction has therapeutic implications. Based largely on past experience managing tumors in the salivary gland, MEC is characterized by a poor response to adjuvant chemotherapy and radiotherapy; notwithstanding a poor response to surgery, this treatment approach remains the mainstay of management.
INTRODUCTION
Mucoepidermoid carcinoma (MEC) is the most common malignant neoplasm in the major and minor salivary glands[1]. It may also arise in other organs, including the bronchi, lacrimal sac, thyroid gland and, rarely, the esophagus. While MEC is characterized by variable proportions of malignant squamoid, mucous and intermediate cells, several histopathological variants, including the clear cell, oncocytic, sebaceous, spindle cell and psammomatous types, have been documented[2]. More recently, sclerosing MEC with an intense sclerosing pattern has been documented[3]. In this subtype, some tumors may also demonstrate an infiltrate of eosinophils, which have been labelled “sclerosing MEC with eosinophilia”. Primary MEC of the esophagus is reported uncommonly, accounting for less than 1% of all primary esophageal cancers[4].
While the rarely described, sclerosing variant of esophageal MEC has been reported in 4 patients[5], to date, sclerosing MEC of the esophagus with “tissue eosinophilia” is undocumented in the literature. In reporting for the first time a primary esophageal sclerosing mucoepidermoid carcinoma with “tissue eosinophilia” (SMCE), we describe the clinicopathological features and also compare and contrast the features of the index tumor with those of other sclerosing esophageal MECs in the world literature. In addition, the pathogenesis of sclerosis and stricture formation in SMCE are discussed.
CASE REPORT
Clinical features
A 51-year-old Indian female presented to our institution with a 2 mo history of progressive dysphagia. She was able to tolerate a soft diet on presentation. Barium swallow revealed a malignant-appearing stricture in the mid-thoracic region (Figure 1A). Endoscopic assessment revealed an ulcerated lesion at 31 cm. Biopsy of the lesion demonstrated a mucoepidermoid carcinoma of the esophagus. The tumor was deemed resectable on computed tomography scan, and the patient was found to be medically fit for an esophageal resection. She was subjected to an exploration. Intra-operatively, tumor adherence to the descending aorta was noted. A 3-stage total esophagectomy was performed by shaving the tumor off the aorta, followed by performing a gastric pull-up and esophago-gastrostomy in the neck. The tumor bed was clipped with titanium clips. Our patient had an uneventful recovery post operatively and was referred to our local oncology unit for further management.
Figure 1.
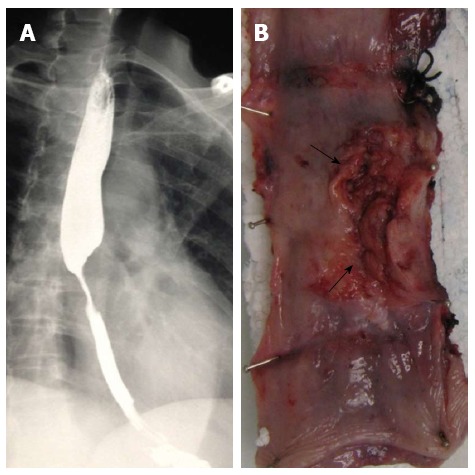
Clinical features. A: Contrast swallow with mid-esophageal stenosis; B: Partially ulcerated annular tumor (arrows).
Pathological features
The resected esophagectomy specimen contained a fungating tumor that measured 52 and 70 mm in the cross-sectional and longitudinal dimensions (Figure 1B). Luminal stenosis and mural thickening were noted in the area of the tumor. A single serosal lymph node was identified. Microscopic assessment confirmed an infiltrative tumor that was composed of a variable admixture of squamoid (Figure 2A), glandular (Figure 2B) and intermediate cells (Figure 2C). High-grade foci demonstrated pleomorphic solid islands of cells with a squamoid appearance characterized by intercellular bridges, focal squamous pearls and dyskeratotic cells, prominent apoptosis and brisk, including atypical, mitotic activity. Intraepithelial neutrophilic aggregates and mucin pools were noted (Figure 2C). Intracytoplasmic and luminal mucin production was confirmed on Southgate mucicarmine staining (Figure 2D). Rupture of the mucin pools and stromal extravasation of the mucin were noted. Discrete, separate glandular structures were not observed. In addition, there were large areas of fibrosis, keloid-like sclerosis (Figure 2E), desmoplasia and an inflammatory infiltrate composed of a prominence of eosinophils (Figure 2A), neutrophils, plasma cells and lymphoid aggregates. Perineurial invasion was observed, but lymphovascular invasion was absent. The diagnosis of the sclerosing variant of mucoepidermoid carcinoma with “tissue eosinophilia” was confirmed.
