Abstract
Adenosine is an endogenous purine metabolite whose concentration in human blood plasma rises from nanomolar to micromolar during stress or hypoxia. Leukocytes express seven-transmembrane adenosine receptors whose engagement modulates Toll-like receptor-mediated cytokine responses, in part via modulation of intracellular cyclic adenosine monophosphate (cAMP). Adenosine congeners are used clinically to treat arrhythmias and apnea of prematurity. Herein we consider the potential of adenosine congeners as innate immune response modifiers to prevent and/or treat infection.
Keywords: adenosine, innate immunity, toll-like receptors, cAMP, CD39 and CD73
Introduction
Immune cells dispose of pathogens directly or by the production of inflammatory mediators and if left unchecked may damage local host tissues. The inflammatory response is therefore regulated to prevent damage to vital organs [1]. Adenosine is an endogenous purine metabolite (Figure 1A) with immunomodulatory properties that accumulates in the extracellular space of tissues under inflammatory or hypoxic conditions [1,2]. Basal plasma adenosine concentrations range from 30-300nM, whereas during hypoxia, inflammation or sepsis extracellular adenosine concentrations increase to the μM range [3]. Extracellular adenosine accumulates as a result of cellular production of adenosine and transport via membrane nucleoside transporters or by extracellular metabolism of ATP to adenosine by the ecto-nucleotidases CD39 and CD73 that are expressed by leukocytes and other host cells [4-6].
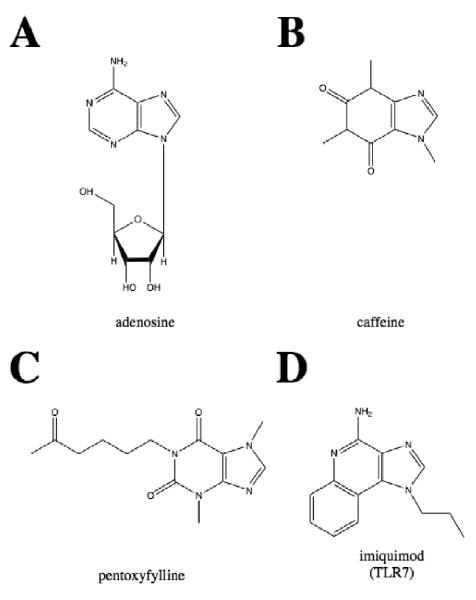
Figure 1. Structures of adenosine congeners in clinical use or development.
(A) adenosine, an endogenous purine metabolite also used to treat arrythmias, (B) caffeine, used to treat apnea of prematurity in preterm newborns and infants, whose administration is also associated with decreased incidence of bronchopulmonary dysplasia (C) pentoxifylline, which has been studied for treatment of neonatal sepsis (D) imiquimod, a TLR7 agonist that is FDA-approved for treatment of human papillioma virus (warts).
A high concentration of ATP can act as a danger signal [6,7]. For example, the oligomerization (and consequent activation) of the nucleotide oligomerization domain (NOD)-like receptor (NLR) family, pyrin domain containing 3 (NLRP3) inflammasome requires ATP or deoxyATP [8]. Binding of extracellular ATP to the purinergic receptor P2X7 is a co-signal required for production of the pro-inflammatory cytokine IL-1β [9-11]. In contrast, the conversion of ATP to adenosine generates a metabolite with inflammation resolving properties [4,6].
Adenosine receptors (ARs) are expressed by all human cells [12]. Expression of ARs by leukocytes serves important immunoregulatory roles [4]. Adenosine, acting via seven-transmembrane G-protein-coupled ARs, including A1R, A2AR, A2BR, and A3R, can modulate cAMP and thereby dampen inflammation, thus protecting tissues from inflammatory damage [13,14]. ARs coupled to stimulatory G proteins (“Gs”) can enhance adenylate cyclase activity and thereby enhance intracellular cAMP levels. ARs are expressed at relatively low levels under basal conditions but expression of ARs, particularly A2A, is increased in response to inflammatory stimuli [15]. The distinct roles of the ARs in cellular responses to adenosine are incompletely characterized [16,17]. A challenge in the adenosine field includes the off-target effects of many adenosine congeners whose specificity can be limited [16]. However, the availability of novel, more selective agonists and antagonists as well as AR-deficient and CD39-deficient mice have strengthened the approaches available in this important field [16,18].
Adenosine in innate immunity
Toll-like receptors
Toll-like receptors (TLRs) are sentinel trans-membrane pattern recognition receptors expressed on leukocytes of humans and other mammals. Engagement of TLRs results in initiation of signaling cascades culminating in host defense responses [19]. The effect of adenosine agonists in modulating TLR-mediated cytokine production depends on the specific TLR agonist used for stimulation [20].
TLR4
Adenosine-receptor stimulation skews TLR4-mediated responses, inhibiting pro-inflammatory Th1-polarizing responses and enhancing Th2-polarizing and anti-inflammatory cytokine production. In general, adenosine inhibits LPS-induced pro-inflammatory/Th1-polarizing cytokines TNF [21,22] and IL-12p70 [23] but enhances production of IL-6 [21], a cytokine with Th2-polarizing and inflammation-resolving properties [24], as well as the anti-inflammatory cytokine IL-10 [23,25]. IL-10 limits inflammation by inhibiting antigen presenting cell (APC) and effector T cell function [26]. IL-6 promotes resolution of inflammation by inhibiting TNF production, limiting neutrophil accumulation and regulating leukocyte apoptosis [24]. Adenosine inhibits LPS-induced production of TNF by murine peritoneal macrophages but increases that of IL-6 [21,27]. A2AR activation, with the selective adenosine congener 2-p-(2-Carboxyethyl)phenethylamino-5′-N-ethyl-carboxamidoadenosine (CGS-21680), inhibits LPS-induced TNF production in human peripheral blood mononuclear cells (PBMCs) [28], monocytes [29], human lung macrophages [30] and murine macrophages [31]. The AR agonist 5′-N-ethylcarboxamidoadenosine (NECA) activated A2AR and increased cAMP in human monocytes thereby inhibiting LPS-induced IL-12p70 while enhancing IL-10 production in human whole blood [23]. Adenosine enhances LPS-induced IL-10 production in murine macrophages through an A2BR-dependent post-transcriptional mechanism [25]. Adenosine did not effect expression of TLR4 and CD14 on human monocytes, suggesting that the effects of adenosine are downstream of the receptor in the signaling pathway [28].
