Abstract
OBJECTIVE
Preeclampsia (PE) is associated with hypertension and elevated endothelin (ET-1), an indicator of endothelial cell activation and dysfunction. Reduction of uteroplacental perfusion (RUPP) in the pregnant rat model of PE is characterized by elevated mean arterial pressure, inflammatory cytokines, and activation of the ET-1 system. We aim to determine whether 17-alpha-hydroxyprogesterone caproate (17-OHPC) or progesterone suppresses these pathways.
STUDY DESIGN
Plasma progesterone was purified from normal pregnant (NP) and PE patients and measured via enzyme-linked immunosorbent assay. Human umbilical vein endothelial cells were exposed to the sera with or without progesterone added and ET-1 was measured. Pregnant rats underwent the RUPP procedure with or without intraperitoneal 17-OHPC. Mean arterial pressure was compared in RUPP vs NP rats. Human umbilical vein endothelial cells were exposed to NP or RUPP sera, with and without progesterone and ET-1 measured.
RESULTS
Progesterone was significantly decreased in PE women compared with NP women. In response to human sera, ET-1 was elevated in PE women compared to NP women, and decreased with addition of progesterone. Mean arterial pressure was significantly elevated in RUPP vs NP rats but was attenuated by 17-OHPC. ET-1 secretion was stimulated significantly by RUPP compared to NP rat sera, but attenuated by progesterone.
CONCLUSION
Circulating progesterone is significantly lower in PE women compared to controls. 17-OHPC attenuates hypertension in response to placental ischemia in RUPP rats. Progesterone blunts vascular ET-1 stimulated at cellular level by sera from PE women or RUPP rats. Decreased circulating progesterone is associated with stimulation of ET-1. 17-OHPC supplementation blunts hypertension and progesterone blunts endothelial cell ET-1 secretion in response to placental ischemia.
Keywords: endothelin, placental ischemia, preeclampsia, progesterone
Preeclampsia (PE), defined as new-onset hypertension with proteinuria after the 20th week of gestation, affects 4–6% of all pregnancies in the United States.1,2 Vascular complications from PE are a leading cause of maternal morbidity and death, and increase the need for premature delivery with resultant neonatal morbidity and mortality. PE is a multisystem syndromic disorder with origins thought to be subsequent to the shallow trophoblastic invasion of the uterine spiral arteries. Thus, a reduction of uteroplacental perfusion (RUPP) results in persistent placental ischemia.3,4
This diffuse dysfunction of the maternal vascular endothelium is associated with the release of inflammatory cytokines such as tumor necrosis factor (TNF)-alpha, interleukin (IL)-1, and IL-6, also known as “the early insult.” These inflammatory cytokines have been shown to be elevated approximately 2-fold in women with PE when compared with normal pregnant (NP) controls.5 Furthermore, the release of TNF-alpha has been shown to mediate endothelial dysfunction characterized by activation of the endothelin (ET-1) system, with blood pressure increases during pregnancy being mediated through the activation of ET-1 type A (ETA) receptors.6–8 Plasma concentrations of ET-1 are increased approximately 2- to 3-fold in patients with PE when compared with NP controls especially late in the disease process, hence a role in disease progression instead of initiation–“the late insult.”5,6
An agent that has been used effectively for the prevention of recurrent preterm birth in singleton pregnancies is 17-alpha-hydroxyprogesterone caproate (17-OHPC).9–12 The mechanism of action pathway is thought to be based on its antiinflammatory properties, with some studies showing inhibition of basal and TNF-alpha-induced apoptosis in fetal membranes.13 A role for progesterone or 17-OHPC in the prevention or treatment of PE has been debated but never clarified. A recent Cochrane Collaboration review demonstrated that there is insufficient evidence for reliable conclusions about the effects of progesterone for preventing PE and its complications.14 Alternatively, a review by Sammour et al15 concluded that progesterone appears to be effective for the treatment of PE. We hypothesize that decreased endogenous progesterone could serve as a stimulus for elevated inflammatory cytokines as a mechanism to chronically activate endothelial cells to secrete ET-1 in PE women compared to NP women.
