Abstract
Most childhood interventions (vaccines, micronutrients) in low-income countries are justified by their assumed effect on child survival. However, usually the interventions have only been studied with respect to their disease/deficiency-specific effects and not for their overall effects on morbidity and mortality. In many situations, the population-based effects have been very different from the anticipated effects; for example, the measles-preventive high-titre measles vaccine was associated with 2-fold increased female mortality; BCG reduces neonatal mortality although children do not die of tuberculosis in the neonatal period; vitamin A may be associated with increased or reduced child mortality in different situations; effects of interventions may differ for boys and girls. The reasons for these and other contrasts between expectations and observations are likely to be that the immune system learns more than specific prevention from an intervention; such training may enhance or reduce susceptibility to unrelated infections. INDEPTH member centres have been in an ideal position to document such additional non-specific effects of interventions because they follow the total population long term. It is proposed that more INDEPTH member centres extend their routine data collection platform to better measure the use and effects of childhood interventions. In a longer perspective, INDEPTH may come to play a stronger role in defining health research issues of relevance to low-income countries.
Keywords: BCG, childhood interventions, DTP, INDEPTH Network, measles vaccine, non-specific effects of vaccines
Key Messages.
Interventions with vaccines and vitamin A have non-specific effects on child survival, i.e. effects not explained by prevention of specific diseases or deficiency.
Live vaccines have beneficial effects which are more important than the specific prevention. Inactivated vaccines may have negative effects particularly for girls.
Non-specific effects often differ for girls and boys.
INDEPTH centres are in an ideal position to monitor non-specific effects of common childhood interventions.
Introduction
Immunization has been advocated as the most successful public health intervention to improve child survival. Each year, immunization averts an estimated 2–3 million deaths from diphtheria, tetanus, pertussis (whooping cough) and measles. However, there is now strong evidence that vaccines have substantial non-specific (heterologous) effects in children in high-mortality regions, i.e. by changing mortality from infections unrelated to the vaccine-targeted infections.1–5 As a consequence. the World Health Organization’s (WHO’s) Strategic Advisory Group of Experts (SAGE) on Immunization has recently initiated a review of the non-specific effects (NSEs) of BCG, diphtheria-tetanus-pertussis (DTP) and measles (MV) vaccines.
The NSEs have a large potential to improve child health and survival and may eventually lead to major evidence-based changes in vaccination and vitamin A supplementation (VAS) policies. Most research on NSEs has had to be observational rather than experimental because it is not considered ethical to perform randomized trials (RCTs) of interventions already recommended by WHO.1,2 Observational studies are usually considered insufficient to prove unequivocally that vaccines have NSEs.1,2,6,7 However, the NSEs were initially detected in RCTs of high-titre measles vaccine (HTMV) conducted at the Bandim and Niakhar INDEPTH member centres.8–10 The present paper reviews briefly the evidence for NSEs of vaccines and other childhood interventions as well as the role of the INDEPTH network in determining the consistency of observation on the NSEs. Furthermore, RCTs of measles vaccine (MV),3 BCG4,5 and inactivated vaccines have been conducted under special circumstances in some INDEPTH centres.
The Bandim Health Project (BHP) group and a few other INDEPTH member centres have tested the hypothesis that vaccines may have non-specific effects (NSE) as shown in Table 1.
Table 1.
