Abstract
Background:
Osteosarcoma is a high grade malignant, osteoid forming, primary bone tumor affecting the metaphysis of long bones. Local recurrence (LR) in osteosarcomas is a sinister. Theoretically, a high tumor volume at the time of presentation will limit surgical margins, involve vital neurovascular bundles and show poor response to chemotherapy thereby causing high rates of amputations (as against limb salvage surgery) and should be associated with poor survival rates. This study evaluated objectively if high tumor volume is a significant predictor of local recurrence (LR) in operated cases of osteosarcomas.
Materials and Methods:
Operated cases of osteosarcoma (presenting to the Orthopedic outpatient or the Medical Oncology outpatient between January 1, 2004 and January 1, 2011 were included in the study. Their preoperative clinical data and investigations along with the operative notes were traced from the medical/departmental records. Details of chemotherapy received in the neo-adjuvant and postoperative periods were noted. Besides, all demographic data were also noted. Tumor volume was calculated using the available magnetic resonance images using the formula: ([π/6] × length × width × depth). Post data extraction, patients were divided in two groups, Groups I (without LR) and Group II (with LR).
Results:
A total of 95 cases of biopsy proven osteosarcomas were identified. Of which 64 were male and 31 females. There were 15 (15.8%) local recurrences. 71% (57/80) patients without LR fell in the age group of 10-20 years, while 66% (10/15) patients with LR were in the age group of 10-20 years. Limb salvage surgery was done in 81.05% (77/95) patients while a total of 18 patients underwent amputation. Of the 80 cases in Group I (without LR), 40 (50%) patients had tumor volume >200 c.c., 30 patients (37.5%) had tumor volume between 50 and 200 c.c. while only 10 patients had tumor volumes <50 c.c. This was in contrast to the tumor volume noted in Group II (with LR) of 15 patients where 8 patients had a tumor volume between 50 and 200 c.c., five had bigger tumor volumes of >200 c.c. and only two patients were smaller in size, with a tumor volume <50 c.c. The mean tumor volume in the group without LR was 406.74 ± 771.67 c.c. as compared with 195.77 ± 226.8 c.c. in the group with local recurrence. Using Mann-Whitney test, the difference between the two groups was found to be statistically insignificant (P = 1.403).
Conclusions:
We conclude that high tumor volume is not a significant predictor of LR in osteosarcomas thus patients with high tumor masses should not be denied limb salvage. However, we recommend that the decision on attempting limb salvage should not only be based on the tumor volume alone.
Keywords: Local recurrence, osteosarcoma, tumor volume
INTRODUCTION
Osteosarcoma is a high grade malignant, osteoid forming, primary bone tumor affecting the metaphysis of long bones. Local recurrence (LR) in osteosarcomas is a sinister. The alternatives for removal of recurrent tumor are limited and amputation is usually the treatment of choice. In these cases, life expectancy is considerably diminished because LR in the most cases is usually followed death due to associated distant metastasis. Thus, local control of the disease remains a critical goal of treatment.1,2,3 Local recurrences in musculoskeletal sarcomas have been extensively studied at various centers across the globe.4,5,6 Despite a large number of studies, there is still a lack of consensus on prognostic factors that determine local recurrence. In developing countries the occurrence of high volume osteosarcomas of the extremities is not uncommon [Figure 1].
Figure 1.
