Highlights
-
•
Biliverdin mitigates LPS-dependent C5aR expression in macrophages in part via mTOR.
-
•
Biliverdin promotes phosphorylation of Akt and PS6.
-
•
Biliverdin decreases LPS-mediated induction of C5aR-associated cytokines.
Abbreviations: ANOVA, analysis of variance; BCA, bicinchoninic acid; FACS, fluorescence-activated cell sorting; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HPRT, hypoxanthine-guanine phosphoribosyltransferase; M-CSF, macrophage-colony stimulating factor; NF-κB, nuclear factor kappa B; qRT-PCR, quantitative real time polymerase chain reaction
Keywords: Macrophage, Inflammation, mTOR
Abstract
Macrophages play a crucial role in the maintenance and resolution of inflammation and express a number of pro- and anti-inflammatory molecules in response to stressors. Among them, the complement receptor 5a (C5aR) plays an integral role in the development of inflammatory disorders. Biliverdin and bilirubin, products of heme catabolism, exert anti-inflammatory effects and inhibit complement activation. Here, we define the effects of biliverdin on C5aR expression in macrophages and the roles of Akt and mammalian target of rapamycin (mTOR) in these responses. Biliverdin administration inhibited lipopolysaccharide (LPS)-induced C5aR expression (without altering basal expression), an effect partially blocked by rapamycin, an inhibitor of mTOR signaling. Biliverdin also reduced LPS-dependent expression of the pro-inflammatory cytokines TNF-α and IL-6. Collectively, these data indicate that biliverdin regulates LPS-mediated expression of C5aR via the mTOR pathway, revealing an additional mechanism underlying biliverdin’s anti-inflammatory effects.
1. Introduction
Biliverdin (BV), a molecule with tetrapyrrole structure, is derived from heme catabolism via heme oxygenase (HO) activity and is rapidly reduced to bilirubin (BR) by biliverdin reductase (BVR) [1,2]. Both BV and BR are antioxidants [3], though, have been regarded previously as waste products. Recent findings, however, have begun to elucidate diverse protective roles for these molecules [4,5]. Biliverdin shows strong cytoprotective activities in various in vitro and in vivo models of vascular injury, ischemia–reperfusion injury and organ transplantation, demonstrating its therapeutic potential [6,7]. We recently reported that BV reduces the expression of toll like receptor-4 (TLR-4) in murine macrophages via nitric oxide-dependent activation of BVR [8]. TLRs transmit signals to induce pro-inflammatory cytokine expression via NF-κB [9] and synergize with C5aR (CD88) to aggravate inflammatory responses to endotoxin [10]. TLR-ligands are dependent on complement activation and C5aR regulates TLR-4 signaling, supporting the importance of C5aR in promoting inflammation [11].
Complement is a major component of innate and adaptive immunity. Similar to TLRs, complement is also activated by pathogen associated molecular patterns, including LPS, among many other mechanisms involved in classical, lectin and alternative activation pathways [12,13]. Complement activation induces pathogen opsonization and generation of the anaphylatoxins: C3a and C5a, which stimulate inflammatory responses by binding to respective C3aR and C5aR receptors [12]. Excessive inflammation mediated by complement activation contributes to various diseases, including sepsis, asthma, Alzheimer’s disease and atherosclerosis [12–14]. Therefore, it is important to identify molecules that regulate or attenuate complement-mediated inflammation. Both BV and BR ameliorate complement-mediated hemolysis by inhibiting the classical pathway of complement activation at the C1 step via physically interacting with complement proteins [15,16]. However, BV’s effect on the expression of complement receptors and mechanisms underlying this regulation remains unknown.
The present study thus assessed the effects of BV and the PI3K/mTOR pathways on C5aR expression in primary and immortalized macrophages. Data reveal that BV inhibits LPS-dependent C5aR expression, in part via mTOR signaling.
2. Material and methods
2.1. Cell culture and treatment
RAW 264.7 mouse macrophage cell line was purchased from ATCC (USA). RAW cells were cultured (<15 passages) in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin (Life Technologies, Grand Island, NY, USA; complete medium). Cells (1.5 × 105 cells/mL) were seeded on 60 mm Sterilin tissue culture plates or 6 well plates (Thermo Scientific, Logan, UT, USA) in 3 mL of complete medium and incubated at 37 °C (5% CO2) for 24 h prior to experimentation. Cells were then untreated or challenged with 100 ng/mL of LPS for 24 h in the absence or presence of freshly prepared biliverdin hydrochloride (10 or 50 μM; Frontier Scientific, Logan UTA, USA) in 0.01% DMSO as previously described [1]. Re595 LPS from Salmonella minnesota (Sigma–Aldrich, St. Louis, MO, USA) was dissolved in DPBS (Life Technologies) and used at a final concentration of 100 ng/mL. Rapamycin (Sigma–Aldrich) was used as selective inhibitor of mTOR [17] and was applied to sub-sets of cells (10 nM in 0.01% DMSO final concentration) 1 h prior to LPS or BV treatment. Biliverdin and related tetrapyrroles are photo sensitive, therefore, all BV containing solutions were protected from light. Appropriate vehicle control experiments were also completed.
