Abstract
Osteosarcoma is the most common primary osseous malignancy excluding malignant neoplasms of marrow origin (myeloma, lymphoma and leukemia) and accounts for approximately 20% of bone cancers. It predominantly affects patients younger than 20 years and mainly occurs in the long bones of the extremities, the most common being the metaphyseal area around the knee. These are classified as primary (central or surface) and secondary osteosarcomas arising in preexisting conditions. The conventional plain radiograph is the best for probable diagnosis as it describes features like sun burst appearance, Codman's triangle, new bone formation in soft tissues along with permeative pattern of destruction of the bone and other characteristics for specific subtypes of osteosarcomas. X-ray chest can detect metastasis in the lungs, but computerized tomography (CT) scan of the thorax is more helpful. Magnetic resonance imaging (MRI) of the lesion delineates its extent into the soft tissues, the medullary canal, the joint, skip lesions and the proximity of the tumor to the neurovascular structures. Tc99 bone scan detects the osseous metastases. Positron Emission Tomography (PET) is used for metastatic workup and/or local recurrence after resection. The role of biochemical markers like alkaline phosphatase and lactate dehydrogenase is pertinent for prognosis and treatment response. The biopsy confirms the diagnosis and reveals the grade of the tumor. Enneking system for staging malignant musculoskeletal tumors and American Joint Committee on Cancer (AJCC) staging systems are most commonly used for extremity sarcomas.
Keywords: Osteosarcoma, imaging, biopsy, Enneking staging
INTRODUCTION
Osteosarcoma is defined as the primary malignant mesenchymal bone tumor where the malignant tumor cells directly form the osteoid or bone or both.1,2,3,4,5,6,7,8,9,10,11,12 Demonstration of osteoid directly formed by the malignant cells in histopathology is essential for making the diagnosis of osteosarcoma.2,3
Although the exact cause of osteosarcoma is still unknown, defects in RB and p53 genes play an important role in the process. Patients with germline mutations in RB have approximately 1000-fold increased risk of osteosarcoma and similarly patients with Li-Fraumeni syndrome (germline p53 mutation) also have greatly elevated incidence of this tumor. Abnormalities in INK4a, which encodes p16 (a cell cycle regulator) and p14 (which aids and abets p53 function) are also seen. It is noteworthy that osteosarcoma occurs more commonly at sites of bone growth, presumably because proliferation makes osteoblastic cells to acquire mutations that could lead to transformation.1 Radiation too has been implicated in causation.1,2 The risk of developing postradiation osteosarcoma correlates with radiation dose and use of electrophilic chemotherapeutic agents.13,14,15 An etiological relationship has not been proven in prosthesis and metal hardware associated osteosarcomas.16
Classification
Osteosarcomas are classified as primary and secondary. Primary are further sub-typed as intramedullry/central and surface osteosarcomas as per World Health Organization classification2 [Box 1].
Box 1.
Classification of osteosarcoma

RADIOLOGICAL INVESTIGATIONS
Common sites of involvement of osteosarcoma are the metaphyseal areas (91%) of long bones of the extremities with its occurrence in (descending order) lower end of femur, upper end of tibia, upper end of humerus and upper end of femur. It can uncommonly occur in the diaphysis (9%) [Figure 1a]. Almost 50% of osteosarcomas occur around the knee. Involvement of nonlong bones like jaw (gnathic), pelvis, scapula, spine, and skull increases with age. Involvement beyond the wrist and ankle (acral sites) is extremely rare.2
Figure 1.

(a) X-ray anteroposterior and lateral views of proximal tibia and knee joint showing diaphyseal osteosarcoma of tibia with sclerosis (arrow), cortical destruction on posteromedial side (arrow heads) and new bone formation in the soft tissues (b) x-ray distal end of femur (anteroposterior and lateral views) showing sclerosis/radio-opacity in sclerosing osteosarcoma
Plain X-ray
The characteristic radiological features are sun-burst appearance, periosteal lifting with formation of Codman's triangle [Figure 2], new bone formation in the soft tissues along with permeative pattern of destruction of bone and other features for specific types of osteosarcoma.2,8,17,18 Osteolysis and expansion in the telangiectatic variety [Figure 3] of bone is observed while more of osteoblastic appearance is seen in the sclerosing type of osteosarcoma [Figure 1b]. The physis (growth plate) may, but not always, act as a barrier to tumor growth. Surface osteosarcomas have typical appearances [Figure 4]. After chemotherapy the tumor becomes well defined, capsulated, and more mineralized [Figure 5]. X-ray chest can detect metastasis in form of cannon ball appearance or nodules in the lungs [Figure 6a], but it is less sensitive than computerized tomography (CT) scan of the thorax [Figure 6b] for early detection of small sub-centimal nodules.19
Figure 2.
