Abstract
SummaryThe PROmotion of Breastfeeding Intervention Trial (PROBIT) is a multicentre, cluster-randomized controlled trial conducted in the Republic of Belarus, in which the experimental intervention was the promotion of increased breastfeeding duration and exclusivity, modelled on the Baby-friendly hospital initiative. Between June 1996 and December 1997, 17 046 mother–infant pairs were recruited during their postpartum hospital stay from 31 maternity hospitals, of which 16 hospitals and their affiliated polyclinics had been randomly assigned to the arm of PROBIT investigating the promotion of breastfeeding and 15 had been assigned to the control arm, in which breastfeeding practices and policies in effect at the time of randomization was continued. Of the mother–infant pairs originally recruited for the study, 16 492 (96.7%) were followed at regular intervals until the infants were 12 months of age (PROBIT I) for the outcomes of breastfeeding duration and exclusivity; gastrointestinal and respiratory infections; and atopic eczema. Subsequently, 13 889 (81.5%) of the children from these mother–infant pairs were followed-up at age 6.5 years (PROBIT II) for anthropometry, blood pressure (BP), behaviour, dental health, cognitive function, asthma and atopy outcomes, and 13 879 (81.4%) children were followed to the age of 11.5 years (PROBIT III) for anthropometry, body composition, BP, and the measurement of fasted glucose, insulin, adiponectin, insulin-like growth factor-I, and apolipoproteins. The trial registration number for Current Controlled Trials is ISRCTN37687716 and that for ClinicalTrials.gov is NCT01561612. Proposals for collaboration are welcome, and enquires about PROBIT should be made to an executive group of the study steering committee (M.S.K., R.M.M., and E.O.). More information, including information about how to access the trial data, data collection documents, and bibliography, is available at the trial website (http://www.bristol.ac.uk/social-community-medicine/projects/probit/).
Why was the cohort set up?
The PROmotion of Breastfeeding Intervention Trial (PROBIT) was established by Professor Michael S Kramer of McGill University and the PROBIT I team to assess the effects of breastfeeding promotion on breastfeeding duration and exclusivity, gastrointestinal and respiratory infections, atopic eczema, and growth among infants.1,2 Prior evidence that breastfeeding is beneficial for infant and child health in industrialized countries was based almost exclusively on observational studies, which are prone to confounding and other biases.3,4 Yet randomising healthy term-infants to breast- versus bottle-feeding is infeasible and probably unethical. The PROmotion of Breastfeeding Intervention Trial was designed as a cluster-randomised controlled trial in which 31 maternity hospitals and one each of their affiliated polyclinics (outpatient clinics where children are followed for routine health care) across the Republic of Belarus were randomised to a control group at 15 of the hospitals and clinics that continued the breastfeeding practices and policies in effect at the time of randomisation or to the experimental intervention arm, at 16 of the hospitals and clinics, which was modelled on the Baby-Friendly Hospital Initiative designed to increase the duration and exclusivity of breastfeeding5 (Figure 1). Eligible maternity hospitals were not located in one of the geographic areas contaminated by radionuclides in the wake of the Chernobyl disaster in the Ukraine, because women from those areas were often advised by paediatricians not to breastfeed.1
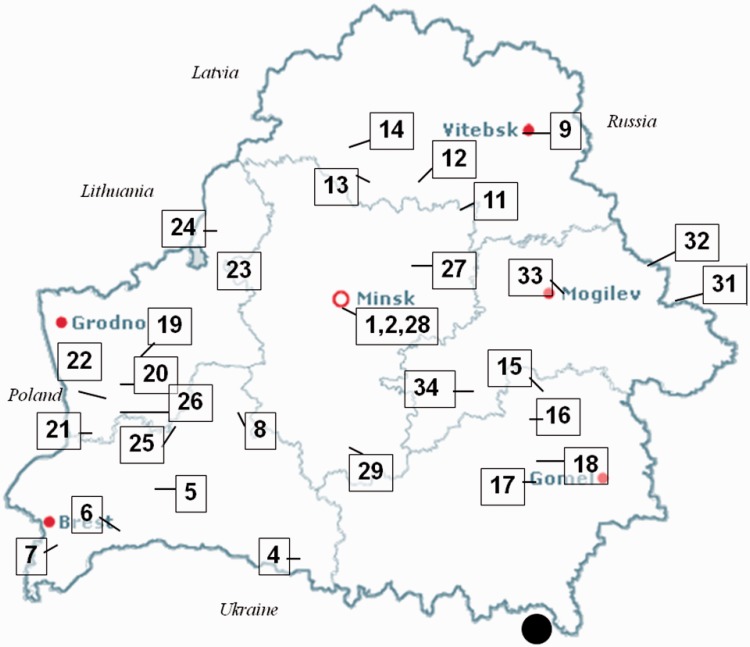
Figure 1.
