Abstract
Wnt proteins are lipid modified glycoproteins that play a central role in development, adult tissue homeostasis and disease. Secretion of Wnt proteins is mediated by the Wnt-binding protein Wntless (Wls), which transports Wnt from the Golgi network to the cell surface for release. It has recently been shown that recycling of Wls through a retromer-dependent endosome-to-Golgi trafficking pathway is required for efficient Wnt secretion, but the mechanism of this retrograde transport pathway is poorly understood. Here, we report that Wls recycling is mediated through a novel retromer pathway that is independent of the retromer sorting nexins SNX1-SNX2 and SNX5-SNX6. We found that the unrelated sorting nexin, SNX3, has an evolutionarily conserved function in Wls recycling and Wnt secretion and show that SNX3 interacts directly with the cargo-selective sub-complex of the retromer to sort Wls into a morphologically distinct retrieval pathway. These results demonstrate that SNX3 is part of an alternative retromer pathway that functionally separates the retrograde transport of Wls from other retromer cargo.
The classical retromer complex consists of a membrane-bound coat formed by the sorting nexins SNX1-SNX2 and SNX5-SNX6 (referred to as SNX-BAR sorting nexins)1-4 and a cargo-selective sub-complex comprised of the subunits VPS26, VPS29 and VPS355, 6, which binds to a sorting motif in the cytoplasmic tail of cargo proteins7. The SNX-BAR sorting nexins are recruited to cargo containing endosomes through a phosphatidylinositol-3-monophosphate (PI3P) binding Phox homology (PX) domain, and utilize the carboxy-terminal Bin-amphiphysin-Rvs (BAR) domain to drive membrane deformation and to generate membrane tubules. In recruiting the cargo-selective sub-complex to the forming tubules, the SNX-BAR coat complex is thought to traffic cargo into a tubular-based endosomal sorting pathway8. One of the principal functions of this pathway is to mediate retrograde transport between endosomes and the trans-Golgi network (TGN), as has been established for cargo proteins such as Vps10p in yeast9 and the cation-independent mannose 6-phosphate receptor (CI-MPR) in mammals7, 10, 11. It has recently been shown that Wls (also known as Evi or Sprinter)12-14 is also a retromer cargo15-19. Wls binds to the cargo-selective sub-complex and in mutants of the cargo-selective subunits, Wls is missorted and degraded in lysosomes, leading to a strong defect in Wnt secretion and downstream signaling15-19.
To further examine the function of the retromer complex in the Wnt secretion pathway, we studied the function of the SNX-BAR coat components in Wls recycling. We made the surprising discovery that the SNX-BAR sorting nexins, which are required for the retromer-dependent trafficking of all retromer cargo proteins that have been studied to date1, 6, 20-22, are fully dispensable for Wls recycling and Wnt secretion. We show that the unrelated sorting nexin, SNX3, has an evolutionarily conserved function in the Wnt secretion pathway. SNX3 directly interacts with the cargo-selective subunits of the retromer in a complex that does not contain the SNX-BAR coat components. Furthermore, we show that the SNX3 retromer pathway sorts Wls into a retrieval pathway that is morphologically distinct from the SNX-BAR retromer pathway. Our results demonstrate that Wls recycling is mediated by a novel retromer pathway that separates the recycling of Wls from cargo proteins that take the classical SNX-BAR dependent retromer pathway. We propose that such uncoupling may be essential to achieve the tight regulation of Wnt secretion that is necessary for normal development and adult tissue homeostasis.
RESULTS
The SNX-BAR retromer sorting nexins are dispensable for Wls recycling and Wnt signaling in C. elegans and Drosophila
In C. elegans, mutation of the cargo-selective subunits of the retromer induces defects in several Wnt dependent processes23, 24, including the EGL-20 (Wnt) dependent posterior migration of the left Q neuroblast descendants (QL.d). In contrast, we found that mutation of the single SNX1-SNX2 ortholog snx-123 and the single SNX5-SNX6 ortholog snx-625 did not induce defects in QL.d positioning (Fig. 1A). A comprehensive analysis of other Wnt dependent processes did not reveal defects either (Fig. S1D, Table S1), indicating that snx-1 and snx-6 are not required for Wnt signaling in C. elegans. Consistently, we found that snx-1 and snx-6 are dispensable for the retromer-dependent recycling of the C. elegans Wls ortholog MIG-14 (Fig. 1D). In contrast, recycling of the retromer cargo protein CED-1 was fully dependent on snx-1 and snx-6 (Fig. S2C)25.
Fig. 1.