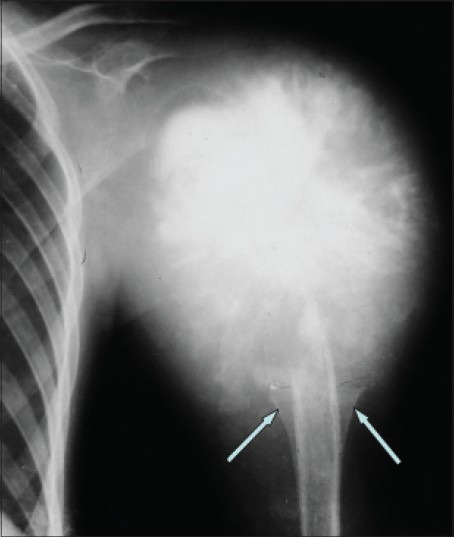
X-ray of humerus anteroposterior view showing osteosarcoma of the proximal humerus- typical sun burst or sun ray appearance, new bone formation in soft tissues, and Codman's triangles (arrows)
Figure 3.

Telangiectatic type of osteosarcoma of the proximal tibia: (a) X-ray anteroposterior and lateral views showing lysis and expansion (b) MRI showing fluid levels
Figure 4.
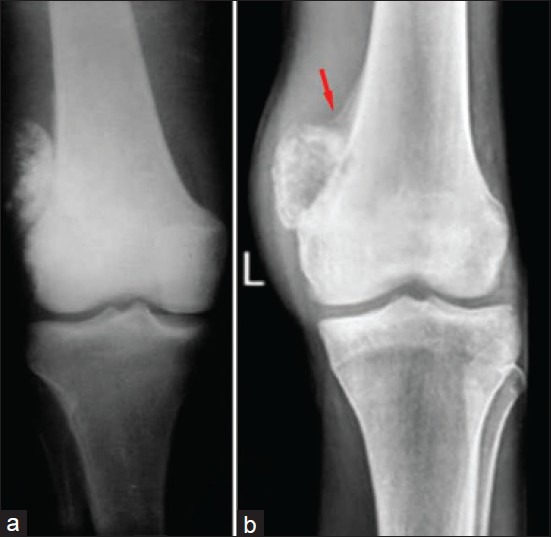
X-ray of knee joint anteroposterior views showing surface osteosarcoma: (a) parosteal (b) periosteal. See the under lying cortex is visibly intact in ‘a’ and lifting of periosteum in ‘b’ (red arrow). However, both are on the surface of the bone
Figure 5.
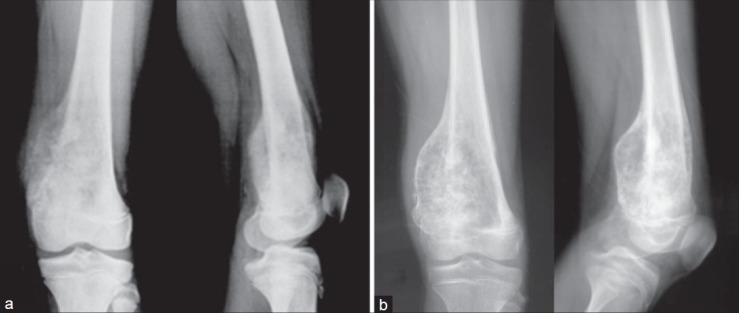
X-ray anteroposterior and lateral views showing that after chemotherapy the tumor becomes well defined with better capsulation: (a) before chemotherapy and (b) after chemotherapy
Figure 6.

