Abstract
The endocytic network is morphologically characterized by a wide variety of membrane bound compartments that are able to undergo dynamic re-modeling through tubular and vesicular structures. The precise molecular mechanisms governing such re-modeling, and the events that co-ordinated this with the major role of the endocytic network, cargo sorting, remain unclear. That said, recent work on a protein family of sorting nexins (SNX) – especially a subfamily of SNX that contain a BAR domain (SNX–BARs) – has begun to shed some much needed light on these issues and in particular the process of tubular–based endosomal sorting. SNX–BARs are evolutionary conserved in endosomal protein complexes such as retromer, where they co–ordinate membrane deformation with cargo selection. Furthermore a central theme emerges of SNX–BARs linking the forming membrane carrier to cytoskeletal elements for transport through motor proteins such as dynein. By studying these SNX–BARs, we are gaining an increasingly detailed appreciation of the mechanistic basis of endosomal sorting and how this highly dynamic process functions in health and disease.
Keywords: Sorting nexin, Endosome, Phosphoinositide, Dynein, Retromer, Endocytosis
1. Introduction
The BAR (Bin/Amphiphysin/Rvs) domain is a protein dimerization motif made up of three α-helices that dimerize to form a rigid banana-shaped structure [1,2], of which the concave surface contains a number of basic residues that allow association with the phospholipid bilayer through electrostatic interactions [3]. Given the rigid nature of the BAR domain, it has been argued that these electrostatic interactions are optimally configured to associate with curved rather than flat membranes [4]. In this way, BAR domains can sense membrane curvature. In addition, BAR domains are also able to drive membrane deformation by forming higher ordered helical arrays, thereby stabilizing the formation of high curvature membrane tubules and vesicles [5].
BAR proteins are encoded in most, if not all eukaryotic genomes [6]. Many of these proteins are evolutionary conserved from budding yeast to human, spanning a wide variety of cellular functions including cell division, apoptosis, phagocytosis, endocytosis, exocytosis, cytoskeleton dynamics and transcriptional regulation (reviewed recently by Ref. [7]). Some BAR proteins contain additional membrane-binding modules to control their membrane association. One such module is the phosphoinositide-binding phox homology (PX) domain [8]. In mammalian cells, there are 12 proteins that contain both PX and BAR domains [9-11]. These PX–BAR or SNX–BAR proteins appear to play essential roles in the regulation of tubular-based sorting events within the endocytic network [11].
2.0 The SNX–BAR family
2.1 SNX–BARs – a sub-family of sorting nexins
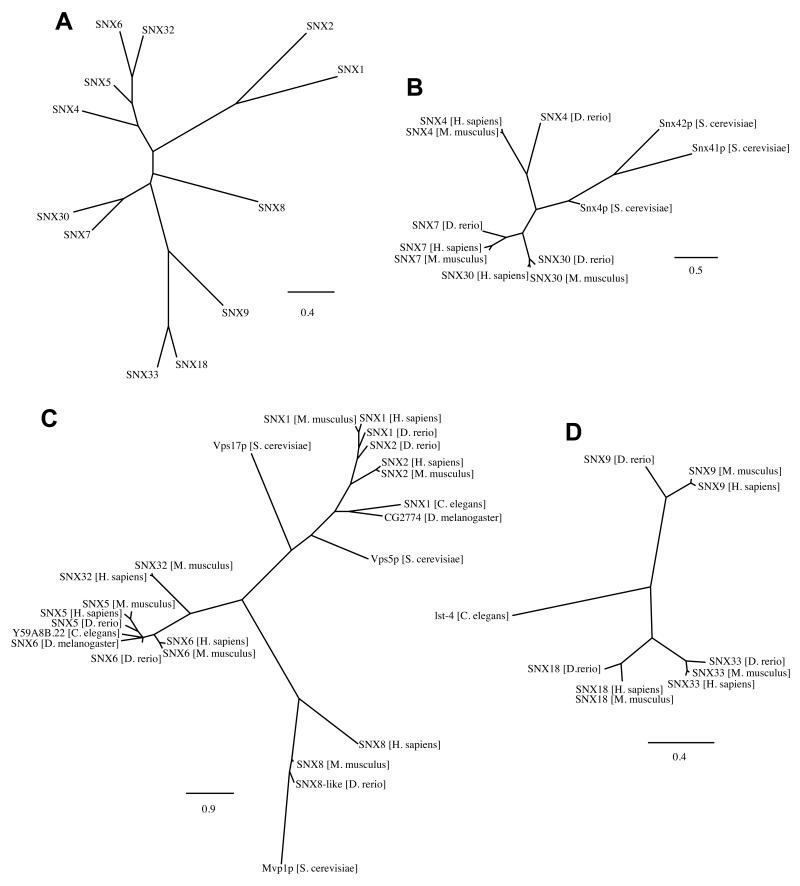
SNX–BARs are a subset of a larger protein family called the sorting nexins (SNXs): these proteins are grouped solely on the basis that they all contain a SNX–PX domain [10-12]. So far, 33 different SNXs have been annotated in the mammalian genome, of which 12 variants contain a C-terminal BAR domain (SNX1, SNX2, SNX4, SNX5, SNX6, SNX7, SNX8, SNX9, SNX18, SNX30, SNX32 and SNX33, see Figure 1A and Ref. [11]). For those SNX–BARs that have been studied, they are localized to tubular and vesicular membrane profiles throughout the endocytic network and have been described to be involved in clathrin-dependent and –independent endocytosis as well as an increasing array of endosomal sorting events [11].
Figure 1. Evolutionary conservation and homology of the different SNX–BAR proteins.
(A) Phylogenic tree of all SNX–BAR proteins annotated in the human genome. The following phylogenic trees show the relation between human SNX–BAR with known homologues in Mus musculus, Danio rerio, Drosophila melanogaster, Caenorhabditis elegans and Saccharomyces cerevisiae of (B) SNX–BARs involved in endosomal recycling SNX4, and possibly SNX7 and SNX30, (C) SNX–BARs involved in endosome-to-TGN traffic (SNX1, SNX2, SNX5, SNX6 and SNX8, and possibly SNX32) and (D) SNX–BARs with a SH3 domain, possibly involved in clathrin-mediated endocytosis and endosomal sorting (SNX9, SNX18 and SNX33). Bars indicate substitutions per site.
2.2 Co-incidence detection in membrane targeting of SNX–BARs
SNX–BARs are peripheral membrane proteins that cycle through a dynamic cytosol-to-membrane equilibrium that is governed by the kinetics of membrane association versus disassociation. For membrane association SNX–BARs combine at least two membrane-binding properties. The first is the binding of the PX domain to specific phosphoinositides that form part of the ‘identity code’ of different endocytic compartments [13]. PX domains display a common structure of four α-helixes and three β-strands, folded into a “baseball-glove” that binds the phosphoinositides in the pocket formed by β1, β2, α2 and their linking loops [14]. Subtle variations in the residues lining the pocket leads to specific phosphoinositide binding, with the insertion of a hydrophobic loop into the membrane serving to further enhance membrane association [15]. Thus, the PX domain of SNX9 associates with phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), thereby aiding the targeting of this SNX–BAR to PI(4,5)P2-enriched regions of forming endocytic pits [16-19]. In contrast, the PX domain of SNX1 associates with the early and late endosomal phosphoinositides, phosphatidylinositol 3-monophosphate (PI(3)P) and phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2) respectively, aiding the targeting of this protein to maturing early endosomes (Figure 4 and Refs. [20,21]).