2.2. Isolation of bone marrow-derived macrophages
7–8 week old C57BL/6 mice were purchased from Jackson Laboratories (Jackson Laboratories, Bar Harbour, ME, USA). All animals were held under pathogen free conditions. Prior to completion, experiments were approved by the Beth Israel Deaconess Medical Centre (BIDMC) Animal Care and Use Committee. Bone marrow-derived macrophages (BMDMs) were isolated as previously described [1]. Macrophages were harvested after 5 days and were then cultured for 24 h in RPMI medium supplemented with 10% FBS and 5% Antibiotic–Antimycotic (Life Technologies) prior to experimentation. Cells were then treated with 50 μM BV and 100 ng/mL LPS for 24 or 48 h.
2.3. RNA Extraction and qRT-PCR
Total RNA was isolated from cultured cells using RNeasy® Plus Mini Kits (Qiagen, Chadstone, VIC, Australia) according to manufacturer’s instructions. One microgram of RNA was reverse transcribed into cDNA using a first strand cDNA synthesis kit (Thermo Scientific). HPRT and GAPDH were used as reference genes based on their stability of expression determined by geNorm analysis as described below. Primers for mouse GAPDH, HPRT, C5aR, TNF-α, and IL-6 were designed using Primer Quest Software (Table 1, Sigma–Aldrich). qRT-PCR was performed with Applied Biosystems Stepone™ and Stepone Plus™ Real-Time PCR Systems (Life Technologies). Each sample was run in triplicate and cycle threshold (CT) values were imported into Microsoft Excel for geNorm analysis.
Table 1.
Primer sequences and amplicon sizes of housekeeping (GAPDH and HPRT) and target genes (C5aR, TNF-α and IL-6) expressed in RAW 264.7 cells.
| Gene target | Forward sequence | Reverse sequence | Amplicon size (bp) |
|---|---|---|---|
| GAPDH | TCAACAGCAACTCCCACTCTTCCA | ACCCTGTTGCTGTAGCCGTATTCA | 115 |
| HPRT | AGGAGTCCTGTTGATGTTGCCAGT | GGGACGCAGCAACTGACATTTCTA | 134 |
| C5aR | TCATCCTGCTCAACATGTACGCCA | TCTGACACCAGATGGGCTTGAACA | 93 |
| TNF-α | TCTCATGCACCACCATCAAGGACT | ACCACTCTCCCTTTGCAGAACTCA | 92 |
| IL-6 | ATCCAGTTGCCTTCTTGGGACTGA | TAAGCCTCCGACTTGTGAAGTGGT | 134 |
2.4. qRT-PCR calculation using genorm analysis
qRT-PCR data was normalized by the use of geNorm algorithm as described by Vandesompele et al. [18]. Briefly, the geNorm application determines the most stably expressed and thus most accurate reference genes for the normalization of qRT-PCR data. The geometric mean of ΔCT expression for GAPDH and HPRT was calculated to obtain the normalization factor for each sample. The expression of each candidate gene for each sample was normalized to the combined reference genes. The ΔCT (difference between cycle threshold values) expression was then calculated for each gene in each sample. The relative expression for each candidate gene was calculated by dividing the ΔCT of target gene for each sample by the normalization factor of GAPDH and HPRT within the same sample.
2.5. Sources of antibodies
The following antibodies were used for western blotting analyses where indicated: rabbit anti-phospho-Akt (Ser473), rabbit anti-Akt, rabbit anti-phospho-S6 Kinase (Ser235/236), anti-rabbit IgG and anti-mouse IgG (Cell Signaling, Beverly, MA, USA) and mouse anti-β-actin (Sigma–Aldrich). For flow cytometry experiments, PE-conjugated anti-mouse CD88 antibody (C5aR) and PE-labeled anti-rat IgG (Biolegend, San Diego, CA, USA) were used.
2.6. Flow cytometry
After harvesting and washing RAW 264.7 or BMDM cells with DPBS, cells were stained with anti-mouse CD88 antibody or anti-rat IgG at 1 μg/106 cells for 30 min at 4 °C. Cells were immediately analyzed using a FACS Caliber flow cytometer (Becton and Dickinson, San Jose, CA, USA) using the FL-2 channel. Mean fluorescence intensity (MFI) was calculated using CellQuest Pro™ software (Becton and Dickinson).
2.7. Western blot
Cell lysates were prepared in ice-cold RIPA buffer (50 mM Tris–HCl, [pH 7.4], 50 mM sodium fluoride, 150 mM NaCl, 1% Nonident P40, 0.5 M EDTA [pH 8.0]) and the protease inhibitor cocktail Complete Mini (Roche, Indianapolis, IN, USA). Samples were centrifuged at 14,000g at 4 °C for 20 min and supernatants were collected. Protein concentrations of supernatants were measured using a BCA protein assay kit (Thermo Scientific). Forty micrograms of each protein sample was then electrophoresed on NuPAGE 4–12% Bis-Tris Gel (Life Technologies) in NuPAGE MES SDS running buffer (Life Technologies) for 90 min at 100 V. The membranes were blocked with 5% non-fat dry milk in 1× Tris buffered saline buffer (TBS; Boston Bio Products, Ashland, MA, USA) for 1 h and then probed with appropriate primary antibodies (diluted at 1:1000 in 1× TBS with 5% non-fat milk) overnight at 4 °C. Membranes were then washed in 1× TBS buffer and thereafter membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies at a dilution of 1:5000 in 1× TBS with 5% non-fat milk for 1 h at room temperature. Membranes were visualized using Super Signal West Pico chemiluminescent substrate (Thermo Scientific) or Femto Maximum Sensitivity Substrate (Thermo Scientific), followed by exposure to autobioradiography film (BioExpress, Kaysville, UT, USA). Precision Plus Protein™ Kaleidoscope™ protein standard (Bio Rad, Hercules, CA, USA) was used to confirm the molecular size of target proteins.