Observations on non-specific effects of childhood interventions tested at INDEPTH member centres
| Observations | Supported by studies from INDEPTH member centresa | Estimated size of effect |
|---|---|---|
| Negative effect of high-titre measles vaccine (HTMV) | Bandim,8 Niakhar9–11 | Meta-analysis:11 HTHV increased the mortality rate by 33% compared with controls |
| MV has beneficial non-specific effects not explained by prevention of measles infection | Bandim,1,3 Bandafassi,13 Niakhar,14 Farafenni,15 Navrongo16, Matlab17, Ballabgard,18 Vadu19 | Observational studies and RCTs:3,20 standard MV compared with unvaccinated controls reduces the mortality rate by 30–50% as long as DTP is not given with or after MV |
| MV has a better effect for girls than for boys | Bandim,21,22 Niakhar,14 Bandafassi,13 Farafenni,15 Navrongo (unpublished) | Among MV-vaccinated children, girls have 30–40% lower mortality rate than boys as long as DTP is not given after MV16,17,30 |
| BCG has beneficial non-specific effects not explained by prevention of tuberculosis | Bandim,4,5,23 Vadu19 | RCTs:4,5 BCG-at-birth compared with delayed BCG reduces the neonatal mortality rate by 40% |
| OPV | Bandim24 | OPV campaign associated with lower mortality rate among the youngest children24 |
| Vaccinia | Bandim25–28 | Observational studies:25,26 having a smallpox vaccination scar associated with 40% lower mortality rate than not having a scar |
| DTP has negative non-specific effects, particularly for girlsb | Bandim,2,21,22,29 Niakhar, Farafenni,15 Navrongo,30 Vadu,19 Ballabgard31 | Observational studies:2 DTP-vaccinated girls compared with DTP-unvaccinated girls have at least 50% higher mortality rate until a different vaccine is received |
| DTP administered with MV or after MV is associated with higher mortality than MV administered alone | Bandim,2 Niakhar, Navrongo (unpublished), Vadu,19 Matlab32 | Observational studies and RCTs:2,10 DTP and other inactivated vaccines after MV compared with MV alone as most recent vaccine is associated with 50–100% higher mortality rate among girls but made little difference to boys |
| BCG administered with DTP reduces the negative effects of DTP | Bandim,33,34 Niakhar,33 Vadu,19 Matlab (unpublished) | Observational studies:19,31,34 first, girls have 30–40% lower mortality rate than boys when BCG is administered with DTP1; and second, the mortality rate before 9 months of age (i.e. before MV) is lower if the vaccination schedule started with BCG + DTP1 rather than with the WHO-recommended schedule of BCG and then DTP1 |
| Vitamin A supplementation (VAS) interacts with vaccines | Bandim,35–37 Navrongo38 | Observational studies and RCTs: VAS may enhance the beneficial effects of vaccines but also amplify the negative effects of some vaccines |
aThese centres are located in Guinea-Bissau (Bandim), Senegal (Niakhar, Bandafassi), The Gambia (Farafenni), Ghana (Navrongo), India (Ballabgard, Vadu) and Bangladesh (Matlab).
bStudies from Niakhar40 and Matlab32 found beneficial effect of DTP. However, these studies did not take into consideration that nearly all children had received BCG and DTP simultaneously even though the official WHO recommendation is to give BCG first, at birth, and then DTP later. The effect of combined BCG and DTP is quite different in reducing female mortality. When this is taken into consideration, the data from Niakhar and Matlab (unpublished) no longer contradict the principle that DTP alone is associated with increased female mortality.
High-titre measles vaccine (HTMV)
HTMV was tested in RCTs in the late 1980s, comparing HTMV at 4–5 months of age with standard MV at 9 months of age. The HTMV was protective against measles infection and was recommended by WHO in 1989 for general use in low-income countries with a high incidence of measles infection.8–10 A meta-analysis of studies from Bissau, Gambia and Senegal showed that this vaccine was associated with 33% increased mortality rate between 4 and 60 months of age.11 The excess mortality was among girls, whereas the new vaccine compared with the traditional MV had no differential effect on survival for boys. These results were subsequently confirmed in RCTs from Sudan and Haiti, and WHO withdrew the 1989 recommendation for HTMV in 1992.10 These RCTs showed:
first, that a fully protective vaccine can have negative NSE
second, that these effects can be sex-differential and
third, that NSE can have major effects on child mortality patterns; had the vaccine not been withdrawn, a 33% excess mortality rate between ages 4 and 60 months would at the time have meant at least an additional half-million female deaths annually, in Africa alone.
Live vaccines: measles vaccine
The main effect of MV on child survival may be NSE; in other words, the NSE of MV may be considerably more important for child survival than the specific prevention of acute measles infection. These beneficial effects have been found both in observation studies and in RCTs.1,3,17,20 In a RCT comparing early MV vs DTP3, MV was associated with a marked reduction in the risk of hospital admission for respiratory infection.41 The beneficial effect of MV is much stronger for girls than for boys. The beneficial effect for MV-children compared with measles-unvaccinated children is particularly strong when measles is given before 12 months of age. In the four studies reporting data for children both below and above 12 months of age, the reduction in mortality before 12 months was 74% (51–86%)but only 29% (8–46%) after 12 months of age.12,42–44
Why should earlier vaccination be better than later vaccination? In Guinea-Bissau, early MV in the presence of maternal measles antibodies had a much stronger beneficial effect on child survival than when MV was provided in the presence of no maternal antibodies.45 These observations clearly contrast with the current MV policy, which is based on the idea that the production of measles-specific antibodies (seroconversion) is best when maternal antibodies have disappeared; and it is therefore recommended to delay MV to after 12 months of age, when measles infection has come under control. To the extent MV has beneficial NSEs, delaying MV will increase child mortality.