(a) Plain X-ray chest of a patient of osteosarcoma showing multiple metastatic lung nodules (b) CT scan (axial section) demonstrating multiple metastases in both lungs (c) Tc-99m bone scan of osteosarcoma in the proximal humerus with hot spot at this site and in spine, ribs and a focus in the skull bone
Computerized tomography scan
CT scan delineates the bony anatomy/architecture like cortical integrity more clearly and picks up pathological fracture and is helpful in assessing ossification and calcification (chondroid component) more accurately.20,21 However, the soft tissue component and medullary extent is best defined by an MRI.22,23,24
Magnetic resonance imaging
MRI is the most accurate tool for determining the limits of tumor within and outside the bone.7,22,23,24 MRI should include the whole of the involved bone with one joint above and below so that skip lesions are not missed in the same bone and across the joint. MRI accurately and precisely delineates (1) extent of the tumor into the soft tissues and the medullary canal, (2) involvement of joint, (3) crossing of the lesion through and/or around the growth plate, (4) any skip lesion in the same bone and across the joint in other bone, (5) proximity and/or encasement of the neurovascular bundle by the tumor [Figure 7]. Recently, even the response of chemotherapy is being judged by MRI as the neo-angiogenesis decreases with chemotherapy, necrosis occurs, and the tumor shrinks with better capsulation. This is done by performing a contrast enhancement and diffusion MRI.25,26 MRI is also being coupled with Positron Emission Tomography for detection of the systemic involvement by the tumor, local recurrence, and metastasis after treatment. In view of the nonspecific findings of an MRI, it should always be correlated with the patient's x-ray.
Figure 7.

Osteosarcoma in the distal end of femur: (a) X-ray thigh with knee anteroposterior view showing big soft tissue component on the medial side; (b) MRI-coronal section showing the medullary extent (arrow); (c) MRI-axial section showing the proximity of the popliteal vessels
Radionuclide bone scan
Tc99 methylenediphosphonate (Tc99 MDP) bone scan is the most commonly used investigation for detecting osseous metastasis [Figure 6c]. It is performed by injecting 20 mCi of isotope intravenously and taking images at different intervals, in three phases: (1) the flow phase, (2) the immediate or equilibrium phase, and (3) the delayed phase. The flow phase demonstrates blood flow just like radionuclide angiogram; the equilibrium phase shows the reactive vascular flow and the distribution in the intercellular spaces, and the delayed phase is after 2-4 hours when the radionuclide is excreted in the urine except in the areas of the osteoblastic activities. This radio-isotope has special predilection to the sites of increased osteoblastic activity and highly vascular areas like the sites of metastasis in sarcomas. It is most easily available and cost-effective investigation for detecting bony metastasis in osteosarcoma.27
Positron emission tomography
Positron Emission Tomography (PET), which picks up metabolic activity is evolving with tremendous potential in oncology.28,29,30,31,32,33,34 Further combining the images of ‘form’ i.e. the anatomical structure provided by CT and MRI and those of ‘function’ i.e. metabolic or biochemical activity, provided by PET can be precisely aligned or correlated. MRI combined with PET facility reduces radiation exposure when compared to a CT.
PET is utilized in: (1) selecting the region of a tumor most likely to yield diagnostic information for biopsy, (2) staging known malignancies, (3) monitoring the effect of therapy, (4) to establish the cause of suspected recurrence seen on other imaging modalities. It differentiates between fibrosis and recurrent tumor (5) detecting tumor recurrence, especially in the presence of elevated levels of tumor markers, (6) differentiating benign from malignant lesions, (7) searching for an unknown primary tumor with metastasis of unknown origin, (8) guiding radiation therapy planning.33
The main drawback is the difficulty and cost of producing and transporting the radiopharmaceuticals used for PET imaging, which are usually extremely short-lived. The half life of radioactive fluorine18 used to trace glucose metabolism (using fluorodeoxyglucose, FDG) is 2 hours only. Its production requires a very expensive cyclotron as well as a production line for the radiopharmaceuticals. It can give false negative and positive results and is still considered the investigation under continuing research.33,34
Biochemical markers
The role of biochemical markers like serum alkaline phosphatase (ALP) and lactate dehydrogenases (LDH) for diagnosis, prognosis and response to treatment is pertinent to mention. Levels of alkaline phosphatase are elevated in osteosarcoma due to increased osteoblastic activity. Higher levels are associated with heavy tumor burden and poor prognosis. The response of therapy can be monitored with the levels of these enzymes. High levels after treatment may persist with residual disease or recurrence and in the presence of metastasis.8
Biopsy
Biopsy should be performed after complete history, clinical examination and imaging. It confirms the diagnosis, reveals specific type and furnishes the grade of the tumor. It is performed by either an open (incisional) or a closed method. Closed biopsy is performed as fine needle aspiration cytology (FNAC) and core needle biopsy.7 Open or incisional biopsy is performed through a small incision [Figure 8] and has the major advantage of obtaining adequate amount of sample for histopathology as well as for ancillary studies like immunohistocytochemistry (IHC) and genetic studies. But it takes more time and requires operation theatre set-up with instruments. There are more chances of contamination of normal soft tissue by tumor cells through an impending hematoma and also other complications like infection and wound problems posing greater morbidity. Further, there is more cost to the patient as it may require short stay in the hospital. However, if performed meticulously and properly, the complications can be reduced markedly almost comparable to those of a core needle biopsy.7 Percutaneous core needle biopsy has now evolved as a better, safe and accurate method for diagnosing of bone tumors. It is performed through a small stab using the Jamshidi needle and taking multiple cores from the representative part of the tumor [Figure 9]. It is less extensive and less time consuming outpatient procedure performed safely and quickly under local anesthesia and is cost effective. There is minimal soft tissue trauma with less contamination of normal tissue by the tumor cells around the tract of the needle which is easily excisable during the limb salvage surgery. It is very suitable for deep and difficult areas like the pelvis and spine.5,6 The efficacy and accuracy can be further increased by performing this under image guidance i.e. under CT scan, MRI or ultrasonography. The recent literature advocates core needle biopsy as it provides adequate amount of sample for the diagnosis and the ancillary studies, and has less number of complications.35,36,37,38,39,40,41,42,43,44,45,46,47,48
Figure 8.