Figure 2.
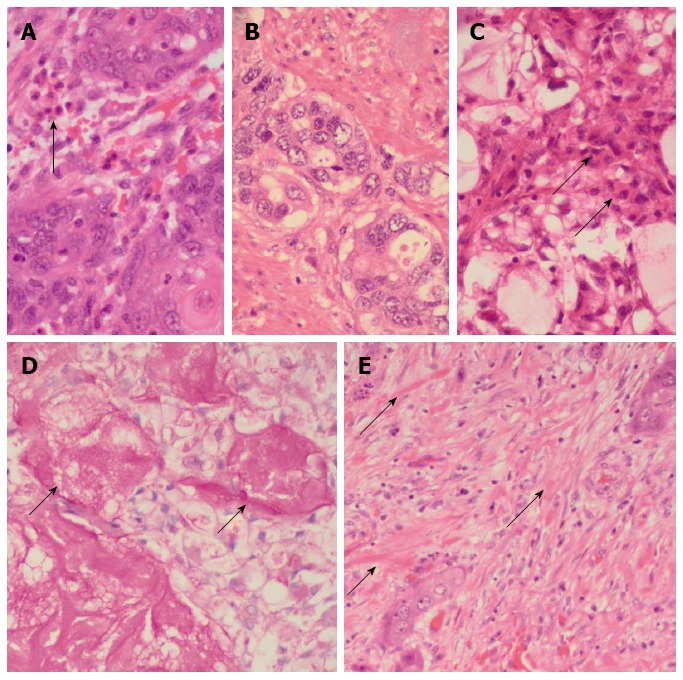
Pathological features. A: Islands of malignant squamoid cells with surrounding stromal eosinophils (arrow) [hematoxylin and eosin (HE), × 480]; B: Glandular foci (HE, × 480); C: Foci of intermediate (arrows) and clear cells and of the cystically dilated mucinous component (HE, × 480); D: Southgate mucicarmine stain demonstrating the luminal (arrows) and intracytoplasmic mucin (southgate mucicarmine, × 480); E: Sclerotic stroma with keloid-like areas (arrows) (HE, × 480).
DISCUSSION
Esophageal MEC occurs mainly in men in their sixth decades. Characterized by the predominant involvement of the middle and lower thirds of the esophagus[6], the exact micro-anatomic origins of these tumors are debatable. The hypothesis that these tumors arise from the esophageal glandular or ductal epithelium[7] is supported by their submucosal location, microscopic findings of normal stratified squamous epithelium overlying the MEC[8] and the common embryological derivation of esophageal and salivary glands[6]. In contrast, the view of some authors, that MEC arises through dysplasia in metaplastic surface squamous epithelia, is supported by documented dysplastic alterations in the surface epithelium[5].