2.8. ELISA analysis
The concentrations of cytokines were measured in cell culture media using commercially available ELISA kits from eBioscience (Kensington, SA, Australia) for IL-6 and R&D Systems (Gymea, NSW, Australia) for TNF-α as per manufacturer’s instructions.
2.9. Statistical analysis
All data are reported as mean ± S.E. Statistical analysis was performed using one-way repeated measures ANOVA (posthoc Tukey test; Sigmastat, Ver. 11.0). If the data set lacked either normal distribution or equal variance, data were log10 transformed to obtain normally distributed data. P < 0.05 was considered significant.
3. Results
3.1. Biliverdin inhibits the expression of C5aR in murine macrophages
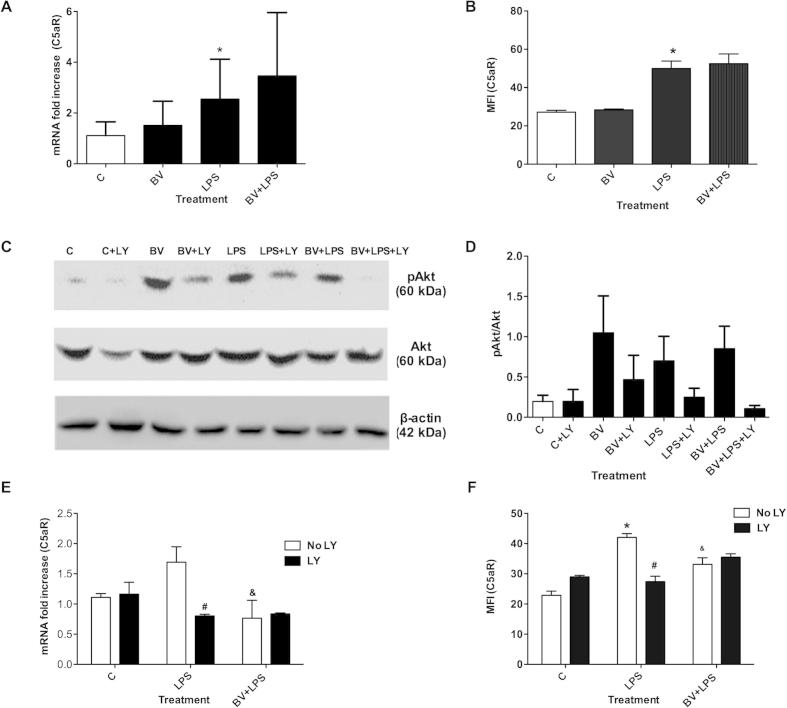
qRT-PCR analysis showed that neither 10 or 50 μM BV modified basal expression of C5aR in RAW 264.7 cells (Fig. S1A and Fig. 1A). However, the LPS-dependent increase in C5aR gene expression at 24 h was significantly decreased by 50 μM BV (Fig. 1A; P < 0.05). Treatment with 10 μM BV at the time of LPS stimulation failed to significantly block C5aR gene expression at 24 h (Fig. S1A), indicating a concentration-dependent inhibition of LPS induced C5aR expression by BV.
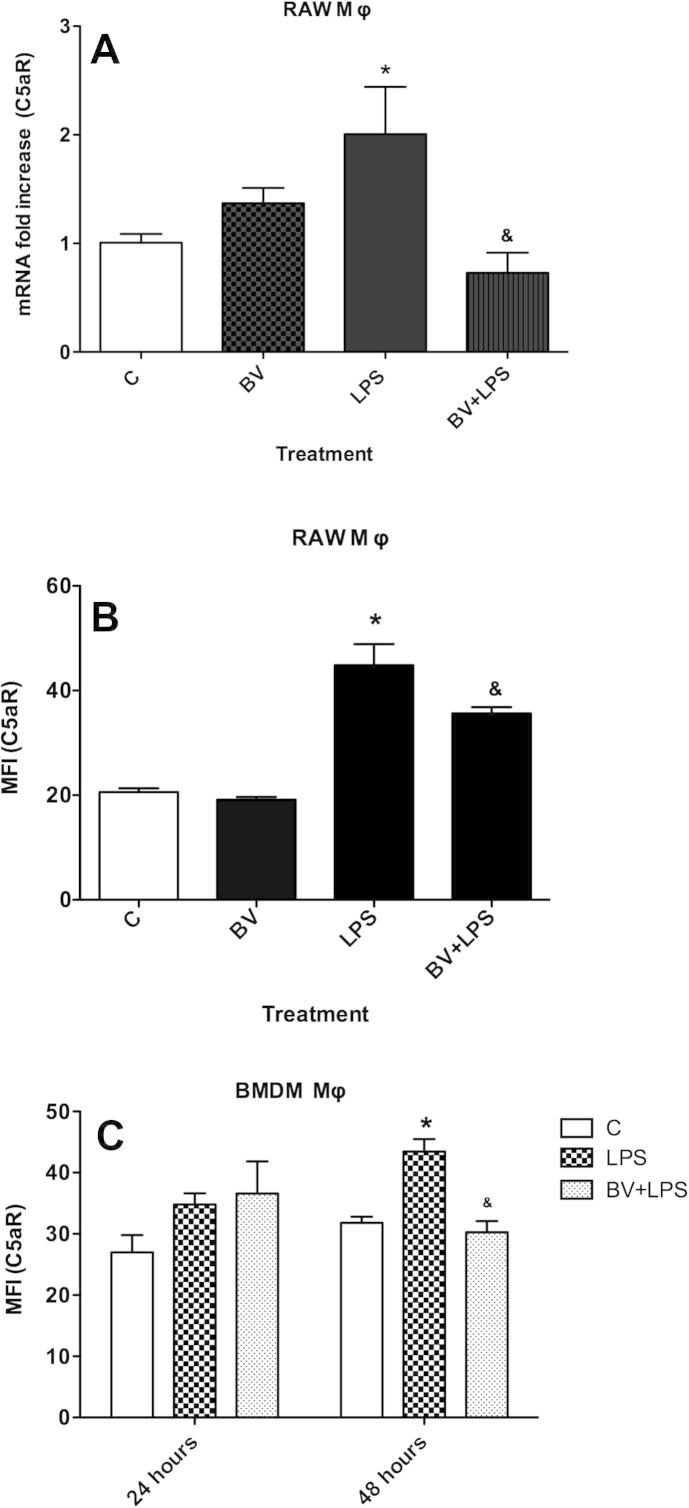
Fig. 1.
Biliverdin inhibits C5aR expression. RAW Mφ were treated BV ± LPS for 24 h. (A) Gene and (B) cell surface expression of C5aR in RAW Mφ. (C) Cell surface expression of C5aR in BMDM Mφ treated with BV and LPS for 24 and 48 h. Data are representatives of three independent experiments. Value represents mean ± S.E. n = 3/group, ∗P < 0.05 vs. non LPS control (0.01% DMSO) at 24 h and 48 h and &P < 0.05 vs. LPS control at 24 and 48 h.
