Abstract
Background:
Periosteal osteosarcoma is an uncommon variant of osteosarcoma which constitutes less than 2% of all osteosarcomas. Whereas adequate surgical excision remains the cornerstone of treatment, the role of chemotherapy in this tumor is still unclear. Existing literature contains very few single center studies on the outcomes for periosteal osteosarcomas and any additional information will help in better understanding of these uncommon lesions. This study aims to evaluate the oncologic and functional outcomes of treatment of periosteal osteosarcoma treated at our institute.
Materials and Methods:
A retrospective analysis of 18 cases of periosteal osteosarcoma treated between January 2001 and December 2010 was carried out. There were 12 males and 6 females. The mean age at presentation was 16.3 years (range 5-26 years). Tibia and femur were the most common sites (n = 8). 16 of 18 patients received chemotherapy, 16 had limb sparing resection, one had an amputation and one had rotationplasty. Of the 16 patients with limb salvage, conventional wide excision was done in 11 cases. In 5 cases tumor was excised with hemicortical excision. Of the 11 cases treated with wide excisions, 4 patients underwent an osteoarticular resection and in 7 patients a joint preserving segmental intercalary resection was done.
Results:
All patients were available for followup. Surgical margins were free in all patients. A good response to chemotherapy was seen in 4/11 cases and poor in 6/11 cases. In one case the histological response was not discernible due to predominant chondromyxoid nature of the tumor. The median followup was 61 months (range: 18-130 months). There were two local recurrences (11%) at 9 and 18 months postsurgery. Pulmonary metastasis subsequently occurred in 4 cases (22%). Fourteen patients are currently alive and continuously disease free. Disease free survival at 5 years was 77.8% and overall survival (OVS) was 83.3%. Patients without marrow involvement had a better OVS at 5 years when compared with patients with marrow involvement (90% vs. 75%) (P = 0.23).
Conclusion:
Surgical excision remains the mainstay of treatment. Intramedullary involvement may suggest aggressive disease biology. The role of chemotherapy is still debatable and multicenter studies are needed to provide guidelines.
Keywords: Hemicortical, intercalary, limb salvage, periosteal osteosarcoma, resection
INTRODUCTION
Osteosarcoma is the most common primary malignant tumor of the bone accounting for approximately 35% of all primary malignant bone tumors.1 Osteosarcoma of the bone has been further classified into eight sub types. Periosteal osteosarcoma is an uncommon variant of osteosarcoma which constitutes less than 2% of all osteosarcomas.1 Even though, the lesion was first recognized by Ewing in 1939 the term “Periosteal osteogenic sarcoma” and its distinct clinicopathological description was given by Unni et al. in 1976.2,3,4,5 Periosteal osteosarcoma is an intermediate grade sarcoma usually arising from the surface in the diaphysio-metaphyseal region of a long bone. Tibia and femur are the most common bones involved. The affection of flat bones and short tubular bones is rare.4 A typical periosteal osteosarcoma has characteristic radiological and histopathological features which differentiate it from other counterparts. Radiologically the epicenter of the tumor is seen at the periosteal surface of bone. On microscopy, the typical appearance of periosteal osteosarcoma is that of chondroblastic osteosarcoma of intermediate grade. The cartilaginous component is predominant and appears as lobules of cartilage which show varying degree of cytological atypia.
Wide surgical excision is the cornerstone of treatment of periosteal osteosarcoma. Unlike conventional and other high grade sub types, the role of chemotherapy in the management of these lesions is unclear. Published literature contains data both in support and countering the efficacy of chemotherapy in improving outcomes of this uncommon intermediate grade sarcomas.6,7,8,9 As per our existing institutional protocol, periosteal osteosarcoma is treated like conventional high grade osteosarcoma. This includes excision of the lesion with adequate margins and administration of multiagent chemotherapy. In this series, we present oncologic and functional outcomes in the management of 18 cases of periosteal osteosarcoma.