Sclerosing MEC, which was first described in 1987 in the salivary gland[9], is typified by its occurrence across a wide age range and a male predilection. While intratumoral eosinophils and peripheral lymphoid aggregates are variable findings in sclerosing MEC, the widespread and obliterative keloidal-type sclerosis sets it apart from other sclerosing conditions in this location[2,3]. Sclerosing MEC of the esophagus has been reported in 4 patients, but sclerosing SMCE of the esophagus is unreported to date. All reported sclerosing MECs and the index case have mid-esophageal involvement, with variable proximal or distal extension in two patients. Dysphagia and stenosing lesions were common findings in all patients. While all reported tumors and the index case demonstrated stromal sclerosis, the exact etiopathogenesis of this sclerosing phenomenon is unresolved. Some authors have speculated that the sclerosing process is a bystander effect[10], while others have suggested an ischaemic pathomechanism[9]. In addition, the presence of extravasated mucin pools throughout the tumor underpins the mucin extravasation theory that posits the temporal sequence of mucin extravasation, an associated inflammatory reaction and subsequent fibrosis[9,11]. More recently eosinophils have been identified in sclerosing MEC of the salivary and thyroid gland, in association with lymphocytic thyroiditis[12]. In the former location, a role for immunoglobulin G4 (IgG4)-positive plasma cells has also been proposed with pathogenetic extension to include a role for IgG-4 related chronic sclerosing inflammation[13].
The exact etiology and pathogenesis of eosinophilic infiltration of cancer in general, and MEC in particular, remain unclear[10,14,15]. The neoplastic milieu contains tumor-associated secretions and released factors that orchestrate qualitative differences in inflammatory cells, recruited stem cells and reparative angiogenic and stromagenic responses[14]. The regulation of the recruitment of eosinophils in cancer is controversial. Some workers hypothesize an interplay of innate and adaptive immune reactions, mainly those elicited by the cytokine activity of T-helper 2 and mast cells[16,17]. Although “tumor-associated tissue eosinophilia” (TATE) is defined as eosinophilic infiltration of a tumor not associated with tumor necrosis or ulceration[18], some workers advocate that necrotic foci within tumors release eosinophil-chemotactic factors[19], including eotoxin, a selective eosinophil chemo-attractant, that mediates eosinophil recruitment[15-17]. Sclerosing MEC with eosinophilia of the thyroid gland is associated with lymphocytic thyroiditis. In this setting, it remains to be seen whether the eosinophils are part of an auto-immune process or are tumor-induced. Fadare et al[3] hypothesized that the stromal fibrocellular response was characterized by a “temporal evolutionary spectrum”[3] in which a predominance of inflammatory cells and sclerosis represented the early and late stages of the spectrum, respectively. In the index esophageal MEC, ruptured mucinous cysts, extravasation of mucin and an associated inflammatory reaction were noted focally, but the sclerosing stromal response with eosinophils that intimately associated with and surrounded the high-grade tumor nests lacked stromal mucin when analyzed using routine and special stains. Based on the theory of Fadare et al[3], we hypothesized that, even within a single SMEC with eosinophilia, including the index esophageal SMEC, the “temporal evolutionary spectrum” applies, with mucin being identified in the lower grade areas with less stromal fibrosis, while mucin is absent in the densely sclerotic foci with inflammatory cells, including eosinophils.
The relationship between TATE and prognosis is debatable and varies in tumors within a single anatomical location and in different locations[18,20-23]. Some authors have demonstrated an association between TATE and advanced patient age in laryngeal carcinoma, stromal invasion in advanced clinical stage oral squamous carcinoma[22] and an association among specific histopathological carcinoma subtypes[24]. TATE has also been associated with variable patient survival outcomes[20-23]. The prognostic significance not only of eosinophils but also of the sclerosis in SMEC is poorly elucidated. This is mainly a function of the rarity of MECs with these histomorphological attributes[3]. Urano et al[10] suggested that the eosinophilic infiltrate in SMEC was responsible for the stromal fibrosis and the decrease in the epithelial component. The former was attributed to the impact of eosinophils on transforming growth factor (TGF)-β1-accelerated synthesis of DNA by fibroblasts. Additionally, eosinophils produce angiogenic factors, including vascular endothelial growth factor, fibroblast growth factor-2 and TGF-α, that induce stromal neovascularization[10,23]. Eosinophils influence interleukin-4-mediated tumor destruction in mice[25]. It remains debatable whether eosinophils in cancers, including in the index SMEC, represent part of the host’s immunosurveillance armamentarium against the tumor or whether they promote cancer growth by immunoregulation and remodeling of the stromal-epithelial interface[23].