Furthermore, MMR (measles-mumps-rubella) used in high-income countries may have similar beneficial effects. In a study of 475 000 Danish children, MMR was associated with a significant lower risk {14% [95% confidence interval (CI): 12–16%]} of hospital admission compared with children who still had DTaP-IPV-Hib3 as their most recent vaccination. If the inactivated DTaP-IPV-Hib3 was given after MMR, the risk of admission increased by 62% (29–105%).46 Also in Denmark, the protective effect against hospital admission was strongest for respiratory infections.
Live vaccines: BCG, OPV, vaccinia and others
Several observational studies suggest that BCG has beneficial NSEs.29,47 It has been possible to test the effect of BCG in a RCT in low-birth-weight (LBW) children because they often do not received BCG-at-birth; in two RCTs in Guinea-Bissau, children randomized to BCG-at-birth had a marked reduction in the neonatal mortality rate.4,5 Furthermore, reanalysis of BCG trials from the USA and the UK in the 1940–50s, in which prevention of TB was the main outcome, showed that BCG vaccination was associated with a 25% reduction in non-TB and non-accident deaths.48 Among the children vaccinated with BCG, those having a scar or a positive TST test have much lower mortality than those who have not responded (see Figure 1).
Figure 1.
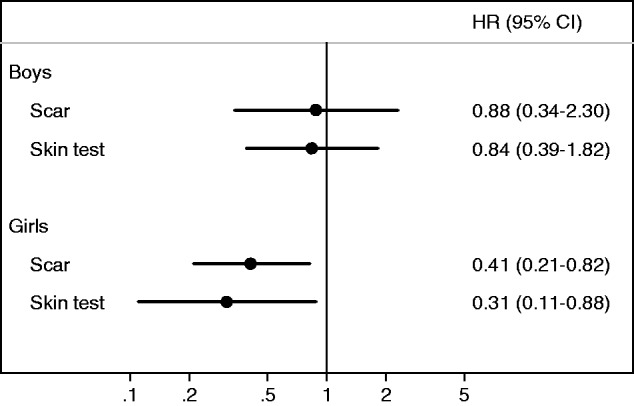
Hazard ratios (HR) for BCG scar-positive vs scar-negative individuals and for tuberculin-skin-test- (TST)-positive vs TST-negative individuals among BCG-vaccinated children in urban Guinea-Bissau, 2000–0223.
Oral polio vaccine (OPV) is difficult to study because it is WHO policy to give OPV with DTP. However, it seems that OPV may have beneficial NSE.24,49 When OPV was introduced in the 1960s in Chile, a virologist noted that OPV enhanced the interferon-gamma response and reduced the risk of other entero-pathogens and diarrhoeal deaths.49 If OPV has beneficial NSEs, this has important consequences because most global health researchers want to stop OPV and replace it with inactivated polio vaccine (IPV). The only reason this has not happened yet is that IPV is much more expensive than OPV.
Inactivated vaccines: DTP, HBV and IPV
There is only one study of what happened when DTP was introduced in low-income countries 2–3 decades ago.47 As seen in Figure 2, the mortality rate was 2-fold higher for DTP-vaccinated compared with DTP-unvaccinated children when DTP was introduced in rural areas of Guinea-Bissau in the 1980s. The DTP-unvaccinated children were travelling or were too sick to get vaccinated. Hence, if anything, the DTP-unvaccinated children should have had a higher mortality rate than the healthier DTP-vaccinated children.47 There is consistent evidence that DTP given after MV is associated with increased female mortality. Five RCTs in the 1980s had a cross-over design in which the children were randomized at 4–5 months of age to receive early MV or a control vaccine (inactivated).11 At 9–10 months of age, the children were crossed over: the control group received the standard MV and the early MV group received the control vaccine (DTP, IPV or meningococcal vaccine). Hence, these trials compared inactivated vaccine (after MV) vs MV as most recent vaccination from 9 months to 3–5 years of age. The overall effect was a 38% (95% CI: 5–83%) higher mortality rate not related to prevention of measles infection since all children had been vaccinated against measles infection; the negative effect was found only among girls who had 89% (27–180%) higher mortality when they had received inactivated vaccine after MV.
Figure 2.