MATERIALS AND METHODS
A retrospective review of our prospectively maintained data base of more than 6000 cases operated between January 2001 and December 2010 was carried out. 986 of these were osteosarcoma. These included 18 cases (1.8%) of periosteal osteosarcoma. The case file records, imaging, histopathology, oncologic and functional status were reviewed. For this study, the histopathological diagnosis of periosteal osteosarcoma was reconfirmed in all cases by an experienced musculoskeletal pathologist.
There were 12 male and 6 female patients. The mean age at presentation was 16.3 years (range 5-26 years). Tibia and femur were affected equally in each sex [Table 1].
Table 1.
Clinical details of patients
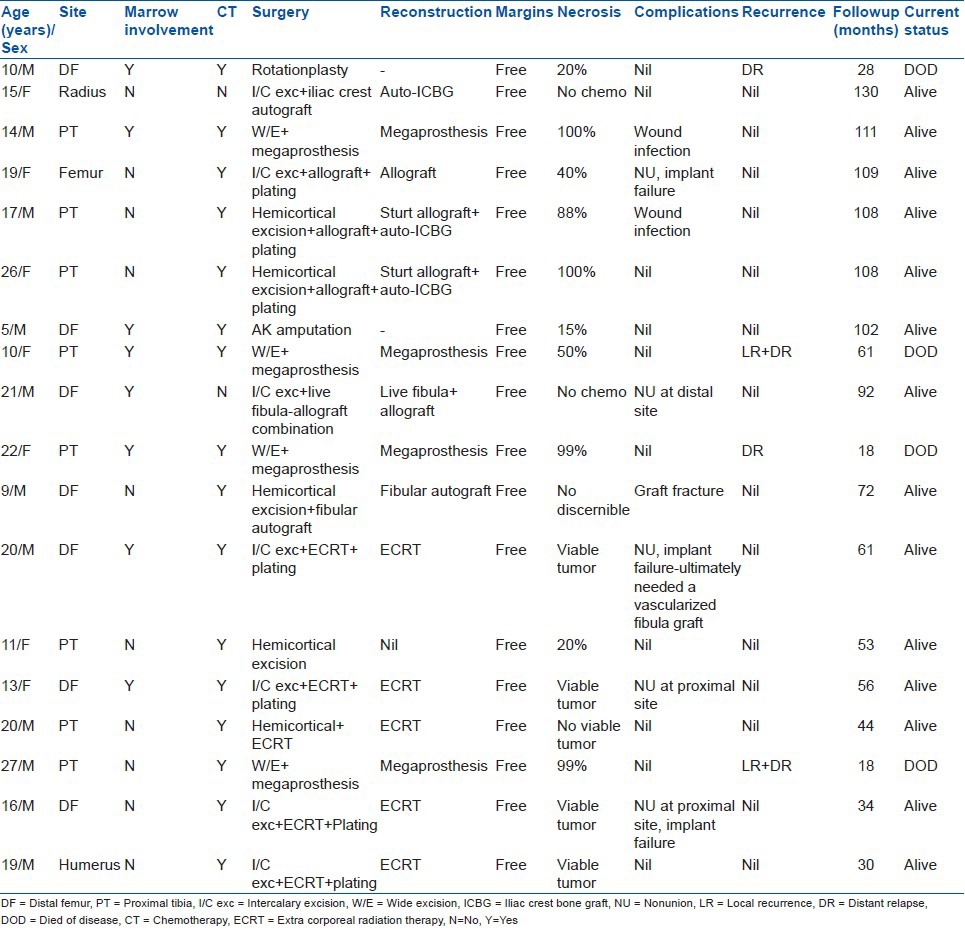
Primary lesion was imaged with a plain radiograph and magnetic resonance imaging for treatment planning. The extent of the lesion, its location, medullary involvement [Figure 1], extra osseous soft tissue component and relationship to the neurovascular bundle and adjacent joints were specifically evaluated. Staging studies included a computed tomogram (CT) scan of the chest and total body scintigraphy. All the patients were nonmetastatic at presentation.