The main diagnostic hurdles include diagnostic confirmation and distinction of MEC from adenosquamous carcinoma. Because esophageal MEC is not recognizable as a distinct entity clinically, esophageal biopsy and histopathological appraisal are the gold standard for diagnosis. In a review of 20 patients with esophageal MEC diagnosed over a 20 year period, Chen et al[6] demonstrated a 100% false pre-operative diagnoses; 18 were misdiagnosed as squamous cell carcinoma, and the remaining 2, as adenosquamous carcinoma. The ductoglandular origin and deeper submucosal location of MEC pose challenges to endoscopic sampling, adequate tumor representation and the interpretation of the architectural and cellular features. Diagnostic difficulties are attributed to poor attention to the exact anatomic location of the tumor, surface epithelial dysplastic alterations and the range of terminology encompassing tumors that contain squamous and glandular elements. A proposal that tumors with squamous and glandular differentiation be labelled “squamous cell carcinoma with prominent mucin-secreting components” does not address the histogenesis of the tumors. In addition, the understanding and management of esophageal MEC has been stymied by the rarity of reported cases. Grouping tumors as “squamous cell carcinoma with prominent mucin-secreting components” will not address these shortcomings.
The challenges associated with the distinction between adenosquamous carcinoma and MEC are multiple and emerge mainly in the context of salivary glands. In the low-grade component of MECs of the salivary gland, squamoid foci are characterized by nests of cells with a stratified morphology[26]. Intercellular bridges are inconspicuous, and keratinization or keratin pearls are not a usual finding[26]. Higher-grade tumors demonstrate established features of squamous differentiation, including intercellular bridges and single cell keratinization[26]. Recognition of these squamoid and glandular components on endoscopic esophageal biopsies is critical for the identification of the divergent squamoid and glandular differentiation. The identification of intermediate cells may be an unrealistic expectation in small biopsies, and histochemical mucin stains are not helpful in differentiating between MEC and adenosquamous cell carcinoma. In the described extrasalivary MECs, advanced squamous differentiation and pleomorphism of squamous, glandular and solid cell growth have been documented[2,3,5-7].
Helpful morphological features of MEC that may aid their diagnostic distinction from adenosquamous carcinoma include the presence of mucin pools, extravasation of mucin, intermediate cells and the deep location of the tumor. While the absence of surface epithelial dysplasia is a helpful diagnostic feature, its identification does not exclude MEC. Because of the glandular and squamous co-differentiation of MEC, immunohistochemical markers of glandular or squamous origins are not helpful in the distinction. There is variable S100 protein staining in salivary MEC[26]. However, S100 immunopositivity in an esophageal tumor with glandular and squamous elements may favor a mucous glandular origin and a diagnosis of MEC. Technological and molecular advances have advocated the role of fusion genes in the diagnosis and prognosis of MEC from salivary and extrasalivary locations, including the uterine cervix[27]. MEC is typified by a specific translocation, t(11;19) (q12;p13), which results in the fusion of the MECT1 and MAML2 genes. The CRTC family includes 3 human genes: CRTC1 (MECT1), CRTC2 at 1q21 and CRTC3 at 15q26. MAML2 (mastermind-like 2) is a 125kD protein that is involved in Notch signaling pathways[28]. The incidence of this fusion varies, but more than 50% of MEC demonstrate MECT1-MAML2 fusion. Other fusions include MECT1-MAML2 and CRTC3-MAML2. MECT1-MAML2 fusion transcript is present less frequently in high-grade than in low- or intermediate-grade tumors and may be regarded as a diagnostic and prognostic biomarker for MEC. Gene fusion studies were not undertaken in the index case.
In the index case, not only were glandular and squamoid components identified in the initial biopsy, but solid cellular nests with focal intracytoplasmic mucin production were also observed. Intermediate cells, however, were absent. In the resected tumor, the absence of adjacent surface epithelial dysplasia, spectrum of low intermediate and high grade features, focal representation of intermediate cells, mucinous pools, extravasated mucus and focal S100 protein immunopositivity supported the diagnosis of esophageal MEC. In addition, the presence of stromal sclerosis with foci of keloid-like sclerosis and eosinophils facilitated the definitive diagnosis.