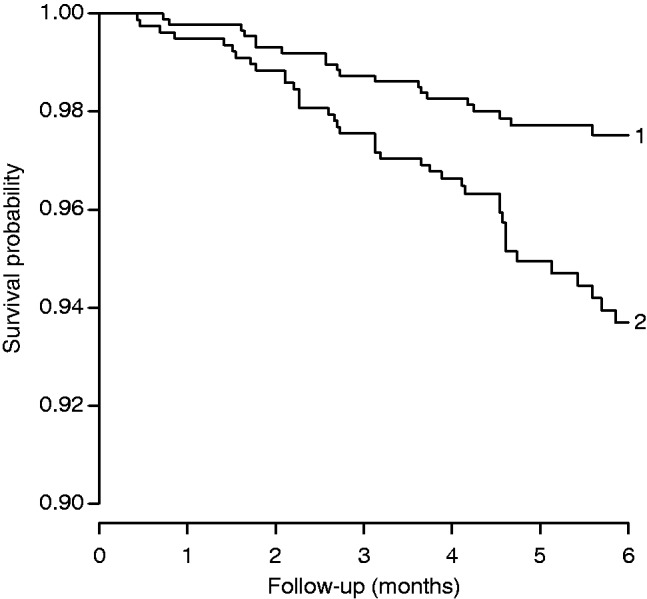
Kaplan–Meier survival curves for unvaccinated children and recipients of DTP in rural areas of Guinea-Bissau, 1984–87.47 Note: the graph shows mortality during 6 months of follow-up for DTP-vaccinated (trace 2) and DTP-unvaccinated (trace 1) children aged 2–8 months at the initial visit to their villages. Unvaccinated children received no DTP because they were travelling on day of vaccination, were too sick to get vaccinated and had lower nutritional status than DTP vaccinated children, or were visited on days when the team for logistic reasons had no vaccines. The adjusted mortality rate ratio for DTP-vaccinated (trace 2) vs DTP-unvaccinated (trace 1) children was 1.92 (1.04–3.52).
Vitamin A supplementation (VAS)
VAS is assumed to be associated with a 25% reduction in child mortality due to the prevention of vitamin A deficiency.50 However, VAS appears to be particularly good when given with live vaccines like BCG and MV but potentially having negative effects when given with inactivated vaccines like DTP. RCTs of neonatal VAS have found a significant negative effect of 41% increased mortality rate after VAS for girls whereas the effect tended to be beneficial among boys.35,36 The negative effect for girls only started when the children received DTP at around 6 weeks of age.37
These trials have showed that effects can be sex-differential and that VAS can affect the mortality pattern long after the initial supplementation. Hence, VAS primes the immune system in more general ways, which were not taken into consideration when the WHO policy to provide vitamin A with vaccines was formulated. In a recent RCT testing of the effect of providing VAS with vaccines, there was no overall benefit of VAS but results were significantly different for boys (harmful) vs girls (beneficial). Recently, the Lancet published a cluster-randomized trial of bi-annual VAS campaigns to 1 million children in India; the study found only a 4% (−3–11%) effect of VAS.51 Given these results, there should be a clear interest in further examining for which sex and with which vaccines VAS may have a beneficial effect.
Sex-differential effects
In global health there are virtually no studies of boys and girls, everything is ‘children’. However, as indicated above, once we ask the question there are very often marked sex-differential effects. Live vaccines like MV, vaccinia and probably also BCG tend to be more beneficial for females than for males, whereas inactivated vaccines like DTP, IPV and HBV have a negative effect which is also stronger for girls.2,15,52,53 Strong sex-differential effects have been found for vitamin A and also for other micronutrients.55 This may not be inherent to the micronutrients but more a question of the micronutrients amplifying the NSE of the vaccines.
By only looking at ‘children’ we may find no effect, whereas it is in reality a beneficial effect for one sex and a negative effect for the other sex. A focus on sex differences could also suggest that in some situations the optimal solution for both sexes might be that they are treated differently according to their genetic constitution.
Interactions
A recurrent theme in these studies has been that interventions may interact. Changing the sequence of vaccinations may change the effect completely—as when early HTMV was associated with increased female mortality because the children got inactivated vaccines after HTMV. Giving a live and inactivated vaccine at the same time can also change the effects completely. Adding VAS or micronutrients can amplify the effect of the intervention. Something that was once a good intervention may no longer be so if new interventions are added. There are likely to be many other interactions with immune-enhancing interventions or conditions which have not been explored. For example, we have very often found that effects differed between the dry and the rainy season.41
Therefore, it seems important to test the possible interactions with the most likely other interventions. INDEPTH is in a unique position to do so and to explore the interactions, because they follow the whole population and could document all the interventions.