Figure 1.
Plain radiograph of leg with knee joint anteroposterior and lateral views (a) and axial magnetic resonance imaging (b) showing periosteal osteosarcoma with no intramedullary extension
Histopathological diagnosis was obtained or reviewed in all cases. We prefer a core needle biopsy for diagnosis. The procedure yields adequate tissue and can be done as an outpatient procedure under local anesthesia with minimal risk of tissue contamination and infection. Seven patients with a prior biopsy outside our institute had their slides/blocks reviewed and the diagnosis confirmed.
All the patients were discussed in a multidisciplinary tumor board. Treatment was planned with 3-4 cycles of neoadjuvant multiagent chemotherapy followed by surgery and subsequent adjuvant chemotherapy.
A total of 16 cases received chemotherapy as per the existing hospital protocol. In one patient chemotherapy was deferred due to age and associated co-morbidities and another patient was unwilling for chemotherapy.
The primary goal of surgery was complete excision of the tumor with adequate margins. In 16 patients a limb sparing resection could be performed. One patient had an amputation in view of previous unplanned excision and contamination and 1 patient had a rotationplasty. Of the 16 patients with limb salvage, conventional wide excision was done in 11 cases. In 5 cases, the tumor was excised with hemicortical excision with adequate margins. Of the 11 cases treated with wide excisions, 4 patients underwent an osteoarticular resection and in 7 patients a joint preserving segmental intercalary resection was done [Table 1].
Various reconstruction modalities were used based on the type of resection, amount of residual bone, individual surgeon preference and the functional desires of the patient as assessed during preoperative counseling. Endoprosthetic reconstruction was done in all four patients with osteoarticular resection. Seven intercalary resections were reconstructed using autografts, allografts or extra corporeal radiotherapy (ECRT) of the tumor bone with reimplantation. Suitable internal fixation was used to stabilize the reconstruction. Of the 5 hemicortical excisions, no reconstruction was required in 1 case, fibular autograft was used in 1 case and ECRT with reimplantation in 1 case and strut allograft with iliac crest cancellous autograft in 2 cases [Table 1]. Postoperative rehabilitation was tailored according to the mode of reconstruction.
The resected specimens were assessed for surgical margins, marrow involvement and response to chemotherapy. The surgical margins were reported as free or involved. Wherever applicable, the response to chemotherapy was assessed using Huvo's grading system.10 The response to chemotherapy was considered good when the tumor necrosis was more than 90% (Huvo's Grade 3 and 4) and poor when the necrosis was less than 90% (Huvo's Grade 1 and 2). In cases of ECRT, the excised soft tissue component of the tumor was evaluated to assess the presence of viable tumor.
Patients were asked to followup every 3 months for the first 2 years, every 6 months for the next 3 years and annually thereafter. A CT of the chest was carried out every 6 months in the first 2 years and yearly for the next 3 years. An annual bone scan was also done for the first 2 years. The functional status was determined at final followup using the Musculoskeletal Tumor Society scoring system. This was based on the analysis of three factors (pain, functional activities and emotional acceptance) pertinent to the patient as a whole and three factors specific to either the upper limb or lower limb. For the upper limb, positioning of the hand, manual dexterity and lifting ability were assessed while for the lower limb use of supports for ambulation, walking ability and gait were assessed. For each of the six factors, values of 0-5 were assigned based on established criteria. The result was expressed as a sum total with a maximum score of 30 and as a percentage of the maximum score.
Survival rates were analyzed with the Kaplan-Meier method. Overall survival (OVS) was taken from the date of diagnosis to the last date when the patient was documented to be alive or the date of death. Disease free survival (DFS) was defined as the time from the date of diagnosis to recurrence of disease at local, regional, or distant sites.