In common with the reported outcome of MEC, radiation and chemotherapy are not highly effective as adjunctive therapies in the management of MEC in the salivary glands[29]. It is thus not surprising that the response to radiation and chemotherapy in the esophagus is poor or that the prognosis for this tumor remains dismal[30]. Therefore, surgery remains the best option for a reasonably improved survival in a patient who is physically fit enough to withstand an esophagectomy[31]. In an attempt to appraise independent prognostic factors influencing survival, Chen et al[6] reviewed 36 patients with MECs of the esophagus over a 20-year period in their institution. Their findings were as follows: most tumors occurred in the middle third of the thoracic esophagus; the median length of the tumor was 5.0 cm; 22% had lymph node metastases; the median survival time was 29 mo; and the 5-year survival rate post-resection was 25%. The median survival time was higher in patients without lymph node metastases compared to those with lymph node metastases. The median survival was also higher in patients who underwent a radical operation compared to those who had a palliative procedure. The age, gender, length of tumor, location of tumor and post-operative radiotherapy did not show a statistically significant correlation with prognosis.
In conclusion, primary MEC of the esophagus, including the sclerosing variant, is rarely reported, but sclerosing MEC with “tissue eosinophilia” has never been reported in this location. The rarity of this situation has precluded a thorough understanding of esophageal MEC. While the deeper, submucosal location of the tumor poses challenges in diagnostic sampling to adequately represent the spectrum of histopathological features, a heightened recognition of MEC in this location is also necessary to distinguish MEC from squamous carcinoma, adenocarcinoma or adenosquamous carcinoma. This distinction has therapeutic implications. Based largely on past experience managing tumors in the salivary gland, MEC is characterized by a poor response to adjuvant chemotherapy and radiotherapy; notwithstanding a poor response to surgery, this procedure remains the mainstay of management. Heightened clinicopathological recognition of the occurrence of MEC in the esophagus and fastidious reporting of the clinicopathological profiles of additional cases are pivotal, not only to facilitate improved understanding of the biological features of this tumor in this location but also to supplement the global diagnostic and management approaches to esophageal MEC, including rare variants.
COMMENTS
Case characteristics
Progressive dysphagia of 2 mo duration in a 51-year-old patient.
Clinical diagnosis
A mid-thoracic malignant-appearing stricture was identified on endoscopic assessment.
Differential diagnosis
The differential diagnosis, that included malignant causes for the stricture, would require biopsy and histopathological appraisal for definitive diagnosis.
Laboratory diagnosis
Histopathological assessment of biopsied tissue demonstrated features of mucoepidermoid carcinoma, stromal sclerosis and increased eosinophils.
Imaging diagnosis
Barium swallow demonstrated a malignant-appearing stricture in the mid-thoracic region.
Pathological diagnosis
The features of a sclerosing mucopepidermoid carcinoma with “tissue eosinophilia” on routine haematoxylin and eosin-stained sections, that encompassed squamoid, glandular and intermediate cells in a sclerosing stromal background rich in eosinophils was supported by positive Southgate mucicarmine demonstration of intracytoplasmic and luminal mucin production.
Treatment
A 3-stage total oesophagectomy that included shaving the tumour of the aorta, performance of a gastric pull-up and oesophago-gastrostomy in the neck, was undertaken.
Related reports
Oesophageal mucoepidermoid carcinoma must be differentiated from adenosquamous carcinoma. The ductoglandular origin and deep submucosal location of the oesophageal mucoepidermoid carcinoma poses challenges to endoscopic sampling, adequate tumour representation and interprestation of architectural and cellular details on pathological appraisal. The presence of intermediate cells, mucin pools and deep tumour location aid the distinction from adenosquamous carcinoma.
Term explanation
“Tissue eosinophilia” refers to eosinophilic infiltration of a tumour not associated with tumour necrosis and ulceration.
Experiences and lessons
Mucoepidermoid carcinoma is diagnosed rarely in the oesophagus but heightened awareness of the entity is pivotal to optimal diagnosis, which in turn underpins appropriate management.