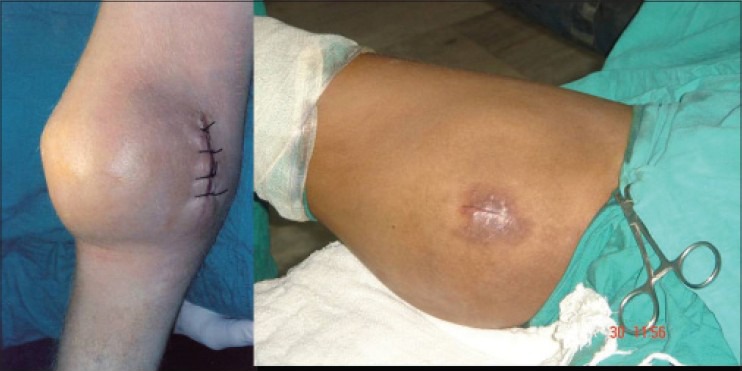
Open biopsies taken through small two cm incisions without making different planes. The incisions were placed such that these can be well resected with definitive resection of the tumor
Figure 9.

Core needle biopsy: (a) Jamshidi needle with trochar and stylet. (b) Biopsy being taken through stab incision. (c) Five good cores taken
FNAC does not have much role in majority of bone and soft tissue sarcomas as only few cells are inadequate for making a specific diagnosis and conducting ancillary studies. Ideally one should not start oncological treatment on the basis of a cytological diagnosis.7
Whether performed open or close, there are set principles for biopsy of musculoskeletal tumors which holds true for osteosarcoma too. Where, who, and how the biopsy should be performed are important issues. The biopsy is such an instrumental step that if not performed properly, the end result of definitive treatment can be affected significantly. We have noticed poorly performed biopsies in patients referred from periphery by nonspecialized general orthopedic surgeons and by un-experienced junior surgeons even at our institute. The incisions for biopsy were wrongly placed [Figure 10], patients had infection at the biopsy site due to big hematoma, and there were nondiagnostic samples due to inadequate material taken from nonrepresentative areas of the tumor. Due to these improper biopsies, the optimal treatment plan required alteration. The importance of biopsy has been well emphasized in literature by Mankin et al. who observed that because of wrong biopsies unnecessary amputations were performed in 4.5% of patients and the prognosis and outcome was altered in 8.5% of their patients. They found 18.2% major errors in diagnosis and 10.3% of biopsies being nonrepresentative. It has been emphasized that ideally the biopsy should be performed at the center where the definitive treatment of the tumor is to be performed under the guidance of a welltrained oncologist, taking all precautions, and following the basic principles.46,47,48,49,50 If the principles are followed properly the final outcome will obviously be better with lesser complications. This will prevent the repetition of biopsy and the treatment delay with reduction of overall cost42 [Box 2].
Figure 10.

Poorly performed biopsies: (a) Avoid transverse incision in the extremity because this is difficult to excise with definitive resection. (b) Never biopsy through buttock as this is the flap for the coverage in the hind quarter amputation if required. (c) Never biopsy through rectus femoris; very important for knee extension (d) Poor biopsy: Long incision and widely placed sutures marks will require excision of wide area of skin and under lying tissues if salvage surgery is contemplated and the wound closure may be compromised
Box 2.