Next, we tested whether BV inhibited C5aR protein expression. RAW 264.7 cells were treated with 10 or 50 μM BV ± 100 ng/mL LPS for 24 h and cell surface expression of C5aR was analyzed. Biliverdin at 10 μM did not significantly affect LPS-dependent C5aR gene and cell surface expression (Fig. S1A and B), however, BV at 50 μM significantly inhibited LPS-induced C5aR expression (Fig. 1B, P < 0.05). These data are in agreement with other published reports showing that 50 μM BV is necessary to induce anti-inflammatory effects [1,8]. Therefore, a concentration of 50 μM was chosen for investigating BV’s effect on cell signaling and LPS-mediated inflammation. To confirm BV’s effects in primary macrophages, BMDMs from mice were also incubated with 50 μM BV and 100 ng/mL LPS for 24 and 48 h. LPS significantly increased C5aR expression by ∼40% at 48 h compared to control and BV abrogated this effect (Fig. 1C, P < 0.05). In summary, BV consistently decreased both C5aR gene (24 h) and protein expression (24–48 h) in primary and immortalized macrophages.
One mechanism by which BV exerts effects in macrophages is via PI3K/Akt signaling [1]. We, therefore, next tested whether the inhibitory effect of BV on C5aR expression was PI3K-dependent. To block PI3K signaling, cells were pre-incubated with LY294002 (LY, 10 μM) for 30 min prior to 50 μM BV or LPS stimulation. To confirm that LY inhibits downstream targets of PI3K, pAkt expression was determined in RAW 264.7 cells treated with 50 μM BV or LPS for 30 min. As shown in Fig. S1C and D, BV/LPS-induced phosphorylation of Akt was blocked by LY. To assess the effects of LY on C5aR expression, experiments were performed over 24 h due to strong effects of BV at this time point (Fig. 1A and B). However, LY blocked the LPS-dependent induction of C5aR gene and protein (Fig. S1E and F), indicating PI3K may play an integral role in mediating C5aR expression in response to LPS. The role of PI3K on BV-mediated changes on C5aR gene and protein expression in the presence of LPS could thus not be determined (Fig. S1E and F; P = 0.286 and P = 0.083, respectively).