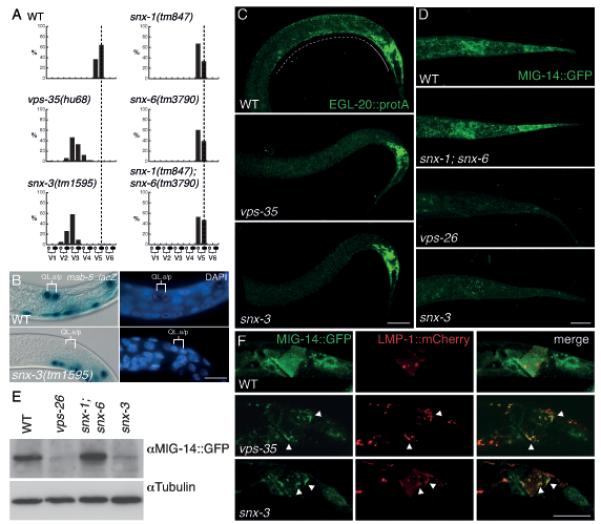
SNX-3 is required for EGL-20 (Wnt) signaling and MIG-14 (Wls) recycling in C. elegans. (A) The final positions of the QL.paa and QL.pap cells relative to the invariant positions of the seam cells V1 to V6 (n>100). Both snx-1(tm847) and snx-6(tm3790) are viable as single or double mutants and could be propagated as homozygous strains, excluding a contribution of maternally provided protein in our assays. (B) Expression of the EGL-20 target gene mab-5 in the QL descendants QL.a and QL.p. Cell nuclei are shown by DAPI staining. Scale bar is 10 μm. (C) Staining of EGL-20::proteinA with rabbit anti-goat-Cy523 in wild type, vps-35(hu68) and snx-3(tm1595). Expression is visible within the egl-20 expressing cells (closed line) and as a punctate posterior to anterior gradient (dotted line). In all images, anterior is to the left and dorsal is up. Scale bar is 10 μm. (D) Confocal images of MIG-14::GFP (huSi2) at identical exposure settings in wild type and in snx-1(tm847); snx-6(tm3790), vps-26(tm1523) and snx-3(tm1595). Scale bar is 10 μm. (E) Western blot quantification of MIG-14::GFP (huSi2) protein levels. (F) Confocal images of MIG-14::GFP (huIs71) (green) and LMP-1::mCherry (red) in wild type, vps-35(hu68) and snx-3(tm1595). Arrowheads indicate examples of co-localization between MIG-14::GFP and LMP-1::mCherry. Scale bar is 10 μm.
To extend these observations, we knocked down the single SNX5-SNX6 ortholog Dsnx6 in the posterior compartment of the Drosophila wing imaginal disc by transgene mediated RNAi. The wing pouch is patterned along the dorsoventral axis by the Wnt protein Wingless (Wg)26, which is expressed by cells that are located at the dorsoventral boundary of the disc (Fig. 2A). In the absence of Dvps35, Wg secretion is strongly reduced, resulting in accumulation of Wg in the producing cells and a loss of expression of the Wg target gene senseless15, 16, 18. We found that knock down of Dsnx6 did not induce accumulation of Wg (Fig. 2G) and that it also did not reduce the expression of senseless (Fig. 2D). Furthermore, knock down of Dsnx6 did not affect the levels of endogenous Wls (Fig. 2J), whereas in the absence of Dvps35, Wls levels are strongly reduced in Wg producing cells15, 16, 18. Taken together, we conclude that the C. elegans and Drosophila SNX-BAR orthologs are dispensable for Wls trafficking and Wnt signaling. To our knowledge this is the first example of the cargo-selective sub-complex of the retromer functioning independently of the SNX-BAR retromer sorting nexins.
Fig. 2.
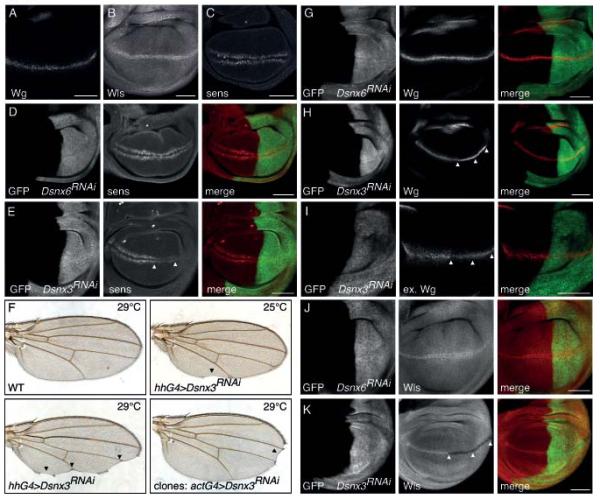
DSnx3 is required for Wg secretion and Wls recycling in the Drosophila wing imaginal disc. (A, B, C) Immunostaining of Wg, Wls and Senseless in wild type wing disc. (D, E, G, H, I, J, K) Expression of Dsnx6 or Dsnx3 RNAi transgenes was induced in the posterior compartment of the wing disc (marked by mCD8-GFP in green) using a hhGal4 driver (see Fig. S3A, B for quantification of knock down efficiency). (D, E) Immunostaining of Senseless (red). Arrowheads indicate loss of senseless expression in the Dsnx3 RNAi expressing posterior compartment. (F) Dsnx3 RNAi was induced in the posterior compartment using hhGal4 or in clones using an actinGal4 driver. Arrowheads indicate notches and loss of sensory bristles. (G, H) Immunostaining of total Wg (red). Arrowheads indicate Wg accumulation in the Dsnx3 RNAi expressing posterior compartment. (I) Immunostaining of extracellular Wg (red). Arrowheads indicate loss of extracellular Wg staining. (J, K) Immunostaining of Wls (red). Arrowheads indicate loss of Wls in wg expressing cells in the Dsnx3 RNAi expressing posterior compartment. Scale bars, 50 μm.
The PX domain-only sorting nexin SNX3 is required for Wnt signaling
In a genome-wide RNAi screen in C. elegans (Table S3), we found that the PX domain-only sorting nexin, snx-3, which is closely related to yeast Grd19p, Drosophila DSnx3 and vertebrate SNX3 and SNX12 (Fig. S1B)8, is required for the EGL-20 (Wnt) dependent posterior migration of the QL.d, a result that we confirmed using the predicted snx-3 null allele tm1595 (Fig. 1A, S1C). EGL-20 induces posterior migration of the QL.d by activating the target gene mab-527, 28. In snx-3(tm1595) mutants, mab-5 expression was lost in the QL lineage (Fig. 1B), consistent with the notion that snx-3 is required for the EGL-20 dependent activation of mab-5. This conclusion is supported by the rescue of the QL.d migration defect of snx-3 by EGL-20 independent activation of mab-5. Thus, the QL.d localize at their normal posterior positions in double mutants between snx-3 and the mab-5 gain of function allele e175128 and in double mutants with the Axin ortholog pry-1 (Table S2)29, 30. In addition to the defect in QL.d migration, snx-3(tm1595) showed a range of other Wnt related phenotypes. Thus, the final position of the QR.d and the HSN neurons was shifted towards the posterior and the polarity of the ALM and PLM mechanosensory neurons was lost or reversed (Table S1, Fig. S1D). Taken together, these results show that snx-3 is required for several Wnt dependent processes and that the range and penetrance of these phenotypes is similar to mutations in components of the cargo-selective retromer sub-complex23, 24.