Adenosine, the A2AR agonist CGS-21680, and the cAMP analog, dibutyryl cAMP (dbcAMP) all increase levels of cAMP in peripheral blood mononuclear cells and thereby inhibit LPS-induced TNF production and ICAM-1 expression [28]. cAMP also inhibits LPS-induced IL-12 production in murine peritoneal macrophages [32]. Of note, adenosine modulation of LPS-induced cytokine production is relevant in vivo. Intra-peritoneal (i.p.) injection of mice with AR agonists (2-chloro-N(6)-cyclopentyladenosine (CCPA), CGS or 1-Deoxy-1-[6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-y l]-N-methyl-b-D-ribofuranuronamide (IB-MECA)) 30 min before LPS injection (i.p.) enhanced LPS-induced plasma IL-10 levels but decreased production of TNF and nitric oxide [33].
Phosphodiesterase inhibitors such as caffeine (Figure 1B), theophylline and pentoxifylline (Figure 1C) increase intracellular cAMP and inhibit LPS-induced TNF production [34-36]. Caffeine increased cAMP in human monocytes. Pretreatment with the PKA-inhibitor Rp-8-Br-cAMPS in human whole blood blocked the caffeine-induced suppression of LPS-induced TNF production [36].
TLR2
Relatively less is known regarding interactions between TLR2 and adenosine signaling pathways. Human neonatal cord blood mononuclear cells (CBMCs) exhibit an adenosine- and cAMP-dependent impairment in TLR2-induced TNF but not IL-6 production that is corrected by inhibiting A3R with the selective A3R antagonist MRS-1220 [37]. Of note, the role of A3R in modulating cytokine production by neonatal CB monocytes appears relatively selective to TLR2 as opposed to TLR4 agonists [37]. Interestingly, an A3R congener enhanced cAMP in CBMCs, suggesting positive coupling of A3R to cAMP production in neonatal leukocytes. The basis for the selective modulation of TLR2 vs. TLR4 pathways is worthy of future study.
Mechanisms of cAMP mediated suppression of the immune response
The second messenger cAMP is a major mediator of adenosine-induced inhibition of pro-inflammatory immune responses (Figure 2). cAMP decreases LPS-induced TNF but enhances IL-10 in human monocytes [38]. Although IL-10 can down-regulate the synthesis of TNF, cAMP decreased TNF production by a post-transcriptional mechanism that was independent of IL-10 [38].
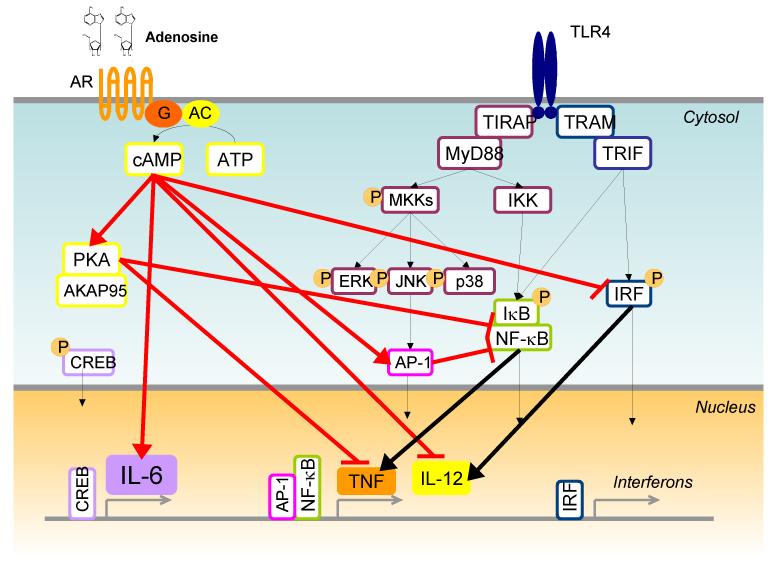
Figure 2. Mechanisms of adenosine-mediated modulation of endotoxin-induced cytokine production: inhibition of TNF but enhancement of IL-6 and IL-10.
Endotoxin-induced activation of the TLR4 receptor complex results in activation of MyD88 and TRIF signaling cascades, leading to production of multiple cytokines, including TNF and interferons. Adenosine interferes with both these cascades. Adenosine binding to the A2A receptor increases intracellular cAMP, which induces expression of PKA. Targeting of PKA to NF-κB, through its scaffold AKAP95, inhibits NF-κB activation, and results in decreased transcription of TNF and increased production of IL-10. In addition, cAMP inhibits phosphorylation of p38 MAPK and activation of IRFs, which are required for production of pro-inflammatory cytokines. Several transcriptional and post-transcriptional mechanisms have been described by which cAMP enhances LPS-induced IL-10. It is not yet clear whether these effects are mediated through PKA or other downstream effectors. Ultimately, adenosine polarizes TLR4-mediated cytokine responses to a Th2-type response characterized by decreased production of pro-inflammatory cytokines such as TNF and IL-12p70 and increased production of IL-6 and IL-10.