3.2. Biliverdin induces the phosphorylation of Akt and S6 and inhibits C5aR expression in macrophages in part via mTOR signaling
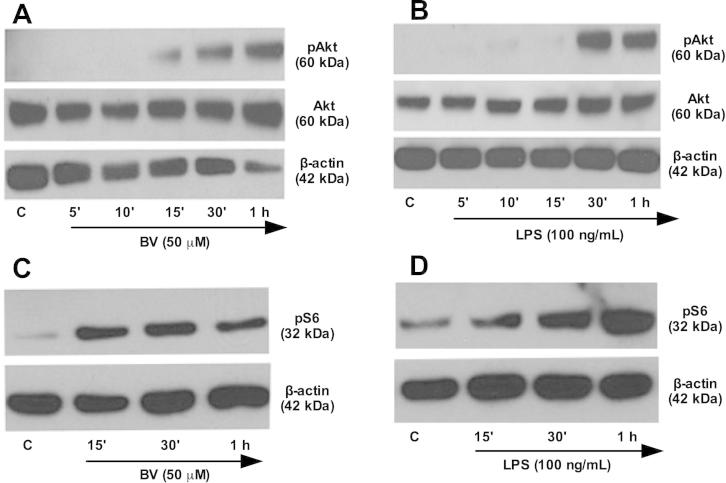
Having established that BV activates the PI3K-Akt signaling axis [1], we next evaluated the activation of pAkt and pS6 (downstream of mTOR) in response to BV in RAW 264.7 macrophages. As shown in Fig. 2A–D, both 50 μM BV and 100 ng/mL LPS increased Akt and S6 phosphorylation in a time-dependent manner.
Fig. 2.
Biliverdin enhances phosphorylation of Akt and S6. RAW 264.7 Mφ were treated with BV and LPS for different time points and protein expression of pAkt, Akt (A and B) and pS6 (C and D) were analyzed. Blots are representative of at least two independent experiments.
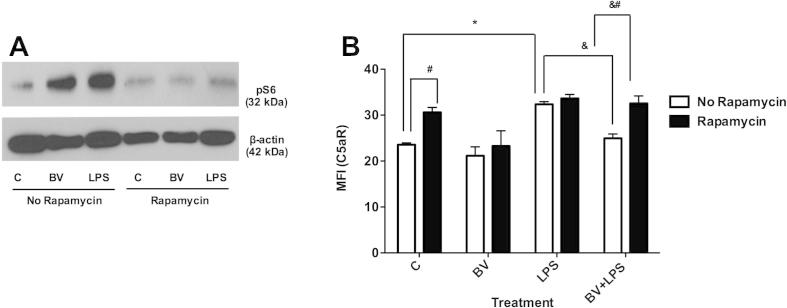
Next, we sought to determine whether inhibition of the mTOR pathway with rapamycin would modulate the effects of BV on C5aR expression. RAW 264.7 cells were incubated with 10 nM rapamycin for 1 h prior to treatment with 50 μM BV or LPS. As shown in Fig. 3A, phosphorylation of S6 in response to BV and LPS was blocked in the presence of rapamycin. Furthermore, rapamycin treatment increased the basal expression of C5aR (Fig. 3B), indicating the possibility that S6 negatively regulates C5aR expression. LPS significantly increased C5aR expression and this effect was not dependent on mTOR signaling (Fig. 3B). However, BV decreased LPS-induced C5aR expression in a rapamycin-dependent manner (Fig. 3B), implicating mTOR signaling in BV’s inhibitory effect. In summary, BV stimulates signaling downstream of PI3K and mTOR. Although some similarities in LPS and BV signaling exist, blocking mTOR signaling attenuates BV’s inhibitory effect on C5aR gene expression.
Fig. 3.
Biliverdin modulates C5aR expression in part via mTOR signaling. RAW 264.7 Mφ were pre-incubated with rapamycin for 1 h and thereafter treated with BV or LPS for 15 min or 24 h for pS6 and C5aR expression, respectively. (A) Protein expression of pS6 and (B) cell surface expression of C5aR in RAW 264.7 cells. The data are representative of three independent experiments. Value represents mean ± S.E. n = 3/group, #P < 0.05 vs. no rapamycin control (0.01% DMSO), ∗P < 0.05 vs. no rapamycin and no LPS control (0.01% DMSO), &P < 0.05 vs. no rapamycin and LPS control and &#P < 0.05 vs. no rapamycin BV + LPS group.
3.3. Biliverdin suppresses the release and expression of complement-associated pro-inflammatory cytokines
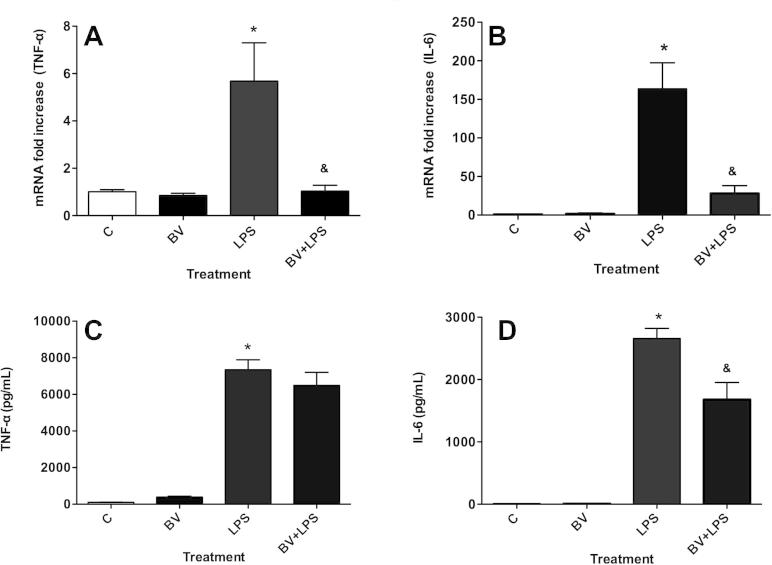
We next evaluated the effects of BV on the expression of the pro-inflammatory cytokines (TNF-α and IL-6) in RAW 264.7 macrophages. LPS significantly increased TNF-α and IL-6 mRNA expression (∼6- and ∼200-fold, respectively) at 24 h, and these responses were significantly inhibited by BV (Fig. 4A and B, P < 0.05).
