Abstract
We present a patient with dermal argyria as a cutaneous manifestation of a silver-coated megaprosthesis used for a distal femoral osteosarcoma. Histological and electron microscopic analyses also showed silver deposition in the dermis.
Keywords: Dermal argyria, distal femur, osteosarcoma, silver
INTRODUCTION
Reconstruction using a megaendoprosthesis is the method of choice for achieving best possible level of function in patients undergoing limb salvage surgery following sarcoma.1,2 This kind of endoprosthesis are used for young patient with malignant bone tumors.3
However, the large surface area of the implant is predisposed to bacterial colonization.4 In addition to bacterial colonizing, a biofilm can be formed that enables bacteria to evade the host defense system and local or systemic antibiotic treatment.5 In such cases the only viable option is removal of the prosthesis and radical debridement, which is harmful for the patient.
Among metals with antimicrobial activity, silver has garnered much interest due to its excellent antimicrobial activity coupled with low toxicity.6 Toxicological side-effects in patients receiving silver-coated tumor endoprostheses have been excluded in previous studies7 and the high antimicrobial activity of silver-coated prostheses has been demonstrated in both human and animal studies.8,9 We describe such a case with cutaneous manifestation (dermal argyria) of a silver-coated femoral megaprosthesis 1 year postimplantation, which was confirmed via histological and electron microscopic analyses. Silver-coated prostheses are applied mainly due to the following two indications: (1) For infection prophylaxis in tumor endoprosthetics and (2) as the last option for patients after extensive trauma-related infection.3
CASE REPORT
A 14 year old girl with distal femoral osteosarcoma was treated with limb salvage surgery [Figure 1a]. A silver coated proximal tibial, distal femoral composite prosthesis (Mutars®, Implantcast, Ltd., Buxtehude, Germany) was used [Figure 1b]. She had a negative history of other diseases.
Figure 1.
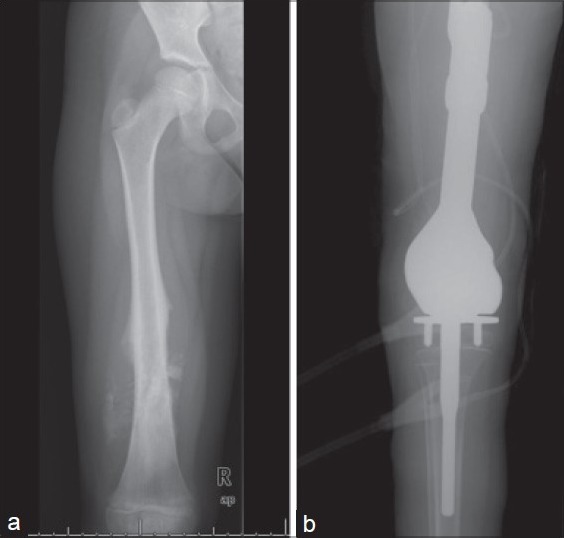
(a) Preoperative X-ray showing femoral osteosarcoma. (b) Postoperative X-ray after limb salvage surgery
There were no complications postoperatively or during routine followup visits. She noticed a blue-gray discolored pattern on the skin over the incision site at the proximal tibia [Figure 2] after 1 year postsurgery. Complete blood count, C-reactive protein, the erythrocyte sedimentation rate and kidney and liver function test results were normal. A diagnosis of dermal argyria was considered. A 0.5-cm skin biopsy specimen was obtained from the discolored area under local anesthesia. Histological analysis revealed silver deposition in dermal layers [Figure 3]. The same specimen was also analyzed via electron microscopic mapping, which also showed the presence of silver [Figure 4]. No additional procedures were considered, as the patient was asymptomatic. At 3 years followup (since the patient's dermal argyria was diagnosed) she was clinically well with normal blood parameters, although the peri-incisional discoloration persisted.
Figure 2.