The effects of cAMP on TLR-mediated cytokine production can be dependent on cell type. cAMP inhibits LPS-induced IL-12p40 production by murine peritoneal macrophages via a mechanism mediated by p38 but independent of NF-κB [32]. However, in murine dendritic cells (DCs), cAMP inhibition of LPS-induced IL-12p70 is p38- and NF-κB-independent and interferon regulatory factor (IRF)-1- and IRF-8-dependent [39]. IRFs are transcription factors that are up-regulated in response to viral infection and enhance expression of interferons (IFNs). Treatment of DCs with adenylate cyclase toxin (CyaA) or prostaglandin E2 (PGE2) increased cAMP levels and inhibited LPS-induced IRF-1 and -8 expression at the mRNA and protein levels [39]. Thus, inhibition of LPS-induced IRF-1 and -8 by cAMP may decrease IL-12p70 production.
In bone marrow derived-DCs (BMDCs) and murine macrophages, cAMP-mediated suppression of pro-inflammatory cytokine production, including that of TNF, is via c-Fos [40]. c-Fos is a transcription factor that makes up part of AP-1 [40]. Inhibitory kappa B kinase β (IKKβ) phosphorylates and stabilizes c-Fos [40]. The effects of cAMP are cytokine-specific, possibly because c-Fos directly interacts with p65 of NF-κB reducing binding of p65 to the TNF gene promoter region, while cAMP enhances binding of p65 to the IL-6 gene promoter region [40].
The anti-inflammatory effect of cAMP on cytokine production from murine macrophages is mediated, in part, by protein kinase A (PKA) [41]. In RAW 264.7 murine macrophages, inhibition of LPS-induced expression of cytokines by cAMP was reversed by a double knockdown in PKA-Cα and PKA-Cβ [41]. The effects of PKA are controlled by its cellular localization which is controlled by interactions of PKA with A kinase-anchoring proteins (AKAPs) [42]. PGE2-mediated suppression of LPS-induced up-regulation of TNF mRNA was impaired in bone marrow derived macrophages (BMDM) from mice with a mutant AKAP95 that does not bind to PKA, suggesting that interaction between AKAP95 and PKA may mediate cAMP-dependent suppression of LPS-induced TNF. AKAP95 interaction with PKA inhibits LPS-induced NF-κB activation via phosphorylation of p105. LPS induced phosphorylation of p105 at Ser935 in WT BMDMs was attenuated by PGE2, but not in AKAP95 mutant BMDMs. Phosphorylation of p105 at Ser940 by PKA decreases IκB kinase (IKK)-dependent phosphorylation of p105 at Ser935, diminishing NF-κB activation. Therefore, p105 may be important for PKA- and cAMP-dependent suppression of LPS-induced cytokine expression [41].
CD39 and CD73
CD39 is an ectonucleotidase that converts extracellular ATP and ADP to AMP. AMP is then converted by the ectonucleotidase CD73 to adenosine [16]. Early reports examining CD39 expression suggest that CD39 is not expressed on resting blood, T, B, or natural killer (NK) cells, neutrophils or monocytes but was found on activated NK cells, B cells and subsets of T cells [43]. However, recent reports do indicate the presence of CD39 on macrophages and dendritic cells (DCs) [44-47].
CD39 is the dominant ectonucleotidase expressed by murine peritoneal macrophages [48]. Recently, it has been found that cAMP up-regulates CD39 transcription in murine macrophages [44]. cAMP increases CD39 mRNA expression in the murine macrophage cell line RAW 264.7 dependent on PKA, phosphoinositide 3-kinase (PI3K) and ERK [44]. cAMP increased the binding of transcription factors cAMP-response element-binding protein (CREB) and activating transcription factor (ATF) to the CD39 promoter [44]. CD39 regulates early P2X7-activated macrophage cytokine production. Peritoneal macrophages that were CD39-deficient were pre-stimulated for 3 h with a TLR4 agonist (LPS) or a TLR2 agonist (Pam3CSK4) but not TLR5 (flagellin) or TLR3 (poly I:C) and then for 1 h with ATP were found to release more IL-1β than WT cells [48]. Therefore, CD39 on macrophages is important in regulating TLR-mediated cytokine production.
Human monocyte-derived DCs (moDCs) express mRNA for both CD39 and CD73 [45]. Extracellular degradation of ATP and AMP suggested that moDCs express functional CD39 and CD73 at the cell surface [45]. Murine epidermal DCs express CD39 at the mRNA and protein level [46]. Murine bone marrow derived dendritic cells (BMDCs) constitutively express CD39 but do not normally express CD73, even after exposure to TLR agonists, and are therefore unable to convert AMP to adenosine [47]. TGFβ induces CD73 expression in DCs, enabling these cells to generate adenosine generation within an immune regulatory microenvironment [47]. Accordingly, CD73-deficient mice had significantly more joint swelling after Borrelia infection than WT mice indicating that dysregulation in adenosine generation may play a role in persistence of bacterial infection and development of arthritis [49].
Imidazoquinolines
Imidazoquinolines (IMQs) are low molecular weight adenosine congeners with immune response modifying and anti-viral activity that activate TLRs 7 and/or TLR8 [50-52]. In addition to their activation via TLRs, one report suggested that IMQs may also interact with the adenosine system. An IMQ named Imiquimod (Figure 1D) that is approved by the Food and Drug Administration for the topical treatment of human papilloma virus infection (warts), antagonized ARs on human epithelial cells in vitro [53]. The antagonistic binding of imiquimod to the A2AR decreased activation of adenylate cyclase thereby decreasing cAMP levels and thereby enhanced immune responses [53]. Further studies are needed to define the interactions of IMQs with the adenosine system on primary leukocytes.