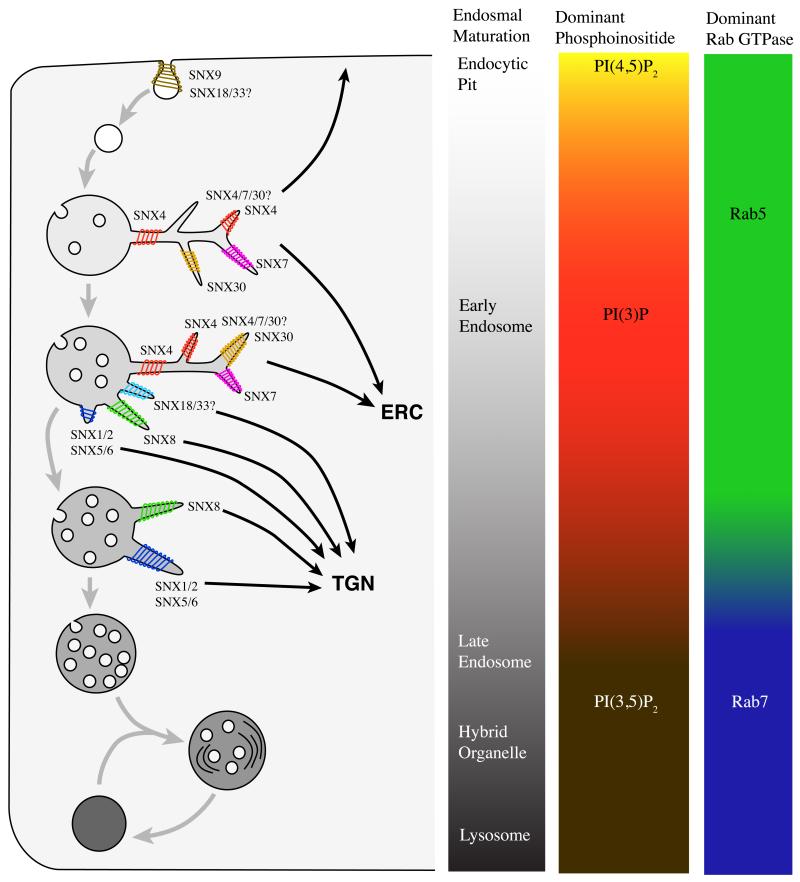
Figure 4. SNX–BAR regulated sorting in the maturing endosome.
Cartoon illustrating the maturation of endosomes from endocytosis through to fusion with the lysosome, and how different SNX–BARs may regulate distinct tubular-based sorting events. The maturation of the endosome coincides with changes in phosphoinositide composition, the presence of different Rab proteins and the increase in number of intraluminal vesicles. In brief, membrane is internalized in PI(4,5)P2-enriched pits. SNX9 plays an important role in this internalization (see text Section 3.4). The endocytic vesicle fuses with the early endosome. In this compartment, proteins are sorted for different destinations while the early endosomal vacuole matures into a late endosome. Proteins targeted for degradation are collected in intraluminal vesicles, which the endosome accumulates during maturation. However, several proteins are retrieved away from this pathway. The transferrin receptor is recycled, at relatively early stages of endosomal maturation, to the plasma membrane either through fast recycling or more slowly via the endosomal recycling compartment (ERC). SNX4 has been established to play a role in regulating tubular-based sorting into the ERC [22], while the roles of SNX7 and SNX30 in this and other recycling pathways are unclear. It also remains to be determined whether SNX4, SNX7 and SNX30 form a restricted series of homo- and/or heterodimeric interactions. The retromer SNX–BARs SNX1, SNX2, SNX5 and SNX6 regulate the retrieval of cation-independent mannose 6-phosphate receptors to the trans-Golgi network (TGN) in a later stage of maturation [33]. SNX8 is also involved in endosome-to-TGN transport, but little is known about its cargo or timing in the maturing endosome [62]. The role(s) of SNX18 and SNX33 remain to be defined ([24,79]. See text (Sections 3.1-3.4) for more detailed discussion of the function of these SNX–BARs.
The second membrane-binding activity of SNX–BARs stems from the ability of their BAR domains to sense membrane curvature [2]. Mutagenic studies targeting key residues in the electrostatic association of the SNX1 BAR domain to curved membranes has revealed that, even though the PX domain of SNX1 retains the ability to bind PI(3)P (and PI(3,5)P2), the mutant protein displays a cytosolic localization [12]. Similarly, a SNX1 PX domain mutant that lacks the ability to bind these phosphoinositides, while retaining a wild-type BAR domain, is also entirely cytosolic [12]. Together these data establish that optimal targeting of SNX1 to the endosomal membrane requires simultaneous binding to phosphoinositides and an ability to sense membrane curvature. A similar dual requirement has also been documented for SNX4 and SNX9 [16,22]. The crystal structure of SNX9 has revealed that the PX domain is situated at the lateral side of the BAR dimer through a flexible linker such that the phosphoinositide-binding pocket lies in the same orientation as the basic concave side of the BAR domain (Figure 2 and Ref. [16]). On basis of these findings, it has been suggested that SNX–BARs utilize a process of ‘co-incidence detention’ to target to sub-domains of the endocytic network that are composed of specific phosphoinositides and an appropriate degree of membrane curvature (Figure 3 and Ref. [23]).
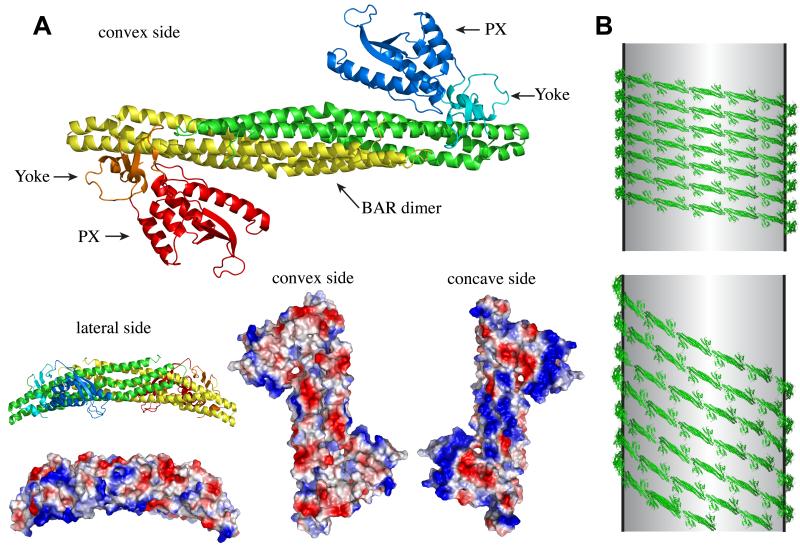
Figure 2. Membrane association/deformation by SNX–BARs.