To investigate whether SNX3 is required for Wnt signaling in Drosophila, we knocked down the single SNX3 ortholog Dsnx3 in the posterior compartment of the wing imaginal disc. As shown in Fig. 2E, there was a strong reduction in expression of the high-threshold Wg target gene senseless. Furthermore, knock down of Dsnx3 in the posterior compartment or in clones frequently resulted in typical wg loss of function defects in the adult wing, such as notches and a loss of sensory bristles at the wing periphery (Fig. 2F). These results establish that SNX3 has an evolutionarily conserved function in Wnt signaling.
SNX3 is required in Wnt producing cells for Wnt secretion
We found that snx-3 is ubiquitously expressed in C. elegans, with prominent expression in coelomocytes, the pharynx and in rectal epithelial cells, which include the cells that produce and secrete EGL-20 (Fig. S2A). We have previously shown that the cargo-selective sub-complex of the retromer is specifically required in Wnt producing cells23. To investigate whether snx-3 has a similar site of action, we tested whether specific expression of wild type snx-3 in the EGL-20 producing cells of snx-3(tm1595) mutants restores the EGL-20 dependent posterior migration of the QL.d. We found that egl-20 promoter directed expression of snx-3 significantly rescued QL.d migration (Table S2). In contrast, expression of snx-1 did not restore QL.d migration. We conclude that the presence of snx-3 in Wnt producing cells is necessary and sufficient for its function in Wnt signaling. Furthermore, these results show that snx-3 and the cargo-selective retromer sub-complex do not only produce similar mutant phenotypes, but also share a common site of action.
The requirement of snx-3 in Wnt producing cells suggests a function of SNX-3 in Wnt secretion. To investigate this possibility, we tested whether the secretion of EGL-20 is affected in snx-3 mutants. EGL-20 (visualized using the immunoglobulin binding domain of protein A as a tag) forms a punctate concentration gradient that ranges from the egl-20 expressing cells in the tail to the mid-body region (Fig. 1C)23. In vps-35 mutants, this gradient is strongly reduced or absent. We found that the EGL-20 gradient was similarly reduced in snx-3(tm1595) (Fig. 1C), indicating that snx-3 is necessary for EGL-20 secretion. To determine whether DSnx3 is also required for Wnt secretion and gradient formation in Drosophila, we depleted Dsnx3 in the posterior compartment of the wing imaginal disc and stained for endogenous Wg. As shown in Fig. 2H and S3C, D, knock down or mutation of Dsnx3 resulted in a strong accumulation of Wg within the stripe of wg expressing cells along the dorsoventral boundary, indicating that Wg secretion is strongly reduced. Indeed, staining of extracellular Wg showed that there was a strong reduction in the level of Wg outside of the wg expressing cells (Fig. 2I). Taken together, these results are consistent with a conserved function of SNX3 in Wnt secretion.
To address the specificity of SNX3 for Wnt secretion, we tested whether knock down of Dsnx3 affects the secretion of two other morphogens in the wing disc: the lipid-modified Hedgehog (Hh) protein31 and the Drosophila BMP ortholog decapentaplegic (Dpp)32. Depletion of Dsnx3 in the dorsal compartment or in large clones spanning the hh expressing domain of the wing imaginal disc did not interfere with Hh secretion, as determined by monitoring endogenous Hh protein (Fig. S3D). To examine effects on Dpp secretion, Dpp signaling activity was measured by staining of phosphorylated Mad protein (pMad), a downstream effector in the Dpp pathway33, but again no effects were observed when Dsnx3 was depleted (Fig. S3E). Although these experiments assay only a limited set of secreted proteins, these results clearly indicate that DSnx3 is not generally required for protein secretion. This conclusion is further supported by the snx-3 mutant phenotype in C. elegans: apart from a defect in Wnt signaling, snx-3 mutants have no other obvious developmental defects (data not shown).
SNX3 has a conserved function in regulating Wls stability
To examine the function of SNX-3 in the Wnt secretion pathway, we tested whether depletion of snx-3 has an effect on the subcellular localization or stability of MIG-14 (Wls)12-14. As shown in Fig. 1D and E, there was a striking decrease in MIG-14 protein levels in snx-3(tm1595), which was similar to the reduction observed in the cargo-selective sub-complex mutant vps-26. In contrast, steady state levels of the retromer cargo protein CED-1 were not significantly changed in the absence of snx-3 (Fig. S2C). Also in Drosophila, depletion of Dsnx3 resulted in a loss of endogenous Wls protein (Fig. 2K, S3C, D). Interestingly, Wls levels were only reduced within the wg expressing cells, an effect that was also observed upon knock down of Dvps3518. We conclude that SNX3 has a conserved function in maintaining Wls protein levels.
To determine whether the reduction in MIG-14 protein levels is the result of lysosomal degradation, we performed co-localization studies between MIG-14 and the late endosomal and lysosomal marker LMP-1::mCherry in C. elegans19. In wild type animals, no significant co-localization between MIG-14::GFP and LMP-1::mCherry could be observed in egl-20 expressing cells (Fig. 1F). However, in snx-3(tm1595) mutants, the remaining MIG-14::GFP was mostly localized to LMP-1::mCherry positive structures. These results indicate that MIG-14 is missorted into the lysosomal degradation pathway in snx-3 mutants, consistent with a function of SNX-3 in the retromer dependent endosome-to-TGN transport of MIG-14.