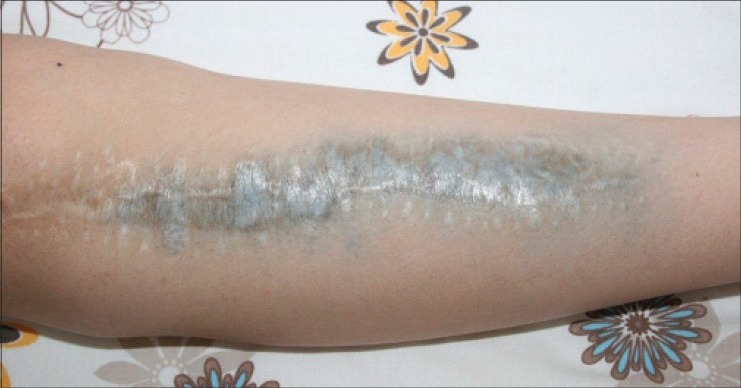
Clinical photograph of the patient's leg shows blue-grayish discoloration
Figure 3.
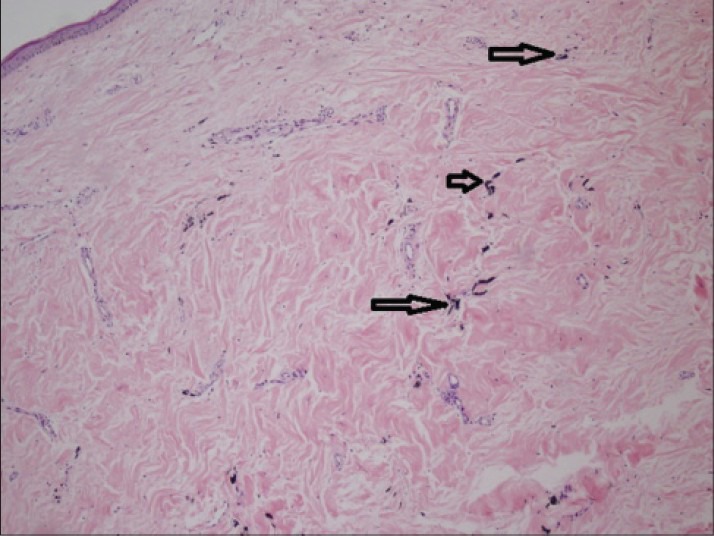
Histological analysis shows silver deposition in the dermis (hollow arrows)
Figure 4.
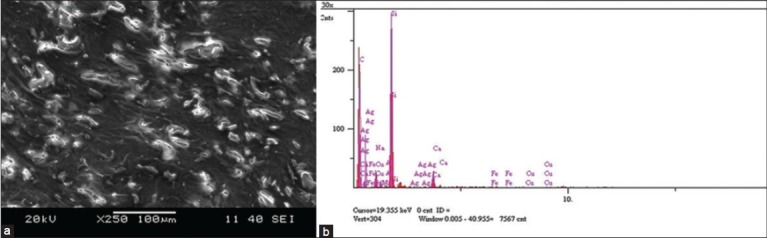
Electron microscopic analysis of the biopsy specimen showing dermal deposits
DISCUSSION
Coating orthopaedic implants with silver to prevent infection is not a new idea.10 In literature most of the studies are focused on silver coating external fixator pins and prosthesis.9,11,12,13,14 The findings of in vivo studies on the efficacy of silver for preventing infection are inconsistent.11,12,13 Hardes et al.9 in their study have reported that silver coating of tumor megaprostheses effectively prevented infection. Their study states that in silver coated prosthesis group the rates of infection and amputation rates are low. In some cases dermal argyria could not be diagnosed due to already discolored area in that region possibly because of varicose veins and additional analyses, such as skin biopsy (as performed in the presented case) were not performed. One study reported that silver-coated prostheses were safe, based on the lack of observable signs of local or systemic argyria and serum silver levels that were below the toxic level.7
Argyria is deposition of silver products in the connective tissue of the dermis, but not the epidermis. These inert silver deposits are intracellular (lysosomal bound) or intercellular, and are long lasting or permanent. There is no association between cellular damage and altered sensory perception in the skin, the mechanism is not fully understood, but is thought to be associated with imbalances in the concentrations of soluble and insoluble silver complexes in the dermis and the action of lysosomal reductase. Argyremia (or elevated blood silver) is not always correlated with dermal manifestations.15
It seems logical to inform patients about the possible consequences of silver-coated megaprostheses, such as dermal argyria, when they are superficially located (i.e. the proximal tibia). The patient should be informed about the complications like the typical toxic side effects of silver, such as dermal argyria (i.e. blue or bluish-grey colored skin), ocular argyrosis, gastroenteritis or fever. At 3 years postdiagnosis of dermal argyria in the presented case was not associated with additional pathology and/or cellular damage, indicating that simple followup of the patient was sufficient.