(A) Crystal structure of the SNX9 BAR-PX dimer; one SNX9 molecule is shown in red/orange/yellow, the other in green/cyan/blue. Red/blue indicate the two PX domains, yellow/green the BAR dimerization domain and orange/cyan the so-called yoke domain, which links the PX domain to the BAR domain [16]. Space-fill models showing the electrostatic distribution on the surface of the SNX9 dimer, red indicates acidic, negative residues while blue indicates positive, basic residues. Note the dominant blue patches on the concave surface, binding the lipid bilayer [16]. (B) A highly speculative view for the oligomerization of SNX–BARs based on the tip-to-tip and lateral contact model for F-BARs [5]. With the presence of the PX domains on either side of the BAR dimer, lateral interactions may occur through two possible routes: via association of a PX domain with its neighbouring lateral BAR domain (top), or through PX domain lateral contact with a neighbouring PX domain (bottom).
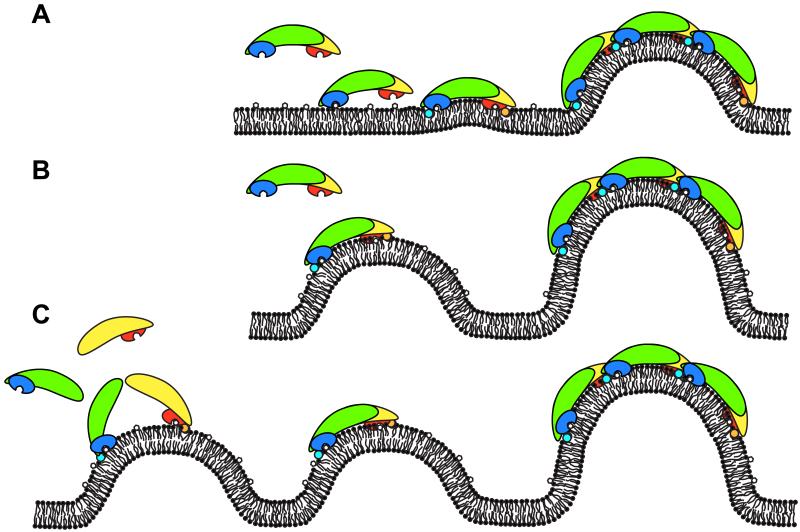
Figure 3. Hypotheses on how SNX–BARs could deform membranes.
Cartoon of the different models how SNX–BARs could deform membranes, the SNX–BAR domains are shown in similar colours as figure 2. (A). Detection of phosphoinositides in the flat membrane allow the PX domains of the SNX–BAR dimer to bind and amphipathic helixes to insert into the lipid bilayer, which produces local curvature. This local curvature is stabilized and extended to form a deformation by oligomerization of the SNX–BAR domains. (B). Co-incidence detection of both local curvature and phosphoinositides by the PX domain and the BAR domain of the SNX–BAR dimer. This local curvature is extended to global deformation by oligomerization of the SNX–BAR domains. (C). Co-incidence detection of phosphoinositides by the PX domain and curvature by the amphipathic helix (shown as cyan/orange circles) [27]. On the curved membrane BAR domains dimerize to stabilize and extend the local curvature.
2.3 SNX–BARs induce membrane deformation leading to membrane tubulation
Like other BAR domain-containing proteins, the dose-dependent addition of recombinant SNX–BARs to artificial liposomes leads to the deformation of vesicles into high curvature membrane tubules in vitro [12,16,24]. The BAR domains of SNX–BARs share little sequence homology with other BAR domains, but their structure and degree of curvature is related to the “classical” BAR/N-BARs (for N-terminal amphipathic helix-BARs) such as endophilin and amphiphysin [2,9,16]. For N-BARs, the amphipathic helix is a flexible structure that forms in the aqueous-lipid interface: hydrophobic residues are clustered at one side of the helix that embeds into the region of fatty acyl chains of the phospho-bilayer while the hydrophilic residues of the helix face the aqueous surface at the level of the polar head groups [2,25]. The insertion of the helix in the bilayer functions as a wedge, pushing the lipids aside in one layer of the membrane. The discrepancy in tension between the layers in the bilayer leads to ‘local’ curvature that is further stabilized by the BAR domain (figure 3 and Ref. [26]). Several SNX–BARs are now predicted to contain similar amphipathic helixes (e.g. SNX1, SNX2, SNX4 and SNX9) [16,27]. Mutations in the predicted amphipathic helix of SNX9, results in reduced membrane affinity and reduced tubulation capacity of lipid vesicles in vitro [16]. A similar type of analysis for the other SNX–BARs should shed more light on the role of these predicted helices in membrane tubulation.
How is ‘local’ membrane deformation translated into a ‘global’ deformation that drives tubule formation? Work on a different class of BAR proteins (so-called F-BARs) has revealed that the BAR dimer can coat membrane tubules in a highly ordered fashion, by which the tips of the BAR dimer interact to form a spiral coat while lateral contacts regulate the angle of the BAR dimer relative to the tubule axis (Fig. 2B and Ref. [5]). For SNX9, the only SNX–BAR so far crystallized with a complete PX-BAR unit, tip-to-tip interactions are observed in the crystals consistent with the observations from F-BARs [5,17]. The lateral interactions described in F-BAR sheets are, however, unlikely to play a role in SNX–BAR oligomerization as the PX domain is located at this site of the BAR dimer (see figure 2B and Ref. [16]). Whether other contacts allow for lateral interactions between the PX domain and adjacent PX–BAR unit remains an important unanswered question. Equally important is the issue of how many distinct tubular profiles are driven by SNX–BARs. If homodimeric interactions prevailed within this family one would predict that each SNX–BAR would coat a distinct tubular profile and hence that 12 SNX–BAR decorated tubular events would be observed. However this appears not to be the case. While SNX9, SNX18 and SNX33 have been reported to form homodimers (although also see Section 3.4 below), SNX1, SNX2, SNX5 and SNX6 form a series of restricted heterodimers that coat a single common tubular profile (described in more detail in Section 3.1.1.) [28,29]. This is quite unusual for BAR proteins as they generally form homodimers, although other examples of heterodimerization are found, e.g. between APPL1 and APPL2 [9,30]. An emerging concept therefore is that SNX–BARs display ‘self-similar assembly’ where, for example, SNX9 drives the formation of a SNX9 restricted tubule through forming a homodimeric helical array, while SNX1 seeds the formation of a heterodimeric helical array comprising specific combinations of SNX1, SNX2, SNX5 and SNX6 [11]. One possible advantage of this heterodimerization is that it allows the recruitment of a wider diversity of proteins to spatially separate domains of the SNX–BAR decorated tubule. While we still need to understand a great deal of the mechanistic detail, it is clear that an overall inherent property of SNX–BARs is an ability to elicit the formation of distinct membrane tubules. A model therefore emerges where through co-incidence detection and self-similar assembly, specific combinations of SNX–BARs can induce the formation of molecularly distinct endosomal tubules. Given that membrane tubules have historically been implicated as sorting stations for a wide variety of endosomal sorting events [31], the study of SNX–BARs has therefore opened up the possibility of describing tubular-based sorting at a molecular level.
3.0 The role of SNX–BARs in endocytosis and endosomal sorting
3.1 Retromer SNX–BARs: SNX1, SNX2, SNX5, SNX6
3.1.1 The retromer components
The best-characterized role for SNX–BARs in tubular-based endosomal sorting involves a protein complex called retromer [32,33]. Retromer regulates endosome to trans-Golgi network (TGN) trafficking of transmembrane proteins, allowing their retrieval away from the endo-lysosomal degradative pathway [34,35]. The best-characterized retromer cargoes are the acid hydrolase receptors such as mannose 6-phosphate receptors (MPRs) in mammalian cells and vacuolar protein sorting (Vps) 10 in yeast [36-38]. The retromer does, however, transport many other cargoes including Wntless (a ‘receptor’ for Wnt morphogens), SNAREs and SorLA, a receptor for the amyloid precursor protein (reviewed by Ref. [33]). Retromer retrieval is also exploited by different pathogens, e.g. Salmonella enterica Serovar Typhimurium, and is required for the delivery of bacterial exotoxins such as Shiga toxin produced by Shigella dysenteriae [39-41].