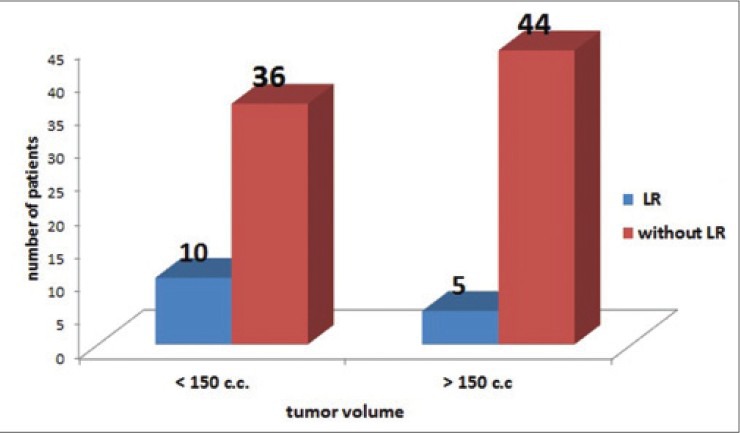
Bar diagram illustrating the relationship between number of patients (with and without local recurrence) and tumor volume in our series
Theoretically, a high tumor volume at the time of presentation will limit surgical margins, involve vital neurovascular bundles and show poor response to chemotherapy thereby causing high rates of amputations (as against limb salvage surgery) and should be associated with poor survival rates. With the same premise, we can conclude that the high volume of initial tumor will also affect the LR rates in osteosarcomas. Studies evaluating high tumor volume as a prognosticator for LR in patients undergoing limb salvage surgeries are few and with conflicting results.7,8,9
This retrospective study reviewed a 7 year data on osteosarcomas at a tertiary care institute and evaluated whether high tumor volume was significantly associated with LR or not. It was important to evaluate this as a factor for LR since a large number of cases in our country present late with high tumor volume and even with fungation.
MATERIALS AND METHODS
This retrospective, observational study was conducted after obtaining approval from the ethics committee of the institute. All patients of osteosarcoma who have undergone limb salvage surgery or amputation as a primary procedure for their disease, between January 1, 2004 and January 1, 2011 and Patients who presented with recurrence during the study period were included in the study. The cases excluded were; all cases with surgery (limb salvage or amputation) done outside our hospital, patients who received radiotherapy or chemotherapy outside our hospital, patients with metastasis at the time of presentation, patients who died within 3 months postoperatively and patients with incomplete clinicopathological/radiological data.
Their preoperative clinical data and investigations along with the operative notes were traced from the medical/departmental records. Details of chemotherapy received in the neo-adjuvant and postoperative periods were noted. Besides, all demographic data were noted on a prefixed proforma. The time of recurrence from surgery and any associated distant metastasis was noted.
The tumor volume was calculated using the available magnetic resonance (MR) images as described by Göbel et al.10 using the formula: ([π/6] × length × width × depth). We used the preoperative MR films for the same. Post data extraction, patients were divided in two groups, Groups I (without local recurrence) and Group II (with local recurrence).
Statistical analysis
The relevant medical, radiological, and oncological secondary data extracted from medical files, using a defined protocol was assembled in a database (Microsoft Excel 2010, Redmond, Washington. Statistical analysis was performed using the SPSS, version 16, statistical software (SPSS Inc., Chicago). Descriptive analysis was performed to determine means, medians, and percentages. Prognostic significance of tumor volume was compared between the two groups (i.e., with and without local recurrence) using the Mann-Whitney test. Chi-square test was finally applied between the two groups using 150 c.c. tumor volume as a cut off. Conclusions were drawn on the basis of significance of the results (P < 0.05).
RESULTS
A total of 95 cases of biopsy proven osteosarcomas were identified from our database. 64 (67.37%) were male and 31 (32.63%) females. Of these, 15 (15.8%) had local recurrences. 71% (57/80) patients without LR fell in the age group of 10-20 years while 66% (10/15) patients with LR were in the age group of 10-20 years. Distal end of femur was the most common site of involvement. Limb salvage surgery was done in 81.05% (77/95) patients, while a total of 18 patients underwent amputation. In Group I, without LR, 68.75% (55/80) were males and 31.25% (25/80) were females. In Group II, with LR, 60% (9/15) were males and 40% (6/15) were females. Though, there was higher incidence of LR in females, the difference between both genders as a risk factor for LR was statistically insignificant (P = 0.507). The average follow up in our study was 26.9 months (range 4-81 months).
Of the 80 osteosarcoma cases in Group I (without LR), 40 (50%) patients had tumor volume >200 c.c., 30 patients (37.5%) had tumor volume between 50 and 200 c.c. while only 10 patients had tumor volumes <50 c.c. This was in contrast to the tumor volume noted in Group II (with LR) of 15 patients where eight patients had a tumor volume between 50 and 200 c.c., five had bigger tumor volumes of >200 c.c. and only two patients were smaller in size, with a tumor volume <50 c.c. The mean tumor volume in the group without LR was 406.74 ± 771.67 c.c. as compared to 195.77 ± 226.8 c.c. in the group with local recurrence. Using Mann-Whitney test, the difference between the two groups was found to be statistically insignificant (P = 1.403).