RESULTS
The mean duration of surgery was 4 h (range 1.5-10 h) and mean blood loss was 630 ml (range 100-3500 ml). Surgical margins were free in all patients. On histopathology, intramedullary involvement was found in 7 patients (44%). Histological response to chemotherapy was assessed in 11 cases. A good response to chemotherapy was seen in 4 cases (Huvo's Grade 3 and 4) and poor response in 6 cases (Huvo's Grade 1 and 2). In one case the histological response was not discernible due to predominant chondromyxoid nature of the tumor. In the 5 cases of ECRT, the soft tissue showed evidence of viable tumor in 4 and complete tumor necrosis in one case.
Two patients developed surgical site infection. One of these cases with endoprosthetic replacement developed infection immediately after surgery. The infection settled after repeated wound lavage. Another case of hemicortical excision required removal of the allograft and implant. This was later reconstructed using the opposite fibula after infection was controlled at 3 months.
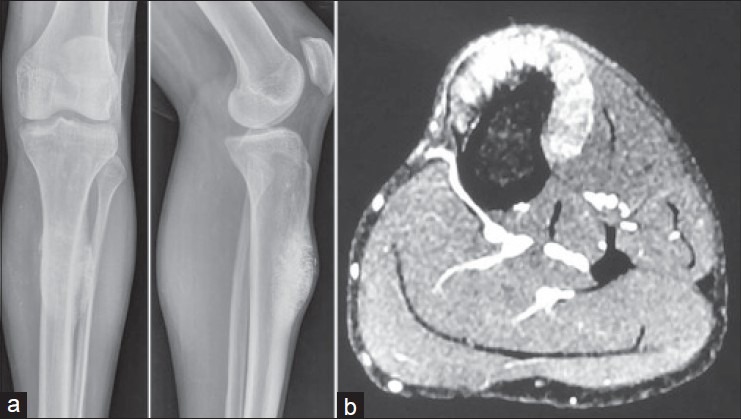
Of the 11 patients reconstructed with biological methods of reconstruction; hemicortical excision (4) and intercalary resection (7), union related complications were seen in 4 cases. Delayed union in a case of intercalary resection of distal femur reconstructed with combination of allograft and vascularized fibula required bone grafting (auto graft) at the metaphyseal (distal) osteotomy. Two cases of ECRT developed nonunion and implant failure at the diaphyseal (proximal) osteotomy site and required open reduction and repeat internal fixation and bone grafting (allograft) at 22 and 32 months respectively [Figure 2]. In spite of repeated bone grafting one case of ECRT did not unite and was ultimately reconstructed with a vascularized fibula at 50 months after removal of the radiated bone graft. One case of hemicortical excision, reconstructed with a fibular autograft, sustained fracture 7 months after index surgery. This required bone grafting (allograft) and plating. All the cases eventually united.
Figure 2.
(a) Plain radiographs of distal femur anteroposterior view showing intercalary excision and reconstruction with extra corporeal radiotherapy and plating (b) Followup radiograph showing nonunion at 20 months (c) Followup radiograph showing union after revision plating and bone grafting
The only case of intercalary resection reconstructed with an isolated allograft, developed allograft fracture 8 years after index surgery and was reconstructed with a combination of an allograft and vascularized fibula stabilized with internal fixation.
All patients were available for followup. The mean followup was 69 months, median 61 months (range 18-130 months).
There were two local recurrences (11%) at 9 and 18 months postsurgery. Both the cases had an open biopsy done elsewhere before presenting to our institute. In 1 case this was the isolated site of recurrence and was treated with an above knee amputation. The patient eventually developed unresectable pulmonary metastasis and died 57 months after the initial diagnosis. The other case with local recurrence had simultaneous unresectable pulmonary metastasis and was offered best supportive care. He died 13 months after index surgery.