Location of the 31 hospitals and affiliated polyclinics involved in the Promotion of Breastfeeding Intervention Trial within the Republic of Belarus (numbered 1–34). The large black circle indicates the location of the Chernobyl nuclear reactor in the Ukraine
The Republic of Belarus was chosen as the location for the trial because postpartum infant care practices in its maternity hospitals and polyclinics in the 1990's were similar to those in North America and Western Europe 20–30 years earlier, providing a large potential contrast between the intervention and control sites and thereby allowing study of the potential health effects of prolonged and exclusive breastfeeding. The conventional practices of postpartum infant care at the hospitals and clinics in the trial included routine separation of mothers and their infants; delayed onset of breastfeeding; scheduled feedings; routine use of water, formula or other liquids in the newborn diet; and early introduction of solid foods.3 Yet Belarus, part of the Union of Soviet Socialist Republics (USSR) for almost 70 years until its independence in 1991, resembles developed Western countries in many important respects, including an uncontaminated water supply, readily accessible health services (even in rural areas), high rates of immunization and adult literacy, similar compositions of infant formula, and relatively low rates of infant and child mortality.1 At the time PROBIT was designed, more than 95% of mothers in Belarus initiated breastfeeding, but few breastfed exclusively, and half discontinued breastfeeding completely (weaned) by 3 months postpartum.
Who is in the cohort and how often have they been followed-up?
Mother–infant pairs were recruited for the trial during their postpartum hospital stay. Mothers were eligible for participation if they initiated breastfeeding on admission to the postpartum ward, had no illnesses that would contraindicate breastfeeding or severely compromise its success, and had given birth to a healthy singleton infant of 37 weeks or longer gestation, with a birth weight of 2500 g or more, and an Apgar score 5 or higher at 5 minutes after delivery. The staff of the trial estimated that only 1–2% of eligible women declined participation. All of the mothers in the trial were Russian- or Belarussian-speaking.
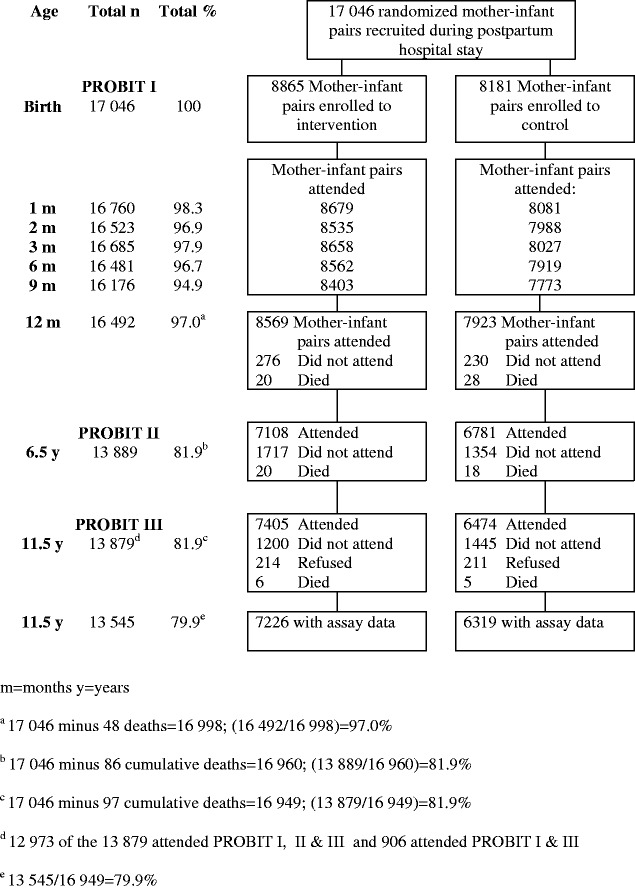
At recruitment (baseline) between June 1996 and December 1997, 17 046 healthy mother–infant pairs were enrolled in the trial and were interviewed and examined at the time of birth of the infant and at 1, 2, 3, 6, 9, and 12 months thereafter (PROBIT I). Of these original mother–infant pairs, 16 492 (96.7%) completed the 12-month follow-up visit, 13 889 (81.5%) completed the age 6.5-year follow-up visit, and 13 879 (81.4%) completed the 11.5-year follow-up visit (Figures 2 and 3). Monitoring visits and data audits were conducted during recruitment and follow-up in all phases of PROBIT to ensure compliance with study protocols and as a check on the validity and inter-observer reproducibility of the measurements of the trial outcome data.1,6,7
Figure 2.
Recruitment and follow-up phases of the Promotion of Breastfeeding Intervention Trial with data for infants and children
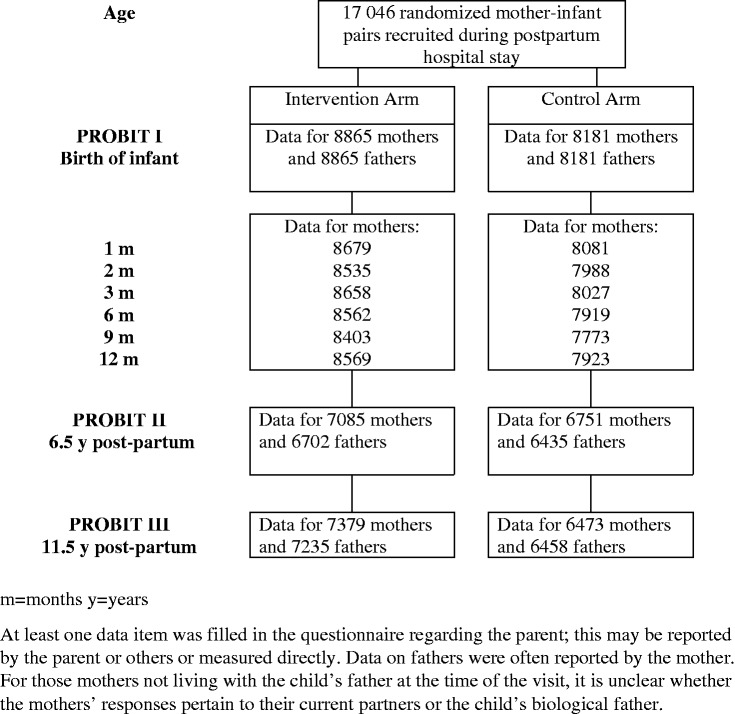
Figure 3.