The retromer was first described in yeast, where it is composed of two sub-complexes: a cargo recognition sub-complex containing Vps26p, Vps29p and Vps35p and a membrane deformation sub-complex consisting of two SNX–BARs (Vps5p and Vps17p) [34,35]. While the later sub-complex functions to deform the donor membrane into a tubular profile that has been termed the ‘endosome-to-TGN transport carrier’ (ETC) [42], the Vps26p–Vps29p–Vps35p sub-complex recognizes specific cargo through mechanisms that remain to be fully defined, but include the direct association of Vps35p to several recognition motifs on the cytosolic region of cargo molecules [37,38,43,44]. Together these two sub-complexes effectively couple the process of membrane deformation and carrier formation with the sorting of specific cargo into a tubular-based endosome-to-TGN retrieval pathway.
The retromer is an evolutionary conserved protein complex of which the mammalian cargo recognition complex comprises close homologues to the yeast retromer: Vps26, Vps29 and Vps35 [34,45,46]. In contrast, the membrane deformation sub-complex in vertebrae differs from less complex organisms in the number of possible SNX–BAR dimer combinations: yeast, Caenorhabditis elegans and Drosophila melanogaster have one pair of SNX–BARs (Vps5p and Vps17p homologues) while zebrafish, mice and human cells can contain at least four different variants of SNX–BAR dimers composed of SNX1 or SNX2 in association with either SNX5 or SNX6 (figure 1C and Refs. [28,29]). At this point it is not known whether SNX32, which shows high sequence similarity with SNX6, is also part of the retromer complex (figure 1). To what extend these different combinations of SNX–BARs differ in cellular function is also not clear. Null mutant studies showed that mice lacking either SNX1 or SNX2 were viable and fertile, while littermates lacking both genes died during mid-gestation [47,48]. This indicates that the function of SNX1 and SNX2 is independent and redundant, and that at least one functional membrane deformation sub-complex of retromer is required for embryonic survival.
3.1.2 Retromer-mediated sorting in the context of endosomal maturation
Membrane flux from early-to-late endosomes occurs via a process of compartment maturation, which is exemplified by the switching of Rab5 on early endosomes to Rab7 on late endosomes (Figure 4 and Ref. [49]). Proteins targeted for degradation are collected in intraluminal vesicles, which accumulate in the endosome during maturation. The number of intraluminal vesicles can therefore be used as a morphological marker for endosomal maturation, where eight or more intraluminal vesicles correspond to the transition from early-to-late endosomes in HepG2 cells [42]. Retromer-mediated sorting events occur with highest frequency on endosomes containing five to six intraluminal vesicles, in other words at the interface of early-to-late endosomal conversion [42]. Supporting this, endosomal association of the cargo recognition sub-complex is achieved not only through association with the SNX–BARs, but also through interaction with Rab7 [50-52]. Together these data clearly place retromer-mediated sorting at the interface between early and late endosomes (figure 4). How such timing is achieved within a highly dynamic endosomal network is entirely unclear. Perhaps this stems from the activation statues of Rab GTPases, and/or from the arrival and concentration of cargo into a relatively mature early endosome, for example, from AP1-GGA-dependent delivery of the CI-MPR from the TGN [53].
3.1.3 Retromer-mediated sorting – towards a thorough mechanistic understanding
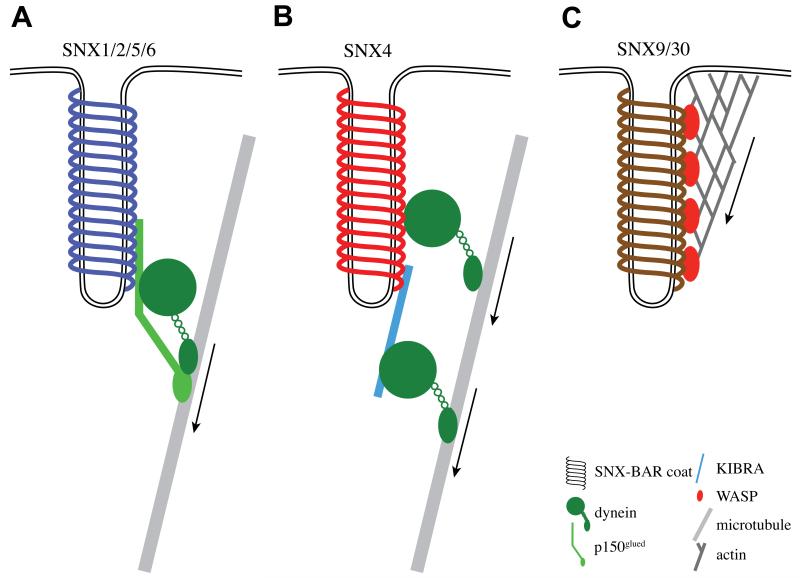
In addition to tubular-based sorting at the donor endosomal membrane, the isolated retromer decorated carrier – the ETC – must undergo long range movement towards the recipient TGN compartment and have an ability to specifically recognize this as the correct recipient compartment for cargo delivery through SNARE-mediated ETC:TGN fusion. To achieve these events, the membrane bound retromer SNX–BAR sub-complex recruits several other proteins to the newly formed sorting tubule to regulate the trafficking between the endosome and the TGN. Apart from the initial membrane deformation, the SNX–BAR coat may assist in membrane carrier fission. SNX1 associates with EHD1 (Eps15 homology domain protein 1) which, through a mechanism similar to dynamin, may mediate membrane carrier fission: EHD proteins oligomerise on membrane tubules in a ring-like structure which stimulates its ATPase activity [54-56]. The family of EHD proteins (four isoforms in mammals) are involved in various membrane trafficking pathways, amongst which is the endosome-to-TGN route [54,56,57]. Possibly assisting EHD proteins in the efficiency of carrier fission from the donor membrane is the association of SNX5 and SNX6 with p150glued, a component of the dynein/dynactin minus-end directed microtubule motor complex [29,58]. Such an association generates mechanical, longitudinal force that may assist the efficiency of carrier fission [59]. Indeed, retromer tubules become excessively long in cells where the coupling of retromer to the dynein/dynactin motor has been disrupted through suppression of p150glued (figure 5A and Ref. [29]). Besides aiding the efficiency of tubule fission, the association to the dynein motor complex is also important for the long range microtubule-dependent transport of retromer carriers towards the TGN, which is generally localized near the perinuclear microtubule organization centre. Consistent with this, knockdown of p150glued results in impaired retromer function and an altered dynamic distribution of retromer-labelled endosomes [29].
Figure 5. SNX–BAR recruit motor proteins to assist in fission and long-range transport.