Principles of biopsy

Staging
The common staging systems for malignant bone tumors are: Enneking system for staging malignant musculoskeletal tumors and the American Joint Committee on Cancer (AJCC) System for staging bone sarcomas.6 The former is based on the histological grade of the tumor, its local extent and the presence or absence of metastasis [Table 1]. Low grade lesions are stage-I, are well differentiated, have few mitoses and exhibit only moderate cytological atypia with low risk of metastasis (less than 25%). High grade lesions are stage-II are poorly differentiated, have high mitotic rate, and high cell to matrix ratio. On the basis of the involvement of the anatomical compartment (as determined by the natural anatomical barriers to tumor growth like cortical bone, articular cartilage, fascial septa, or joint capsules) these are further sub-divided as A and B. Stage–IA and IIA are contained in well defined compartment (intracompartmental) and stage–IB and IIB lesions extend beyond the compartment of origin (extracompartmental). Stage –III are lesions with metastasis (lymph node or distant) regardless of the size and grade.2,7
Table 1.
Enneking system for staging malignant musculoskeletal tumors

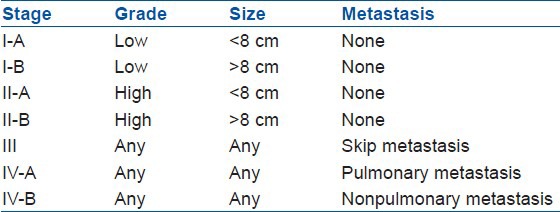
The AJCC system for bone sarcomas is based on tumor grade, size, presence, and location of metastases [Table 2]. Stage-I tumors are low grade and stage-II are high grade, which are subdivided based on tumor size. Stages I-A and II-A are 8 cm or less in their greatest linear measurement; stage I-B and II-B are larger than 8 cm. Stage III tumors have “skip metastases”, which are defined as discontinuous lesions within the same bone. Stage IV-A involves pulmonary metastases, whereas Stage IV-B involves nonpulmonary metastases. The stage IV is subdivided because patients with nonpulmonary metastases from osteosarcoma have worse prognosis than those with only pulmonary metastases.7,51,52, 53
Table 2.
American joint committee on cancer system for staging bone sarcomas
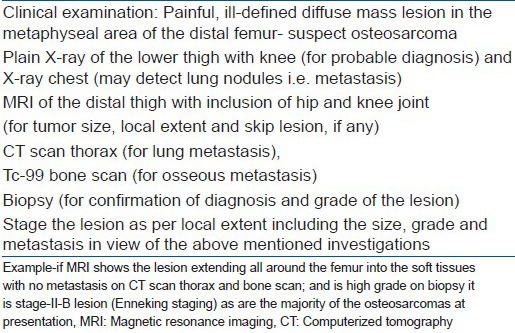
CONCLUSION
The plain radiograph provides the best clue to the diagnosis and MRI the local extent. Thorax CT scan and Tc99 bone scan are used for the detection of lung and bony metastasis respectively. The biopsy confirms the diagnosis and reveals the grade of the lesion. The basic principles of biopsy should be followed precisely and meticulously. After clinical, radiological and the histopathological examinations the tumor can be staged adequately [Box 3]. It is pertinent to mention that the patient should be immediately referred to the treating specialist centre for early diagnosis and treatment as this can make limb salvage possible in large number of patients. The urgent need of the MDT (multidisciplinay team) for the better out come in all musculoskeletal sarcomas can not be over-emphasised. However, the biological behavior of osteosarcoma is yet to be fully understood.
Box 3.
Algorithm for staging osteosarcoma in distal femur
Footnotes
Source of Support: Nil.
Conflict of Interest: None.