Recruitment and follow-up phases of the Promotion of Breastfeeding Intervention Trial with data for parents
The characteristics by trial arm of the mother–infant pairs that were not followed-up and of those who were followed-up in PROBIT I, II, and III are given in Table 1. Mothers who did not attend the follow-up visit of PROBIT II were slightly younger at the time of birth of their infant (24.6 years and 24.5 years vs. 24.9 years and 25.0 years in the intervention and control arms, respectively), were slightly less likely to have partly completed university or advanced secondary education (46% and 48.1% vs. 47.8% and 54.5%, respectively), and were more likely to have smoked during pregnancy (3.5% and 2.6% vs. 2.6% and 1.6%, respectively), and the study child was more likely to have been their first child (64.1% and 63.6% vs. 58.8% and 54.5%, respectively). The characteristics of mothers not followed-up at the PROBIT III visit were similar to those of the mothers at the PROBIT II visit. The data shown in Figures 2 and 3 and in Table 1 vary minimally from previously published figures owing to the updating of some variables during collection of the PROBIT III data.
Table 1.
Comparison of baseline characteristics of attenders and non-attenders at each phase of the Promotion of Breastfeeding Intervention Trial by intervention or control arm
| PROBIT I |
PROBIT II |
PROBIT III |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recruitment |
Attenders |
Non-attenders |
Attenders |
Non-attenders |
|||||||
| Trial arm | Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | |
| N | 8865 | 8181 | 7108 | 6781 | 1757 | 1400 | 7405 | 6474 | 1460 | 1707 | |
| Maternal education (%) | |||||||||||
| Completed university | 14.1 | 13 | 13.5 | 12.9 | 16.5 | 13.5 | 14.1 | 13 | 14 | 13.1 | |
| Advanced secondary or partial university | 47.4 | 53.4 | 47.8 | 54.5 | 46 | 48.1 | 48.1 | 55 | 44.2 | 47.3 | |
| Common secondary | 33.9 | 30.4 | 34.3 | 29.7 | 32.2 | 34.3 | 33.6 | 29.2 | 35.4 | 35.1 | |
| Incomplete secondary | 4.6 | 3.2 | 4.4 | 3 | 5.4 | 4.1 | 4.2 | 2.8 | 6.4 | 4.5 | |
| Paternal education (%) | |||||||||||
| Completed university | 13.8 | 13.1 | 12.5 | 12.3 | 19 | 16.7 | 13.4 | 12.2 | 16 | 16.4 | |
| Advanced secondary or partial university | 40.9 | 49.6 | 41.7 | 50.4 | 38 | 46 | 41.6 | 51.1 | 37.5 | 44.2 | |
| Common secondary | 39.4 | 31.7 | 40.1 | 31.7 | 36.6 | 31.3 | 39.7 | 31.5 | 37.8 | 32.2 | |
| Incomplete secondary | 2.7 | 1.9 | 2.7 | 1.9 | 3 | 2.1 | 2.5 | 1.8 | 4 | 2.5 | |
| Stratum-level variable (%) | |||||||||||
| East/Urban | 34.9 | 30.9 | 34.2 | 30.6 | 37.7 | 32.4 | 33.8 | 30.2 | 40.4 | 33.6 | |
| East/Rural | 14.5 | 17.4 | 15.8 | 16.7 | 9.2 | 20.5 | 14.7 | 16.1 | 13.6 | 22.3 | |
| West/Urban | 30.6 | 20.4 | 29.1 | 18.9 | 36.5 | 27.6 | 31.5 | 19.3 | 25.8 | 24.4 | |
| West/Rural | 20.1 | 31.4 | 20.9 | 33.8 | 16.6 | 19.5 | 20 | 34.4 | 20.1 | 19.7 | |
| Other children living in the household at birth | |||||||||||
| 0 | 59.8 | 56.1 | 58.8 | 54.5 | 64.1 | 63.6 | 59 | 54.5 | 64 | 61.9 | |
| 1 | 32.3 | 34.9 | 33.3 | 36.1 | 28.1 | 28.7 | 33.3 | 36.4 | 27.1 | 29.2 | |
| ≥2 | 7.9 | 9.1 | 7.9 | 9.4 | 7.7 | 7.6 | 7.7 | 9.1 | 8.9 | 8.9 | |
| Maternal smoking in pregnancy (%) | 2.8 | 1.8 | 2.6 | 1.6 | 3.5 | 2.6 | 2.5 | 1.6 | 4.2 | 2.2 | |
| Male (%) | 51.8 | 52 | 51.5 | 52.1 | 52.9 | 51.4 | 50.9 | 52.1 | 56 | 51.8 | |
| Mean (SD) maternal age | 24.9(4.9) | 24.9(4.9) | 24.9(5.0) | 25.0(4.9) | 24.6(4.8) | 24.5(4.8) | 25.0(5.0) | 25.0(4.9) | 24.2(4.7) | 24.6(4.9) | |
| Mean (SD) paternal age | 27.4(5.2) | 27.4(5.1) | 27.4(5.2) | 27.5(5.1) | 27.1(5.4) | 26.9(5.1) | 27.5(5.2) | 27.5(5.1) | 26.9(5.3) | 27.1(5.2) | |
| Mean (SD) birthweight (g) | 3438(418) | 3438(421) | 3440(418) | 3441(423) | 3428(418) | 3421(411) | 3443(417) | 3442(423) | 3414(419) | 3421(413) | |
What has been measured?