The RUPP rat model of PE induces a state of chronic placental ischemia that is associated with inflammatory cytokines and activation of the ET-1 system.16 As shown in prior studies, RUPP-induced hypertension is associated with increases in circulating TNF-alpha, IL-6, and agonistic autoantibodies to the angiotensin II type 1 receptor, as well as decreases in endothelial-dependent relaxation factors. The overexpression of these inflammatory cytokines also occurs in placental explants from PE women.17 Previously our laboratory has demonstrated that hypertension in response to TNF-alpha excess in pregnant rats is associated with increased ET-1 that is mediated through activation of ETA.6–8 Blockade of ETA receptors in rats with placental ischemia abolishes hypertension in response to RUPP.7 Furthermore, we showed that 17-OHPC blunts hypertension and inflammatory cytokines, TNF-alpha and IL-6, associated with RUPP. Administration of 17-OHPC blunted renal ET-1 but had no effect on placental ET-1.18 In addition, administration of 17-OHPC attenuated TNF-alpha-induced hypertension and decreased renal ET-1, without affecting ET-1 in the placenta.19 In the present study we first sought to determine differences in endogenous progesterone among NP and PE women. We next wanted to determine if progesterone supplementation in vitro would affect endothelial cell activation as measured by stimulated ET-1. This set of studies was designed to further investigate beneficial effects of progesterone or 17-OHPC supplementation to suppress endothelial cell activation that occurs in response to chronic placental ischemia in PE.
Materials and Methods
All studies were performed in timed pregnant Sprague-Dawley rats purchased from Harlan (Indianapolis, IN). Animals were housed in a temperature-controlled room (23°C) with a 12-/12-hour light/dark cycle. All of the experimental procedures executed in this study were in accordance with National Institutes of Health guidelines for use and care of animals, and the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center approved all protocols.
The human serum protocol was submitted and approved by the Committee on Human Investigation at the University of Mississippi Medical Center. Obstetric patients were admitted to Winfred Wiser Hospital for Women and Infants (Jackson, MS) with evidence of mild or severe PE, either primary disease or secondary to underlying essential hypertension. Patients were consented to have blood obtained for PE markers under investigation. The patients underwent impedance cardiography (BioZ, Cardiodynamics Sonosite; Cardiodynamics International, San Diego, CA), followed immediately by collection of blood via venipuncture in collection tubes for serum and plasma and then patients were prepared for delivery. The sample was immediately centrifuged for 10 minutes and the serum stored at −20°C.
NP women had blood drawn immediately before scheduled cesarean section. Circulating progesterone was isolated from plasma collected from NP and PE patients. Briefly, 100 μL of plasma was pipetted into a glass tube and 1 mL of petroleum ether was added, vortexed for 30 seconds, and then allowed to separate into phases. The organic phase was transferred into a clean glass tube and the solvent evaporated with a stream of N2. The residue was dissolved in 500 μL of diluted extraction buffer, vortexed, and assayed in 1:4 dilution with appropriate extraction buffer supplied by the manufacturer and measured in duplicate via enzyme-linked immunosorbent assay (ELISA) (Oxford Biomedical Research, Rochester Hills, MI).