Pulmonary metastasis occurred in 4 cases (22%) at 57 months, 12 months, 11 months and 9 months followup. Two of these patients had local recurrence as discussed above. Intramedullary involvement was seen in 3 of these 4 cases. Two patients had good response to chemotherapy and 2 patients had poor response to chemotherapy. All four patients had unresectable pulmonary metastasis and eventually succumbed to the disease.
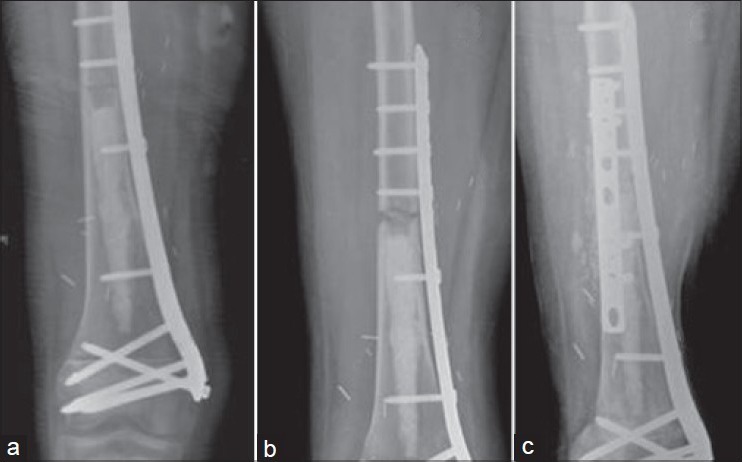
A total of 14 patients are currently alive and continuously disease free. The median followup of survivors was 82 months (range 30-130 months). DFS at 5 years was 77.8% and OVS was 83.3% [Figure 3]. Patients without marrow involvement had a better OVS at 5 years when compared with patients with marrow involvement (90% vs. 75%), however the log-rank test showed no significant difference (P = 0.23) [Figure 4]. There was no difference in survival in patients with good or poor response to chemotherapy (P = 0.500).
Figure 3.
Kaplan Meier curve depicting overall survival
Figure 4.
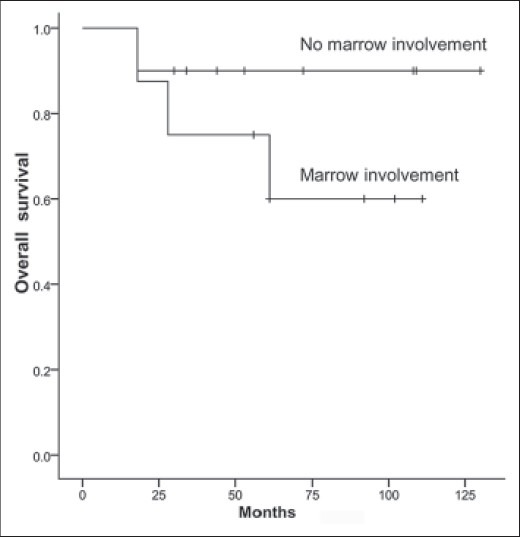
Kaplan Meier curve depicting survival based on intramedullary extension
DISCUSSION
Periosteal osteosarcoma is a relatively uncommon variant of osteosarcoma. Similar to other published reports, less than 2% of the osteosacomas operated at our institute were periosteal osteosarcoma.11,1
Surgical excision with wide margins remains the mainstay of achieving adequate local control [Table 2]. It is essential to achieve free margins as local recurrence is associated with poor survival. Grimer et al. reported a local recurrence rate of 7% with wide or radical margins in a series of 57 patients and stated that local recurrence is the most important prognostic factor for survival. 5 of 8 patients with local recurrence died of disease in their series.9 Revell et al. achieved tumor free margins in 15 of 17 cases. They had a 6% local recurrence rate in their series.12 We were able to achieve tumor free margins in all cases. In spite of that local recurrence was seen in 2 cases (11%). Both cases with local recurrence eventually succumbed to their disease reinforcing the importance of achieving adequate local control.