A summary of data recorded at each phase of PROBIT is given in Table 2. In PROBIT I, information was collected on the baseline characteristics of the infant (e.g. sex; delivery date, method and complications; gestational age; Apgar score; weight (g), length (mm), and head circumference (mm); number of siblings; and type of infant feeding) and the parents (date of birth; education; occupation; marital status; atopic history (asthma, hay fever and eczema); and maternal smoking and alcohol consumption during pregnancy). At regular, scheduled polyclinic visits at 1, 2, 3, 6, 9, and 12 months, the study paediatrician or midwife collected data via maternal interviews and infant measurement that included the following information about the infant: all home and clinic visits for acute illnesses; weight (g), length (mm), and head circumference (mm); vaccinations; feeding method (breastfeeding, mother’s and/or donor’s expressed milk, formula, cow milk, or other milk; water, juice, or other liquids; cereals and other solid foods); times per day of feedings or millilitres per day of intake, as appropriate; and occurrence of and hospitalisations for gastrointestinal illness, respiratory illness, and atopic eczema. Information was also collected about maternal smoking and alcohol consumption.
Table 2.
Summary of data items collected in the Promotion of Breastfeeding Intervention Trial
| Method | Measurements |
|---|---|
| PROBIT I | |
| June 1996–December 1997 | |
| At birth | |
| N = 17 046 | |
| Study paediatrician/ midwife- recorded from medical notes and interview with mother |
|
| July 1996–January 1999 | |
| Age 1, 2, 3, 6, 9, and 12 months | |
| N = 16 492 at 12 months | |
| Paediatrician measured/recorded |
|
| PROBIT II | |
| December 2002––April 2005 | |
| Age 6.5 years | |
| N = 13 889 | |
| Parent-reported: 91% mother 8% father/guardian 1% unknown |
|
| Paediatrician measured in duplicate | Child’s:
|
| Paediatrician administered | |
| Paediatrician reviewed medical records since the child was age 12 months |
|
| Parent-reported child behaviour; questionnaires |
|
| Teacher-reported child behaviour; questionnaires |
|
| PROBIT III | |
| January 2008–December 2010 | |
| Age 11.5 years | |
| N = 13 879 | |
| Parent-reported: 72% mother 17% father/guardian 1% both parents 10% child/unknown |
|
| Child-reported | |
| Paediatrician measured | If available, mother’s:
|
| Paediatrician collected |
|
| Laboratory assays |
|
| Paediatrician- reviewed medical records from age 7 years to current date |
|
| Paediatrician- reviewed medical records for those children who did not participate in PROBIT II from age 12 months to 7 years |
|
Abbreviations: PROBIT, Promotion of Breastfeeding Intervention Trial; WHO, World Health Organization
To assess the accuracy of PROBIT I data, 20 polyclinic charts per study site were selected at random and reviewed, and were compared in terms of data with the PROBIT polyclinic visit forms. Among the 20 charts reviewed, interviews were conducted with 10 of the mothers. This audit compared the occurrence of 1 or more gastrointestinal-tract infections and 2 or more respiratory-tract infections in the infants, as well as breastfeeding at 3 months. Agreement was high for all 3 outcomes, with no difference in the degree of under- or over-reporting by trial arm.1
When the infants in PROBIT I had grown into children with a median age of 6.6 years [interquartile range (IQR) 6.5–6.7; range 5.6–8.5 years], the following data were collected about them in PROBIT II: measures of adiposity [e.g. weight (kg); triceps and subscapular skinfold thicknesses (mm) and waist circumference (cm)]; standing and sitting height (cm);6 blood pressure (BP);6 measures of cognitive development (Wechsler Abbreviated Scales of Intelligence (WASI));8 mother- and teacher-reported behaviour and results of the Strengths and Difficulties Questionnaire (SDQ);9 measures of atopy (the International Study of Asthma and Allergies in Childhood (ISAAC)) questionnaire and the results of skin-prick tests);10 and measures of dental caries.11 For 91% of the participants, the following information was reported by the mother, and in the remaining 9% was reported by the father or guardian of the child or by unknown sources: parental weight and height, marital status, family relationships, and smoking and alcohol consumption.