To investigate whether MIG-14 becomes limiting for Wnt signaling in snx-3 mutants, we tested whether mig-14 overexpression can rescue EGL-20 signaling. Overexpression of a functional MIG-14::GFP fusion protein from a multi-copy transgene fully rescued the QL.d migration defect of snx-3(tm1595) (Table S2), supporting the hypothesis that the Wnt secretion defect of snx-3 mutants is caused by a decrease in MIG-14 (Wls) protein level.
Human SNX3 co-localizes with Wls and VPS26 on endosomes and facilitates membrane association of the cargo-selective retromer sub-complex
To investigate how SNX-3 and the cargo-selective sub-complex functionally interact in Wls recycling, we examined human SNX3 in HeLa cells, which express SNX3 but lack detectable expression of the related sorting nexin SNX12 (Fig. S4A, B). Using a lentivirally expressed GFP-SNX3 fusion protein, we found that SNX3 co-localizes with the cargo-selective retromer subunit VPS26 on endosomes (Fig. 3A), as was confirmed by immuno-electron microscopy (EM) (Fig. 3B). Furthermore, we found that GFP-SNX3 co-localizes with Wls-mCherry (Fig. 4A, and Fig. S2B for co-localization in C. elegans). Knock down of SNX3 led to a significant reduction in endogenous human Wls protein levels (Fig. 4D), consistent with the conserved function of SNX3 in maintaining Wls protein levels. SNX3 depletion also induced a striking reduction in co-localization between Wls-mCherry and endogenous VPS26 (Fig. 4B, C). As the VPS26 staining pattern appeared more cytoplasmic than in control siRNA treated cells, we tested whether SNX3 is required for membrane association of VPS26. Similar to knock down of Rab7, which has been shown to mediate membrane recruitment of the cargo-selective sub-complex to late endosomes34, 35, depletion of SNX3 induced a decrease in membrane associated VPS26 and a corresponding increase in cytoplasmically localized VPS26 (Fig. 4E). These results are consistent with a function of SNX3 in aiding the association of the cargo-selective subunits to Wls containing endosomes. Whether this function is independent of other retromer recruitment mechanisms, such as mediated by Rab734, 35 and Hrs36, remains to be established.
Fig. 3.
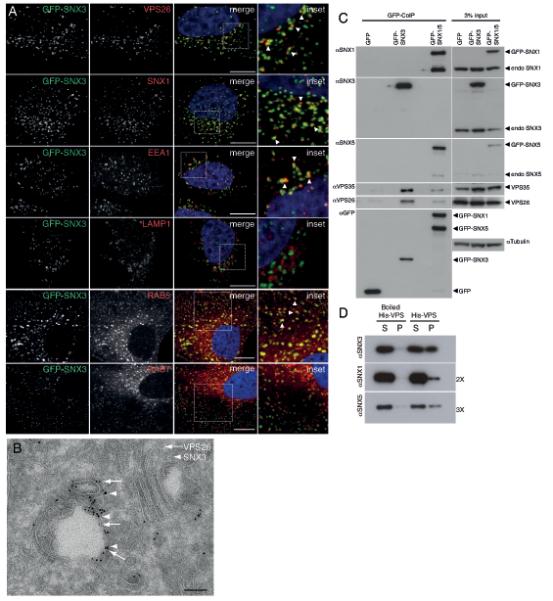
Co-localization and physical interaction of SNX3 with the cargo-selective sub-complex of the retromer. (A) SNX3 partially co-localizes with VPS26-positive early endosomes. HeLa cells lentivirally transduced to express GFP-SNX3 (green) were fixed and stained for VPS26, SNX1, EEA1 or LAMP1 (red). Co-localization between GFP-SNX3 and VPS26, SNX1, EEA1, LAMP1, Rab5 and Rab7 was quantified as 0.43 ± 0.05, 0.55 ± 0.04, 0.38 ± 0.02 and 0.07 ± 0.04, 0.61 ± 0.02, and 0.34 ± 0.02, respectively (Pearson’s coefficient, mean ± SD, n=3 with 30 cells per condition, for Rab5 and Rab7, n=20 cells). Scale bar, 11 μm. (B) At the ultrastructural level, SNX3 and VPS26 localize to common vesicular endosomal profiles. GFP-SNX3 is labeled with 10 nm gold and mCherry-VPS26 with 6 nm gold. The image is representative of that observed from the analysis of 5 other endosomal vacuoles. Scale bar, 100 nm. (C) SNX3 interacts with the cargo-selective sub-complex of the retromer. Cell extracts derived from HeLa cells lentivirally transduced with GFP, GFP-SNX3 or both GFP-SNX1 and GFP-SNX5 (GFP-SNX1/5), were subjected to GFP-nanotrap. The classical retromer SNX-BARs form heterodimeric complexes leading to the presence of both endogenous SNX1 and SNX5 in the GFP-SNX1/5 IPs3. (D) 3xFLAG-VPS26-VPS29-VPS35-His6 complex (His-VPS) was isolated from BL21 E. coli onto TALON resin and incubated with 2 μM of recombinant SNX3, SNX1 or SNX5 for 2 hours at 4°C. Supernatant (S) and TALON containing resin (P) were isolated. SNX3 directly associates with His-VPS as do SNX1 and SNX5 although this is less well pronounced (longer exposures are shown). Control: boiled His-VPS resin.
Fig. 4.
