Abstract
Venomous snakebites are an important health problem in tropical and subtropical countries. King cobra (Ophiophagus hannah) is the largest venomous snake found in South and Southeast Asia. In this study, the O. hannah venom proteome and the venom components cross-reactive to N. kaouthia monospecific antivenin were studied. O. hannah venom consisted of 14 different protein families, including three finger toxins, phospholipases, cysteine-rich secretory proteins, cobra venom factor, muscarinic toxin, L-amino acid oxidase, hypothetical proteins, low cysteine protein, phosphodiesterase, proteases, vespryn toxin, Kunitz, growth factor activators and others (coagulation factor, endonuclease, 5’-nucleotidase). N. kaouthia antivenin recognized several functionally different O. hannah venom proteins and mediated paratherapeutic efficacy by rescuing the O. hannah envenomed mice from lethality. An engineered human ScFv specific to N. kaouthia long neurotoxin (NkLN-HuScFv) cross-neutralized the O. hannah venom and extricated the O. hannah envenomed mice from death in a dose escalation manner. Homology modeling and molecular docking revealed that NkLN-HuScFv interacted with residues in loops 2 and 3 of the neurotoxins of both snake species, which are important for neuronal acetylcholine receptor binding. The data of this study are useful for snakebite treatment when and where the polyspecific antivenin is not available. Because the supply of horse-derived antivenin is limited and the preparation may cause some adverse effects in recipients, a cocktail of recombinant human ScFvs for various toxic venom components shared by different venomous snakes, exemplified by the in vitro produced NkLN-HuScFv in this study, should contribute to a possible future route for an improved alternative to the antivenins.
Keywords: antivenin, human ScFv (HuScFv), paraspecificity, Naja kaouthia, Ophiophagus hannah, proteome
1. Introduction
Snake envenomation is an important health problem and occupational hazard among outdoor workers, such as farmers, plantation workers and agricultural harvesters, in tropical and subtropical areas, where venomous snakes have a habitat predilection [1,2,3]. It has been estimated that more than 50,000 deaths occur yearly from the snakebites [3]. The majority of cases were from rural areas where access to healthcare facilities is limited and antivenins are usually not available [1,2,3,4,5]. The treatment mainstay of the venomous snakebites in Thailand relies on the horse-derived antivenins produced by Queen Saovabah Memorial Institute (QSMI), Bangkok. The antivenins may be either monospecific for cases when the causative snakes are known or polyspecific, which neutralizes more than one venom species when biting snakes are not identified [5]. The therapeutic efficacy of the latter depends highly on the amounts of the antibodies that could neutralize the heterologous causative venom components (paraspecificity) [6,7]. Therefore, insight into the venom proteomes of individual venomous snake species that inhabit common geographical areas/localities, like O. hannah and N. kaouthia, which produce similar clinical features [7,8] and the identification of common components shared among their venoms should be useful information for treatment indication when homologous and polyspecific antivenins are not available. In this study, the O. hannah venom proteome was characterized, and the components cross-reactive to horse derived-monospecific N. kaouthia antivenin were determined. Paraspecific immunity mediated by the antivenin was evaluated also in mice.
Basically, treatment with the horse-derived antivenins is highly effective. Nevertheless, there have been limitations in the production, supply and use of the remedies. The production of immune sera in large animals requires adequate and appropriate animal husbandry, including pasture for grazing, shelter, an animal care taker and a veterinarian. For immunization, the snake venom or a mixture of venoms of several snake species in the immunological adjuvant is injected into the animal at multiple sites. Several boosters are required over an extended period of time (6–12 months or longer) in order to expect the satisfactory serum antibody levels. The quality of the antivenin is subjected to batch-to-batch/animal-to-animal variation. The amount of immune globulin obtained from individual animals at each bleeding time is limited. As such, in some regions of the world where antivenin is not available, many snake bitten subjects receive only traditional panaceas and/or palliative treatment. Besides, the preparations also contain a large fraction of nonspecific proteins. Thus, the antivenin dosage for the treatment of snakebites has never been certain and can be based only on the degree of envenomation [9]. Approximately 20% of the recipients develop either immediate hypersensitivity, including allergy and anaphylaxis and/or late serum sickness, due to the human anti-animal isotype response [6]. Although the early adverse reactions are readily managed in clinical settings with the use of adrenaline, anti-histamines and steroids, the late anti-animal isotype response is difficult to avoid. With contemporary technology, such as the phage display technique, the production of standardized recombinant antibodies to any required target antigen is possible in vitro without the prolonged animal immunization process and in vivo immune regulations by using the antibody phage display library as a biological tool [10].
Recently, human single-chain variable antibody fragments (HuScFv) specific to N. kaouthia long neurotoxin (NkLN-HuScFv) were produced in vitro [10]. The engineered HuScFv could rescue the N. kaouthia envenomized mice from lethality. Moreover, humanized-camel single-domain antibodies (sdAb) specific to N. kaouthia phospholipase A2 (PLA2) prepared from a humanized-camel VH/VHH (nanobody) phage display library have been shown to neutralize the enzymatic activity of the detrimental enzyme [11]. Therefore, it is envisioned that a cocktail of human/humanized-small antibodies, which are devoid of Fc fragments (thus, not causing an additional inflammatory response) and specific to venomous components should be a possible future road for anti-snake venom design. In this study, the ability of NkLN-HuScFv in rescuing the O. hannah envenomed mice from lethality was determined as an example of recombinant-specific antibodies that can mediate paraspecificity.