Potential biological mechanisms
The perceived lack of biological plausibility has been a major obstacle in recognizing and further investigating non-specific effects. Hence, it is important to consider immunological mechanisms that may mediate such effects. Novel insights in understanding both the adaptive immune system and innate immunity has provided arguments that exposure to a pathogen leads not only to specific immunological memory (represented by memory T- and B-cells), but also to T-cell mediated cross-reactivity, as well as training of the innate immune system.55
T-cell mediated cross-reactivity—‘heterologous immunity’
Each individual has a unique lifelong history of infections and vaccinations, and each exposure leaves an imprint on the immune system that can affect future innate and adaptive immune responses to new pathogens.56 This concept of ‘heterologous immunity’ could explain the observation that vaccines may have non-specific effects, because the vaccines encode antigens that cross-react with other pathogens. In some scenarios, beneficial heterologous immunity can provide partial protective immunity and be the difference between life and death. In other scenarios, detrimental heterologous immunity can lead to severe immunopathology. Hence, T-cell mediated heterologous immunity provides a plausible biological mechanism by which vaccines may affect the immune response to a subsequent unrelated infection and also explains how, in certain situations, a vaccine could have detrimental effects on the outcome of secondary infections.
Training of the innate immune system
Activation of cross-reactive T-cell responses, as seen in heterologous immunity, might explain some of the non-specific effects of vaccination. However, there is also evidence suggesting that the altered resistance to subsequent infections after vaccination or infection with an unrelated pathogen cannot be attributed to adaptive immune responses alone, and that innate immune responses result in a heightened state of activation. Vaccination of volunteers with BCG showed that in addition to induction of specific T-cell responses, non-specific innate immune responses to unrelated pathogens were also increased for at least 3 months after the vaccination.57 This ‘trained immunity’ was associated in humans with epigenetic reprogramming of monocytes at the level of H3K4 trimethylation. Hence, these data suggest a picture in which the innate immune system is characterized by adaptive features, and can be trained to provide a partial protection against infection independent of the classical T- and B-cell adaptive immunity.
These immunological mechanisms do support the biological plausibility by demonstrating that the encounter with one pathogen may alter the immune response to subsequent completely unrelated pathogen challenges, and this may result in improved outcomes, but also at times be detrimental. So far this has been demonstrated most convincingly for BCG.
Conclusion
Observational studies have been consistent in showing NSEs in spite of many different study designs being used, and several key observations have been confirmed in RCTs.3–5,8,9,35,36 The epidemiological data indicate that vaccines have non-specific effects which may be just as important, or even more important, for childhood survival than their specific effects (Box 1). Existing studies suggest a general pattern, namely that the live vaccines (BCG, measles vaccine, OPV and vaccinia) are associated with beneficial non-specific effects, leading to reduced all-cause mortality, whereas the inactivated, alum-adjuvated DTP vaccine is associated with increased susceptibility to other unrelated infections, particularly in females.2
Box 1 The focus on NSEs of interventions has provided several lessons for future research and policy making, as follows.
The NSEs are usually more important for overall morbidity/mortality than the targeted effects.1–5
Though we always plan for beneficial effects in health, we may actually produce the opposite.2,8–10,24,47,49
Effects are often sex-differential, emphasising that male and female immune systems differ and the results of their training from interventions may therefore differ.
Since different interventions train the same immune system, the interventions may interact in unpredictable ways as when vitamin A supplementation (VAS) increases female mortality.35,37,38 As a consequence, something which was shown to be effective when first introduced, e.g. VAS when tested in the 1980s, may later lose its effect due to interactions with other interventions.
The INDEPTH Network has already played a key role in testing the NSE hypotheses. This role is likely to be even more important in the future. So far there are no studies of the possible NSEs of the many new vaccines, including rotavirus vaccine, PCV, yellow fever, conjugated meningococcal vaccin, or malaria vaccine. Only one study24 has examined the possible NSEs of vaccination campaigns with OPV or MV. Studies at INDEPTH member centres may help to explain how the immunological profile can change so quickly between different vaccines, why live vaccines have beneficial effects and inactivated vaccines have negative effects, and why the reactions of boys and girls differ. Furthermore, if the NSEs are accepted by the global health community, there will be a need to conduct many new trials to guide global policies. For example, multicentre community trials of OPV and MV vaccination campaigns should clarify whether these trials have played a role in the major reductions in child mortality which have occurred during the last 10–15 years. Trials to assure that BCG is delivered at birth and not much later (by 4 weeks of age), as is current practice in many African countries, could help reduce neonatal mortality; trials may examine whether co-administration of BCG and DTP reduces the negative effect of DTP as suggested by all the observational studies; trials of not giving DTP with or after MV, even though the child may be missing some doses of DTP, could help establish the principle that live-vaccine-last is the best general vaccination schedule; and trials should also test the hypotheses that vaccination in the presence of maternal antibodies and boosting with live vaccines enhances the beneficial NSEs.
Funding
The INDEPTH vaccine network was supported by the Danish Council for Development Research, the European Union FP7 support for OPTIMUNISE (grant: Health-F3-2011-261375) and the Danish National Research Foundation (DNRF108). P.A. holds a research professorship grant from the Novo Nordisk Foundation. INDEPTH funded the workshop which formed the basis of this paper. O.S. and J.B.O.E. are funded by core support grants from Sida, Wellcome Trust, William & Flora Hewlett Foundation.
