Abstract
Parathyroid hormone analogue PTH(1–34), used clinically to treat osteoporosis, forms a stable complex with its receptor and prolongs cAMP production even after internalization and recruitment to endosomes. New data suggests this signaling cascade is stimulated by β-arrestins and terminated by retromer.
The parathyroid hormone receptor (PTHR) is a G protein-coupled receptor (GPCR) present in bone and kidney cells that regulates calcium levels in the blood and homeostasis between bone resorption and growth. A synthetic analogue of the parathyroid hormone, PTH(1–34), is clinically used to stimulate bone formation in some patients suffering from osteoporosis. Treatment is dependent on the dosage of PTH(1–34) as intermittent administration results in bone growth, continuous administration stimulates bone resorption. PTH(1–34) forms an unusually stable complex with the PTHR, resulting in prolonged activation of the receptor and elevated levels of the second messenger cAMP, even after internalization of the PTHR-PTH(1–34) complex. Feinstein and colleagues now address how signaling of this receptor-ligand complex is terminated, given that removal from the plasma membrane does not seem to suffice1. Canonical GPCR signaling is silenced by β-arrestins (Fig 1A), however the authors find that β–arrestins actually prolong PTHR-PTH(1–34) signaling, and that this signal is terminated by retromer.
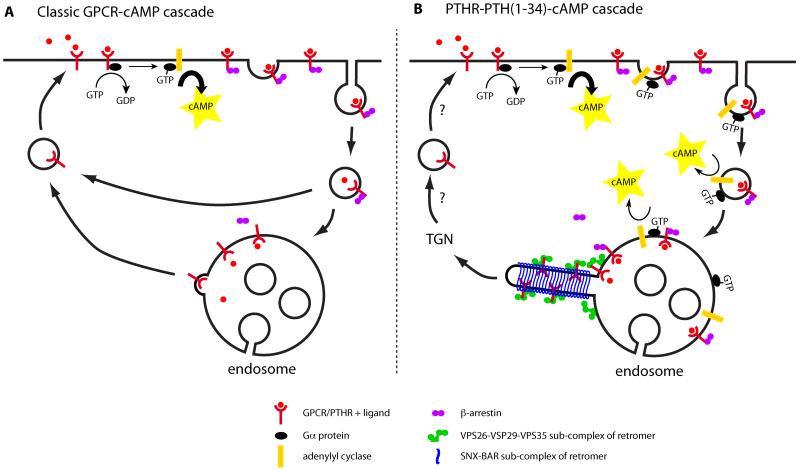
Figure 1. Canonical and non-canonical GPCR-cAMP signaling.
A) The classic model of GPCR signaling starts with the binding of ligand to the receptor, which induces the exchange of GDP for GTP on Gα subunit of heterotrimeric G proteins on the inner leaflet of the plasma membrane. Active GTP-Gα subsequently stimulates adenylyl cyclase to produce second messenger cAMP. cAMP in its turn activates protein kinase A, which has many downstream targets to affect the cell’s behaviour. The second messenger signal is terminated by the association of β-arrestins to the receptor-ligand complex, competing with Gα binding. β-arrestins associate with clathrin to trigger internalization of the receptor-ligand complex, which removes the active receptor from the plasma membrane. B) The PTHR-PTH(1–34) complex is unusually stable and continues to produce cAMP after β-arrestin binding and internalization in the presence of G-protein and adenylyl cyclase. The cAMP signal is terminated by exchange of β-arrestin for retromer, upon which the silenced PTHR is transported to the TGN.
Using FRET to monitor the level of cAMP in live cells, Feinstein et al. provide evidence that β-arrestins actually enhance cAMP signaling by PTHR-PTH(1–34). Time-lapse co-localization studies show that β-arrestin1 is recruited to the plasma membrane upon PTH(1–34) stimulation and remains co-localized with the PTHR after internalization, forming a dynamic complex on endosomes as indicated by FRAP experiments. Twenty-five minutes after stimulation, the co-localization of the receptor and β-arrestin1 drops, which coincides with an increase of PTHR co-localization and interaction with the retromer complex. Co-localization of retromer with PTHR is decreased or delayed by co-expression of mutant β-arrestin1[VI-AA] with increased affinity for the PTHR, suggesting that β-arrestin1 and retromer compete for PTHR binding. It is argued that retromer association with the active PTHR negatively regulates its cAMP signal: overexpression of retromer components attenuate the cAMP level upon PTH(1–34) stimulation and depletion of the retromer component VPS35 prolongs the cAMP signal. The retromer-mediated attenuation of cAMP levels can be partly counteracted by the co-expression of β-arrestin1[IV-AA], consistent with β-arrestin1 and retromer competing for PTHR binding. However, β-arrestin1[IV-AA] may also prolong cAMP levels indirectly through the inhibition of phosphodiesterase 4, an enzyme involved in cAMP breakdown. So how does retromer switch-off cAMP signaling through the internalized PTHR-PTH(1–34) complex?