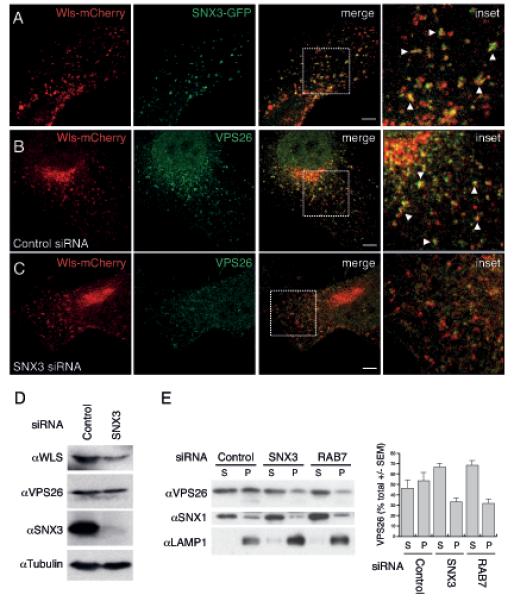
SNX3 co-localizes with Wls and facilitates membrane association of the cargo-selective sub-complex of the retromer. (A) Co-localization between SNX3-GFP (green) and Wls-mCherry (red) in HeLa cells was quantified as 0.25 ± 0.02% (Pearson’s coefficient; mean ± SEM, n=2 with 23 and 11 cells). Arrowheads show examples of co-localization. Scale bar, 10 μm. (B, C) Co-localization between Wls-mCherry and endogenous VPS26 (green) in HeLa cells treated with control or SNX3 siRNA was quantified as 0.19 ± 0.02% and 0.08 ± 0.02%, respectively (Pearson’s coefficient; mean ± SEM, n=4 with 7-10 cells each). Arrowheads show examples of co-localization. (D) HeLa cells were transfected with control or SNX3 siRNA and assayed for endogenous Wls, VPS26, SNX3 and tubulin protein levels. (E) HeLa cells treated with control, SNX3 or RAB7 siRNA were separated into a supernatant (S) fraction containing cytosol and a pellet fraction (P) containing membranes34 and were stained for endogenous VPS26, SNX1 and LAMP1. The amount of VPS26 in the supernatant and pellet fractions was quantified using densitometry and is shown as percentage of the total. Data are presented as mean ± SEM and represent three independent experiments. There was no significant change in SNX1 membrane association upon SNX3 knock down (17.8 ± 3.8% versus 22 ± 8.1%). Knock down of RAB7 was included as a positive control34.
SNX3 physically interacts with the cargo-selective retromer subunits in a complex that does not contain the SNX-BAR sorting nexins
To investigate whether SNX3 and the retromer physically interact, we performed co-immunoprecipitation (CoIP) experiments with GFP-SNX3. As shown in Fig. 3C, there was significant CoIP between SNX3 and both endogenous VPS35 and VPS26, indicating that SNX3 and the cargo-selective retromer sub-complex can associate in vivo. This interaction is direct, as recombinant full length SNX3 co-precipitated with purified VPS26-VPS29-VPS35 trimeric complex (Fig. 3D and S4C). Next, we compared IPs of SNX3 with IPs of the SNX-BAR sorting nexins SNX1 and SNX5 (Fig. 3C). Although in both cases the cargo-selective subunits were co-precipitated (note however that the interaction with SNX1-SNX5 is weaker than with SNX3), we failed to detect endogenous SNX1 and SNX5 in the SNX3 IP and endogenous SNX3 in the SNX1 and SNX5 IP. Based on these results, we conclude that there are two distinct retromer complexes: a SNX-BAR containing retromer complex and a novel complex in which SNX3 interacts with the cargo-selective retromer sub-complex.
SNX3 localizes to early endosomes and segregates Wls into a retrieval pathway that is morphologically distinct from the SNX-BAR retromer pathway
To examine the spatial distribution of SNX3 and the SNX-BAR sorting nexins along the endosomal maturation pathway, we expressed GFP-SNX3 and GFP-SNX1 in HeLa cells. Using markers for early (EEA1 and Rab5) and late endosomes and lysosomes (LAMP1 and Rab7), we found that GFP-SNX3 primarily localizes to early endosomes (Fig. 3A), in agreement with the previously reported localization of SNX3 in A431 cells37. In contrast, the classical SNX-BAR retromer sorting nexins are most abundant on endosomes that are at the early-to-late endosomal transition point22 (J.R.T. van Weering and P.J. Cullen, unpublished). Consistent with this distinct but overlapping distribution, co-localization between SNX1 and SNX3 was only partial (Fig. 3A), supporting the notion that SNX3 and SNX1 display an element of spatial segregation along the endosomal maturation pathway (see Fig. S6).
The SNX-BAR retromer sorting nexins contain a membrane curvature sensing BAR domain which drives membrane deformation to segregate cargo into a tubular-based endosomal trafficking pathway8. SNX3 lacks a BAR domain, indicating that it may direct Wls and the cargo-selective retromer sub-complex into a morphologically distinct sorting pathway. Using live cell confocal imaging to define the dynamic relationship between SNX3, the SNX-BAR sorting nexins and Wls, we found that Wls is not sorted into SNX-BAR labeled tubular endosomal profiles. Thus, in cells expressing GFP-SNX1 and Wls-mCherry, Wls was not enriched in SNX1 decorated tubules emerging from endosomes labeled for both proteins (Fig. 5A, B, S4D) (18/22 tubules were negative for Wls, while 4/22 were weakly positive). Instead, we observed the emergence of small GFP-SNX3 and Wls-mCherry labeled transport vesicles from endosomes co-labeled for both proteins (Fig. 5C). These results are consistent with our observation that the SNX-BAR sorting nexins are dispensable for Wls trafficking and indicate that Wls exits SNX3-labelled early endosomes through vesicular carriers rather than through SNX-BAR decorated tubular profiles. This conclusion is further supported by the observation that SNX3 interacts with clathrin38, a result that we have confirmed here through endogenous CoIP (Fig. S5A). Although SNX3 has been suggested to contain an inverted clathrin box38, the interaction appears indirect, as recombinant SNX3 did not associate with recombinant clathrin (residues 1-579) either in the absence or presence of PI3P-containing liposomes (Fig. S5B, C). Immunostaining of endogenous clathrin revealed that a small population of SNX3 decorated endosomes co-localizes with clathrin (Fig. S5D). At the resolution of immuno-EM, these appear as clathrin coated, small 50-75 nm vesicles (Fig. S5E) that are morphologically distinct from the previously characterized retromer decorated endosome-to-TGN transport carriers (ETC’s), which appear as clathrin-negative, non-branched tubules (average length 170-230 nm and diameter 20-50 nm)22. These results suggest that SNX3 may direct the Wls-retromer complex into a clathrin-dependent vesicular transport pathway. Future studies will examine how Wls trafficking is mediated by the SNX3 retromer complex.