Conflict of interest: Osman Sankoh is the Executive Director of the INDEPTH Network; Jacques BO Emina is the Science Programme Manager of the INDEPTH Network.
References
- 1.Aaby P, Martins CL, Garly ML, Rodrigues A, Benn CS, Whittle HC. The optimal age of measles immunization in low-income countries: A secondary analysis of the assumptions underlying the current policy . BMJ Open 2012;2:e000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aaby P, Benn CS, Nielsen J, Lisse IM, Rodrigues A, Ravn H. Testing the hypothesis that diphtheria-tetanus-pertussis vaccine has negative non-specific and sex-differential effects on child survival in high-mortality countries. BMJ Open 2012;2:e000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aaby P, Martins CL, Garly ML, et al. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: Randomised controlled trial. BMJ 2010;341:c6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aaby P, Roth A, Ravn H, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis 2011;204:245–52 [DOI] [PubMed] [Google Scholar]
- 5.Biering-Sørensen S, Aaby P, Napirna BM, et al. Small randomised trial among low-birth-weight children of Bacillus Calmette-Guérin vaccination at first health center contact. Pediatr Inf Dis J 2012;31:306–08 [DOI] [PubMed] [Google Scholar]
- 6.Global Advisory Committee on Vaccine Safety. Wkly Epidemiol Rec 2004;79:269–72 [PubMed] [Google Scholar]
- 7.Meeting of Global Advisory Committee on Vaccine Safety, 18–19 June 2008. Wkly Epidemiol Rec 2008;83:287–92 [PubMed] [Google Scholar]
- 8.Aaby P, Knudsen K, Whittle H, et al. Long-term survival after Edmonston–Zagreb measles vaccination in Guinea-Bissau: Increased female mortality rate. J Pediatr 1993;122: 904–08 [DOI] [PubMed] [Google Scholar]
- 9.Aaby P, Samb B, Simondon F. Sex-specific differences in mortality after high-titre measles immunization in rural Senegal. Bull World Health Organ 1994;72:761–70 [PMC free article] [PubMed] [Google Scholar]
- 10.Aaby P, Jensen H, Samb B, et al. Differences in female-male mortality after high-titre measles vaccine and association with subsequent vaccination with diphtheria-tetanus-pertussis and inactivated poliovirus: re-analysis of West African studies. Lancet 2003;361:2183–88 [DOI] [PubMed] [Google Scholar]
- 11.Knudsen KM, Aaby P, Whittle H, et al. Child mortality following standard, medium or high titre measles immunization in West Africa. Int J Epidemiol 1996;25;665–73 [DOI] [PubMed] [Google Scholar]
- 12.Aaby P, Bukh J, Lisse IM, Smits AJ. Measles vaccination and reduction in child mortality: a community study from Guinea–Bissau. J Infect 1984;8:13–21 [DOI] [PubMed] [Google Scholar]
- 13.Desgrées du Loû A, Pison G, Aaby P. The role of immunizations in the recent decline in childhood mortality and the changes in the female/male mortality ratio in rural Senegal. Am J Epidemiol 1995;142:643–52 [DOI] [PubMed] [Google Scholar]
- 14.Aaby P, Samb B, Simondon F, et al. Divergent mortality for male and female recipients of low–titer and high–titer measles vaccines in rural Senegal. Am J Epidemiol 1993;138:746–55 [DOI] [PubMed] [Google Scholar]
- 15.Aaby P, Jensen H, Walraven G. Age-specific changes in female-male mortality ratio related to the pattern of vaccinations: an observational study from rural Gambia. Vaccine 2006;24:4701–08 [DOI] [PubMed] [Google Scholar]
- 16.Nyarko P, Pence B, Debpuur C. Immunization status and child survival in rural Ghana.Working papers No. 147. New York: Population Council, 2001 [Google Scholar]
- 17.Aaby P, Bhuyia A, Nahar L, Knudsen K, de Francisco A, Strong M. The survival benefit of measles immunization may not be explained entirely by the prevention of measles disease: a community study from rural Bangladesh. Int J Epidemiol 2003;32:106–15 [DOI] [PubMed] [Google Scholar]
- 18.Kapoor SK, Reddaiah VP. Effectiveness of measles immunization on diarrhea and malnutrition related mortality in 1-4 year olds. Indian J Pediatr 1991;58:821–23 [DOI] [PubMed] [Google Scholar]
- 19.Hirve S, Bavdekar A, Juvekar S, Benn CS, Nielsen J, Aaby P. Non-specific and sex-differential effects of vaccinations on child survival in rural western India. Vaccine 2012;30:7300-08 [DOI] [PubMed] [Google Scholar]