2. Materials and Methods
2.1. Animals
Male Institute of Cancer Research (ICR) mice, 5 weeks old, were from The National Laboratory Animal Center, Mahidol University, Nakhonpathom, Thailand. Animal husbandry and manipulation were performed following the guideline of the National Research Council of Thailand. Animal experiments were approved by the Siriraj Animal Care and Use Committee (SiACUC), Faculty of Medicine, Siriraj Hospital, Mahidol University (COA No. 004/2556).
2.2. O. hannah Venom, Horse-Derived N. kaouthia Antivenin and HuScFv-Specific to N. kaouthia Long Neurotoxin (NkLN-HuScFv)
O. hannah holovenom and horse-derived monospecific N. kaouthia antivenin (purified equine F(ab)’2) were obtained in lyophilized form from the Queen Saovabah Memorial Institute (QSMI). The lyophilized antivenin was dissolved in ten mL of ultrapure sterile distilled water (UDW), while the venom was dissolved in one mL of normal saline solution (NSS). Protein concentrations of the preparations were determined by using Bradford’s reagent (Bio-Rad, Hercules, CA, USA).
For preparing NkLN-HuScFv, the gene sequence coding for the HuScFv (huscfv) in phagemid transformed E. coli clone no. P8/22/3 [10] was subcloned into pET23b+, and the recombinant plasmids were put into BL21 (DE3) E. coli. The transformed bacteria were grown under IPTG induction condition, and soluble NkLN-HuScFv was purified from the bacterial lysate by using Ni-NTA affinity resin (Thermoscience, Rockford, IL, USA). The E. coli-derived NkLN-HuScFv has been shown to neutralize N. kaouthia neurotoxin and rescued the N. kaouthia envenomed mice from lethality [10]. By using the phage peptide mimotope search and multiple alignments, the HuScFv was found to bind to amino acids in loop 3 of N. kaouthia long neurotoxin (accession No. 229777), which is the venom binding site to the neuronal acetylcholine receptor (AchR) [10].
2.3. Characterization of O. hannah Venom Proteome by 1DE-ESI-LC-MS/MS
O. hannah venom was denatured by heating at 95 °C for 5 min in sample buffer (60 mM Tris-HCl, pH 6.8, 2% (w/v) SDS, 10% (v/v) glycerol, 1% (v/v) β-mercaptoethanol and bromophenol blue). The sample was subjected to 12% SDS-PAGE and stained with Coomassie Brilliant Blue R-250 (CBB) dye. The SDS-PAGE gel was cut horizontally into 10 equal pieces, destained in 100 μL of 50% (v/v) acetonitrile in ammonium bicarbonate and 100 µL of 4 mM dithiothreitol (DTT), kept at 60 °C for 15 min, alkylated by adding 7 µL of 250 mM iodoacetamide and kept in the dark for 40 min. Excess iodoacetamide was quenched with 3 µL of 4 mM dithiothreitol (DTT). All preparations were dehydrated by using acetonitrile, rehydrated with trypsin solution and incubated at 37 °C overnight. Peptides were extracted from each gel by adding acetonitrile; the supernatant was collected, and the acetonitrile was removed by using speed-vac (Eppendorf, Hamburg, Germany). The samples were subjected to mass spectrometric analysis using ESI-LC-MS/MS (a micrOTOF-Q instrument, Bruker Daltonics, Bremen, Germany). Each peptide preparation was acidified before injecting into an EASY-nLC system (Bruker Daltonics), and the separation was done at a flow rate 300 nL/min. The eluent was sprayed using a capillary voltage of 22 to 28 kV into a nano-electrospray source of the QToF. The cone was at 100 V; the source temperature was 85 °C, and the microchannel plate detector (MCP) was 2300 V. The MS scan mode covered m/z 400–2000. Three most abundant precursors were selected to fragment for 3 s. The MS/MS spectra covered m/z 50–1500. For data analysis, the LC-MS/MS data files were searched against Mascot version 2.4.1 (Matrix Science, London, UK) [12], which contained 37,848,116 sequence entries, respectively, was used. Bony vertebrate was set for the taxonomy filter. Missed cleavage was set to 1 with peptide tolerance set to 200 ppm and tandem MS tolerance set to 0.6 Da. Fixed modification was set to carbamidomethyl on cysteine. Variable modifications were set to include methionine oxidation. Only peptides identified above 95% confidence were reported in this study. Each identified peptide was searched against Basic Local Alignment Search Tool (BLAST) [13] for considering isoforms of proteins [14].