Fig. 4.
Biliverdin attenuates complement associated pro-inflammatory cytokines. mRNA expression of TNF-α (A) and IL-6 (B) and protein concentration of TNF-α (C) and IL-6 (D) were analyzed in RAW 264.7 macrophages, incubated with BV ± LPS for 24 h. The data are representative of two independent experiments. Value represents mean ± S.E. n = 3/group, ∗P < 0.05 vs. no LPS control (0.01% DMSO) and &P < 0.05 vs. LPS control.
ELISA analysis of both cytokines showed that LPS significantly increased TNF-α and IL-6 concentrations in cell culture supernatants at 24 h (P < 0.05), while, BV only reduced IL-6 levels in response to LPS (Fig. 4D, P < 0.05).
4. Discussion
The present study provides novel insights into the anti-inflammatory effects of BV, demonstrating that BV consistently decreases LPS-mediated C5aR gene and protein expression in RAW 264.7 cells and BMDMs. This inhibitory effect of BV was partially mediated via the mTOR pathway and was accompanied by decreased expression of complement associated pro-inflammatory cytokines.
PI3K/Akt negatively regulates LPS signaling and inhibition of the PI3K pathway augments LPS-induced responses, including the activation of NF-κB and TNF gene expression [19]. A novel and unexpected finding of this report is that pharmacological inhibition of PI3K with LY attenuated LPS-induced increases in C5aR expression, suggesting that PI3K signaling may be necessary for C5aR expression. Two studies show that inhibition of PI3K with LY inhibits C5a induced chemotactic migration of macrophages [20,21], which may be related to inhibition of C5aR expression as reported here. However, LY’s inhibitory effects exist beyond PI3K signaling [22]. Therefore, it is also possible that LY blocked C5aR expression via a PI3K-independent mechanism. Since LY’s effects are rather non-specific, we chose a more specific downstream inhibitor of PI3K signaling [17,23], rapamycin, to determine whether BV’s effect on C5aR was PI3K/mTOR dependent.
Rapamycin pre-treatment blocked BV and LPS-mediated phosphorylation of S6 (a downstream signaling molecule of mTOR, which plays an important role in protein synthesis) [23]. However, inhibition of mTOR signaling did not influence LPS-induced C5aR expression, indicating that LPS likely regulates C5aR through a different signaling pathway, such as NF-κB signaling [24]. On the other hand, BV inhibition of LPS-induced C5aR was partially mitigated in the presence of rapamycin, suggesting that BV inhibits C5aR in part via activation of the mTOR pathway.
The C5a-C5aR axis cross-talks with TLR-4 [11] and C5a via C5aR concentration-dependently increases LPS-induced secretion of pro-inflammatory cytokines, including IL-6 and TNF-α in human monocytes [25]. Therefore, the effects of BV on TNF-α and IL-6 were also explored. While BV significantly downregulated LPS-induced mRNA expression of both cytokines at 24 h, only IL-6 and not TNF-α protein levels were reduced by BV. TNF-α gene expression and synthesis/release are regulated via different pathways [26]. Activation of macrophages with LPS leads to rapid cytosolic accumulation of TNF-α mRNA via activation of the NF-κB pathway [27]. However, TNF-α is initially expressed as pro-TNF-α and release of mature TNF-α from leukocytes relies on matrix metalloproteinase (MMP) activation, which promotes cleavage of mature TNF-α from pro-TNF-α [28]. Furthermore, TNF-α mRNA is short-lived (∼46 min) and does not contribute to rapid increases in TNF-α release by RAW macrophages upon LPS activation [26]. Therefore, it is likely that BV inhibits TNF-α transcription, via inhibition of NF-κB [6,29], yet does not prevent activation of MMP-induced cleavage and release of TNF-α. These data and conclusions are consistent with reported in vivo findings, which show that BV only decreases mRNA expression of TNF-α and does not influence serum levels of TNF-α in endotoxin/transplantation challenged animals [6,7]. However, BV significantly decreased IL-6 expression and secretion. We suggest that BV may decrease IL-6 by inhibiting activation of C5aR since C5aR antagonists reportedly decrease LPS-mediated release of cytokines including IL-6 by monocytes, macrophages and thymocytes [25,30].
Both LPS and BV induce BVR, which rapidly converts BV to BR [1]. Both in vitro and in vivo studies show rapid conversion of BV to BR over time [1,6]. Furthermore, in vivo studies suggest that BV may inhibit LPS-induced responses via BR generation [6]. However, BR may heighten inflammation at higher concentrations (>17.1 μM) [31,32]. Whether BV’s anti-inflammatory effects are influenced by BR are still debated and require further investigation. In this study, 50 μM BV consistently inhibited effects of LPS on C5aR gene expression after 24 h of incubation, with effects of 50 μM BV statistically significant. These effects are consistent with inhibition of C5aR protein expression at 24 and 48 h. We speculate that the lower 10 μM concentration of BV is more rapidly reduced to BR [1], reducing BV availability for BVR activity/signaling. At the higher 50 μM concentration, BV induces prolonged S6 phosphorylation and modulation of C5aR expression. These data suggest a threshold concentration of BV of 50 μM is necessary to activate kinase signaling and evoke changes in protein synthesis [1,33].