Fig. 5.
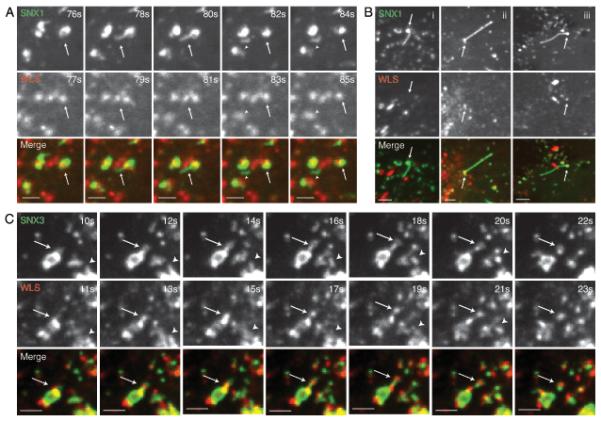
Wls is contained within SNX3 positive vesicular carriers but is absent from SNX1 retromer decorated tubular carriers. (A) RPE-1 cells were transiently co-transfected with pEGFP-SNX1 (green) and Wls-mCherry (red) and cells were subsequently imaged live after a 16 hour incubation period. Frames depicting the formation and scission of a GFP-SNX1 tubule from a vesicle positive for both SNX1 and Wls are shown (arrows indicate the dual expressing vesicle, while arrowheads indicate the carrier post scission) (see Supplementary Information, Movie 1). Scale bars represent 6 μm. Of the 100 SNX1 decorated tubulating endosomes that were analyzed, 22 were positive for Wls; 18/22 tubules emanating from these endosomes were negative for Wls, while 4/22 were weakly positive. Quantification of Wls-mCherry and GFP-SNX1 levels in an endosome and corresponding tubule is shown in Fig. S4D. (B) Further examples of SNX1 retromer tubules negative for Wls. (i) An example of a SNX1 retromer positive endosome and tubule both of which are negative for Wls. (ii and iii) Further examples of SNX1-labelled endosomes positive for Wls, but with tubules negative for Wls. Scale bars represent 6 μm. (C) RPE-1 cells were transiently co-transfected with pEGFP-SNX3 (green) and Wls-mCherry and cells were subsequently imaged live after a 16 hour incubation period. Frames depicting the formation and scission of GFP-SNX3 labeled buds from vesicles positive for both SNX3 and Wls are shown. Note the 1 second delay between acquisitions for a given image pair. Arrows and arrowheads show two examples of buds positive for both Wls and SNX3 (see Supplementary Information, Movie 2). Scale bars represent 6 μm.
DISCUSSION
The identification of the Wnt sorting receptor Wls has shown that the secretion of Wnt proteins is mediated by a specialized trafficking pathway that provides an important layer of regulation to Wnt signaling39, 40. A key step in this pathway is the retromer-dependent transport of Wls from endosomes to the TGN15-19. Here, we report that Wls retrieval is mediated by a novel retromer pathway that functions independently of the SNX-BAR retromer coat components.
The retromer consists of a cargo-selective sub-complex that interacts with sorting nexins of the SNX-BAR family to segregate cargo into a tubular endosomal sorting pathway8. Our results show that the cargo-selective sub-complex also interacts with SNX3 as part of an alternative retromer pathway that mediates the recycling of Wls. Three lines of evidence suggest that these are functionally distinct retromer pathways. First, genetic analysis in C. elegans showed that snx-3 and the SNX-BAR sorting nexins function in parallel pathways. Thus, retrieval of the phagocytic receptor CED-125 is dependent on the SNX-BAR sorting nexins but independent of SNX3, whereas Wls recycling requires SNX3 but not the SNX-BAR sorting nexins. Second, CoIP experiments showed that the interaction of the cargo-selective sub-complex of the retromer with the SNX-BAR sorting nexins and SNX3 is mutually exclusive. Finally, live cell imaging revealed that the SNX3 retromer pathway sorts Wls into a vesicular retrieval pathway that is morphologically distinct from the SNX-BAR dependent tubular endosomal sorting pathway8. Based on these results we conclude that the SNX-BAR and SNX3 pathways are independent and mechanistically distinct retromer pathways.
Studies in yeast have shown that the SNX3 ortholog Grd19p also functions in retromer-dependent endosome to Golgi retrieval21, 41-43, but in contrast to SNX3, Grd19p functions together with the SNX-BAR sorting nexins Vps5p and Vps17p in the retrieval of cargo proteins such as the iron transporter Fet3p-Ftr1p. Grd19p physically interacts with a sorting sequence in the cytoplasmic tail of Ftr1p and with the SNX-BAR retromer complex, which led to the hypothesis that Grd19p acts as a cargo-specific adaptor that links Ftr1p to the SNX-BAR retromer complex43. We did not observe an interaction between SNX3 and Wls in CoIP experiments (data not shown) and also did not find co-precipitation of SNX3 with the SNX-BAR sorting nexins. Furthermore, we found that mutation of the SNX-BAR sorting nexins did not affect the SNX3 dependent retrieval of Wls, indicating that the function of SNX3 is fundamentally different from Grd19p in yeast.