REFERENCES
- 1.Rosenberg AE. Robins and cotran pathologic basis of disease. 8th ed. Philadelphia: WB Saunders; 2010. Bone, joints and soft tissue tumors; pp. 1203–56. [Google Scholar]
- 2.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. Lyon: IARC Press; 2013. World Health Organization, classification of tumours: Pathology and genetics of tumors of soft tissue and bone. [Google Scholar]
- 3.Wold LE, Adler CP, Sim FH, Unni KK. 2nd ed. Philadelphia-London: Saunders; 2003. Atlas of orthopaedic pathology; pp. 179–85. [Google Scholar]
- 4.Jaffe HL. Philadelphia: Lea and Febiger; 1958. Tumors and tumors like conditions of the bones and joints. [Google Scholar]
- 5.Unni KK. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2010. Inwards CY Bone tumors: General aspects and data on 10,165 cases. [Google Scholar]
- 6.Lichtenstein L. 4th ed. Mosby: St Louis; 1972. Bone tumors. [Google Scholar]
- 7.Heck RK., Jr Canale ST, Beaty JH. Campbell's Operative Orthopedics. Missouri: Mosby Year Book; 2008. Malignant bone tumors; pp. 901–38. [Google Scholar]
- 8.Dorfman HD, Czerniak B. Mosby: St. Louis; 1998. Bone tumors. [Google Scholar]
- 9.Campanacci M. 2nd ed. Vienna: Springer-Verlag; 1999. Bone and soft tissue tumours: Clinical features, imaging, pathology and treatment. [Google Scholar]
- 10.Bacci G, Ferrari S, Lari S, Mercuri M, Donati D, Longhi A. Osteosarcoma of the limb: Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg. 2002;84:88–92. doi: 10.1302/0301-620x.84b1.12211. [DOI] [PubMed] [Google Scholar]
- 11.Bacci G, Ferrari S, Longhi A. Preoperative therapy versus immediate surgery in nonmetastatic osteosarcoma. J Clin Oncol. 2003;21:4662–6. doi: 10.1200/JCO.2003.99.157. [DOI] [PubMed] [Google Scholar]
- 12.Bacci G, Longhi A, Varsari M. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15 years experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–64. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 13.Huvos AG, Woodard HQ, Cahan WG, Higinbotham NL, Stewart FW, Butler A, et al. Postradiation osteogenic sarcoma of bone and soft tissues. A clinicopathologic study of 66 patients. Cancer. 1985;55:1244–55. doi: 10.1002/1097-0142(19850315)55:6<1244::aid-cncr2820550617>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Le Vu B, de Vathaire F, Shamsaldin A, Hawkins MM, Grimaud E, Hardiman CA, et al. Radiation dose, chemotherapy and risk of osteosarcoma after solid tumor during childhood. Int J Cancer. 1998;77:370–7. doi: 10.1002/(sici)1097-0215(19980729)77:3<370::aid-ijc11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Tucker MA, D’Angio GJ, Boice JD, Jr, Strong LC, Li FP, Stovall M, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Eng J Med. 1987;317:588–93. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 16.Visuri T, Pulkkinen P, Paavolainen P. Malignant tumors at the site of total hip prosthesis. Analytic review of 46 cases. J Artharoplasty. 2006;21:311–23. doi: 10.1016/j.arth.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 17.Resnick D, Kransdorf MJ. Bone and joint imaging. 3rd ed. Philadelphia Pennsylvania: Elsevier Saunders; 2005. Tumors and tumor-like lesions of bone: Radiographic principles; pp. 1109–98. [Google Scholar]
- 18.Resnick D. Tumors and tumor-like lesions of bone: Radiographic principles. In: Resnick D, editor. Diagnosis of bone and joint disorders. 4th ed. Philadelphia Pennsylvania: Saunders; 2002. pp. 3745–4128. [Google Scholar]
- 19.Vanel D, Henry AM, Lumbroso J, Lemalet E, Couanet D, Piekarski JD, et al. Pulmonary evaluation of patients with osteosarcoma: Role of standard radiography, tomography, CT, Scintigraphy and tomoscintigraphy. Am J Roentgenol. 1984;143:519–23. doi: 10.2214/ajr.143.3.519. [DOI] [PubMed] [Google Scholar]