In conclusion, this is the first report to show that BV significantly inhibits LPS-induced C5aR expression in primary and immortalized macrophage cell lines, an effect that is partially mediated via mTOR signaling. Biliverdin also reduced pro-inflammatory cytokine expression, which may be related to C5aR inhibition. We propose that inhibition of C5aR by BV provides a previously unknown anti-inflammatory mechanism, supporting BV’s role as an endogenous anti-inflammatory molecule that serves to re-establish homeostasis and protect against transplant rejection and endotoxic shock. Taken together, we propose that BV may offer unique therapeutic avenues for treating sepsis and shock.
Acknowledgments
This research was supported by the FWF-Austrian Science Fund (P21162 – K.-H.W. and A.B.). This work was also supported in part by NIH-United States grants: HL-071797 and HL-07616 (L.O.) and AHA-United States grants 10SDG2640091 (B.W.). We thank the Julie Henry Fund at the Transplant Centre of the BIDMC and Eleanor Shore Foundation (B.W.) for their support.
Appendix A. Supplementary data
Supplementary Fig. S1.
References
- 1.Wegiel B., Baty C.J., Gallo D., Csizmadia E., Scott J.R., Akhavan A., Chin B.Y., Kaczmarek E., Alam J., Bach F.H., Zuckerbraun B.S., Otterbein L.E. Cell surface biliverdin reductase mediates biliverdin-induced anti-inflammatory effects via phosphatidylinositol 3-kinase and Akt. J. Biol. Chem. 2009;284:21369–21378. doi: 10.1074/jbc.M109.027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulmer A.C., Coombes J.S., Blanchfield J.T., Toth I., Fassett R.G., Taylor S.M. Bile pigment pharmacokinetics and absorption in the rat: therapeutic potential for enteral administration. Br. J. Pharmacol. 2011;164:1857–1870. doi: 10.1111/j.1476-5381.2011.01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molzer C., Huber H., Steyrer A., Ziesel G., Ertl A., Plavotic A., Wallner M., Bulmer A.C., Wagner K.H. In vitro antioxidant capacity and antigenotoxic properties of protoporphyrin and structurally related tetrapyrroles. Free Radic. Res. 2012;46:1369–1377. doi: 10.3109/10715762.2012.715371. [DOI] [PubMed] [Google Scholar]
- 4.Wegiel B., Otterbein L.E. Go green: the anti-inflammatory effects of biliverdin reductase. Front. Pharmacol. 2012;3:47. doi: 10.3389/fphar.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon A.C., Hawkins C.L., Bisht K., Coombes J.S., Bakrania B., Wagner K.H., Bulmer A.C. Reduced circulating oxidized LDL is associated with hypocholesterolemia and enhanced thiol status in Gilbert syndrome. Free Radic. Biol. Med. 2012;52:2120–2127. doi: 10.1016/j.freeradbiomed.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarady-Andrews J.K., Liu F., Gallo D., Nakao A., Overhaus M., Ollinger R., Choi A.M., Otterbein L.E. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L1131–L1137. doi: 10.1152/ajplung.00458.2004. [DOI] [PubMed] [Google Scholar]
- 7.Kosaka J., Morimatsu H., Takahashi T., Shimizu H., Kawanishi S., Omori E., Endo Y., Tamaki N., Morita M., Morita K. Effects of biliverdin administration on acute lung injury induced by hemorrhagic shock and resuscitation in rats. PLoS One. 2013;8:e63606. doi: 10.1371/journal.pone.0063606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wegiel B., Gallo D., Csizmadia E., Roger T., Kaczmarek E., Harris C., Zuckerbraun B.S., Otterbein L.E. Biliverdin inhibits Toll-like receptor-4 (TLR4) expression through nitric oxide-dependent nuclear translocation of biliverdin reductase. Proc. Natl. Acad. Sci. U.S.A. 2011;108:18849–18854. doi: 10.1073/pnas.1108571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vink A., de Kleijn D.P., Pasterkamp G. Functional role for toll-like receptors in atherosclerosis and arterial remodeling. Curr. Opin. Lipidol. 2004;15:515–521. doi: 10.1097/00041433-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Holst B., Raby A.C., Hall J.E., Labeta M.O. Complement takes its Toll: an inflammatory crosstalk between Toll-like receptors and the receptors for the complement anaphylatoxin C5a. Anaesthesia. 2012;67:60–64. doi: 10.1111/j.1365-2044.2011.07011.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X., Kimura Y., Fang C., Zhou L., Sfyroera G., Lambris J.D., Wetsel R.A., Miwa T., Song W.C. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarma J.V., Ward P.A. The complement system. Cell Tissue Res. 2011;343:227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrnthaller C., Ignatius A., Gebhard F., Huber-Lang M. New insights of an old defense system: structure, function, and clinical relevance of the complement system. Mol. Med. 2011;17:317–329. doi: 10.2119/molmed.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manthey H.D., Thomas A.C., Shiels I.A., Zernecke A., Woodruff T.M., Rolfe B., Taylor S.M. Complement C5a inhibition reduces atherosclerosis in ApoE−/− mice. FASEB J. 2011;25:2447–2455. doi: 10.1096/fj.10-174284. [DOI] [PubMed] [Google Scholar]