Adenosine and angiogenesis
There is synergy between TLR and A2AR signaling that switches macrophages from an inflammatory to an angiogenic phenotype, which plays a role in wound healing in vivo [54]. The A2AR agonist, NECA, strongly inhibited TNF production in murine macrophages treated with TLR2, -3, -4, -7, or 9 agonists [55]. A novel signaling pathway was identified in murine macrophages involving synergy between TLRs 2, 4, 7, and 9 and A2ARs, that up-regulates vascular endothelial growth factor (VEGF), acts on endothelial cells to promote neo-vascularization, and down-regulates expression of TNF, thus representing an angiogenic switch that may be important for wound healing and ischemia [55].
A2AR-activation protects against lung ischemia-reperfusion injury by attenuating inflammatory cytokine production [56]. Treatment with the A2AR agonist (ATL313) during reperfusion inhibited the production of TNF, KC (CXCL1), MIP-2 (CXCL2) and RANTES (CCL5), neutrophil recruitment and lung injury [56].
Inflammasome
The inflammasome (INFL) is a multi-protein cytosolic complex that is assembled during cellular activation [8,57-59]. P2X7 receptors bind extracellular ATP and can participate in activation of the INFL and processing of procaspase-1 to active caspase-1, which, in turn, cleaves pro-IL-1β to mature IL-1β [9-11,60]. To our knowledge there are not yet any published reports assessing whether adenosine and/or the ARs modulate cytokine responses mediated by NOD-like receptors (NLRs) that are components of the inflammasome [8,57,58], a topic worthy of future study.
Adenosine system deficiencies
Adenosine deaminase deficiency
Adenosine deaminase is the enzyme that converts adenosine to inosine. Individuals with a defect in adenosine deaminase have severe combined immunodeficiency (SCID) characterized by non-functional T and B lymphocytes. These patients have high levels of plasma adenosine from birth [61], which is thought to play a role in the observed impaired immune responses in these patients. Lymphocytes are sensitive to the accumulation of the ADA substrates adenosine and 2′-deoxyadenosine [62]. High levels of adenosine can induce aberrant adenosine receptor signaling that can affect cellular viability or function [62]. Several review articles address adenosine deaminase deficiency and current treatment [63-65].
CD39-deficiency/polymorphisms
CD39 promotes an anti-inflammatory/pro-resolving response since it is involved in the conversion of ATP to adenosine [16]. Mice that are CD39-deficient have higher expression of pro-inflammatory mediators such as IFNγ, IL-1β and TNF [66]. CD39-deficient mice were highly susceptible to an experimental mouse model of colitis using dextran sodium sulfate (DSS) compared to wild-type controls [67]. In humans, genetic polymorphisms associated with low expression of CD39 are associated with inflammatory bowel disease [67].
P2X7-polymorphisms
Polymorphisms in the P2X7 gene have been linked to an increased risk of tuberculosis [68-70]. A polymorphism in P2X7 of 1513A->C inhibits ATP-induced mycobacterial killing in adult human macrophages [69,70]. Recently, the 1513A->C polymorphism has been associated with extrapulmonary tuberculosis in children [68]. Interestingly, the -762T->C single nucleotide polymorphism (SNP) in the promoter region of P2X7 appears to protect against tuberculosis in the Gambian population [71] but not in other populations [68,72,73].
Translational efforts
Several adenosine analogues, including agonists and antagonists, are currently in clinical use and multiple others are in pre-clinical and clinical evaluation.
Apnea of prematurity
Caffeine is widely used to treat apnea of prematurity, a condition of preterm newborns characterized by cessation of breathing [74,75]. Caffeine is an adenosine congener (Figure 1B) that can antagonize the A1R and A2AR [76] and also acts as a phosphodiesterase inhibitor to increase cytosolic cAMP concentrations [36], that stimulates ventilation [74,75]. A large clinical study indicated that newborns receiving caffeine for apnea of prematurity unexpectedly also had lower rates of bronchopulmonary dysplasia (BPD) [75]. There were over 2000 infants with birth weights between 500 to 1250 g enrolled during the first 10 days of life to receive either caffeine or placebo, until drug therapy was discontinued because apnea of prematurity had resolved. BPD was defined by the need for supplemental oxygen at a postmenstrual age of 36 weeks [75]. Although the mechanisms for caffeine’s beneficial effects in preventing BPD are not yet known, it may reflect the ability of caffeine to block A1R, increase intracellular cAMP and inhibit LPS-induced TNF production in human neonatal (but not adult) monocytes [77].
Adenosine pathway involvement in sepsis
Dysregulation of the adenosine system may contribute to the pathophysiology of sepsis. Adenosine has a diminished capability during sepsis to inhibit the generation of oxygen radicals by neutrophils in an in vitro sepsis model and in neutrophils of sepsis patients [78]. Coupling to adenylate cyclase was impaired and the potency of adenosine to stimulate cAMP accumulation was decreased. Despite an increase in A2AR expression, receptor function declines due to diminished ligand-binding affinity.
Administration of an A2AR agonist (ATL313) i.p. every 6h starting 8 h post infection provided a significant survival benefit in mice infected i.p. with live E. coli or S. aureus [79]. A2AR agonist administration every 6 h starting 30 min after LPS injection was associated with diminished plasma concentrations of TNF, MIP-1α, MCP-1, IFN-γ and IL-17 and increases in IL-10 as well as higher circulating white blood cell concentrations. Thus, AR agonists may be potentially useful in treating sepsis by dampening the inflammatory cascade [79]. Another approach to modulating the adenosine system to improve sepsis outcomes involves use of adenosine deaminase inhibitors such as pentostatin. In a rat model [80], administration of pentostatin was associated with reduced TNF production in serum, liver and spleen and improved survival [80].
A substantial literature documents that pentoxifylline, a phosphodiesterase inhibitor that raises intracellular cAMP concentrations [34,81], can reduce LPS-induced TNF production in vitro and may have some efficacy in treating sepsis in vivo [82,83]. Pentoxifylline in combination with either adenosine or the A2AR agonist CGS21680 further inhibited the generation of H2O2 in human neutrophils [78]. The increase in cAMP by adenosine was potentiated by pentoxifylline in human LPS treated neutrophils [78].