- 20.Aaby P, Samb B, Simondon F, CollSeck AM, Knudsen K, Whittle H. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ 1995;311:481–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aaby P, Jensen H, Garly ML, Balé C, Martins C, Lisse I. Routine vaccinations and child survival in war situation with high mortality: effect of gender. Vaccine 2002;21:15–20 [DOI] [PubMed] [Google Scholar]
- 22.Veirum JE, Sodemann M, Biai S, et al. Routine vaccinations associated with divergent effects on female and male mortality at the paediatric ward in Bissau, Guinea-Bissau. Vaccine 2005;23:1197–203 [DOI] [PubMed] [Google Scholar]
- 23.Roth A, Sodemann M, Jensen H, et al. Tuberculin reaction, BCG scar, and lower female mortality. Epidemiology 2006:17:562–68 [DOI] [PubMed] [Google Scholar]
- 24.Aaby P, Hedegaard K, Sodemann M, et al. Childhood mortality after oral polio immunisation campaign in Guinea-Bissau. Vaccine 2005;23:1746–51 [DOI] [PubMed] [Google Scholar]
- 25.Aaby P, Gustafson P, Roth A, et al. Vaccinia scars associated with better survival for adults. An observational study from Guinea-Bissau. Vaccine 2006;24:5718–25 [DOI] [PubMed] [Google Scholar]
- 26.Jensen ML, Dave S, van der Loeff MS, et al. Vaccinia scars associated with improved survival among adults in rural Guinea-Bissau. PLoS One 2006;1:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bager P, Westergaard T, Rostgaard K, Nielsen NM, Melbye M, Aaby P. Smallpox vaccination and risk of atopy and asthma. J Allergy Clin Immunol 2003;111:1227–31 [DOI] [PubMed] [Google Scholar]
- 28.Sørup S, Villumsen M, Ravn H, et al. Smallpox vaccination and all-cause infectious disease hospitalization: a Danish register-based cohort study . Int J Epidemiol 2011;40:955–63 [DOI] [PubMed] [Google Scholar]
- 29.Kristensen I, Aaby P, Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ 2000;321:1435–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welega P, Nielsen J, Adjuik M, et al. Non-specific effects of diphtheria-tetanus-pertussis and measles vaccinations? An analysis of surveillance data from Navrongo, Ghana. Trop Med Int Health 2012;17:1492–505 [DOI] [PubMed] [Google Scholar]
- 31.Krishnan A, Srivastava R, Dwivedi P, Ng N, Byass P, Pandav CS. Non-specific sex-differential effect of DTP vaccination may partially explain the excess girl child mortality in Ballabgarh, India. Trop Med Int Health 2013;18:1329–37 [DOI] [PubMed] [Google Scholar]
- 32.Breiman RF, Streatfield PK, Phelan M, Shifa N, Rashi M, Yunus M. Effect of infant immunization on childhood mortality in rural Bangladesh: analysis of health and demographic surveillance data. Lancet 2004;364:2204–11 [DOI] [PubMed] [Google Scholar]
- 33.Aaby P, Jensen H, Rodrigues A, et al. Divergent female-male mortality ratios associated with different routine vaccinations among female-male twin pairs. Int J Epidemiol 2004;33:367–73 [DOI] [PubMed] [Google Scholar]
- 34.Aaby P, Ravn H, Roth A, et al. Early diphtheria-tetanus-pertussis vaccination associated with higher female mortality and no difference in male mortality in a cohort of low birthweight children: an observational study within a randomised trial. Arch Dis Child 2012:13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benn CS, Diness BR, Roth A, et al. Effect of 50,000 IU vitamin A given with BCG vaccine on mortality in infants in Guinea-Bissau: randomised placebo controlled trial. BMJ 2008;336:1416–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benn CS, Fisker A, Napirna BM, et al. Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: two by two factorial randomised controlled trial. BMJ 2010;340:c1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benn CS, Rodrigues A, Yazdanbakhsh M, et al. The effect of high-dose vitamin A supplementation administered with BCG vaccine at birth may be modified by subsequent DTP vaccination. Vaccine 2009;27:2891–98 [DOI] [PubMed] [Google Scholar]
- 38.Benn CS, Aaby P, Nielsen J, Binka FN, Ross DA. Does vitamin A supplementation interact with routine vaccinations? An analysis of the Ghana vitamin A supplementation trial. Am J Clin Nutr 2009;90:629–39 [DOI] [PubMed] [Google Scholar]