Peer review
This is an interesting case of a primary oesophageal sclerosing mucoepidermoid carcinoma with eosinophilia.
Footnotes
P- Reviewers: Chatzistamou I, Ray S S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Pinkston JA, Cole P. Incidence rates of salivary gland tumors: results from a population-based study. Otolaryngol Head Neck Surg. 1999;120:834–840. doi: 10.1016/S0194-5998(99)70323-2. [DOI] [PubMed] [Google Scholar]
- 2.Veras EF, Sturgis E, Luna MA. Sclerosing mucoepidermoid carcinoma of the salivary glands. Ann Diagn Pathol. 2007;11:407–412. doi: 10.1016/j.anndiagpath.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Fadare O, Hileeto D, Gruddin YL, Mariappan MR. Sclerosing mucoepidermoid carcinoma of the parotid gland. Arch Pathol Lab Med. 2004;128:1046–1049. doi: 10.5858/2004-128-1046-SMCOTP. [DOI] [PubMed] [Google Scholar]
- 4.Hagiwara N, Tajiri T, Tajiri T, Miyashita M, Sasajima K, Makino H, Matsutani T, Tsuchiya Y, Takubo K, Yamashita K. Biological behavior of mucoepidermoid carcinoma of the esophagus. J Nippon Med Sch. 2003;70:401–407. doi: 10.1272/jnms.70.401. [DOI] [PubMed] [Google Scholar]
- 5.Mafune K, Takubo K, Tanaka Y, Fujita K. Sclerosing mucoepidermoid carcinoma of the esophagus with intraepithelial carcinoma or dysplastic epithelium. J Surg Oncol. 1995;58:184–190. doi: 10.1002/jso.2930580309. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Chen Y, Yang J, Yang W, Weng H, Li H, Liu D. Primary mucoepidermoid carcinoma of the esophagus. J Thorac Oncol. 2011;6:1426–1431. doi: 10.1097/JTO.0b013e31821cfb96. [DOI] [PubMed] [Google Scholar]
- 7.Tamura S, Kobayashi K, Seki Y, Matsuyama J, Kagara N, Ukei T, Uemura Y, Miyauchi K, Kaneko T. Mucoepidermoid carcinoma of the esophagus treated by endoscopic mucosal resection. Dis Esophagus. 2003;16:265–267. doi: 10.1046/j.1442-2050.2003.00342.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu ZJ, Sun SY, Guo JT, Wang S, Ge N, Liu X, Wang GX, Yang XH. A primary esophageal mucoepidermoid carcinoma mimicking a benign submucosal tumor. Dis Esophagus. 2012;25:178–179. doi: 10.1111/j.1442-2050.2010.01158.x. [DOI] [PubMed] [Google Scholar]
- 9.Chan JK, Saw D. Sclerosing mucoepidermoid tumour of the parotid gland: report of a case. Histopathology. 1987;11:203–207. doi: 10.1111/j.1365-2559.1987.tb02623.x. [DOI] [PubMed] [Google Scholar]
- 10.Urano M, Abe M, Horibe Y, Kuroda M, Mizoguchi Y, Sakurai K, Naito K. Sclerosing mucoepidermoid carcinoma with eosinophilia of the salivary glands. Pathol Res Pract. 2002;198:305–310. doi: 10.1078/0344-0338-00259. [DOI] [PubMed] [Google Scholar]
- 11.Muller S, Barnes L, Goodurn WJ. Sclerosing mucoepidermoid carcinoma of the parotid. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:685–690. doi: 10.1016/s1079-2104(97)90320-6. [DOI] [PubMed] [Google Scholar]
- 12.Baloch ZW, Solomon AC, LiVolsi VA. Primary mucoepidermoid carcinoma and sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland: a report of nine cases. Mod Pathol. 2000;13:802–807. doi: 10.1038/modpathol.3880140. [DOI] [PubMed] [Google Scholar]
- 13.Tian W, Yakirevich E, Matoso A, Gnepp DR. IgG4(+) plasma cells in sclerosing variant of mucoepidermoid carcinoma. Am J Surg Pathol. 2012;36:973–979. doi: 10.1097/PAS.0b013e318258f018. [DOI] [PubMed] [Google Scholar]