In PROBIT II, 190 randomly selected children (5 per paediatrician) were re-examined by one of the Minsk-based study paediatricians (auditors) to assess the reproducibility of the children’s measurements.6 To avoid selection bias, the audit was done after completion of the primary data collection so that all of the children seen during follow-up were eligible for selection for the audit. Primary data collection took 28 months. Audit examinations were conducted at an average of 17.7 months (range 5.3–32.6 months) after the primary examination. Owing to the time elapsed between the audit and primary examinations, the results were compared through use of the Pearson correlation coefficient. The auditors were blinded to the initial measurements taken by the polyclinic paediatricians. The test–retest correlation was high for height (Pearson correlation coefficient r = 0.84), body mass index (r = 0.89), and mid-upper–arm (r = 0.85) and waist circumferences (r = 0.84), but was modest for leg length (r = 0.74), hip (r = 0.72), head (r = 0.65), and mid-thigh (r = 0.55) circumferences and for subscapular (r = 0.65) and triceps (r = 0.59) skinfold thicknesses and systolic (r = 0.55) and diastolic (r = 0.45) BP.
In the third phase of the trial, PROBIT III, children were followed-up at a median age of 11.5 years (IQR 11.3–11.8; range 10.2–14.5 years). The measurements made on the children were: measures of adiposity [e.g. weight (kg); triceps and subscapular skinfold thicknesses (mm); waist circumference (cm); percent body fat, fat mass (kg), and fat-free mass (kg), measured by foot-to-foot bio-impedance]; standing and sitting height (cm); BP; and whole-blood insulin,12 adiponectin, insulin-like growth factor-I (IGF-I), and apolipoproteins A1 and B, measured after at least 8 hours of fasting and collected via finger prick onto specially developed Whatman 903 (Whatman plc, Maidstone, UK) filter-paper cards. Fasted whole blood glucose was also measured in a finger prick blood specimen with a hand-held glucometer (Advantage Accu-Chek Glucometer; Roche Diagnostics Limited, Burgess Hill, UK). The children self-completed the Children’s Eating Attitude Test (ChEAT), a brief questionnaire that elicits information about attitudes toward eating.13,14 If the mother of the child was present at the study visit or attended later, the paediatrician also measured her height (cm), weight (kg), percent body fat, fat mass (kg), fat-free mass (kg), and diastolic or systolic BP or both (mm Hg). Of the children’s mothers, 93.7% had at least one measurement made, and 66.3% had all seven measurements. Answers to questions about the father’s medical history, by the parent or guardian that accompanied the child, were also recorded.
As with PROBT II, a re-measurement audit was done on 143 randomly selected children (1 to 5 per paediatrician) in PROBIT III by one of five blinded auditors at 2.1–28.8 months (mean 16.0 months) after the initial examination. The test–retest correlation was high for height (Pearson correlation coefficient r = 0.90) and weight (r = 0.91), fat mass (r = 0.87), and fat-free mass (r = 0.87); and was moderate for fat percentage (r = 0.82), subscapular (r = 0.82) and triceps (r = 0.73) skinfold thicknesses, leg (r = 0.78) and upper-arm length (r = 0.74), and mid-upper–arm (r = 0.86), hip (r = 0.83), waist (r = 0.77), and head (r = 0.50) circumferences.
Funding has been obtained and preparations are underway for the interview and examination of the children in PROBIT at age 16 years (PROBIT IV). The main outcomes in this phase of the trial are computer-administered neurocognitive measures (including verbal and non-verbal cognitive ability); visual acuity; eczema; and lung function as measured with spirometry.
What has been found? Key findings and publications
The key findings from the PROBIT trial are summarized in the following sections of this paper, divided into intention-to-treat analyses (effects of the randomised breastfeeding promotion intervention) and observational analyses (ignoring the randomisation). A complete list of PROBIT publications can be found at: http://www.bristol.ac.uk/social-community-medicine/projects/probit/publications/.
Effects of the intervention for breastfeeding promotion analysed according to intention-to-treat
In comparison to the control arm, the breastfeeding promotion intervention substantially increased the duration and exclusivity of breastfeeding [according to the definitions of these variables by the World Health Organization (WHO)].15 At 3 months, infants in the intervention arm were 7 times more likely (43.3% vs. 6.4%) to be exclusively breastfed and twice as likely (51.9% vs. 28.3%) to be predominantly breastfed, and were breastfed to any degree at higher rates throughout infancy, although at 6 months the rates of both exclusive (7.9% vs. 0.6%) and predominant (10.6% vs. 1.6%) breastfeeding were low.1 During infancy, the experimental intervention reduced the risk of one or more gastrointestinal-tract infections (9.1% vs. 13.2% for the intervention vs. control groups, respectively; adjusted odds ratio, OR 0.60; 95% CI 0.40–0.91) and of atopic eczema (3.3% vs. 6.3%; adjusted OR 0.54; 95% CI 0.31–0.95), but there was no important reduction in the risk of 2 or more respiratory-tract infections (39.2% vs. 39.4%; adjusted OR 0.87; 95% CI 0.59–1.28).1,16 We found greater weight and length gains in the first 3 months of life in the experimental group than in the control group. By 12 months of life, however, there were no detectable weight or length differences in the two randomised groups.3
At 6.5 years, children randomised to the breastfeeding promotion intervention in PROBIT had a 7.5-point advantage in verbal IQ (95% CI 0.8 to 14.3), a 2.9-point higher performance IQ (–3.3 to 9.1), and a 5.9-point higher full-scale IQ (–1.0 to 12.8) than those in the control arm. Smaller but consistently positive differences of 2–3 IQ points were seen in an audit of 190 children and blinded teacher rated academic performance.17 No differences were observed in children in the breastfeeding-promotion and control arms of the trial in the risks of childhood asthma and allergy,16,18 measures of adiposity,6,19,16 stature,6,16 BP,6,16 dental caries,11,16 or child behaviour.20 Findings made in PROBIT III7 are currently in preparation for publication.