Cartoon of different SNX–BAR coats recruiting motor proteins to link the forming membrane carrier to cytoskeletal elements. (A). SNX5 and SNX6 associate with p150glued, which is part of the dynactin complex that activates the minus-end directed transport on microtubules by the dynein motor thereby generating longitudinal force. (B). Illustrates the association of the SNX4 coat with the dynein motor [22,72], again generating longitudinal force. (C). SNX9 and SNX33 coats associate with N-WASP, which activates the Arp2/3 complex to polymerize actin filaments. By stimulating this actin polymerization, longitudinal force is generated that may assist the efficiency of membrane fission.
Upon arrival at the TGN, the ETC must tether and dock to the TGN before it can fuse and deliver the cargo. The SNX–BAR dimer also plays a role in the tethering process as it interacts with Rab6 Interacting protein 1 (Rab6IP1) [29]. Rab6IP is an effector molecule of the Rab6 GTPase that is located at the TGN, from where it has been suggested to function as a tether [60]. Knock-down of Rab6IP1 interferes with retromer function and redistributes retromer-labelled endosomes to the periphery [29]. The Rab6IP1–SNX1 complex may therefore serve as a tether by which incoming ETC are recognized by the TGN prior to carrier fusion. Currently, however one cannot discount a role for other well established TGN tethers, such as the Golgins, in the recognition of incoming ETCs from the retromer pathway [61].
Overall, mechanistic studies of retromer components, and in particular the SNX–BAR membrane bound sub-complex, have revealed new insight into how not only tubular-based cargo sorting can be orchestrated at the donor endosomal membrane, but how these events can be choreographed with carrier fission and long-range movement, and carrier recognition by the recipient compartment. In the following we expand upon these concepts by describing the role of other SNX–BAR complexes in tubular-based endosomal sorting.
3.2 SNX8 – further regulation of endosome-to-TGN transport
Recent evidence indicates that sorting nexin-8 (SNX8), a SNX–BAR that shows little homology to the retromer SNX–BARs (figure 1C), is involved in endosome-to-TGN transport [62]. Like the retromer, SNX8 is also conserved in yeast where its proposed homologue Mvp1p is involved in endosomal sorting of Kex2p [63,64]. In mammalian cells, light microscopy-based experiments have established that SNX8 shares a partial co-localization with retromer components [62], although the key issue of how SNX8 relates to retromer decorated tubular endosomal sub-domains remains unclear. Equally, it has yet to be established how SNX8 and retromer dynamically relate in the context of endosomal maturation. RNAi-mediated knock-down studies have yielded confusing results: SNX8 suppression stimulates the uptake and retrograde endosome-to-TGN trafficking of the retromer cargo Shiga toxin, while Ricin trafficking is only slightly impaired [62]. Presently therefore, the endosomal biology of SNX8 remains poorly characterized. Does it regulate a parallel endosome-to-TGN retrieval pathway entirely distinct to retromer, or alternatively is SNX8 functioning upstream/downstream of retromer in a sequential tubular-based retrograde transport pathway?
3.3 Recycling to the cell surface: SNX4, SNX7, SNX30
In yeast, an endosomal recycling pathway genetically distinct to the retromer route is regulated by a set of SNX–BARs: the Snx4p, Snx41p and Snx42p complex [65]. Like the retromer and SNX8, these proteins appear evolutionary conserved, comprising SNX4, SNX7 and SNX30 in mammalian cells [66]. In these cells, the best-studied marker for endosome-to-plasma membrane recycling is the transferrin receptor, which traffics though a fast, direct pathway from early endosomes to the plasma membrane and a slower, indirect pathway via the perinuclear endosomal recycling compartment (ERC) [31,67,68]. Depletion of SNX4 by siRNA or expression of competitive peptides results in missorting of the transferrin receptor to lysosomes and disruption of the ERC [22,69]. This suggests that SNX4 is mainly involved in recycling from endosomes to the perinuclear ERC (figure 4). However, the ERC is not the only perinuclear target as SNX4 has also been implicated in regulating endosome-to-TGN trafficking of Ricin in mammalian cells and Yif1 in yeast [70,71]. Little is known about the mammalian homologues of Snx41p and Snx42p: SNX7 and SNX30.
The molecular make-up of the SNX4 sub-family is similar to the retromer SNX–BARs, containing no conserved domains other than the PX–BAR tandem at the C-terminus of the protein. In vivo, SNX4 forms tubular profiles from endosomes that can be observed to be spatially and temporally distinct to those of retromer (figure 4 and Ref. [22]): again this requires co-incidence detection mediated via the PX and BAR domains of SNX4 [22]. Whether this sub-family of SNX–BARs utilize self-similar assembly – either through SNX4, SNX7 and SNX30 homo- or heterodimerization - has not been reported.
SNX4 has been described to interact with several proteins involved in membrane traffic: amphiphysin 2, KIBRA, dynein, clathrin heavy chain and tubulin [22,69,72]. Interestingly, the interaction of SNX4 with dynein, clathrin and tubulin is perturbed after wortmannin incubation – an inhibitor of 3-phosphoinositide formation – suggesting that these interactions require 3-phosphoinositide-mediated membrane association of SNX4 [72]. Similar to the retromer SNX–BARs, SNX4 links membrane carrier formation to motor driven movement along microtubules in the minus-end direction. SNX4 associates with the dynein motor directly, and indirectly through the dynein-interactor KIBRA [22,72]. Indeed, knockdown of either SNX4 or KIBRA inhibits the transport of transferrin receptor-enriched carriers from early endosomes to the ERC [22]. Again, as discussed for the retromer SNX–BAR association with dynein, SNX4 may assist carrier fission by recruiting the dynein motor to the forming membrane carrier (figure 5B and Ref. [29]).
3.4 SNX9 and clathrin-mediated endocytosis
SNX9 (previously annotated as SH3PX1), SNX18 and SNX33 are SNX–BARs that contain an additional SH3 (Src Homology 3) domain at their N-terminus [11]. SH3 domains are protein–protein interaction domains, which in the case of SNX9 bind dynamin, synaptojanin, N-WASP and Cdc42-associated kinase (ACK) [19,73-76]. The linker region between the SH3 and the PX domain (called the low-complexity region) contains sequences that bind clathrin and AP-2 [74]. All of these proteins play a role in clathrin-mediated endocytosis at the plasma membrane, which suggests that SNX9 plays an important role in this process. Indeed, SNX9 depletion inhibits fluid phase endocytosis, transferrin receptor internalization and synaptic vesicle endocytosis [18,19,77]. Furthermore, CD28 endocytosis for T-cell activation is dependent on functional SNX9 [78].
SNX9 and SNX18 are able to deform membranes in vitro and in vivo [16,24], which in the case of SNX9 is shown to require co-incidence detection through both BAR and PX domains [16]. Whether the BAR domains of SNX9, SNX18 and SNX33 form hetero-dimers is not clear, as conflicting results have been presented in terms of co-immuno-precipitation and co-localization studies [24,79]. The PX domain of SNX9 and SNX18 associates with a wide variety of phosphoinositides but has the highest affinity for the plasma membrane phosphoinositide PI(4,5)P2 [16,24,80]. This is consistent with SNX9’s proposed role in membrane deformation of clathrin-coated pits at the plasma membrane (figure 4).