Colitis and cirrhosis
The intestinal epithelial A2BR is an important mediator of pro- and anti-inflammatory responses in murine colitis [84-86]. The A2BR antagonist (ATL-801) reduced colitis in both a DSS model and in IL-10-deficient mice treated with piroxicam (a non-steroidal anti-inflammatory drug) [84]. A2BR-deficient mice demonstrate reduced colonic inflammation compared to controls in models of DSS-, trinitrobenzene sulfonic acid (TNBS)- or Salmonella typhimurium-induced colitis [85]. In contrast, Frick et al. show that colitis in A2BR-deficient mice was more severe than controls in a DSS model. The A2BR inhibitor (PSB1115) given to WT mice increased the severity of DSS-induced colitis [86]. Possible reasons for the differences observed in these studies include differences in the mouse strains studied, details of the colitis model, and potential differences in the colonizing murine gut flora.
Pentoxifylline, a phosphodiesterase inhibitor that inhibits LPS-induced TNF production in vitro and in vivo, reduced complications in patients with advanced cirrhosis [87]. A large, multisite, randomized, double-blind, placebo-controlled study examined the effects of pentoxifylline on survival in patients with advanced cirrhosis and the development of complications. Pentoxifylline did not decrease short-term mortality in patients with advanced cirrhosis, however, there was a decrease in complications [78]. Pentoxifylline treated patients had lower risk of liver-related complications, bacterial infection, developing renal insufficiency and hepatic encephalopathy [78].
Acute lung injury
The A2BR decreases murine endotoxin-induced acute lung injury (ALI) [88]. In a murine LPS inhalation model, inhibition or genetic ablation of the A2BR increased lung inflammation as manifested by higher levels of pro-inflammatory cytokines, myeloperoxidase levels and more severe lung pathology after ALI. Chimeric mice demonstrated that pulmonary A2BR signaling is important for endotoxin-induced ALI. Pretreatment with A2BR agonist (BAY 60-6583) abrogated myeloperoxidase, IL-6 and lung fluid levels in endotoxin-induced ALI [88]. These data suggest that targeting the A2BR may be a beneficial treatment option for preventing or reducing endotoxin-induced ALI.
Conclusion
There have been substantial recent advances in the understanding of how the adenosine system regulates immune responses. Animal models with selective gene deficiencies in adenosine system pathways have demonstrated the relevance of these molecules in vivo. Translational efforts have demonstrated the efficacy of many adenosine modulators in murine models. In human clinical trials caffeine, an adenosine congener, demonstrated reduced bronchopulmonary dysplasia, a condition that reflects lung inflammation.
The adenosine system, comprised of adenosine and its receptors represents an attractive target to prevent and/or mitigate inflammatory diseases. AR antagonists block adenosine-induced immunosupression, which may be beneficial in up-regulating the immune response in the immunocompromised. AR agonists and/or agents that modulate intracellular cAMP levels may also be beneficial for modulating immune responses in the context of infection or sepsis. A major challenge will be to optimally target and manipulate this ubiquitous system, which is expressed by virtually all organ systems, without causing unacceptable side effects.
Five-year view
Within the next five years our understanding of the adenosine system will continue to advance. As agents that modulate the adenosine system are in human trials our understanding on their impact on inflammation will be improved. Study of these agents and their mechanisms of action in vitro and in vivo will enable us to properly combine them with other treatments to effectively treat inflammatory disorders.
Key issues.
Adenosine is a ubiquitous purine metabolite produced by all cells of the body.
Leukocytes express adenosine receptors that modulate cellular function, often via modulation of cellular cAMP concentrations and the function of protein kinase A.
In general, adenosine is an anti-inflammatory/pro-resolving agent.
Adenosine modifies cytokine responses to TLR agonists, inhibiting production of pro-inflammatory/Th1-poalrizing cytokines such as TNF and IL-12p70 while preserving or enhancing production of IL-6 and IL-10.
CD39, an ecto-enzyme that converts ATP to AMP, a key step in adenosine generation, promotes an anti-inflammatory/pro-resolving response.
Genetic polymorphisms of adenosine system genes are associated with risks of infection (eg, P2X7 and mycobacterium tuberculosis) and inflammatory bowel disease (eg, CD39).
Caffeine an adenosine homologue and phosphodiesterase inhibitor is used to treat apnea of prematurity and also reduces bronchopulmonary dysplasia, an inflammatory condition.
Approaches to modulate the adenosine system to treat sepsis include adenosine agonists, as well as agents that modulate adenosine levels and/or intracellular cAMP levels (pentoxifylline).
References
Papers of special note have been highlighted as:
• Of interest
•• Of considerable interest
- 1.Ohta A, Sitkovsky M. The adenosinergic immunomodulatory drugs. Curr Opin Pharmacol. 2009;9(4):501–506. doi: 10.1016/j.coph.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sitkovsky MV, Lukashev D, Apasov S, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annual Review of Immunology. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 3.Martin C, Leone M, Viviand X, Ayem ML, Guieu R. High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Critical Care Medicine. 2000;28(9):3198–3202. doi: 10.1097/00003246-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends in Immunology. 2004;25(1):33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3(1-2):171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112(2):358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Di Virgilio F, Boeynaems JM, Robson SC. Extracellular nucleotides as negative modulators of immunity. Curr Opin Pharmacol. 2009;9(4):507–513. doi: 10.1016/j.coph.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119(12):3502–3511. doi: 10.1172/JCI40599. • Excellent review on inflammasomes
- 9.Chen L, Brosnan CF. Regulation of immune response by P2X7 receptor. Crit Rev Immunol. 2006;26(6):499–513. doi: 10.1615/critrevimmunol.v26.i6.30. [DOI] [PubMed] [Google Scholar]
- 10.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175(11):7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari D, Pizzirani C, Adinolfi E, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176(7):3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 12.Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacological reviews. 2001;53(4):527–552. • Description of the adenosine receptors.