Effect of progesterone on arterial pressure in RUPP rat model (protocol I)
Experiments were performed in the following groups of rats: NP (n = 9) and RUPP pregnant rats (n = 12). All of the pregnant rats undergoing surgical procedures were anesthetized with 2% isoflurane (W.A. Butler Co., Middletown, PA) delivered by an anesthesia apparatus (vaporizer for isoflorane anesthetic; Ohio Medical Products, Gurnee, IL). The 17-OHPC (Marty’s Compounding Pharmacy, Jackson, MS) was diluted in normal saline and administered intraperitoneally as 0.5 cm3 solution of 3.32 mg/kg 17-OHPC to pregnant rats. We chose the 1-time 17-OHPC dose to be the weight equivalent of a typical human dose for the prevention of preterm labor. The rats were anesthetized on day 14 of pregnancy and underwent one of the following: (1) examination under anesthesia; (2) RUPP (the lower abdominal aorta was isolated and clipped [0.203 mm in diameter] above the iliac bifurcation–branches of both the right and left ovarian arteries were clipped [0.100 mm in diameter]); (3) injection of intraperitoneal 17-OHPC; or (4) RUPP + injection of intraperitoneal 17-OHPC. On day 18, carotid artery catheters were placed in each rat; on day 19 mean arterial pressure, maternal weight, and pup weight were measured and serum and plasma were collected. Study groups were: NP, NP + 17-OHPC, RUPP, and RUPP + 17-OHPC.
Effect of progesterone on ET-1 secretion from human umbilical vein endothelial cell
In vitro experimental protocols
To determine the properties of progesterone on ET-1 synthesis, we directly examined the effect of progesterone (Sigma Aldrich, St. Louis, MO) after the human umbilical vein endothelial cell (HUVEC) culture was initially exposed to serum media collected from women diagnosed with PE and NP controls (protocol II), and to serum media from NP and RUPP rats (protocol III).
Protocol II, sera of women with PE vs NP
The experimental media contained 50%/50% culture media with and without 1 μmol/L progesterone/10% human serum. Study groups (n = 11 each) were: NP, NP + progesterone, PE, and PE + progesterone.
Protocol III, sera of RUPP rats vs NP
The experimental media contained 50%/50% culture media with and without 1 μmol/L progesterone/50% rat serum. Study groups (n = 8 each) were: NP, NP + progesterone, RUPP, and RUPP + progesterone.
Endothelial cell culture
HUVECs, passage 2, were cultured in 50%/50% Dulbecco modified Eagle medium/medium 199 (Sigma-Aldrich) with 10% fetal bovine serum (Hyclone, Logan, UT) and 1% antimycotic-antibiotic (Gibco, Billings, MT) at 37°C in a humidified atmosphere of 5% CO2–20% O2–75% N2.
Experimental protocol
For 48 hours, 70% confluent monolayers were incubated in serum-free media before the experimental media were added. HUVECs were exposed to 2 mL of experimental media for 24 hours then removed. Fresh serum-free media were added, and the cells were cultured for an additional 24 hours. At 6-hour and 24-hour intervals, during the last culture period, 1 mL of media were collected for further ELISA. Cells were trypsinized and total protein collected.
Assay methods
Measurement of ET-1 concentration
ET-1 was determined using 100 μL of medium collected and measured using the ET-1 Quantikine ELISA kit (R&D Systems, Minneapolis, MN). The assay displayed a sensitivity of 0.031–0.207 pg/mL, interassay variability of 6.3%, and intra-assay variability of 2.8%.
Isolation of total protein
Total protein was isolated and used to standardize immunoassay results. After trypsinization, cells were collected by centrifugation, washed with 200 μL Dulbecco phosphate buffer solution, and centrifuged. A total of 200 μL of protein lysis buffer was added, and cells were disrupted by vortexing. The lysate was placed on ice for 5 minutes, and cell debris was collected by centrifugation for 2 minutes. The protein lysate was extracted and placed in a clean tube. Total protein was quantitated using the BCA protein quantitation kit from Pierce (Thermo Fisher Scientific Inc, Rockford, IL).
Statistical analysis
All of the data are expressed as mean ± SEM. Comparisons of control with experimental groups were analyzed by analysis of variance. A value of P < .05 was considered statistically significant.