Table 2.
Review of literature on periosteal osteosarcoma
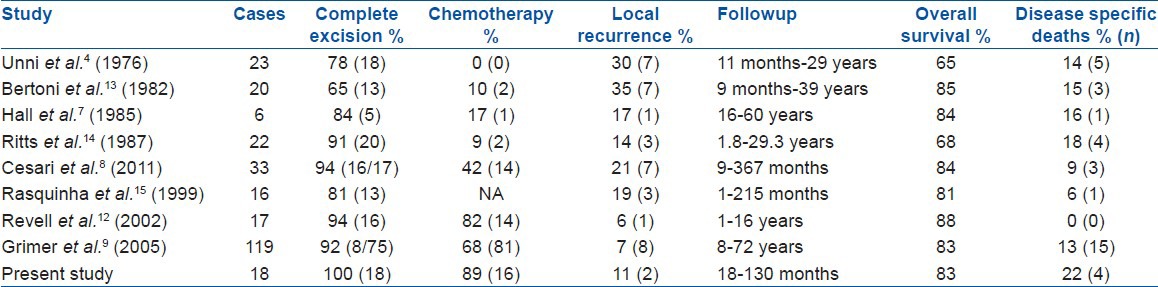
Current recommendations are unclear regarding the use of chemotherapy in the treatment of periosteal osteosarcoma [Table 2]. Two large series refute the role of chemotherapy in improving overall or DFS.8,9 Cesari et al. in a series of 33 patients had a similar OVS in patients receiving or not receiving chemotherapy.8 Similarly, Grimer et al. in a multicentric European review of 119 patients showed no benefit in survival in patients receiving chemotherapy.9 Though in our series 90% of patients received chemotherapy, there was no difference in survival of patients based on the response to chemotherapy unlike that observed in conventional high grade osteosarcoma.
Even though, Unni et al. have remarked that intramedullary extension is a point against the diagnosis of periosteal osteosarcoma, medullary and cortical involvement has been recognized by Spjut et al.6 and Hall et al.7 Whether intramedullary extension suggests an aggressive biology or a natural progression in course of the disease is uncertain.
Cesari et al. had seen medullary involvement in 69% of cases. In their series, distant relapse occurred only in patients with medullary involvement. The 10 year DFS was 61% in patients with medullary invasion while 86% when it was absent and the 10 year OVS rates were 74% and 86%, respectively.8 However, Revell et al. in a series of 17 patients had a medullary involvement in 4 cases (23%) and no case of distant relapse was seen.12 In our series medullary involvement was seen in 44% of cases. Three of the four cases (75%) with distant relapse had medullary involvement. Patients with medullary involvement had a poorer 5 year survival when compared with the patients without medullary involvement (OVS 89% vs. 75%). Currently, the jury is still out regarding the significance of medullary involvement in periosteal osteosarcoma and whether this is a subgroup of patients that may benefit with more aggressive treatment is debatable.
Second malignancies are an area of concern in most large series of periosteal osteosarcoma. At a median followup of 10 years Cesari et al. showed a higher incidence of secondary malignancies (9%) in periosteal osteosarcoma when compared with classic osteosarcoma. Most common second malignancies are leukemia and brain tumors.8,9,12 One of the possible hypotheses suggests that secondary neoplasms are related to inherited tumor syndromes like Li Fraumeni syndrome and are the result of a natural longer survival in periosteal osteosarcoma. It may be possible that these could be also related to the use of chemotherapy.
Our study has its limitations. It is a retrospective study. It is difficult to opine on the role and effect of chemotherapy as response to chemotherapy could not be assessed in 30% of cases (5/16) because the bone was reimplanted back (ECRT). The followup duration too is relatively short. In spite of these shortcomings, we believe that this study does add to the increasing understanding of the outcome of this uncommon variant of osteosarcoma. Its strength lies in the fact that it presents the results of a group of patients who were managed by the same multidisciplinary team. The cases were seen and treated at a specialist oncology center over a relatively short period of time where treatment philosophy was consistent and not affected by the availability of various investigational and therapeutic modalities in different eras.15
Footnotes
Source of Support: Nil.