How do the two distinct SNX3- and SNX-BAR-retromer complexes regulate sorting of different endosomal cargo? One simple model to answer this question relies on the spatial segregation of SNX3 and the SNX-BAR sorting nexins along the endosomal maturation pathway. While there is significant co-localization between these sorting nexins, SNX3 is predominantly localized to early endosomes by its high affinity interaction with PI3P44, while the SNX-BAR retromer sorting nexins reside at the interface between early and late endosomes22. Endocytosed Wls therefore initially enters SNX3-labeled early endosomes where it engages the VPS26-VPS29-VPS35 trimeric complex, recruited to this compartment by the interaction with SNX3 (Fig. S6). Through a vesicular pathway, possibly dependent upon indirect binding to clathrin as well as additional membrane remodeling proteins, the SNX3 retromer complex sorts Wls for retrieval back to the TGN. In the absence of SNX3, Wls can be missorted into intraluminal vesicles and hence lysosomal degradation, or can be recycled through SNX-BAR retromer back to the TGN. The relative flux through these two distinct pathways therefore determines the steady-state level of Wls. As the level of Wls is severely reduced upon loss of SNX3, the flux into the lysosomal degradative pathway appears to be dominant. Thus, while a proportion of Wls may undergo SNX-BAR retromer-mediated recycling in the absence of SNX3, this is insufficient to maintain the required level of Wls for Wnt gradient formation during iterative rounds of Wnt secretion and Wls retrieval from the cell surface.
Interestingly, the steady-state trafficking of the classical SNX-BAR retromer cargo CI-MPR is primarily defined by intracellular cycling between the TGN and late endosomes with retrieval back to the TGN via the SNX-BAR retromer. The spatial segregation model therefore suggests that the lack of effect of SNX3 suppression on steady-state CI-MPR distribution arises from CI-MPR entering the endosomal network at a point downstream of SNX33. That said, the complexity of CI-MPR trafficking - a proportion of this receptor traffics to the plasma membrane before undergoing endocytosis and retrograde transport to the TGN45-47 - suggests that such a simple spatial segregation model may be an oversimplification. We therefore speculate that alongside spatial segregation, cargo binding to the VPS26-VPS29-VPS35 complex may be an important element in selecting the sorting nexin coat that specifies the subsequent retrograde trafficking route. Thus, binding of VPS26-VPS29-VPS35 to Wls may favor association with SNX3, while engagement with CI-MPR favors binding to the SNX-BAR coat complex.
Supplementary Material
Acknowledgements
We thank Dr. Catherine Rabouille for critically reading the manuscript, Dr Matthew Seaman for invaluable advice, Dr. Ding Xue (University of Colorado, Boulder) for smIs34, Dr Mitsuaki Tabuchi (Kawasaki Medical School, Okayama, Japan) for the bacterial construct expressing 3xFLAG-tagged retromer complex, Dr. Shohei Mitani (National Bioresource Project for the Nematode, Tokyo, Japan) for deletion mutants, Dr. Andrew Fire for expression vectors and the Caenorhabditis Genetic Center (University of Minnesota, Minneapolis) for strains. This work was funded by the Dutch Cancer Society (HUBR 2008-4114), the EU FP6 Program Cells into Organs and NWO VIDI (016.076.317) (H.C.K.), an NWO VENI fellowship (M.J.L), a Boehringer Ingelheim Foundation fellowship (M.H.), the Swiss National Science Foundation and the Forschungskredit of the University of Zürich (F.P. and K.B.) and the Wellcome Trust (089928/Z/09/Z and 085743) (P.J.C.). I.J.M. is a Wellcome Trust-funded PhD student (086777/Z/08/Z).
References
- 1.Carlton J, et al. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr Biol. 2004;14:1791–1800. doi: 10.1016/j.cub.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 2.Carlton JG, et al. Sorting nexin-2 is associated with tubular elements of the early endosome, but is not essential for retromer-mediated endosome-to-TGN transport. J Cell Sci. 2005;118:4527–4539. doi: 10.1242/jcs.02568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wassmer T, et al. A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J Cell Sci. 2007;120:45–54. doi: 10.1242/jcs.03302. [DOI] [PubMed] [Google Scholar]
- 4.Wassmer T, et al. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell. 2009;17:110–122. doi: 10.1016/j.devcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seaman MN. Recycle your receptors with retromer. Trends Cell Biol. 2005;15:68–75. doi: 10.1016/j.tcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Attar N, Cullen PJ. The retromer complex. Adv Enzyme Regul. 2009;50:216–236. doi: 10.1016/j.advenzreg.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Seaman MN. Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J Cell Sci. 2007;120:2378–2389. doi: 10.1242/jcs.009654. [DOI] [PubMed] [Google Scholar]
- 8.Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9:574–582. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- 9.Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165:111–122. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–133. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banziger C, et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 13.Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Goodman RM, et al. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- 15.Belenkaya TY, et al. The retromer complex influences Wnt secretion by recycling Wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Franch-Marro X, et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan CL, et al. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell. 2008;14:132–139. doi: 10.1016/j.devcel.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Port F, et al. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- 19.Yang PT, et al. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Nothwehr SF, Hindes AE. The yeast VPS5/GRD2 gene encodes a sorting nexin-1-like protein required for localizing membrane proteins to the late Golgi. J Cell Sci. 1997;110(Pt 9):1063–1072. doi: 10.1242/jcs.110.9.1063. [DOI] [PubMed] [Google Scholar]