Summary of observational results
Several studies have analysed PROBIT as an observational study, combining the 2 arms of the trial into a single cohort. Key findings are presented below, grouped under similar themes.
Infant feeding and growth
In PROBIT, we obtained frequent measurements of infant feeding and growth during the first year of life, and of growth up to 11.5 years of age. This feature, along with the large sample size and high follow-up rates in the trial, has led to several PROBIT publications relating infant feeding to growth.3,21–23 Although our observational analyses have suggested slower trajectories of growth in infants with prolonged and exclusive breastfeeding than in those without this,3,23 those analyses are subject to the same sorts of bias as are previous observational studies. Another analysis, which examined the association between infant size and subsequent feeding decisions, found that smaller infant size was strongly associated with an increased risk of weaning and of discontinuing breastfeeding, especially between 2 and 6 months of infant age.22
Infants exclusively breastfed for 6 months had a higher level of adiposity at age 6.5 years than those exclusively breastfed for 3 months (e.g. the difference in BMI was 0.3 kg/m2; 95% CI 0.1 to 0.4).24 However, this observation may reflect reverse causality rather than a causal effect of prolonged exclusive breastfeeding, whereby mothers with infants on a faster weight gain trajectory are more confident or encouraged or both to continue breastfeeding for a longer period.24
Breastfeeding duration, growth trajectories, and health outcomes
Infants exclusively breastfed for 6 months had a lower risk of gastrointestinal infection during the period from 3–6 months postnatally than those exclusively breastfed for 3 months who then continued partial breastfeeding to 6 months (adjusted incidence density ratio: 0.35; 95% CI 0.13–0.96), but the two groups showed no important differences in the risk of respiratory infection or atopic eczema.21 These data suggest that exclusive breastfeeding from the age of 3–6 months reduces an infant’s risk of gastrointestinal infection during the period of exclusive breastfeeding; moreover, no adverse health effects of this feeding practice were observed.21
We have used multilevel linear spline models to analyse repeated measures of length/height and weight during infancy and childhood, and studied these growth trajectories in relation to the occurrence of rashes25 and to BP,26 cognition,27 and mental health.27 We found no strong or consistent evidence to support the hypothesis that there is a critical or sensitive period in infancy that determines the risk of these outcomes in early childhood.
Gestation, size at birth, and later behaviour/cognitive ability
We observed that cognitive ability at age 6.5 years was lower in infants born at 37 and 38 weeks of gestation than in those born at 39–41 weeks, and that this was also true for infants that were small for gestational age (<10th percentile, 10th–50th percentile, and >50th–90th percentile) as compared with those born large for gestational age (>90th percentile).28 A lower birthweight-for-gestational age was also associated with problematic behaviour at age 6.5 years.29
Socioeconomic patterning of adiposity and linear growth
The effects of socioeconomic position on health and growth outcomes in transitional countries of middle-income, such as Belarus, have previously received little attention. We found that children aged 6.5 years who had at least one parent with a non-manual job were more likely to be overweight or obese than those from backgrounds entirely of manual labour. Fathers in a family in which either they or their spouse had a non-manual job were also more likely to be overweight or obese, but mothers with the same background were less likely to be overweight or obese.30 Children aged 6.5 years and their parents who came from more advantaged backgrounds (non-manual work or a university education) were taller than those from less advantaged backgrounds.31
What are the study’s main strengths and weaknesses?
A major strength of the PROBIT cohort is that it provides a rich data set with follow-up of a large number of contemporary children and their parents living in a middle-income, transitional economy, with high response rates (>80%) in all phases of data collection. Data in the trial were collected on several occasions, and included information on infant feeding, growth, and the occurrence of gastrointestinal and respiratory infections and on health outcomes during childhood. Some parental measurements were also included. The major advantage of the PROBIT cohort is that it is nested in a randomised controlled trial of breastfeeding promotion. The data can therefore be used to compare and contrast the results of intention-to-treat with those of observational analysis, as has been done for both infant growth3 and long-term behavioural outcomes.32
If we were establishing the PROBIT cohort again, we believe it would be valuable to collect at birth data on maternal pre-pregnancy weight, weight gain during gestation, and changes in weight during the first year postpartum, as well as data on paternal smoking and drinking during the mother’s pregnancy. We could also, at follow-up at 6.5 years, have asked more questions about paternal behaviours. Additionally, it is unclear whether the responses of mothers not living with their childrens’ fathers at the time of the 6.5-year visit pertained to their then-current partners or to their childrens’ biological fathers, and we would have clarified this.