Conflict of Interest: None.
REFERENCES
- 1.Fletcher DM, Unni K, Mertens F, editors. Lyon, France: IARC Press; 2002. Pathology and Genetics of Tumours of Soft Tissue and Bone. [Google Scholar]
- 2.Rose PS, Dickey ID, Wenger DE, Unni KK, Sim FH. Periosteal osteosarcoma: Long term outcome and risk of late recurrence. Clin Orthop Relat Res. 2006;453:314–7. doi: 10.1097/01.blo.0000229341.18974.95. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein L. Tumors of periosteal origin. Cancer. 1955;8:1060–9. doi: 10.1002/1097-0142(1955)8:5<1060::aid-cncr2820080533>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Unni KK, Dahlin DC, Beabout JW. Periosteal osteogenic sarcoma. Cancer. 1976;37:2476–85. doi: 10.1002/1097-0142(197605)37:5<2476::aid-cncr2820370541>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.Ewing J. A review of the classification of bone tumours. Bull Am Coll Surg. 1939;24:290–5. [Google Scholar]
- 6.Spjut HJ, Ayala AG, de Santos LA, Murray JA. Management of Primary Bone and Soft Tissue Tumors. Chicago, IL: Year Book Medical Publishers; 1977. Periosteal osteosarcoma; pp. 79–95. [Google Scholar]
- 7.Hall RB, Robinson LH, Malawar MM, Dunham WK. Periosteal osteosarcoma. Cancer. 1985;55:165–71. doi: 10.1002/1097-0142(19850101)55:1<165::aid-cncr2820550126>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Cesari M, Alberghini M, Vanel D, Palmerini E, Staals EL, Longhi A, et al. Periosteal osteosarcoma: A single-institution experience. Cancer. 2011;117:1731–5. doi: 10.1002/cncr.25718. [DOI] [PubMed] [Google Scholar]
- 9.Grimer RJ, Bielack S, Flege S, Cannon SR, Foleras G, Andreeff I, et al. Periosteal osteosarcoma: A European review of outcome. Eur J Cancer. 2005;41:2806–11. doi: 10.1016/j.ejca.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 10.Huvos AG. Bone Tumors: Diagnosis, Treatment, and Prognosis. 2nd ed. Philadelphia: W. B. Saunders; 1991. Osteogenic sarcoma: Pathologic assessment of preoperative (neoadjuvant) chemotherapy; pp. 122–8. [Google Scholar]
- 11.Campanacci M, editor. Wien: Springer Verlag; 1999. Bone and Soft Tissue Tumours. [Google Scholar]
- 12.Revell MP, Deshmukh N, Grimer RJ, Carter SR, Tillman RM. Periosteal osteosarcoma: A review of 17 cases with mean followup of 52 months. Sarcoma. 2002;6:123–30. doi: 10.1080/1357714021000066368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertoni F, Boriani S, Laus M, Campanacci M. Periosteal chondrosarcoma and periosteal osteosarcoma. Two distinct entities. J Bone Joint Surg Br. 1982;64:370–6. doi: 10.1302/0301-620X.64B3.7096408. [DOI] [PubMed] [Google Scholar]
- 14.Ritts GD, Pritchard DJ, Unni KK, Beabout JW, Eckardt JJ. Periosteal osteosarcoma. Clin Orthop Relat Res. 1987;219:299–307. [PubMed] [Google Scholar]
- 15.Rasquinha VJ, Zambakidis C, Pringle JAS, Saifuddin A, Briggs TWR, Cannon SR. Periosteal osteosarcoma. J Bone Jointt Surg. 1999;81B(Suppl II):186. [Google Scholar]