One of the major interaction partners of SNX9 is dynamin, a GTPase that is involved in the fission of membrane carriers [74]. The mechanism by which dynamin pinches off vesicles is still not completely clear, but it probably goes through a number of self-assembly rounds before the carrier is separated from the donor membrane [81]. SNX9 co-localizes with dynamin to clathrin-coated pits and stimulates self-assembly of dynamin in vitro [18]. This suggests that SNX9 may shift the equilibrium of dynamin-self-assembly locally at clathrin-coated pits towards larger dynamin-complexes, which trigger carrier fission [81]: such concepts are interesting given the suggested link between retromer and the dynamin-related EHD proteins (see above Section 3.1.2). SNX9 also associates with N-WASP (Neural Wiskott–Aldrich Syndrome Protein) through its SH3 domain [19]. Association releases the auto-inhibition of N-WASP to activate Arp2/3 [19], which in turn co-ordinates polymerization of actin filaments (reviewed by Ref. [82]). Linking the sorting tubule to the polymerization of actin may provide longitudinal force to assist in fission of the carrier, as discussed above for the interaction of the retromer SNX–BARs and SNX4 with the dynein motor (figure 5). When SNX9 lacking the SH3 domain is expressed, long sorting tubules are formed, indicative of a defect in efficient tubule fission [76].
4. Concluding remarks
Recent research has shown that SNX–BAR proteins fulfill a central role in different steps of endocytic trafficking. Although the overall sequence homology of the SNX–BARs is poorly conserved, they share a common mechanism of co-incidence detection that allows selective targeting to high curvature sub-domains of phosphoinositide-enriched endosomal compartments. Upon membrane association they deform membranes, driving the formation of tubules onto which they scaffold the formation of sorting complexes that select appropriate cargoes. SNX–BARs co-ordinate these tubular-based sorting events with coupling to entities that allow the generation of longitudinal force, thereby aiding tubule fission and long-range carrier transport. Finally, the SNX–BAR coats may also play a role in recognition of, and tethering to, the correct recipient compartment.
Besides greatly enhancing our appreciation of the molecular basis behind tubular-based endosomal sorting, the study of SNX–BARs is also generating new insight into diseases that arise from defects in endosomal sorting, for example, the canonical Wnt/β-catenin signaling, an important contributor to normal development and tumorigenesis (reviewed in Ref. [83]), and the processing of amyloid precursor protein in late-onset Alzheimer’s disease [84-86]. The continued study of sorting nexins therefore constitutes a productive route in advancing our appreciation of the mechanistic details of endosomal sorting in health and disease.
REFERENCES
- [1].Sakamuro D, Elliott KJ, Wechsler-Reya R, Prendergast GC. BIN1 is a novel MYC-interacting protein with features of a tumour suppressor. Nat Genet. 1996;14:69–77. doi: 10.1038/ng0996-69. [DOI] [PubMed] [Google Scholar]
- [2].Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–9. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- [3].McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–6. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- [4].Zimmerberg J, McLaughlin S. Membrane curvature: how BAR domains bend bilayers. Curr Biol. 2004;14:R250–2. doi: 10.1016/j.cub.2004.02.060. [DOI] [PubMed] [Google Scholar]
- [5].Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman E, De Camilli P, Unger V. Structural basis of membrane invagination by F-BAR domains. Cell. 2008;132:807–17. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Casal E, Federici L, Zhang W, Fernandez-Recio J, Priego EM, Miguel RN, DuHadaway JB, Prendergast GC, Luisi BF, Laue ED. The crystal structure of the BAR domain from human Bin1/amphiphysin II and its implications for molecular recognition. Biochemistry. 2006;45:12917–28. doi: 10.1021/bi060717k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell. 2009;137:191–6. doi: 10.1016/j.cell.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lemmon MA. Phosphoinositide recognition domains. Traffic. 2003;4:201–13. doi: 10.1034/j.1600-0854.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- [9].Habermann B. The BAR-domain family of proteins: a case of bending and binding? EMBO Rep. 2004;5:250–5. doi: 10.1038/sj.embor.7400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Seet L, Hong W. The Phox (PX) domain proteins and membrane traffic. Biochim Biophys Acta. 2006;1761:878–96. doi: 10.1016/j.bbalip.2006.04.011. [DOI] [PubMed] [Google Scholar]
- [11].Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9:574–82. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- [12].Carlton J, Bujny M, Peter B, Oorschot V, Rutherford A, Mellor H, Klumperman J, McMahon HT, Cullen PJ. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Curr Biol. 2004;14:1791–1800. doi: 10.1016/j.cub.2004.09.077. [DOI] [PubMed] [Google Scholar]
- [13].Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- [14].Bravo J, Karathanassis D, Pacold CM, Pacold ME, Ellson CD, Anderson KE, Butler PJ, Lavenir I, Perisic O, Hawkins PT, Stephens L, Williams RL. The crystal structure of the PX domain from p40(phox) bound to phosphatidylinositol 3-phosphate. Molecular Cell. 2001;8:829–39. doi: 10.1016/s1097-2765(01)00372-0. [DOI] [PubMed] [Google Scholar]
- [15].Málková S, Stahelin RV, Pingali SV, Cho W, Schlossman ML. Orientation and penetration depth of monolayer-bound p40phox-PX. Biochemistry. 2006;45:13566–75. doi: 10.1021/bi061133l. [DOI] [PubMed] [Google Scholar]
- [16].Pylypenko O, Lundmark R, Rasmuson E, Carlsson S, Rak A. The PX-BAR membrane-remodeling unit of sorting nexin 9. EMBO J. 2007;26:4788–4800. doi: 10.1038/sj.emboj.7601889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang Q, Kaan HY, Hooda RN, Goh SL, Sondermann H. Structure and plasticity of endophilin and sorting nexin 9. Structure. 2008;16:1574–87. doi: 10.1016/j.str.2008.07.016. [DOI] [PubMed] [Google Scholar]
- [18].Soulet F, Yarar Leonard M, Schmid SNX9 regulates dynamin assembly and is required for efficient clathrin-mediated endocytosis. Mol Biol Cell. 2005;16:2058–67. doi: 10.1091/mbc.E04-11-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yarar D, Waterman-Storer CM, Schmid SL. SNX9 couples actin assembly to phosphoinositide signals and is required for membrane remodeling during endocytosis. Dev Cell. 2007;13:43–56. doi: 10.1016/j.devcel.2007.04.014. [DOI] [PubMed] [Google Scholar]
- [20].Cozier G, Carlton J, McGregor A, Gleeson P, Teasdale RD, Mellor H, Cullen PJ. The phox homology (PX) domain-dependent, 3-phosphoinositide-mediated association of sorting nexin-1 with an early sorting endosomal compartment is required for its ability to regulate epidermal growth factor receptor degradation. J Biol Chem. 2002;277:48730–6. doi: 10.1074/jbc.M206986200. [DOI] [PubMed] [Google Scholar]