- 39.Benn CS, Bale C, Sommerfelt H, Friis H, Aaby P. Hypothesis: Vitamin A supplementation and childhood mortality: amplification of the non-specific effects of vaccines? Int J Epidemiol 2003:32:822–28 [DOI] [PubMed] [Google Scholar]
- 40.Elguero E, Simondon F, Simondon K, Vaugelade J. Non-specific effects of vaccination on survival: a prospective study in Senegal. Trop Med Int Health 2005;10:956–60 [DOI] [PubMed] [Google Scholar]
- 41.Martins CL, Benn CS, Andersen A, et al. A randomized trial of a standard dose of EZ measles vaccine given at 4.5 months of age: Effect on total hospital admissions. J Infect Dis 2014;epub ahead of print. PMID: 24436454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garenne M, Cantrelle P. Rougeole e mortalité au Sénégal: étude de l’impact de la vaccination effectuée à Khombole 1965-1968 sur la survie des enfants. In: Cantrelle P, Dormont S, Fargues P, Goujard J, Guignard J, Rumeau-Rouquette C. (eds). Estimation de la mortalité de jeune enfant (0–5 ans) pour guider les actions de santé dans les pays en développement [Measles and mortality in Senegal: study of the impact of vaccination implemented in Khombole 1965–1968 for the survival of children. IN: Estimation of the mortality of young children (aged 0–5 years) to guide health interventions in developing countries]. INSERM 1986;145:515–32 [Google Scholar]
- 43.Aaby P, Pedersen IR, Knudsen K, et al. Child mortality related to seroconversion or lack of seroconversion after measles vaccination. Pediatr Infect Dis J 1989;8:197–200 [PubMed] [Google Scholar]
- 44.Velema JP, Alihonou EJ, Gandaho T, Hounye FH. Childhood mortality among users and non- users of primary health care in a rural West African community. Int J Epidemiol 1991;20:474–79 [DOI] [PubMed] [Google Scholar]
- 45.Aaby P, Martins CL, Garly ML, et al. Measles vaccination in the presence or absence of maternal measles antibody: Impact on child survival. Clin Infect Dis (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sørup S, Benn CS, Krause T, Aaby P, Ravn H. Vaccination with the live vaccine against measles, mumps, and rubella and the risk of hospital admissions related to non-targeted infections. JAMA 2014;311:826–35 [DOI] [PubMed] [Google Scholar]
- 47.Aaby P, Jensen H, Gomes J, Fernandes M, Lisse IM. The introduction of diphtheria-tetanus-pertussis vaccine and child mortality in rural Guinea-Bissau: an observational study. Int J Epidemiol 2004;33:374–80 [DOI] [PubMed] [Google Scholar]
- 48.Shann F. The non-specific effects of vaccines. Arch Dis Child 2010;95:662–67 [DOI] [PubMed] [Google Scholar]
- 49.Contreras G. Sabin's vaccine used for nonspecific prevention of infant diarrhea of viral etiology. Bull Pan Am Health Organ 1974;8:123–32 [PubMed] [Google Scholar]
- 50.Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ 2011. Aug 25;343:d5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Awasthi S, Peto R, Read S, et al. Vitamin A supplementation every 6 months with retinol in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet 2013;381:1469–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garly ML, Jensen H, Martins CL, et al. Hepatitis B vaccination associated with higher female than male mortality in Guinea-Bissau: an observational study. Pediatr Infect Dis J 2004;23:1086–92 [PubMed] [Google Scholar]
- 53.Aaby P, Garly ML, Nielsen J, et al. Increased female-male mortality ratio associated with inactivated polio and diphtheria-tetanus-pertussis vaccines: Observations from vaccination trials in Guinea-Bissau. Pediatr Infect Dis J 2007;26:247. [DOI] [PubMed] [Google Scholar]
- 54.Benn CB, Lund S, Fisker A, Jørgensen MJ, Aaby P. Should infant girls receive micronutrient supplements? Int J Epidemiol 2009;38:58–90 [DOI] [PubMed] [Google Scholar]
- 55.Benn CS, Netea MG, Selin LK, Aaby P. A small jab – a big effect: non-specific immunomodulation by vaccines. Trends Immunol 2013;34:431–39 [DOI] [PubMed] [Google Scholar]
- 56.Welsh RM, Selin LH. No one is naïve: The significance of heterologous T-cell immunity. Nature Rev 2002;2:417–26 [DOI] [PubMed] [Google Scholar]
- 57.Kleinnijenhuis J, Quintin J, Preijers F, et al. BCG induces NOD2-dependent non-specific protection to reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 2012;109:17537–42 [DOI] [PMC free article] [PubMed] [Google Scholar]