Can I get hold of the data? Where can I find out more?
Proposals for collaboration with PROBIT are welcome, and enquiries and requests for further information about the trial should be made to the executive group of the study steering committee (M.S.K., R.M.M., E.O.), which will review all proposals (e-mail: Michael.Kramer@mcgill.ca, Richard.Martin@bristol.ac.uk, or Emily_Oken@harvardpilgrim.org). More information, including how to access the trial data, data-collection documents, and the trial bibliography, is available on our website: http://www.bristol.ac.uk/social-community-medicine/projects/probit/.
Funding
This work was supported by a grant from the Canadian Institutes of Health Research (MOP-53155 to MSK), the European Union's project on Early Nutrition Programming: Long-term Efficacy and Safety Trials (grant FOOD-DT-2005-007036 to RMM), and the US National Institutes of Health (R01HD050758, K24 HD069408). The Promotion of Breastfeeding Intervention Trial was originally funded by the National Health Research and Development Program (Health Canada), the Thrasher Research Fund, the United Nations Children’s Fund (UNICEF), and the European Regional Office of the World Health Organization (WHO). None of the funding bodies influenced the collection, analysis, or interpretation of data for this paper. The views expressed are those of the authors and not necessarily of any funding body.
Acknowledgements
We are grateful to the cohort members and their parents for their generous participation in the study, to the polyclinic paediatricians who examined the children and ensured a very high follow-up rate, and to the dedication of the Belarussian team members who ensured that this trial could start and who continue to support and guide the study through all stages of the Promotion of Breastfeeding Intervention Trial.
Conflict of interest: None declared.
Key Messages.
The Promotion of Breastfeeding Intervention Trial is the largest randomised trial ever conducted in the area of human lactation. The randomised intervention in this trial has resulted in two groups with substantially different exposures to prolonged, exclusive breastfeeding. The trial included >17 000 randomised mother–infant pairs, more than 80% of which have been followed for 11.5 years, allowing the causal effects of breastfeeding promotion to be estimated through analyses of intention-to-treat.
The breastfeeding promotion intervention in PROBIT was associated with reduced risks of gastrointestinal-tract infections and atopic eczema during infancy, and with a 7.5-point (95% CI 0.8 to 14.3 point) advantage in verbal IQ, a 2.9-point (–3.3 to 9.1 point) higher performance IQ, and a 5.9-point (95% CI –1.0 to 12.8 point) higher full-scale IQ at 6.5 years, as compared with the respective variables in the control arm of the trial.
The breastfeeding promotion intervention was not associated with the risk of respiratory-tract infections during infancy or with asthma, allergy, measures of adiposity, stature, BP, dental caries, or child behaviour at age 6.5 years.
The long-term follow-up of the children, mothers, and fathers in PROBIT has been exploited for several observational analyses. Highlights of this observational research include: (i) Examination of breastfeeding and infant growth, comparing and contrasting the results of observational analysis (with its associated biases) with those of intention-to-treat analysis. (ii) The use of repeated measures of length/height and weight during infancy and childhood to model growth trajectories through multilevel linear spline methods, and examination of these trajectories in relation to the occurrence of rashes and to BP, cognition, and mental health.
References
- 1.Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413–20. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 2.Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of breastfeeding intervention trial (PROBIT): a cluster-randomized trial in the Republic of Belarus. Design, follow-up, and data validation. Adv Exp Med Biol. 2000;478:327–45. [PubMed] [Google Scholar]
- 3.Kramer MS, Guo T, Platt RW, et al. Breastfeeding and infant growth: biology or bias? Pediatrics. 2002;110:343–47. doi: 10.1542/peds.110.2.343. [DOI] [PubMed] [Google Scholar]
- 4.Kramer MS. Does breast feeding help protect against atopic disease? Biology, methodology, and a golden jubilee of controversy. J Pediatr. 1988;112:181–90. doi: 10.1016/s0022-3476(88)80054-4. [DOI] [PubMed] [Google Scholar]
- 5.WHO/UNICEF. Protecting, Promoting and Supporting Breastfeeding: The Special Role of Maternity Services. Geneva: World Health Organization; 1989. [Google Scholar]
- 6.Kramer MS, Matush L, Vanilovich I, et al. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr. 2007;86:1717–21. doi: 10.1093/ajcn/86.5.1717. [DOI] [PubMed] [Google Scholar]