Retromer is a pentameric complex, well conserved from yeast to mammals, whose primary function is sorting receptors away from the degradative pathway of maturing endosomes to the trans-Golgi network (TGN)2. In mammals, retromer is composed of two sub-complexes: a cargo-recognition unit comprising a stable VPS26:VPS29:VPS35 trimer and a membrane-deforming sub-complex consisting of SNX-BAR proteins that re-models endosome membrane into tubular transport carriers3,4. Interest in retromer has recently escalated as the complex has been implicated in a variety of development and disease orientated processes such as the establishment of Wnt gradients during development and normal tissue homeostasis, apoptotic cell clearance and the sorting of enzymes involved in APP processing in Alzheimer’s disease. The work described by Feinstein and colleagues suggests an additional role for retromer-mediated endosome-to-TGN sorting in the regulation of signaling from a medically important receptor-ligand complex. Moreover, the work extends the concept that the endosomes constitute an important intracellular signaling platform for several pathways including cAMP signaling in addition to their classic role in receptor sorting. The textbook view of GPCR induced cAMP signaling describes the inner leaflet of the plasma membrane as the major site of cAMP production, with internalization of the receptor-ligand complex terminating this signal (Fig. 1). Heterotrimeric G-proteins and adenylyl cyclase are found on endosomes and a subset of GPCRs have been described to prolong cAMP signaling from internal compartments5,6,7. Inhibition of internalization even attenuated the maximal cAMP response of the PTHR-PTH(1–34) signaling cascade, consistent with an endosomal association contributing to PTHR-PTH(1–34) signaling6. Delineating the role of endosomes in receptor signaling is one of the major challenges for future research.
A number of additional important questions remain. How does PTHR-β-arrestin signaling through phosphorylation of ERK relate to the rise cAMP level? This question is of medical importance as PTHR signaling though the ERK-MAPK pathway by β-arrestins is suggested to stimulate bone formation selectively, while its signaling through G-proteins promotes bone turn-over8. The molecular details of the PTHR-PTH(1–34) complex interaction with retromer also needs to be clarified. VPS26 shows striking structural homology to arrestins9,10 and would therefore be a good candidate to compete with β-arrestin1 for PTHR association. Examining this possibility would be of particular interest as no functional homology has yet been described for arrestins and VPS26. Information on this association might shine some light on how the retromer silences the PTHR-PTH(1–34) complex. Is this a direct effect of PTHR-retromer binding or is the termination a secondary effect of receptor sorting? Is retromer involved in silencing of other receptor-ligand signaling cascades on endosomes? To what extent is endosomal cAMP signaling, and its regulation by retromer, important for PTHR signaling when stimulated with the natural ligand?
Clearly, understanding the biological activity of PTH(1–34) is clinically relevant as it can help to improve treatment of patients suffering from osteoporosis. Prolonged internal cAMP signaling has been observed by different laboratories5,6,7 and is interesting from a cell biology point of view as it challenges the classic model of GCPR-cAMP signaling (Fig. 1). From the study of Feinstein and colleagues, one would argue that determining the mechanistic role of sorting complexes like retromer will lead to novel insight in the relationship between endosomal sorting and endosomal signaling. It is an area that certainly warrants further study.
ACKNOWLEDGEMENTS
Research in the Cullen lab is supported by the Wellcome Trust.
REFERENCES
- 1.Feinstein T, et al. Nat Chem Biol. 2011;7:278–284. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attar N, Cullen PJ. Adv Enzyme Regul. 2010;50:216–36. doi: 10.1016/j.advenzreg.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Carlton J, et al. Curr Biol. 2004;14:1791–800. doi: 10.1016/j.cub.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 4.Wassmer T, et al. Dev Cell. 2009;17:110–22. doi: 10.1016/j.devcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calebiro D, et al. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrandon S, et al. Nat Chem Biol. 2009;5:734–42. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullershausen F, et al. Nat Chem Biol. 2009;5:428–34. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 8.Gesty-Palmer D, et al. Sci Transl Med. 2009;1:1ra1. doi: 10.1126/scitranslmed.3000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H, Rojas R, Bonifacino JS, Hurley JH. Nat Struct Mol Biol. 2006;13:540–8. doi: 10.1038/nsmb1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins BM, et al. Traffic. 2008;9:366–79. doi: 10.1111/j.1600-0854.2007.00688.x. [DOI] [PubMed] [Google Scholar]