- [21].Carlton JG, Bujny MV, Peter BJ, Oorschot VM, Rutherford A, Arkell RS, Klumperman J, McMahon HT, Cullen PJ. Sorting nexin-2 is associated with tubular elements of the early endosome, but is not essential for retromer-mediated endosome-to-TGN transport. J Cell Sci. 2005;118:4527–39. doi: 10.1242/jcs.02568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Traer C, Rutherford A, Palmer K, Wassmer T, Oakley J, Attar N, Carlton J, Kremerskothen J, Stephens D, Cullen PJ. SNX4 coordinates endosomal sorting of TfnR with dynein-mediated transport into the endocytic recycling compartment. Nat Cell Biol. 2007;9:1370–80. doi: 10.1038/ncb1656. [DOI] [PubMed] [Google Scholar]
- [23].Carlton JG, Cullen PJ. Coincidence detection in phosphoinositide signaling. Trends Cell Biol. 2005;15:540–47. doi: 10.1016/j.tcb.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Haberg K, Lundmark R, Carlsson S. SNX18 is an SNX9 paralog that acts as a membrane tubulator in AP-1-positive endosomal trafficking. J Cell Sci. 2008;121:1495–1505. doi: 10.1242/jcs.028530. [DOI] [PubMed] [Google Scholar]
- [25].Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B. A general amphipathic alpha-helical motif for sensing membrane curvature. Nat Struct Mol Biol. 2007;14:138–46. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- [26].Gallop J, Jao C, Kent H, Butler P, Evans P, Langen R, McMahon HT. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 2006;25:2898–910. doi: 10.1038/sj.emboj.7601174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bhatia VK, Madsen KL, Bolinger PY, Kunding A, Hedegård P, Gether U, Stamou D. Amphipathic motifs in BAR domains are essential for membrane curvature sensing. EMBO J. 2009;28:3303–14. doi: 10.1038/emboj.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wassmer T, Attar N, Bujny M, Oakley J, Traer C, Cullen PJ. A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J Cell Sci. 2007;120:45–54. doi: 10.1242/jcs.03302. [DOI] [PubMed] [Google Scholar]
- [29].Wassmer T, Attar N, Harterink M, van Weering JRT, Traer CJ, Oakley J, Goud B, Stephens DJ, Verkade P, Korswagen HC, Cullen PJ. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell. 2009;17:110–22. doi: 10.1016/j.devcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nechamen CA, Thomas RM, Dias JA. APPL1, APPL2, Akt2 and FOXO1a interact with FSHR in a potential signaling complex. Mol Cell Endocrinol. 2007;260-262:93–9. doi: 10.1016/j.mce.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hopkins CR. Intracellular routing of transferrin and transferrin receptors in epidermoid carcinoma A431 cells. Cell. 1983;35:321–30. doi: 10.1016/0092-8674(83)90235-0. [DOI] [PubMed] [Google Scholar]
- [32].Seaman M. Recycle your receptors with retromer. Trends Cell Biol. 2005;15:68–75. doi: 10.1016/j.tcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- [33].Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–36. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bachhawat AK, Suhan J, Jones EW. The yeast homolog of H < beta > 58, a mouse gene essential for embryogenesis, performs a role in the delivery of proteins to the vacuole. Genes Dev. 1994;8:1379–87. doi: 10.1101/gad.8.12.1379. [DOI] [PubMed] [Google Scholar]
- [35].Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–81. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Seaman MN, Marcusson EG, Cereghino JL, Emr SD. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J Cell Biol. 1997;137:79–92. doi: 10.1083/jcb.137.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Seaman M. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165:111–22. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Arighi C, Hartnell L, Aguilar R, Haft C, Bonifacino J. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–33. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Popoff V, Mardones GA, Tenza D, Rojas R, Lamaze C, Bonifacino JS, Raposo G, Johannes L. The retromer complex and clathrin define an early endosomal retrograde exit site. J Cell Sci. 2007;120:2022–31. doi: 10.1242/jcs.003020. [DOI] [PubMed] [Google Scholar]
- [40].Bujny MV, Popoff V, Johannes L, Cullen PJ. The retromer component sorting nexin-1 is required for efficient retrograde transport of Shiga toxin from early endosome to the trans Golgi network. J Cell Sci. 2007;120:2010–21. doi: 10.1242/jcs.003111. [DOI] [PubMed] [Google Scholar]
- [41].Bujny M, Ewels P, Humphrey S, Attar N, Jepson M, Cullen PJ. Sorting nexin-1 defines an early phase of Salmonella-containing vacuole-remodeling during Salmonella infection. J Cell Sci. 2008;121:2027–36. doi: 10.1242/jcs.018432. [DOI] [PubMed] [Google Scholar]
- [42].Mari M, Bujny M, Zeuschner D, Geerts W, Griffith J, Petersen C, Cullen PJ, Klumperman J, Geuze H. SNX1 defines an early endosomal recycling exit for sortilin and mannose 6-phosphate receptors. Traffic. 2008;9:380–93. doi: 10.1111/j.1600-0854.2007.00686.x. [DOI] [PubMed] [Google Scholar]
- [43].Nothwehr SF, Bruinsma P, Strawn LA. Distinct domains within Vps35p mediate the retrieval of two different cargo proteins from the yeast prevacuolar/endosomal compartment. Mol Biol Cell. 1999;10:875–90. doi: 10.1091/mbc.10.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Seaman MNJ. Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J Cell Sci. 2007;120:2378–89. doi: 10.1242/jcs.009654. [DOI] [PubMed] [Google Scholar]
- [45].Edgar AJ, Polak JM. Human homologues of yeast vacuolar protein sorting 29 and 35. Biochem Biophys Res Commun. 2000;277:622–30. doi: 10.1006/bbrc.2000.3727. [DOI] [PubMed] [Google Scholar]
- [46].Haft CR, de la Luz Sierra M, Bafford R, Lesniak MA, Barr VA, Taylor SI. Human orthologs of yeast vacuolar protein sorting proteins Vps26, 29, and 35: assembly into multimeric complexes. Mol Biol Cell. 2000;11:4105–16. doi: 10.1091/mbc.11.12.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schwarz DG, Griffin CT, Schneider EA, Yee D, Magnuson T. Genetic analysis of sorting nexins 1 and 2 reveals a redundant and essential function in mice. Mol Biol Cell. 2002;13:3588–600. doi: 10.1091/mbc.E02-03-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Griffin C, Trejo J, Magnuson T. Genetic evidence for a mammalian retromer complex containing sorting nexins 1 and 2. Proc Natl Acad Sci U S A. 2005;102:15173–7. doi: 10.1073/pnas.0409558102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–49. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- [50].Nakada-Tsukui K, Saito-Nakano Y, Ali V, Nozaki T. A retromerlike complex is a novel Rab7 effector that is involved in the transport of the virulence factor cysteine protease in the enteric protozoan parasite Entamoeba histolytica. Mol Biol Cell. 2005;16:5294–303. doi: 10.1091/mbc.E05-04-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, Heck AJ, Raposo G, van der Sluijs P, Bonifacino JS. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183:513–26. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Seaman MNJ, Harbour ME, Tattersall D, Read E, Bright N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J Cell Sci. 2009;122:2371–82. doi: 10.1242/jcs.048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Doray B, Ghosh P, Griffith J, Geuze H, Kornfeld S. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science. 2002;297:1700–3. doi: 10.1126/science.1075327. [DOI] [PubMed] [Google Scholar]