- 7.Guthrie L, Oken E, Sterne J, et al. Ongoing monitoring of data clustering in multicenter studies. BMC Med Res Methodol. 2012;12:29. doi: 10.1186/1471-2288-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wechsler D. Wechsler Abbreviated Scales of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 9.Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 2006;38:581–86. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 10.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 11.Kramer MS, Vanilovich I, Matush L, et al. The effect of prolonged and exclusive breast-feeding on dental caries in early school-age children. New evidence from a large randomized trial. Caries Res. 2007;41:484–88. doi: 10.1159/000108596. [DOI] [PubMed] [Google Scholar]
- 12.Martin RM, Patel R, Zinovik A, et al. Filter paper blood spot enzyme linked immunoassay for insulin and application in the evaluation of determinants of child insulin resistance. PLoS ONE. 2012;7:e46752. doi: 10.1371/journal.pone.0046752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maloney MJ, McGuire JB, Daniels SR. Reliability testing of a children's version of the Eating Attitude Test. J Am Acad Child Adolesc Psychiatry. 1988;27:541–43. doi: 10.1097/00004583-198809000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Maloney MJ, McGuire J, Daniels SR, Specker B. Dieting behavior and eating attitudes in children. Pediatrics. 1989;84:482–89. [PubMed] [Google Scholar]
- 15.World Health Organization. Indicators for Assessing Breast-Feeding Practices. Geneva: World Health Organization; 1991. [Google Scholar]
- 16.Kramer MS. “Breast is best”: The evidence. Early Hum Dev. 2010;86:729–32. doi: 10.1016/j.earlhumdev.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Kramer MS, Aboud F, Mironova E, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch Gen Psychiatry. 2008;65:578–84. doi: 10.1001/archpsyc.65.5.578. [DOI] [PubMed] [Google Scholar]
- 18.Kramer MS, Matush L, Vanilovich I, et al. Effect of prolonged and exclusive breast feeding on risk of allergy and asthma: cluster randomised trial. BMJ. 2007;335:815. doi: 10.1136/bmj.39304.464016.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer MS, Matush L, Vanilovich I, et al. A randomized breast-feeding promotion intervention did not reduce child obesity in Belarus. J Nutr. 2009;139:417–21S. doi: 10.3945/jn.108.097675. [DOI] [PubMed] [Google Scholar]
- 20.Kramer MS, Fombonne E, Igumnov S, et al. Effects of prolonged and exclusive breastfeeding on child behavior and maternal adjustment: evidence from a large, randomized trial. Pediatrics. 2008;121:e435–40. doi: 10.1542/peds.2007-1248. [DOI] [PubMed] [Google Scholar]
- 21.Kramer MS, Guo T, Platt RW, et al. Infant growth and health outcomes associated with 3 compared with 6 mo of exclusive breastfeeding. Am J Clin Nutr. 2003;78:291–95. doi: 10.1093/ajcn/78.2.291. [DOI] [PubMed] [Google Scholar]
- 22.Kramer MS, Moodie EEM, Dahhou M, Platt RW. Breastfeeding and infant size: evidence of reverse causality. Am J Epidemiol. 2011;173:978–83. doi: 10.1093/aje/kwq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer MS, Guo T, Platt RW, et al. Feeding effects on growth during infancy. J Pediatr. 2004;145:600–05. doi: 10.1016/j.jpeds.2004.06.069. [DOI] [PubMed] [Google Scholar]
- 24.Kramer MS, Matush L, Bogdanovich N, et al. Health and development outcomes in 6.5-y-old children breastfed exclusively for 3 or 6 months. Am J Clin Nutr. 2009;90:1070–1074. doi: 10.3945/ajcn.2009.28021. [DOI] [PubMed] [Google Scholar]
- 25.Tilling K, Davies NM, Nicoli E, et al. Associations of growth trajectories in infancy and early childhood with later childhood outcomes. Am J Clin Nutr. 2011;94:1808–13S. doi: 10.3945/ajcn.110.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tilling K, Davies N, Windmeijer F, et al. Is infant weight associated with childhood blood pressure? Analysis of the Promotion of Breastfeeding Intervention Trial (PROBIT) cohort. Int J Epidemiol. 2011;40:1227–37. doi: 10.1093/ije/dyr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang S, Tilling K, Martin R, et al. Pre-natal and post-natal growth trajectories and childhood cognitive ability and mental health. Int J Epidemiol. 2011;40:1215–26. doi: 10.1093/ije/dyr094. [DOI] [PubMed] [Google Scholar]
- 28.Yang S, Platt RW, Kramer MS. Variation in child cognitive ability by week of gestation among healthy term births. Am J Epidemiol. 2010;171:399–406. doi: 10.1093/aje/kwp413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S, Fombonne E, Kramer MS. Duration of gestation, size at birth and later childhood behaviour. Paediatr Perinat Epidemiol. 2011;25:377–87. doi: 10.1111/j.1365-3016.2011.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel R, Lawlor DA, Kramer MS, et al. Socio-economic position and adiposity among children and their parents in the Republic of Belarus. Eur J Public Health. 2011;21:158–65. doi: 10.1093/eurpub/ckq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel R, Lawlor DA, Kramer MS, et al. Socioeconomic inequalities in height, leg length and trunk length among children aged 6.5 years and their parents from the Republic of Belarus: evidence from the Promotion of Breastfeeding Intervention Trial (PROBIT) Ann Hum Biol. 2011;38:592–602. doi: 10.3109/03014460.2011.577752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramer MS, Fombonne E, Matush L, et al. Long-term behavioural consequences of infant feeding: the limits of observational studies. Paediatr Perinat Epidemiol. 2011;25:500–06. doi: 10.1111/j.1365-3016.2011.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]