- 15.Basiglio C.L., Arriaga S.M., Pelusa F., Almara A.M., Kapitulnik J., Mottino A.D. Complement activation and disease: protective effects of hyperbilirubinaemia. Clin. Sci. (Lond.) 2010;118:99–113. doi: 10.1042/CS20080540. [DOI] [PubMed] [Google Scholar]
- 16.Kinderlerer A.R., Pombo Gregoire I., Hamdulay S.S., Ali F., Steinberg R., Silva G., Ali N., Wang B., Haskard D.O., Soares M.P., Mason J.C. Heme oxygenase-1 expression enhances vascular endothelial resistance to complement-mediated injury through induction of decay-accelerating factor: a role for increased bilirubin and ferritin. Blood. 2009;113:1598–1607. doi: 10.1182/blood-2008-04-152934. [DOI] [PubMed] [Google Scholar]
- 17.Hay N., Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 18.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guha M., Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 20.Perianayagam M.C., Balakrishnan V.S., King A.J., Pereira B.J., Jaber B.L. C5a delays apoptosis of human neutrophils by a phosphatidylinositol 3-kinase-signaling pathway. Kidney Int. 2002;61:456–463. doi: 10.1046/j.1523-1755.2002.00139.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsai H.R., Yang L.M., Tsai W.J., Chiou W.F. Andrographolide acts through inhibition of ERK1/2 and Akt phosphorylation to suppress chemotactic migration. Eur. J. Pharmacol. 2004;498:45–52. doi: 10.1016/j.ejphar.2004.07.077. [DOI] [PubMed] [Google Scholar]
- 22.Gharbi S.I., Zvelebil M.J., Shuttleworth S.J., Hancox T., Saghir N., Timms J.F., Waterfield M.D. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem. J. 2007;404:15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weichhart T., Costantino G., Poglitsch M., Rosner M., Zeyda M., Stuhlmeier K.M., Kolbe T., Stulnig T.M., Horl W.H., Hengstschlager M., Muller M., Saemann M.D. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Sun Z., Andersson R. NF-kappaB activation and inhibition: a review. Shock. 2002;18:99–106. doi: 10.1097/00024382-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Seow V., Lim J., Iyer A., Suen J.Y., Ariffin J.K., Hohenhaus D.M., Sweet M.J., Fairlie D.P. Inflammatory responses induced by lipopolysaccharide are amplified in primary human monocytes but suppressed in macrophages by complement protein C5a. J. Immunol. 2013;191:4308–4316. doi: 10.4049/jimmunol.1301355. [DOI] [PubMed] [Google Scholar]
- 26.Mijatovic T., Houzet L., Defrance P., Droogmans L., Huez G., Kruys V. Tumor necrosis factor-alpha mRNA remains unstable and hypoadenylated upon stimulation of macrophages by lipopolysaccharides. Eur. J. Biochem. 2000;267:6004–6012. doi: 10.1046/j.1432-1327.2000.01676.x. [DOI] [PubMed] [Google Scholar]
- 27.Shakhov A.N., Collart M.A., Vassalli P., Nedospasov S.A., Jongeneel C.V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J. Exp. Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gearing A.J., Beckett P., Christodoulou M., Churchill M., Clements J., Davidson A.H., Drummond A.H., Galloway W.A., Gilbert R., Gordon J.L. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita K., McDaid J., Ollinger R., Tsui T.Y., Berberat P.O., Usheva A., Csizmadia E., Smith R.N., Soares M.P., Bach F.H. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac allografts. FASEB J. 2004;18:765–767. doi: 10.1096/fj.03-0839fje. [DOI] [PubMed] [Google Scholar]
- 30.Riedemann N.C., Guo R.F., Laudes I.J., Keller K., Sarma V.J., Padgaonkar V., Zetoune F.S., Ward P.A. C5a receptor and thymocyte apoptosis in sepsis. FASEB J. 2002;16:887–888. doi: 10.1096/fj.02-0033fje. [DOI] [PubMed] [Google Scholar]
- 31.Wallner M., Bulmer A.C., Molzer C., Mullner E., Marculescu R., Doberer D., Wolzt M., Wagner O.F., Wagner K.H. Haem catabolism: a novel modulator of inflammation in Gilbert’s syndrome. Eur. J. Clin. Invest. 2013;43:912–919. doi: 10.1111/eci.12120. [DOI] [PubMed] [Google Scholar]
- 32.Weinberger B., Archer F.E., Kathiravan S., Hirsch D.S., Kleinfeld A.M., Vetrano A.M., Hegyi T. Effects of bilirubin on neutrophil responses in newborn infants. Neonatology. 2013;103:105–111. doi: 10.1159/000343097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maines M.D. Potential application of biliverdin reductase and its fragments to modulate insulin/IGF-1/MAPK/PI3-K signaling pathways in therapeutic settings. Curr. Drug Targets. 2010;11:1586–1594. doi: 10.2174/1389450111009011586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.