- [54].Gokool S, Tattersall D, Seaman M. EHD1 Interacts with retromer to stabilize SNX1 tubules and facilitate endosome-to-Golgi retrieval. Traffic. 2007;8:1873–86. doi: 10.1111/j.1600-0854.2007.00652.x. [DOI] [PubMed] [Google Scholar]
- [55].Daumke O, Lundmark R, Vallis Y, Martens S, Butler P, McMahon HT. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature. 2007;449:923–7. doi: 10.1038/nature06173. [DOI] [PubMed] [Google Scholar]
- [56].Grant BD, Caplan S. Mechanisms of EHD/RME-1 protein function in endocytic transport. Traffic. 2008;9:2043–52. doi: 10.1111/j.1600-0854.2008.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Naslavsky N, McKenzie J, Altan-Bonnet N, Sheff D, Caplan S. EHD3 regulates early-endosome-to-Golgi transport and preserves Golgi morphology. J Cell Sci. 2009;122:389–400. doi: 10.1242/jcs.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schroer TA. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- [59].Roux A, Uyhazi K, Frost A, De Camilli P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–31. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- [60].Fernandes H, Franklin E, Recacha R, Houdusse A, Goud B, Khan AR. Structural aspects of Rab6-effector complexes. Biochem Soc Trans. 2009;37:1037–41. doi: 10.1042/BST0371037. [DOI] [PubMed] [Google Scholar]
- [61].Hayes GL, Brown FC, Haas AK, Nottingham RM, Barr FA, Pfeffer SR. Multiple Rab GTPase binding sites in GCC185 suggest a model for vesicle tethering at the trans Golgi. Mol Biol Cell. 2009;20:209–17. doi: 10.1091/mbc.E08-07-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Dyve AB, Bergan J, Utskarpen A, Sandvig K. Sorting nexin 8 regulates endosome-to-Golgi transport. Biochem Biophys Res Commun. 2009;390:109–14. doi: 10.1016/j.bbrc.2009.09.076. [DOI] [PubMed] [Google Scholar]
- [63].Ekena K, Stevens TH. The Saccharomyces cerevisiae MVP1 gene interacts with VPS1 and is required for vacuolar protein sorting. Mol Cell Biol. 1995;15:1671–8. doi: 10.1128/mcb.15.3.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Luo W, Chang A. Novel genes involved in endosomal traffic in yeast revealed by suppression of a targeting-defective plasma membrane ATPase mutant. J Cell Biol. 1997;138:731–746. doi: 10.1083/jcb.138.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hettema EH, Lewis MJ, Black MW, Pelham HR. Retromer and the sorting nexins Snx4/41/42 mediate distinct retrieval pathways from yeast endosomes. EMBO J. 2003;22:548–57. doi: 10.1093/emboj/cdg062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Teasdale RD, Loci D, Houghton F, Karlsson L, Gleeson PA. A large family of endosome-localized proteins related to sorting nexin 1. Biochem J. 2001;358:7–16. doi: 10.1042/0264-6021:3580007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7:897–908. doi: 10.1038/nrm2060. [DOI] [PubMed] [Google Scholar]
- [68].Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Leprince C, Le Scolan E, Meunier B, Fraisier V, Brandon N, De Gunzburg J, Camonis J. Sorting nexin 4 and amphiphysin 2, a new partnership between endocytosis and intracellular trafficking. J Cell Sci. 2003;116:1937–48. doi: 10.1242/jcs.00403. [DOI] [PubMed] [Google Scholar]
- [70].Kama R, Robinson M, Gerst JE. Btn2, a Hook1 ortholog and potential Batten disease-related protein, mediates late endosome-Golgi protein sorting in yeast. Mol Cell Biol. 2007;27:605–21. doi: 10.1128/MCB.00699-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Skånland SS, Wälchli S, Utskarpen A, Wandinger-Ness A, Sandvig K. Phosphoinositide-regulated retrograde transport of ricin: crosstalk between hVps34 and sorting nexins. Traffic. 2007;8:297–309. doi: 10.1111/j.1600-0854.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- [72].Skånland SS, Wälchli S, Brech A, Sandvig K. SNX4 in complex with clathrin and dynein: implications for endosome movement. PLoS ONE. 2009;4:e5935. doi: 10.1371/journal.pone.0005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lundmark R, Carlsson SR. The beta-appendages of the four adaptor-protein (AP) complexes: structure and binding properties, and identification of sorting nexin 9 as an accessory protein to AP-2. Biochem J. 2002;362:597–607. doi: 10.1042/0264-6021:3620597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lundmark R, Carlsson SR. Sorting nexin 9 participates in clathrin-mediated endocytosis through interactions with the core components. J Biol Chem. 2003;278:46772–81. doi: 10.1074/jbc.M307334200. [DOI] [PubMed] [Google Scholar]
- [75].Yeow-Fong L, Lim L, Manser E. SNX9 as an adaptor for linking synaptojanin-1 to the Cdc42 effector ACK1. FEBS Lett. 2005;579:5040–8. doi: 10.1016/j.febslet.2005.07.093. [DOI] [PubMed] [Google Scholar]
- [76].Shin N, Ahn N, Chang-Ileto B, Park J, Takei K, Ahn S, Kim S, Di Paolo G, Chang S. SNX9 regulates tubular invagination of the plasma membrane through interaction with actin cytoskeleton and dynamin 2. J Cell Sci. 2008;121:1252–1263. doi: 10.1242/jcs.016709. [DOI] [PubMed] [Google Scholar]
- [77].Shin N, Lee S, Ahn N, Kim SA, Ahn SG, YongPark Z, Chang S. Sorting nexin 9 interacts with dynamin 1 and N-WASP and coordinates synaptic vesicle endocytosis. J Biol Chem. 2007;282:28939–50. doi: 10.1074/jbc.M700283200. [DOI] [PubMed] [Google Scholar]
- [78].Badour K, McGavin MK, Zhang J, Freeman S, Vieira C, Filipp D, Julius M, Mills Siminovitch KA. Interaction of the Wiskott–Aldrich syndrome protein with sorting nexin 9 is required for CD28 endocytosis and cosignaling in T cells. Proc Natl Acad Sci U S A. 2007;104:1593–8. doi: 10.1073/pnas.0610543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang J, Zhang X, Guo Y, Xu L, Pei D. Sorting nexin 33 induces mammalian cell micronucleated phenotype and actin polymerization by interacting with WiskottAldrich syndrome protein. J Biol Chem. 2009;284:21659–69. doi: 10.1074/jbc.M109.007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–7. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- [81].Pucadyil TJ, Schmid Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell. 2008;135:1263–75. doi: 10.1016/j.cell.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–77. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- [83].Verges M. Retromer and sorting nexins in development. Front Biosci. 2007;12:3825–51. doi: 10.2741/2355. [DOI] [PubMed] [Google Scholar]
- [84].Small S, Kent K, Pierce A, Leung C, Kang M, Okada H, Honig L, Vonsattel J, Kim TW. Model-guided microarray implicates the retromer complex in Alzheimer’s disease. Ann Neurol. 2005;58:909–19. doi: 10.1002/ana.20667. [DOI] [PubMed] [Google Scholar]
- [85].Nielsen MS, Gustafsen C, Madsen P, Nyengaard JR, Hermey G, Bakke O, Mari M, Schu P, Pohlmann R, Dennes A, Petersen C. Sorting by the cytoplasmic domain of the amyloid precursor protein binding receptor SorLA. Mol Cell Biol. 2007;27:6842–51. doi: 10.1128/MCB.00815-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Muhammad A, Flores I, Zhang H, Yu R, Staniszewski A, Planel E, Herman M, Ho L, Kreber R, Honig LS, Ganetzky B, Duff K, Arancio O, Small SA. Retromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proc Natl Acad Sci USA. 2008;105:7327–32. doi: 10.1073/pnas.0802545105. [DOI] [PMC free article] [PubMed] [Google Scholar]