Footnotes
Source of Support: Nil.
Conflict of Interest: None.
REFERENCES
- 1.Hillmann A, Hoffmann C, Gosheger G, Krakau H, Winkelmann W. Malignant tumor of the distal part of the femur or the proximal part of the tibia: Endoprosthetic replacement or rotationplasty. Functional outcome and quality-of-life measurements. J Bone Joint Surg Am. 1999;81:462–8. doi: 10.2106/00004623-199904000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Grimer RJ, Belthur M, Chandrasekar C, Carter SR, Tillman RM. Two-stage revision for infected endoprostheses used in tumor surgery. Clin Orthop Relat Res. 2002;395:193–203. doi: 10.1097/00003086-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Hussmann B, Johann I, Kauther MD, Landgraeber S, Jäger M, Lendemans S. Measurement of the silver ion concentration in wound fluids after implantation of silver-coated megaprostheses: Correlation with the clinical outcome. Biomed Res Int 2013. 2013 doi: 10.1155/2013/763096. 763096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechert T, Steinrücke P, Guggenbichler JP. A new method for screening antiinfective biomaterials. Nat Med. 2000;6:1053–6. doi: 10.1038/79568. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerli W, Lew PD, Waldvogel FA. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Invest. 1984;73:1191–200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobin EJ, Bambauer R. Silver coating of dialysis catheters to reduce bacterial colonization and infection. Ther Apher Dial. 2003;7:504–9. doi: 10.1046/j.1526-0968.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 7.Hardes J, Ahrens H, Gebert C, Streitbuerger A, Buerger H, Erren M, et al. Lack of toxicological side-effects in silver-coated megaprostheses in humans. Biomaterials. 2007;28:2869–75. doi: 10.1016/j.biomaterials.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Gosheger G, Hardes J, Ahrens H, Streitburger A, Buerger H, Erren M, et al. Silver-coated megaendoprostheses in a rabbit model: An analysis of the infection rate and toxicological side effects. Biomaterials. 2004;25:5547–56. doi: 10.1016/j.biomaterials.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Hardes J, von Eiff C, Streitbuerger A, Balke M, Budny T, Henrichs MP, et al. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol. 2010;101:389–95. doi: 10.1002/jso.21498. [DOI] [PubMed] [Google Scholar]
- 10.Darouiche RO. Antiinfective efficacy of silver-coated medical prostheses. Clin Infect Dis. 1999;29:1371–7. doi: 10.1086/313561. quiz 1378. [DOI] [PubMed] [Google Scholar]
- 11.Collinge CA, Goll G, Seligson D, Easley KJ. Pin tract infections: Silver vs uncoated pins. Orthopedics. 1994;17:445–8. doi: 10.3928/0147-7447-19940501-11. [DOI] [PubMed] [Google Scholar]
- 12.Coester LM, Nepola JV, Allen J, Marsh JL. The effects of silver coated external fixation pins. Iowa Orthop J. 2006;26:48–53. [PMC free article] [PubMed] [Google Scholar]
- 13.Massè A, Bruno A, Bosetti M, Biasibetti A, Cannas M, Gallinaro P. Prevention of pin track infection in external fixation with silver coated pins: Clinical and microbiological results. J Biomed Mater Res. 2000;53:600–4. doi: 10.1002/1097-4636(200009)53:5<600::aid-jbm21>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.Bosetti M, Massè A, Tobin E, Cannas M. Silver coated materials for external fixation devices: In vitro biocompatibility and genotoxicity. Biomaterials. 2002;23:887–92. doi: 10.1016/s0142-9612(01)00198-3. [DOI] [PubMed] [Google Scholar]
- 15.Lansdown AB. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv Pharmacol Sci 2010. 2010 doi: 10.1155/2010/910686. 910686. [DOI] [PMC free article] [PubMed] [Google Scholar]