- 21.Hettema EH, Lewis MJ, Black MW, Pelham HR. Retromer and the sorting nexins Snx4/41/42 mediate distinct retrieval pathways from yeast endosomes. EMBO J. 2003;22:548–557. doi: 10.1093/emboj/cdg062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mari M, et al. SNX1 defines an early endosomal recycling exit for sortilin and mannose 6-phosphate receptors. Traffic. 2008;9:380–393. doi: 10.1111/j.1600-0854.2007.00686.x. [DOI] [PubMed] [Google Scholar]
- 23.Coudreuse DY, Roel G, Betist MC, Destree O, Korswagen HC. Wnt gradient formation requires retromer function in Wnt-producing cells. Science. 2006;312:921–924. doi: 10.1126/science.1124856. [DOI] [PubMed] [Google Scholar]
- 24.Prasad BC, Clark SG. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development. 2006;133:1757–1766. doi: 10.1242/dev.02357. [DOI] [PubMed] [Google Scholar]
- 25.Chen D, et al. Retromer is required for apoptotic cell clearance by phagocytic receptor recycling. Science. 2010;327:1261–1264. doi: 10.1126/science.1184840. [DOI] [PubMed] [Google Scholar]
- 26.Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
- 27.Harris J, Honigberg L, Robinson N, Kenyon C. Neuronal cell migration in C. elegans: regulation of Hox gene expression and cell position. Development. 1996;122:3117–3131. doi: 10.1242/dev.122.10.3117. [DOI] [PubMed] [Google Scholar]
- 28.Salser SJ, Kenyon C. Activation of a C. elegans Antennapedia homologue in migrating cells controls their direction of migration. Nature. 1992;355:255–258. doi: 10.1038/355255a0. [DOI] [PubMed] [Google Scholar]
- 29.Korswagen HC, et al. The Axin-like protein PRY-1 is a negative regulator of a canonical Wnt pathway in C. elegans. Genes Dev. 2002;16:1291–1302. doi: 10.1101/gad.981802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maloof JN, Whangbo J, Harris JM, Jongeward GD, Kenyon C. A Wnt signaling pathway controls hox gene expression and neuroblast migration in C. elegans. Development. 1999;126:37–49. doi: 10.1242/dev.126.1.37. [DOI] [PubMed] [Google Scholar]
- 31.Hidalgo A, Ingham P. Cell patterning in the Drosophila segment: spatial regulation of the segment polarity gene patched. Development. 1990;110:291–301. doi: 10.1242/dev.110.1.291. [DOI] [PubMed] [Google Scholar]
- 32.Entchev EV, Schwabedissen A, Gonzalez-Gaitan M. Gradient formation of the TGF-β homolog Dpp. Cell. 2000;103:981–991. doi: 10.1016/s0092-8674(00)00200-2. [DOI] [PubMed] [Google Scholar]
- 33.Teleman AA, Cohen SM. Dpp gradient formation in the Drosophila wing imaginal disc. Cell. 2000;103:971–980. doi: 10.1016/s0092-8674(00)00199-9. [DOI] [PubMed] [Google Scholar]
- 34.Seaman MN, Harbour ME, Tattersall D, Read E, Bright N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J Cell Sci. 2009;122:2371–2382. doi: 10.1242/jcs.048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rojas R, et al. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183:513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popoff V, et al. Analysis of articulation between clathrin and retromer in retrograde sorting on early endosomes. Traffic. 2009;10:1868–1880. doi: 10.1111/j.1600-0854.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Hortsman H, Seet L, Wong SH, Hong W. SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat Cell Biol. 2001;3:658–666. doi: 10.1038/35083051. [DOI] [PubMed] [Google Scholar]
- 38.Skanland SS, Walchli S, Brech A, Sandvig K. SNX4 in complex with clathrin and dynein: implications for endosome movement. PLoS ONE. 2009;4:e5935. doi: 10.1371/journal.pone.0005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenowicz MJ, Korswagen HC. Sailing with the Wnt: charting the Wnt processing and secretion route. Exp Cell Res. 2009;315:2683–2689. doi: 10.1016/j.yexcr.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Port F, Basler K. Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic. 2010;11:1265–1271. doi: 10.1111/j.1600-0854.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 41.Voos W, Stevens TH. Retrieval of resident late-Golgi membrane proteins from the prevacuolar compartment of Saccharomyces cerevisiae is dependent on the function of Grd19p. J Cell Biol. 1998;140:577–590. doi: 10.1083/jcb.140.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nothwehr SF, Ha SA, Bruinsma P. Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J Cell Biol. 2000;151:297–310. doi: 10.1083/jcb.151.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strochlic TI, Setty TG, Sitaram A, Burd CG. Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling. J Cell Biol. 2007;177:115–125. doi: 10.1083/jcb.200609161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu JW, Lemmon MA. All phox homology (PX) domains from Saccharomyces cerevisiae specifically recognize phosphatidylinositol 3-phosphate. J Biol Chem. 2001;276:44179–44184. doi: 10.1074/jbc.M108811200. [DOI] [PubMed] [Google Scholar]
- 45.Duncan JR, Kornfeld S. Intracellular movement of two mannose 6-phosphate receptors: return to the Golgi apparatus. J Cell Biol. 1988;106:617–628. doi: 10.1083/jcb.106.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin M, Sahagian GG, Jr., Snider MD. Transport of surface mannose 6-phosphate receptor to the Golgi complex in cultured human cells. J Biol Chem. 1989;264:7675–7680. [PubMed] [Google Scholar]
- 47.Lin SX, Mallet WG, Huang AY, Maxfield FR. Endocytosed cation-independent mannose 6-phosphate receptor traffics via the endocytic recycling compartment en route to the trans-Golgi network and a subpopulation of late endosomes. Mol Biol Cell. 2004;15:721–733. doi: 10.1091/mbc.E03-07-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