Results
Plasma from women with NP (n = 9) and PE pregnancies (n = 14) were used to measure progesterone and to determine ET-1 dysfunction. There was no significant difference in maternal age between women with NP (range, 16–37 years) and those with PE (range, 18–35 years; 28 ± 1.9 vs 25 ± 1.1 years in PE women, P = .169). The average gestational age at delivery for women with NP was 38.67 ± 0.37 weeks (range, 36–39.6 weeks) compared to 33.13 ± 0.84 weeks (P < .0001) (range, 27–38 weeks) for women with PE. All (100%) of the women with NP delivered via scheduled cesarean section compared to 71% of the women with PE who delivered via scheduled cesarean section (P = .127); the remaining 19% delivered vaginally. All women diagnosed with PE were treated with magnesium sulfate intravenous prophylaxis intrapartum as part of the standard of care at our hospital.20 Three women (21%) with PE were placed on antihypertensives (alpha methyl dopa or labetalol) prior to their blood being drawn.
Plasma progesterone concentrations in PE vs NP
To determine if women with PE have lower circulating levels of progesterone compared to NP women we measured circulating progesterone. As shown in Figure 1, progesterone was significantly decreased in PE women (15 ng/mL, n =14) when compared with NP women (34 ng/mL, n = 9), P <.013.
FIGURE 1. Plasma progesterone concentrations.

Plasma progesterone: NP vs preeclamptic women P < .05 vs NP (asterisk).
NP, normal pregnant.
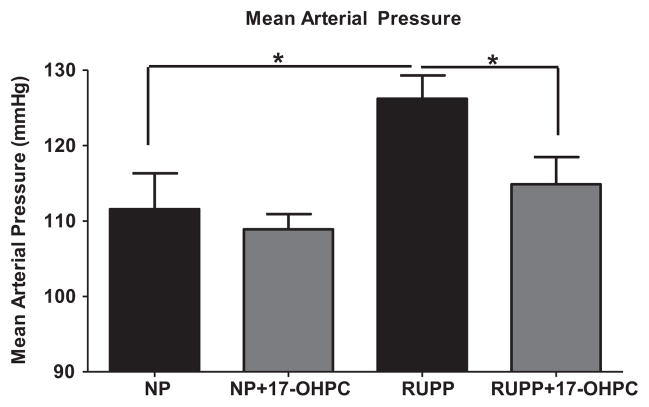
Mean arterial pressure in response to 17-OHPC in RUPP rat model
We have previously shown that 17-OHPC is capable of decreasing inflammation in animal models of inflammation-induced hypertension,18 and wanted to determine if 17-OHPC was also capable of reducing hypertension in an animal model of PE. As shown in Figure 2, mean arterial pressure was significantly higher (P <.05) in RUPP rats (126 ± 3 mm Hg) vs NP rats (110 ± 3.6 mm Hg), and the addition of 17-OHPC significantly (P = .03) blunted mean arterial pressure to 114 ± 3.6 mm Hg in RUPP rats. There was no significant difference in pup weight between RUPP rats (1.92 ± 0.06 g) and RUPP rats treated with 17-OHPC (1.77 ± 0.09 g, P = .252), or NP rats treated with and without 17-OHPC (2.35 ± 0.18 vs 2.26 ± 0.06 g, P =.608). Likewise, there was not a significant difference in litter survival rate between RUPP rats (46.8 ± 8.9%) and RUPP rats treated with 17-OHPC (47.43 ± 11.22%, P = .97) or NP rats treated with and without 17-OHPC (97 ± 1.5% vs 95.56 ± 2.4%, P =.52).
FIGURE 2. Mean arterial pressure in response to 17-OHPC in RUPP rat model.
Mean arterial pressure in NP rats and RUPP rats ± 17-OHPC at day 19 of gestation P < .05 vs NP rats; P < .05 vs RUPP rats (asterisk).
NP, normal pregnant; RUPP, reduction of uteroplacental perfusion; 17-OHPC, 17-alpha-hydroxyprogesterone caproate.