- 14.Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother. 2007;30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- 15.Pereira MC, Oliveira DT, Kowalski LP. The role of eosinophils and eosinophil cationic protein in oral cancer: a review. Arch Oral Biol. 2011;56:353–358. doi: 10.1016/j.archoralbio.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Simson L, Ellyard JI, Dent LA, Matthaei KI, Rothenberg ME, Foster PS, Smyth MJ, Parish CR. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J Immunol. 2007;178:4222–4229. doi: 10.4049/jimmunol.178.7.4222. [DOI] [PubMed] [Google Scholar]
- 17.Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007;70:1–11. doi: 10.1111/j.1399-0039.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 18.Leighton SE, Teo JG, Leung SF, Cheung AY, Lee JC, van Hasselt CA. Prevalence and prognostic significance of tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. Cancer. 1996;77:436–440. doi: 10.1002/(SICI)1097-0142(19960201)77:3<436::AID-CNCR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 19.Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur SI, O’Neill K, Colbert D, et al. Pivotal Advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;79:1131–1139. doi: 10.1189/jlb.0106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowe D, Fletcher CD. Eosinophilia in squamous cell carcinoma of the oral cavity, external genitalia and anus--clinical correlations. Histopathology. 1984;8:627–632. doi: 10.1111/j.1365-2559.1984.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki K, Torisu M, Fujimura T. Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer. 1986;58:1321–1327. doi: 10.1002/1097-0142(19860915)58:6<1321::aid-cncr2820580623>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Tadbir AA, Ashraf MJ, Sardari Y. Prognostic significance of stromal eosinophilic infiltration in oral squamous cell carcinoma. J Craniofac Surg. 2009;20:287–289. doi: 10.1097/SCS.0b013e318199219b. [DOI] [PubMed] [Google Scholar]
- 23.Tostes Oliveira D, Tjioe KC, Assao A, Sita Faustino SE, Lopes Carvalho A, Landman G, Kowalski LP. Tissue eosinophilia and its association with tumoral invasion of oral cancer. Int J Surg Pathol. 2009;17:244–249. doi: 10.1177/1066896909333778. [DOI] [PubMed] [Google Scholar]
- 24.Lowe D, Jorizzo J, Hutt MS. Tumour-associated eosinophilia: a review. J Clin Pathol. 1981;34:1343–1348. doi: 10.1136/jcp.34.12.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tepper RI, Pattengale PK, Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989;57:503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- 26.Cheuk W, Chan JKC. Salivary gland tumours. In: Fletcher CDM, editor. Diagnostic Histopathology of Tumors. Philadelphia: Churchill Livingstone Elsevier; 2007. pp. 239–326. [Google Scholar]
- 27.Lennerz JK, Perry A, Mills JC, Huettner PC, Pfeifer JD. Mucoepidermoid carcinoma of the cervix: another tumor with the t(11; 19)-associated CRTC1-MAML2 gene fusion. Am J Surg Pathol. 2009;33:835–843. doi: 10.1097/PAS.0b013e318190cf5b. [DOI] [PubMed] [Google Scholar]
- 28.Bell D, El-Naggar AK. Molecular heterogeneity in mucoepidermoid carcinoma: conceptual and practical implications. Head Neck Pathol. 2013;7:23–27. doi: 10.1007/s12105-013-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82:1217–1224. doi: 10.1002/(sici)1097-0142(19980401)82:7<1217::aid-cncr2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman MD, Franceschi D, Marsan B, Burt M. Esophageal carcinoma. The unusual variants. J Thorac Cardiovasc Surg. 1994;108:1138–1146. [PubMed] [Google Scholar]
- 31.Turkyilmaz A, Eroglu A, Gursan N. Muco-epidermoid carcinoma of the oesophagus: a case report. Acta Chir Belg. 2009;109:416–418. doi: 10.1080/00015458.2009.11680452. [DOI] [PubMed] [Google Scholar]