- 13.Sitkovsky MV, Lukashev D, Apasov S, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 14.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–492. [PubMed] [Google Scholar]
- 15.Khoa ND, Montesinos MC, Reiss AB, Delano D, Awadallah N, Cronstein BN. Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monocytic THP-1 cells. Journal of Immunology. 2001;167(7):4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- 16.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7(9):759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trincavelli ML, Daniele S, Martini C. Adenosine receptors: what we know and what we are learning. Curr Top Med Chem. 2010;10(9):860–877. doi: 10.2174/156802610791268756. [DOI] [PubMed] [Google Scholar]
- 18.Kumar V, Sharma A. Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol. 2009;616(1-3):7–15. doi: 10.1016/j.ejphar.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. •• Excellent current review on TLRs.
- 20.Ramakers BP, Riksen NP, Rongen GA, van der Hoeven JG, Smits P, Pickkers P. The effect of adenosine receptor agonists on cytokine release by human mononuclear cells depends on the specific Toll-like receptor subtype used for stimulation. Cytokine. 2006;35(1-2):95–99. doi: 10.1016/j.cyto.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Ritchie PK, Spangelo BL, Krzymowski DK, Rossiter TB, Kurth E, Judd AM. Adenosine increases interleukin 6 release and decreases tumour necrosis factor release from rat adrenal zona glomerulosa cells, ovarian cells, anterior pituitary cells, and peritoneal macrophages. Cytokine. 1997;9(3):187–198. doi: 10.1006/cyto.1996.0153. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi HK, Iwagaki H, Hamano R, et al. Effects of adenosine on adhesion molecule expression and cytokine production in human PBMC depend on the receptor subtype activated. Br J Pharmacol. 2007;150(6):816–822. doi: 10.1038/sj.bjp.0707126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Link AA, Kino T, Worth JA, et al. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. Journal of Immunology. 2000;164(1):436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- 24.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175(6):3463–3468. doi: 10.4049/jimmunol.175.6.3463. • Excellent summary of evdence indicating that IL-6 is a pro-resolution cytokine.
- 25.Nemeth ZH, Lutz CS, Csoka B, et al. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175(12):8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9(4):447–453. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouma MG, Stad RK, van den Wildenberg FA, Buurman WA. Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J Immunol. 1994;153(9):4159–4168. [PubMed] [Google Scholar]
- 28.Hamano R, Takahashi HK, Iwagaki H, et al. Stimulation of adenosine A2A receptor inhibits LPS-induced expression of intercellular adhesion molecule 1 and production of TNF-alpha in human peripheral blood mononuclear cells. Shock. 2008;29(2):154–159. doi: 10.1097/shk.0b013e31812385da. [DOI] [PubMed] [Google Scholar]
- 29.Prabhakar U, Brooks DP, Lipshlitz D, Esser KM. Inhibition of LPS-induced TNF alpha production in human monocytes by adenosine (A2) receptor selective agonists. Int J Immunopharmacol. 1995;17(3):221–224. doi: 10.1016/0192-0561(94)00096-7. [DOI] [PubMed] [Google Scholar]
- 30.Buenestado A, Delyle SG, Arnould I, et al. The role of adenosine receptors in regulating production of tumour necrosis factor-alpha and chemokines by human lung macrophages. Br J Pharmacol. 2010;159(6):1304–1311. doi: 10.1111/j.1476-5381.2009.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317(1):172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 32.Feng WG, Wang YB, Zhang JS, Wang XY, Li CL, Chang ZL. cAMP elevators inhibit LPS-induced IL-12 p40 expression by interfering with phosphorylation of p38 MAPK in murine peritoneal macrophages. Cell Research. 2002;12(5-6):331–337. doi: 10.1038/sj.cr.7290135. [DOI] [PubMed] [Google Scholar]
- 33.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157(10):4634–4640. [PubMed] [Google Scholar]
- 34.Strieter RM, Remick DG, Ward PA, et al. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988;155(3):1230–1236. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 35.Endres S, Fulle HJ, Sinha B, et al. Cyclic nucleotides differentially regulate the synthesis of tumour necrosis factor-alpha and interleukin-1 beta by human mononuclear cells. Immunology. 1991;72(1):56–60. [PMC free article] [PubMed] [Google Scholar]
- 36.Horrigan LA, Kelly JP, Connor TJ. Caffeine suppresses TNF-alpha production via activation of the cyclic AMP/protein kinase A pathway. Int Immunopharmacol. 2004;4(10-11):1409–1417. doi: 10.1016/j.intimp.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Levy O, Coughlin M, Cronstein B, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shames BD, McIntyre RC, Jr., Bensard DD, et al. Suppression of tumor necrosis factor alpha production by cAMP in human monocytes: dissociation with mRNA level and independent of interleukin-10. Journal of Surgical Research. 2001;99(2):187–193. doi: 10.1006/jsre.2001.6178. [DOI] [PubMed] [Google Scholar]
- 39.Hickey FB, Brereton CF, Mills KH. Adenylate cycalse toxin of Bordetella pertussis inhibits TLR-induced IRF-1 and IRF-8 activation and IL-12 production and enhances IL-10 through MAPK activation in dendritic cells. J Leukoc Biol. 2008;84(1):234–243. doi: 10.1189/jlb.0208113. [DOI] [PubMed] [Google Scholar]
- 40.Koga K, Takaesu G, Yoshida R, et al. Cyclic adenosine monophosphate suppresses the transcription of proinflammatory cytokines via the phosphorylated c-Fos protein. Immunity. 2009;30(3):372–383. doi: 10.1016/j.immuni.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Wall EA, Zavzavadjian JR, Chang MS, et al. Suppression of LPS-induced TNF-alpha production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Sci Signal. 2009;2(75):ra28. doi: 10.1126/scisignal.2000202. • Characterization of the role of PKA in cAMP mediated suppression of LPS-induced TNF.