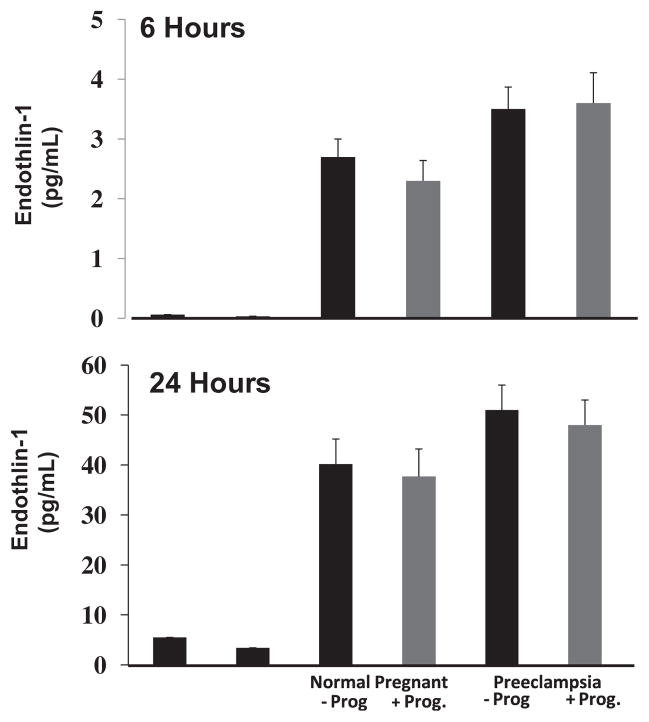
Progesterone supplementation blunts ET-1 secretion in response to PE sera
To determine if decreased circulating progesterone is associated with endothelial dysfunction, we supplemented progesterone in media containing serum from women with PE to prevent an endothelial response. As shown in Figure 3, progesterone decreases ET-1 secretion from HUVECs exposed to serum from both NP (46–43 pg/mL with progesterone) and PE (51–48 pg/mL) women after 24 hours.
FIGURE 3. Progesterone blunts ET-1 in response to PE serum.
Effect of progesterone (Prog) on endothelin (ET-1) after human umbilical vein endothelial cell (HUVEC) exposure to sera from preeclamptic (PE) or normal pregnant (NP) women. ET-1 media concentration 6 hours (top) and 24 hours (bottom) after HUVEC exposure to serum of PE or NP patients ± Prog.
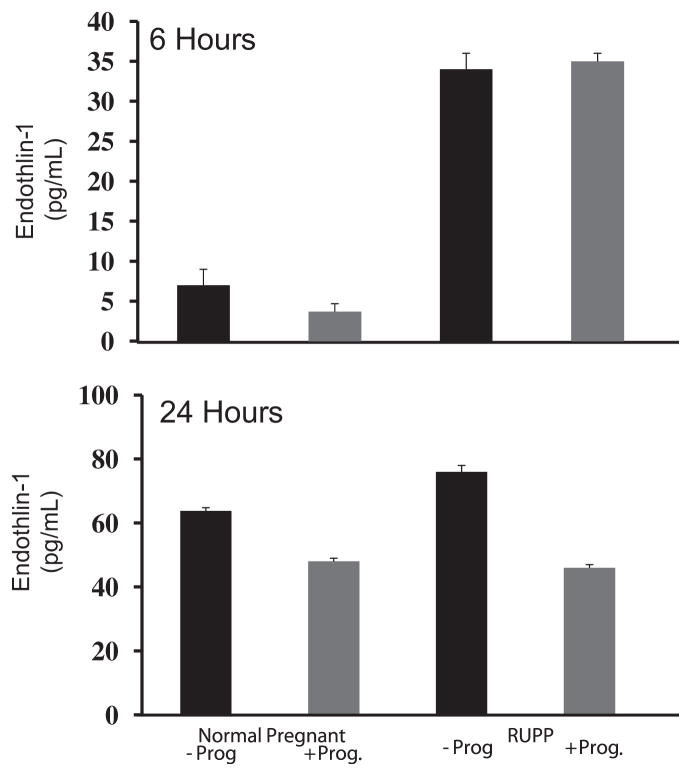
Progesterone supplementation blunts ET-1 secretion in response to RUPP sera
Media containing serum from either NP or RUPP rats was supplemented with progesterone to determine if progesterone supplementation blunts the ET-1 secretion response. In response to RUPP serum there was a sharp increase in ET-1 at both 6-hour and 24-hour exposure times compared to ET-1 secretion in response to serum from NP rats. At 24 hours after exposure to RUPP serum, ET-1 was significantly higher (76 ± 1 pg/mL) when compared to the ET-1 from exposure to NP rat serum (64 ± 1 pg/mL, P <.0001) (Figure 4, B). Progesterone attenuated ET-1 production initially stimulated by the RUPP serum (46 ± 1 pg/mL, P <.0001) (Figure 4, B).