- 20.Brown KT, Kattapuram SV, Rosenthal DI. Computed tomography analysis of bone tumors: Patterns of cortical destruction and soft tissue extension. Skeletal Radiol. 1986;15:448–51. doi: 10.1007/BF00355103. [DOI] [PubMed] [Google Scholar]
- 21.Kenney PJ, Gilula LA, Murphy WA. The use of computed tomography to distinguish osteochondroma and chondrosarcoma. Radiology. 1981;139:129–37. doi: 10.1148/radiology.139.1.6937887. [DOI] [PubMed] [Google Scholar]
- 22.Aisen AM, Martel W, Braunstein EM, McMillin KI, Philips WA, Kling TF. MRI and CT evaluation of primary bone and soft-tissue tumors. Am J Roentgenol. 1986;146:749–56. doi: 10.2214/ajr.146.4.749. [DOI] [PubMed] [Google Scholar]
- 23.Rubin DA. Magnetic resonance imaging: Practical considerations. In: Resnick D, Kransdorf MJ, editors. Bone and joint imaging. 3rd ed. Philadelphia Pennsylvania: Elsevier Saunders; 2005. pp. 118–32. [Google Scholar]
- 24.Bohndorf K, Reiser M, Lochner B, Feaux DL, Steinbrich W. Magnetic resonance imaging of primary tumors and tumor-like lesions of bone. Skeletal Radiol. 1986;15:511–7. doi: 10.1007/BF00361046. [DOI] [PubMed] [Google Scholar]
- 25.Holscher HC, Bloem JL, Vanel D, Hermans J, Nooy MA, Taminiau AH, et al. Osteosarcoma: Chemotherapy induced changes at MR imaging. Radiology. 1992;182:839–44. doi: 10.1148/radiology.182.3.1535905. [DOI] [PubMed] [Google Scholar]
- 26.Uhl M, Saueressig U, van Buiren M, Kontny U, Niemeyer C, Köhler G, et al. Osteosarcoma: Preliminary results of in vivo assessment of tumor necrosis after chemotherapy with diffusion- and perfusion-weighted magnetic resonance imaging. Invest Radiol. 2006;41:618–23. doi: 10.1097/01.rli.0000225398.17315.68. [DOI] [PubMed] [Google Scholar]
- 27.Schneider R. Radionuclide technique. In: Resnick D, Kransdorf MJ, editors. Bone and joint imaging. 3rd ed. Philadelphia Pennsylvania: Elsevier Saunders; 2005. pp. 86–117. [Google Scholar]
- 28.Huang TL, Liu RS, Chen TH, Chen WY, Hsu HC, Hsu YC. Comparison between F-18-FDG positron emission tomography and histology for the assessment of tumor necrosis rates in primary osteosarcoma. J Chin Med Assoc. 2006;69:372–6. doi: 10.1016/S1726-4901(09)70275-8. [DOI] [PubMed] [Google Scholar]
- 29.Hongtao L, Hui Z, Bingshun W, Xiaojin W, Zhiyu W, Shuier Z, et al. 18F-FDG positron emission tomography for the assessment of histological response to neoadjuvant chemotherapy in osteosarcomas: A meta-analysis. Surg Oncol. 2012;21:165–70. doi: 10.1016/j.suronc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Bajpai J, Kumar R, Sreenivas V, Sharma MC, Khan SA, Rastogi S, et al. Prediction of chemotherapy response by PET-CT in osteosarcoma: Correlation with histologic necrosis. J Pediatr Hematol Oncol. 2011;33:271–8. doi: 10.1097/MPH.0b013e31820ff78e. [DOI] [PubMed] [Google Scholar]
- 31.Im HJ, Kim TS, Park SY, Min HS, Kim JH, Kang HG, et al. Prediction of tumour necrosis fractions using metabolic and volumetric 18F-FDG PET/CT indices, after one course and at the completion of neoadjuvant chemotherapy, in children and young adults with osteosarcoma. Eur J Nucl Med Mol Imaging. 2012;39:39–49. doi: 10.1007/s00259-011-1936-4. [DOI] [PubMed] [Google Scholar]
- 32.Cistaro A, Lopci E, Gastaldo L, Fania P, Brach DP, Fagioli F. The role of 18F-FDG PET/CT in the metabolic characterization of lung nodules in pediatric patients with bone sarcoma. Pediatr Blood Cancer. 2012;59:1206–10. doi: 10.1002/pbc.24242. [DOI] [PubMed] [Google Scholar]
- 33.Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, et al. Procedure Guideline for SPECT/CT Imaging 1.0. J Nucl Med. 2006;47:1227–34. [PubMed] [Google Scholar]
- 34.Cook GJ, Maisey MN, Fogelman I. Fluorine-18-FDG PET in Paget's disease of bone. J Nucl Med. 1997;38:1495–7. [PubMed] [Google Scholar]