On taking 150 c.c. as a cut off, 10 (66.66%) cases in Group II had smaller tumor volume of <150 c.c. as compared to 36 (45%) cases in Group I [Figure 2]. Though LR was more common in patients with smaller tumor volume (of <150 c.c.), it was statistically not significant (P = 0.123).
Figure 2.
Clinical photograph of a patient with a high volume osteosarcoma of the proximal tibia of the left leg
Thus, tumor volume per se is not a significant predictor of LR in operated cases of osteosarcomas.
DISCUSSION
A large number of osteosarcomas present late to the treating physician in developing countries. It is established that in Ewing's sarcoma a delay in seeking treatment in India is largely due to socio-economic factors, which causes the tumor size to increase and therefore do poorly when compared to a demographically similar population of the west, even when treated by the same multimodality chemotherapy regimen.11 Unfortunately, such data is lacking on osteosarcomas. A delay in seeking treatment for osteosarcomas is known to increase the tumor size and therefore results in a drastic change in the treatment offered.12 It is thus prudent that the initiation of treatment should be quick in osteosarcomas, so as to avoid large tumor sizes, which can have a bearing on the final surgical treatment offered and the incidence of local recurrence. There is a paucity of objective data from India on the relationship between tumor size and occurrence of LR in patients with osteosarcomas. This study had aimed to fulfill this lacuna.
Tumor volume (size of the primary tumor) as a predictive factor for LR in osteosarcoma has conflicting results in the literature. In a study by Bacci et al., 560 osteosarcomas of the extremities were studied.8 Of these 465 underwent limb salvage while 95 patients had an amputation (or a rotationplasty). The LR rate in their study was 61.5% (34 local recurrences at a median followup of 10.5 years). They took 150 cm3 as the cut off for high tumor volume. On statistical analysis, the tumor volume was not found to be a significant predictor of LR in their study (P = 0.007). Our results were similar to those reported by Bacci et al. but the LR rate in our study was 15.8% as compared to 6.15% in their study. We feel that the high LR in this study was multifactorial and was not dependent on the initial tumor volume as has been seen in the statistical analysis in our study.
In a study from the European Osteosarcoma Intergroup,7 202 patients were assessed for surgical treatment of osteosarcomas. The LR rates at three different European centers (two from United Kingdom and one from Netherlands) were 13.3%, 6.8%, and 2.5%, respectively. The overall incidence of LR was 8%. This was lower than the LR rate seen in our study (14%). They evaluated tumor volume as a predictor of local recurrence. The size and tumor volume varied between those having amputation and those having limb salvage surgery, but local surgery perse (salvage vs. amputation) was not a significant factor for LR in the study. Median length of tumor requiring an amputation in this study was 13 cm, while it was 10.8 cm for those patients undergoing limb salvage surgery. They concluded that high tumor size was a significant risk factor in the occurrence of LR in osteosarcomas (P = 0.003). Diversity in patient population and difference in surgical approaches can be reasons attributed to high tumor volume being a risk factor in LR in this multi-center study. In fact, the authors themselves conclude that the remarkably different LR rates in different centers of the study could be attributed to the difference in surgical margins that were achieved postsurgery at these centers. Interestingly, in this study, though statistically insignificant, LR was more common in tumors with smaller volumes (that is <150 c.c.). We cannot explain this fact completely, but feel that this occurs due to the presence of other factors, which affect LR namely, surgical margins, chemotherapy response, pathological fracture or a delay in surgery post neoadjuvant chemotherapy. The latter being a common occurrence in resource challenged environments like ours.