FIGURE 4. Progesterone blunts ET-1 in response to RUPP serum.
Effect of progesterone (Prog) on endothelin (ET-1) after human umbilical vein endothelial cell (HUVEC) exposure to sera of reduction of uteroplacental perfusion (RUPP) or normal pregnant (NP) rats. ET-1 media concentration 6 hours (top) and 24 hours (bottom) after HUVEC exposure to serum from RUPP or NP rats ± Prog.
Comment
PE affects 2–8% of pregnancies and is a leading cause of maternal and perinatal morbidity and mortality worldwide; it is also a leading cause of preterm birth. The mechanisms responsible for the pathogenesis of PE remain unclear. The syndromic signs and symptoms of PE including hypertension usually develop during the second half of pregnancy and remit after delivery, implicating a central role for the placenta in this disease. Reduced placental perfusion is considered to be an initiating event in the cascade of PE pathogenesis that leads to systemic maternal vascular endothelial dysfunction. The ischemic placenta appears to play an important role in endothelial activation, which leads to a cascade of increased production of the vasoconstrictor ET-1, enhanced synthesis of inflammatory cytokines such as IL-6 and IL-17, vascular endothelial growth factor receptor antagonists (soluble fms-like tyrosine kinase-1), agonistic autoantibodies to the angiotensin II receptor, type 1 receptor, and enhanced vascular reactivity to angiotensin II.5 We were able to mirror the same findings in our laboratory with the RUPP rat model. Numerous studies indicate that the ET-1 system plays an important role in the pathogenesis of PE and that ET-1 production is increased in women with PE.8 Also, we previously demonstrated that hypertension in the RUPP rat model is associated with increased ET-1 production.16,21
In the present investigation, we have confirmed our earlier report that the potent progestin 17-hydroxyprogesterone caproate reduces the hypertensive response to placental ischemia in the RUPP rat model. We have gone further by exploring the effect of progesterone to impede ET-1 production by vascular endothelial cells, which is significantly enhanced at 6 and 24 hours after exposure to RUPP rat sera. Similar findings were demonstrated in pregnant human volunteers with PE. Progesterone was demonstrated to blunt ET-1 production by vascular endothelial cells whether stimulated by RUPP rat serum or serum from human subjects with PE. This raises the possibility that progesterone and 17-OHPC may have inhibitory roles as antiinflammatories and in endothelial cell activation for potential use in the treatment of pregnancy-associated hypertensive disorders such as PE.