- 42.Jarnaess E, Tasken K. Spatiotemporal control of cAMP signalling processes by anchored signalling complexes. Biochem Soc Trans. 2007;35(Pt 5):931–937. doi: 10.1042/BST0350931. [DOI] [PubMed] [Google Scholar]
- 43.Kansas GS, Wood GS, Tedder TF. Expression, distribution, and biochemistry of human CD39. Role in activation-associated homotypic adhesion of lymphocytes. J Immunol. 1991;146(7):2235–2244. [PubMed] [Google Scholar]
- 44.Liao H, Hyman MC, Baek AE, Fukase K, Pinsky DJ. cAMP/CREB-mediated transcriptional regulation of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) expression. J Biol Chem. 2010;285(19):14791–14805. doi: 10.1074/jbc.M110.116905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berchtold S, Ogilvie AL, Bogdan C, et al. Human monocyte derived dendritic cells express functional P2X and P2Y receptors as well as ecto-nucleotidases. FEBS Lett. 1999;458(3):424–428. doi: 10.1016/s0014-5793(99)01197-7. [DOI] [PubMed] [Google Scholar]
- 46.Mizumoto N, Kumamoto T, Robson SC, et al. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8(4):358–365. doi: 10.1038/nm0402-358. •• Defines an important role for CD39 in immunity.
- 47.Cobbold SP, Adams E, Nolan KF, Regateiro FS, Waldmann H. Connecting the mechanisms of T-cell regulation: dendritic cells as the missing link. Immunol Rev. 2010;236:203–218. doi: 10.1111/j.1600-065X.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- 48.Levesque SA, Kukulski F, Enjyoji K, Robson SC, Sevigny J. NTPDase1 governs P2X7-dependent functions in murine macrophages. Eur J Immunol. 2010;40(5):1473–1485. doi: 10.1002/eji.200939741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yegutkin GG, Hytonen J, Samburski SS, Yrjanainen H, Jalkanen S, Viljanen MK. Disordered lymphoid purine metabolism contributes to the pathogenesis of persistent Borrelia garinii infection in mice. J Immunol. 2010;184(9):5112–5120. doi: 10.4049/jimmunol.0902760. [DOI] [PubMed] [Google Scholar]
- 50.Vasilakos JP, Smith RM, Gibson SJ, et al. Adjuvant activities of immune response modifier R-848: comparison with CpG ODN. Cellular Immunology. 2000;204(1):64–74. doi: 10.1006/cimm.2000.1689. [DOI] [PubMed] [Google Scholar]
- 51.Burns RP, Jr., Ferbel B, Tomai M, Miller R, Gaspari AA. The imidazoquinolines, imiquimod and R-848, induce functional, but not phenotypic, maturation of human epidermal Langerhans’ cells. Clinical Immunology. 2000;94(1):13–23. doi: 10.1006/clim.1999.4804. [DOI] [PubMed] [Google Scholar]
- 52.Miller RL, Meng TC, Tomai MA. The antiviral activity of Toll-like receptor 7 and 7/8 agonists. Drug News Perspect. 2008;21(2):69–87. doi: 10.1358/dnp.2008.21.2.1188193. [DOI] [PubMed] [Google Scholar]
- 53.Schon MP, Schon M, Klotz KN. The Small Antitumoral Immune Response Modifier Imiquimod Interacts with Adenosine Receptor Signaling in a TLR7- and TLR8-Independent Fashion. J Invest Dermatol. 2006;126(6):1338–1347. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 54.Macedo L, Pinhal-Enfield G, Alshits V, Elson G, Cronstein BN, Leibovich SJ. Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. The American journal of pathology. 2007;171(6):1774–1788. doi: 10.2353/ajpath.2007.061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinhal-Enfield G, Ramanathan M, Hasko G, et al. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. American Journal of Pathology. 2003;163(2):711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma AK, Linden J, Kron IL, Laubach VE. Protection from pulmonary ischemia-reperfusion injury by adenosine A2A receptor activation. Respir Res. 2009;10:58. doi: 10.1186/1465-9921-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franchi L, Munoz-Planillo R, Reimer T, Eigenbrod T, Nunez G. Inflammasomes as microbial sensors. Eur J Immunol. 2010;40(3):611–615. doi: 10.1002/eji.200940180. [DOI] [PubMed] [Google Scholar]
- 58.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 59.Abdul-Sater AA, Said-Sadier N, Ojcius DM, Yilmaz O, Kelly KA. Inflammasomes bridge signaling between pathogen identification and the immune response. Drugs Today (Barc) 2009;45(Suppl B):105–112. [PMC free article] [PubMed] [Google Scholar]
- 60.Yilmaz O, Sater AA, Yao L, Koutouzis T, Pettengill M, Ojcius DM. ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis. Cell Microbiol. 2010;12(2):188–198. doi: 10.1111/j.1462-5822.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirschhorn R, Roegner V, Rubinstein A, Papageorgiou P. Plasma deoxyadenosine, adenosine, and erythrocyte deoxyATP are elevated at birth in an adenosine deaminase-deficient child. Journal of Clinical Investigation. 1980;65(3):768–771. doi: 10.1172/JCI109725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blackburn MR, Kellems RE. Adenosine deaminase deficiency: metabolic basis of immune deficiency and pulmonary inflammation. Adv Immunol. 2005;86:1–41. doi: 10.1016/S0065-2776(04)86001-2. [DOI] [PubMed] [Google Scholar]
- 63.Gaspar HB, Aiuti A, Porta F, Candotti F, Hershfield MS, Notarangelo LD. How I treat ADA deficiency. Blood. 2009;114(17):3524–3532. doi: 10.1182/blood-2009-06-189209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sauer AV, Aiuti A. New insights into the pathogenesis of adenosine deaminase-severe combined immunodeficiency and progress in gene therapy. Curr Opin Allergy Clin Immunol. 2009;9(6):496–502. doi: 10.1097/ACI.0b013e3283327da5. • Excellent review on ADA deficiency.