We feel that in view of the present available knowledge for osteosarcomas, it is difficult to pinpoint tumor volume as the single, important determinant of LR following surgery. In a recent study, Song et al. showed that increase in tumor volume during preoperative chemotherapy is a prognostic factor for LR in osteosarcomas.9 However, they also mentioned that poor response to preoperative chemotherapy could also be a reason in their study for higher local relapses in patients with high tumor volume since chemotherapy response is an important prognosticator for local recurrence. We did not measure the change in tumor volume postchemotherapy in our study and hence cannot conclude on the same.
The main limitations of this study are its retrospective nature, hospital based design, small sample size and a short follow up. Despite the shortcomings of our study, we conclude that initial tumor volume at presentation per se is not a significant prognostic factor predicting LR in osteosarcomas. We recommend that a high tumor volume osteosarcoma should thus be carefully evaluated before planning surgery. A prechemo MR images should be done and involvement of the neurovascular bundle and surrounding compartments should be carefully assessed and limb salvage surgery planned according to the clinicoradiological profile of the patient. Limb salvage should not be denied to patients merely by the assessment of tumor size.
Footnotes
Source of Support: Nil.
Conflict of Interest: None.
REFERENCES
- 1.Spanier SS, Shuster JJ, Vander Griend RA. The effect of local extent of the tumor on prognosis in osteosarcoma. J Bone Joint Surg Am. 1990;72:643–53. [PubMed] [Google Scholar]
- 2.Wuisman P, Enneking WF. Prognosis for patients who have osteosarcoma with skip metastasis. J Bone Joint Surg Am. 1990;72:60–8. [PubMed] [Google Scholar]
- 3.Stahl M, Ranft A, Paulussen M, Bölling T, Vieth V, Bielack S, et al. Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer. 2011;57:549–53. doi: 10.1002/pbc.23040. [DOI] [PubMed] [Google Scholar]
- 4.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–90. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 5.Hauben EI, Weeden S, Pringle J, Van Marck EA, Hogendoorn PC. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer. 2002;38:1218–25. doi: 10.1016/s0959-8049(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 6.Bacci G, Longhi A, Cesari M, Versari M, Bertoni F. Influence of local recurrence on survival in patients with extremity osteosarcoma treated with neoadjuvant chemotherapy: The experience of a single institution with 44 patients. Cancer. 2006;106:2701–6. doi: 10.1002/cncr.21937. [DOI] [PubMed] [Google Scholar]
- 7.Grimer RJ, Taminiau AM, Cannon SR Surgical Subcommitte of the European Osteosarcoma Intergroup. Surgical outcomes in osteosarcoma. J Bone Joint Surg Br. 2002;84:395–400. doi: 10.1302/0301-620x.84b3.12019. [DOI] [PubMed] [Google Scholar]
- 8.Bacci G, Ferrari S, Lari S, Mercuri M, Donati D, Longhi A, et al. Osteosarcoma of the limb. Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84:88–92. doi: 10.1302/0301-620x.84b1.12211. [DOI] [PubMed] [Google Scholar]
- 9.Song WS, Jeon DG, Kong CB, Cho WH, Koh JS, Lee JA, et al. Tumor volume increase during preoperative chemotherapy as a novel predictor of local recurrence in extremity osteosarcoma. Ann Surg Oncol. 2011;18:1710–6. doi: 10.1245/s10434-010-1536-8. [DOI] [PubMed] [Google Scholar]
- 10.Göbel V, Jürgens H, Etspüler G, Kemperdick H, Jungblut RM, Stienen U, et al. Prognostic significance of tumor volume in localized Ewing's sarcoma of bone in children and adolescents. J Cancer Res Clin Oncol. 1987;113:187–91. doi: 10.1007/BF00391442. [DOI] [PubMed] [Google Scholar]
- 11.Tiwari A, Gupta H, Jain S, Kapoor G. Outcome of multimodality treatment of Ewing's sarcoma of the extremities. Indian J Orthop. 2010;44:378–83. doi: 10.4103/0019-5413.69307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sneppen O, Hansen LM. Presenting symptoms and treatment delay in osteosarcoma and Ewing's sarcoma. Acta Radiol Oncol. 1984;23:159–62. doi: 10.3109/02841868409136005. [DOI] [PubMed] [Google Scholar]