These data indicate that progesterone or 17-OHPC may provide a viable anti-inflammatory venue toward therapy in the multifaceted pathophysiology of PE including treatment of hypertension. 17-OHPC may exert its antihypertensive effects due to its antiinflammatory properties; indeed other investigators have also shown an important role for 17-OHPC in blood pressure regulation.15 Progesterone is an essential reproductive hormone for the maintenance of pregnancy. A role for progesterone supplementation to prevent or reduce the incidence of preterm birth has received much attention recently. In the 1930s, it was first suggested that progesterone deficiency might play a role in the pathogenesis of PE, but the results from several small cases series were not encouraging.14 Dalton22 briefly revived the idea in the 1960s and Sammour et al15 in the 1970s through 1980s but progesterone for the prevention of PE did not achieve widespread clinical acceptance. Although 3 groups of investigators found no deficiency of progesterone in association with PE23,24), a group from Sweden provided evidence that plasma progesterone increased until week 24 in normotensive and hypertensive women with further increase in normotensive women as compared with the hypertensive group.25 Our group found evidence of progesterone deficiency in patients with severe forms of PE and supplementation of sera with progesterone blunted PE-induced endothelial cell activation from vascular endothelial cells. Importantly, administration of 17-OHPC to placental ischemic rats decreased mean arterial pressure without decreasing pup weight or litter size. Exploring pathways whereby progesterone supplementation could illicit antiinflammatory and antihypertensive effects in response to placental ischemia during pregnancy certainly deserves further consideration.
Clinical implications
We have previously demonstrated that 17-OHPC blunts inflammatory cytokine secretion, hypertension, and renal ET-1 in a pregnant rat model of PE. Furthermore 17-OHPC attenuated hypertension in chronic TNF-alpha-treated pregnant rats and progesterone had a significant impact to reduce TNF-alpha-stimulated ET-1 in HUVECs.18 In the current study, we provide evidence that PE is a state of progesterone deficiency.19 Importantly, we demonstrate the efficacy of 17-OHPC to blunt hypertensive actions in response to placental ischemia, without further reducing pup weight, reducing litter size, or inducing fetal malformations. Furthermore, we report that sera isolated from pregnant rats with chronic RUPP and sera from pregnant women with PE enhance ET-1 production from vascular endothelial cells and that the addition of progesterone attenuated that response. These data suggest an important role of progesterone to blunt ET-1 activation and supports the hypothesis that 17-OHPC could be a potential treatment of hypertension associated with placental ischemia, without further causing harm to fetal development and growth. Data from this study emphasize the need to evaluate the use of progesterone and/or 17-OHPC in a larger trial to determine the efficacy of this treatment as a possible therapy for primary or superimposed PE. This intervention has the potential to exert an antihypertensive effect through a decrease in the maternal intravascular inflammatory response and maternal vascular endothelial cell activation with secondary prolongation of pregnancy for maternal and fetal benefit.
Footnotes
The authors report no conflict of interest.
Presented in poster format at the 32nd annual meeting of the Society for Maternal-Fetal Medicine, Dallas, TX, Feb. 6–11, 2012.
References
- 1.Chappell L, Enyse S, Seed P, Briley A, Poston L, Shennan A. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension: a prospective study. Hypertension. 2008;51:1002–9. doi: 10.1161/HYPERTENSIONAHA.107.107565. [DOI] [PubMed] [Google Scholar]
- 2.Davey D, Macgilivray I, James M, Richard J. Classification of hypertensive disorders in pregnancy. Lancet. 1989;334:112–3. [Google Scholar]
- 3.Roberts J, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension. 2003;41:437–45. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 4.Laresgoiti-Servitje E, Gomez-Lopez N. The pathophysiology of preeclampsia involves altered levels of angiogenic factors promoted by hypoxia and autoantibody–mediated mechanisms. [Accessed April 25, 2013];Biology of Reproduction. 2012 87:36. doi: 10.1095/biolreprod.112.099861. Available at: http://dx.doi.org/10.1095/biolreprod.112.099861. [DOI] [PubMed] [Google Scholar]