- 65.Kohn DB. Update on gene therapy for immunodeficiencies. Clin Immunol. 2010;135(2):247–254. doi: 10.1016/j.clim.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Enjyoji K, Kotani K, Thukral C, et al. Deletion of cd39/entpd1 results in hepatic insulin resistance. Diabetes. 2008;57(9):2311–2320. doi: 10.2337/db07-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedman DJ, Kunzli BM, YI AR, et al. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009;106(39):16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tekin D, Kayaalti Z, Dalgic N, et al. Polymorphism in the p2x7 gene increases susceptibility to extrapulmonary tuberculosis in Turkish children. Pediatr Infect Dis J. 2010;29(8):779–782. doi: 10.1097/INF.0b013e3181d9932e. [DOI] [PubMed] [Google Scholar]
- 69.Saunders BM, Fernando SL, Sluyter R, Britton WJ, Wiley JS. A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J Immunol. 2003;171(10):5442–5446. doi: 10.4049/jimmunol.171.10.5442. [DOI] [PubMed] [Google Scholar]
- 70.Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Wiley JS, Britton WJ. Gene dosage determines the negative effects of polymorphic alleles of the P2X7 receptor on adenosine triphosphate-mediated killing of mycobacteria by human macrophages. J Infect Dis. 2005;192(1):149–155. doi: 10.1086/430622. [DOI] [PubMed] [Google Scholar]
- 71.Li CM, Campbell SJ, Kumararatne DS, et al. Association of a polymorphism in the P2X7 gene with tuberculosis in a Gambian population. J Infect Dis. 2002;186(10):1458–1462. doi: 10.1086/344351. [DOI] [PubMed] [Google Scholar]
- 72.Nino-Moreno P, Portales-Perez D, Hernandez-Castro B, et al. P2X7 and NRAMP1/SLC11 A1 gene polymorphisms in Mexican mestizo patients with pulmonary tuberculosis. Clin Exp Immunol. 2007;148(3):469–477. doi: 10.1111/j.1365-2249.2007.03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mokrousov I, Sapozhnikova N, Narvskaya O. Mycobacterium tuberculosis co-existence with humans: making an imprint on the macrophage P2X(7) receptor gene? J Med Microbiol. 2008;57(Pt 5):581–584. doi: 10.1099/jmm.0.47455-0. [DOI] [PubMed] [Google Scholar]
- 74.Davis PG, Schmidt B, Roberts RS, et al. Caffeine for Apnea of Prematurity trial: benefits may vary in subgroups. J Pediatr. 2010;156(3):382–387. doi: 10.1016/j.jpeds.2009.09.069. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 76.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51(1):83–133. [PubMed] [Google Scholar]
- 77.Chavez-Valdez R, Wills-Karp M, Ahlawat R, Cristofalo EA, Nathan A, Gauda EB. Caffeine modulates TNF-alpha production by cord blood monocytes: the role of adenosine receptors. Pediatr Res. 2009;65(2):203–208. doi: 10.1203/PDR.0b013e31818d66b1. [DOI] [PubMed] [Google Scholar]
- 78.Kreth S, Kaufmann I, Ledderose C, Luchting B, Thiel M. Reduced ligand affinity leads to an impaired function of the adenosine A2A receptor of human granulocytes in sepsis. J Cell Mol Med. 2009;13(5):985–994. doi: 10.1111/j.1582-4934.2008.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moore CC, Martin EN, Lee GH, Obrig T, Linden J, Scheld WM. An A2A adenosine receptor agonist, ATL313, reduces inflammation and improves survival in murine sepsis models. BMC infectious diseases. 2008;8:141. doi: 10.1186/1471-2334-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adanin S, Yalovetskiy IV, Nardulli BA, Sam AD, 2nd, Jonjev ZS, Law WR. Inhibiting adenosine deaminase modulates the systemic inflammatory response syndrome in endotoxemia and sepsis. Am J Physiol Regul Integr Comp Physiol. 2002;282(5):R1324–1332. doi: 10.1152/ajpregu.00373.2001. [DOI] [PubMed] [Google Scholar]
- 81.Bell SG. Immunomodulation, part I: pentoxifylline. Neonatal Netw. 2005;24(4):45–48. doi: 10.1891/0730-0832.24.4.45. [DOI] [PubMed] [Google Scholar]
- 82.Haque K, Mohan P. Pentoxifylline for neonatal sepsis. Cochrane Database Syst Rev. 2003;(4):CD004205. doi: 10.1002/14651858.CD004205. [DOI] [PubMed] [Google Scholar]
- 83.Tarnow-Mordi W, Isaacs D, Dutta S. Adjunctive immunologic interventions in neonatal sepsis. Clin Perinatol. 2010;37(2):481–499. doi: 10.1016/j.clp.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 84.Kolachala V, Ruble B, Vijay-Kumar M, et al. Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol. 2008;155(1):127–137. doi: 10.1038/bjp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kolachala VL, Vijay-Kumar M, Dalmasso G, et al. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology. 2008;135(3):861–870. doi: 10.1053/j.gastro.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182(8):4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lebrec D, Thabut D, Oberti F, et al. Pentoxifylline does not decrease short-term mortality but does reduce complications in patients with advanced cirrhosis. Gastroenterology. 2010;138(5):1755–1762. doi: 10.1053/j.gastro.2010.01.040. • Pentoxifylline reduces complications associated with cirrhosis.
- 88.Schingnitz U, Hartmann K, Macmanus CF, et al. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 2010;184(9):5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]