- 35.Bickels J, Jelinek JS, Shmookler BM, Neff RS, Malawer MM. Biopsy of musculoskeletal tumors. Clin Orthop Rel Res. 1999;36:212–9. [PubMed] [Google Scholar]
- 36.Mitsuyoshi G, Naito N, Kawai A, Kunisada T, Oshida A, Yanai H, et al. Accurate diagnosis of musculoskeletal lesions by core needle biopsy. J Surg Oncol. 2006;94:21–7. doi: 10.1002/jso.20504. [DOI] [PubMed] [Google Scholar]
- 37.Ottolenghi CE. Diagnosis of orthopaedic lesions by aspiration biopsy. J Bone Joint Surg Am. 1955;37:443–64. [PubMed] [Google Scholar]
- 38.Murphy WA, Destouet JM, Gilula LA. Percutaneous skeletal biopsy. A procedure for radiologists – results, review and recommendations. Radiology. 1981;139:545–9. doi: 10.1148/radiology.139.3.7232719. [DOI] [PubMed] [Google Scholar]
- 39.Moore TM, Meyers MH, Patzakis MJ, Terry R, Harvey JP., Jr Closed biopsy of musculoskeletal lesions. J Bone Joint Surg Am. 1979;61:375–80. [PubMed] [Google Scholar]
- 40.Skrzynski MC, Biermann JS, Montag A, Simon MA. Diagnostic accuracy and charge savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 1996;78:644–9. doi: 10.2106/00004623-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence Y, Nelson SD, Seeger LL, Eckardt JJ, Eilber FR. Primary musculoskeletal neoplasms: Effectiveness of core needle biopsy. Radiology. 1999;212:682–6. doi: 10.1148/radiology.212.3.r99se19682. [DOI] [PubMed] [Google Scholar]
- 42.Welker JA, Henshaw RM, Jelinek J, Shmookler BM, Malawer MM. The percutaneous needle biopsy is safe and recommended in the diagnosis of musculoskeletal masses. Cancer. 2000;89:2677–86. doi: 10.1002/1097-0142(20001215)89:12<2677::aid-cncr22>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 43.Jelinek JS, Murphey MD, Welker JA. Diagnosis of primary bone tumor with image-guided percutaneous biopsy: Experience with 110 Tumors. Radiology. 2002;223:731–7. doi: 10.1148/radiol.2233011050. [DOI] [PubMed] [Google Scholar]
- 44.Wu JS, Goldsmith JD, Horwich PJ, Shetty SK, Hochman MG. Bone and soft-tissue lesions: What factors affect diagnostic yield of image-guided core-needle biopsy? Radiology. 2008;248:962–70. doi: 10.1148/radiol.2483071742. [DOI] [PubMed] [Google Scholar]
- 45.Carrino JA, Khurana B, Ready JE, Silverman SG, Winalski CS. Magnetic resonance imaging-guided percutaneous biopsy of musculoskeletal lesions. J Bone Joint Surg Am. 2007;89:2179–87. doi: 10.2106/JBJS.F.01230. [DOI] [PubMed] [Google Scholar]
- 46.Pollock RC, Stalley PD. Biopsy of musculoskeletal tumours:Beware. ANZ J Surg. 2004;74:516–9. doi: 10.1111/j.1445-2197.2004.03060.x. [DOI] [PubMed] [Google Scholar]
- 47.Hogendoorn PC, Athanasoul N, Bielack S, De Alava E, Dei Tos AP, Ferrari S, et al. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and followup. Ann Oncol. 2010;21:204–13. doi: 10.1093/annonc/mdq223. [DOI] [PubMed] [Google Scholar]
- 48.Jalgaonkar A, Dawson-Bowling SJ, Mohan AT, Spielberg B, Saifudin A, Pollock R, et al. Identification of the biopsy track in musculoskeletal tumour surgery: A novel technique using India ink. Bone Joint J. 2013;95:250–3. doi: 10.1302/0301-620X.95B2.30123. [DOI] [PubMed] [Google Scholar]
- 49.Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am. 1982;64:1121–7. [PubMed] [Google Scholar]
- 50.Mankin HJ, Mankin CJ, Simon MA. The hazards of biopsy, revisited: Members of Musculoskeletal Society. J Bone Joint Surg. 1996;64:656–63. doi: 10.2106/00004623-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Broders AC. Squamous cell epithelioma of the lip: A study of 537 cases. JAMA. 1920;74:656–64. [Google Scholar]
- 52.Inwards CY, Unni KK. Classification and grading of bone sarcomas. Hematol Oncol Clin North Am. 1995;9:545–69. [PubMed] [Google Scholar]
- 53.Wolf RE, Enneking WF. The staging and surgery of musculoskeletal neoplasms. Orthop Clin North Am. 1996;27:473–81. [PubMed] [Google Scholar]