- 5.Conrad K, Benyo D. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–9. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 6.LaMarca B, Bennett W, Alexander B, Cockrell K, Granger J. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46:1022–5. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 7.Alexander B, Rinewalt A, Cockrell K, Massey M, Bennett W, Granger J. Endothelin type A receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37:485–9. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 8.Roberts L, LaMarca B, Fournier L, Bain J, Cockrell K, Granger J. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47:615–8. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 9.Sfakianaki A, Norwitz E. Mechanisms of progesterone action in inhibiting prematurity. J Matern Fetal Neonatal Med. 2006;19:763–72. doi: 10.1080/14767050600949829. [DOI] [PubMed] [Google Scholar]
- 10.Saghafi N, Khadem N, Mohajeri T, Shakeri M. Efficacy of 17-alpha-hydroxy-progesterone caproate in prevention of preterm delivery. J Obstet Gynaecol Res. 2011;37:1342–5. doi: 10.1111/j.1447-0756.2011.01524.x. [DOI] [PubMed] [Google Scholar]
- 11.Merlob P, Stahl B, Klinger G. 17-alpha-Hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth. Reprod Toxicol. 2012;33:15–9. doi: 10.1016/j.reprotox.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Society for Maternal-Fetal Medicine Publications Committee. Berghella V. Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;5:376–86. doi: 10.1016/j.ajog.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Luo G, Abrahams V, Tadesse S, et al. Progesterone inhibits basal and TNF-alpha-induced apoptosis in fetal membranes: a novel mechanisms to explain progesterone-mediated prevention of preterm birth. Reprod Sci. 2010;17:532–9. doi: 10.1177/1933719110363618. [DOI] [PubMed] [Google Scholar]
- 14.Mehrer S, Duley L. Progesterone for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2006;4:CD006175. doi: 10.1002/14651858.CD006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sammour MB, El-Kabarity H, Khalifa AS. Progesterone therapy in pre-eclamptic toxaemia. Acta Obstet Gynecol Scand. 1975;54:195–202. doi: 10.3109/00016347509157760. [DOI] [PubMed] [Google Scholar]
- 16.Granger J, Alexander B, Llinas M, Bennett W, Khalil R. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38:718–22. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 17.Benyo D, Smarason A, Redman C, Sims C, Conrad K. Expression of inflammatory cytokines in placentas from women with pre-eclampsia. J Clin Endocrinol Metab. 2001;86:2502–12. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 18.Keiser S, Veillon E, Parrish M, et al. Effects of 17-hydroxyprogesterone on tumor necrosis factor-alpha-induced hypertension during pregnancy. Am J Hypertens. 2009;22:1120–5. doi: 10.1038/ajh.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veillon E, Keiser S, Parrish M, et al. 17-Hydroxyprogesterone blunts the hypertensive response associated with reductions in uterine perfusion pressure in pregnant rats. Am J Obstet Gynecol. 2009;201:324.e1–6. doi: 10.1016/j.ajog.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terrone D, Rinehart B, May W, Martin R, Martin JJ. The myth of transient hypertension: descriptor or disease process? Am J Perinatol. 2001;18:73–7. doi: 10.1055/s-2001-13635. [DOI] [PubMed] [Google Scholar]
- 21.LaMarca B, Cockrell K, Sullivan E, Bennett W, Granger J. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46:82–6. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- 22.Dalton K. Early symptoms of pre-eclamptic toxemia. Lancet. 1960;23:198–9. doi: 10.1016/s0140-6736(60)90113-6. [DOI] [PubMed] [Google Scholar]
- 23.Tamimi R, Lagiou P, Vatten L, et al. Pregnancy hormones, pre-eclampsia, and implications for breast cancer risk in the offspring. Cancer Epidemiol Biomarkers Prev. 2003;12:647–50. [PubMed] [Google Scholar]
- 24.Zeisler H, Sator M, Joura E. Serum levels of progesterone in patients with pre-eclampsia. Wien Klin Wochenschr. 2000;112:362–4. [PubMed] [Google Scholar]
- 25.Risberg A, Olsson K, Lyrenas S, Sjoquist M. Plasma vasopressin, oxytocin, estradiol and progesterone related to water and sodium excretion in normal pregnancy and gestational hypertension. Acta Obstet Gynecol Scand. 2009;88:639–46. doi: 10.1080/00016340902919002. [DOI] [PubMed] [Google Scholar]