2.4. Determination of O. hannah Venom Components Cross-Reactive with N. kaouthia Antivenin
O. hannah venom was subjected to two-dimensional gel electrophoresis (2DE) [15]. Three aliquots of 75 µg of the O. hannah venom were each dissolved in a DeStreakTM rehydration solution that contained pH 3–10 NL IPG buffer (the final volume was 125 µL), and each solution was added into a strip holder of the Ettan IPG Phor Electrofocusing System (Amersham Biosciences). An IPG strip was placed into each strip holder containing the venom (right side down) and allowed to rehydrate at 20 °C for 12 h. Electrophoresis of the IPG strips was performed at 300 V for 30 min, 1000 V for 30 min and 5000 V for 72 min. For the second dimension, the focused IPG strips were equilibrated in a reduction buffer (50 mM Tris-HCl, pH 8.8, 6 M urea, 30% (v/v) glycerol, 2% (w/v) SDS, 0.002% bromophenol blue and 1% (w/v) DTT] at 25 °C for 15 min and in an alkylation buffer (50 mM Tris-HCl, pH 8.8, 6 M urea, 30% (v/v) glycerol, 2% (w/v) SDS, 0.002% bromophenol blue and 2.5% (w/v) iodoacetamide) at 25 °C for 15 min. The SDS-PAGE was carried out in a 15% gel cast in Mini PROTEAN® 3 Cell (Bio-Rad) at 10 mAmp/gel during the first 15 min and 20 mAmp/gel until the tracking dye reached the lower gel edge. One gel was stained by CBB dye; the separated components of the other two gels were transferred to two nitrocellulose membranes (NC) for 2DE-immunoblotting. One NC blot was probed with horse monospecific N. kaouthia antivenin (1:100), while another blot was probed with normal horse immunoglobulin (equal protein concentration to 1:100 of antivenin). The O. hannah components bound by the horse antibodies were revealed by using goat anti-horse immunoglobulin-alkaline phosphatase (AP) conjugate (Southern Biotech, Birmingham, AL, USA) and 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT) substrate (KPL, Gaithersburg, MD, USA). The venom spots on the CBB stained 2DE-gel relevant to the spots that reacted to the horse antivenin on the first 2DE immunoblot membrane, but that did not react to the normal horse immunoglobulin on the second immunoblot membrane were excised out and digested with trypsin. Peptides were subjected to protein identification by ESI-LC-MS/MS using a micrOTOF-Q instrument (Bruker Daltonics, Bremen, German). Each identified peptide was searched against the O. hannah nucleotide database.
2.5. Median Lethal Dose (LD50) of O. hannah Venom in Mice
The LD50 of O. hannah venom in mice was determined using a previously established method [16,17,18]. Mice (6 mice per group) were injected with varying amounts of O. hannah venom in 200 µL of sterile NSS intraperitoneally (i.p.). Alternatively, mice were injected intramuscularly (i.m.) with O. hannah venom in 30 µL of sterile NSS in order to simulate the most common route of the snakebites. Control mice received NSS only. The mortality of the mice in all groups was observed, and the experiments were terminated at 48 h post-injection. The LD50 of the i.p. and the i.m. injected venom were calculated from two reproducible experiments [19].
2.6. Cross-Neutralization of O. hannah Venom by N. kaouthia Antivenin
The cross-species neutralization of the N. kaouthia antivenin was performed as described previously [17,18]. Seven groups of 5 mice each (Groups 1–7) were prepared. O. hannah venom (1.5 LD50) was mixed with the N. kaouthia antivenin in 1:5, 1:10, 1:20 or 1:40 (w/w) and kept at 37 °C for 30 min. Individual mice of Groups 1–4 (test groups) were injected i.p. with the venom-antivenin mixtures (500 μL) [16,17,18]. Each mouse of Group 5 received normal horse serum (an equal protein concentration to Group 4) in 500 μL NSS (background neutralization control). Mice of Group 6 received 1.5 LD50 O. hannah venom in 500 μL NSS (non-neutralization control). Mice of Group 7 received N. kaouthia antivenin only (an equal protein concentration to Group 4 in 500 μL NSS) and served as non-envenomed control. The numbers of dead and alive mice at 48 h post-injection were recorded, and the antivenin effective dose (ED50) was calculated [19].
Alternatively, six groups of 5 mice each (Groups 1–6) were injected i.m. individually with 1.5 LD50 of O. hannah venom in 30 µL of NSS (the simulated common route of snakebites). Mice of Group 7 received 30 µL of NSS i.m. Ten minutes later, N. kaouthia antivenin (w/w of venom:antibody 1:5, 1:10, 1:20 and 1:40) in 60 µL NSS was injected intravenously (i.v.) (the simulated antivenin treatment route of snakebites in human) into the envenomed mice of Groups 1–4, respectively. Each mouse of Group 5 received normal horse serum (equal protein concentration to the mice of Group 4). Mice of Groups 6 and 7 received 60 µL NSS i.v. alone and the NSS containing N. kaouthia antivenin (amount of antivenin equal to Group 4), respectively. The numbers of dead and alive mice at 48 h post-venom injection were recorded, and the effective dose (ED50) was calculated [19].
2.7. Cross-Neutralization of O. hannah Venom by NkLN-HuScFv
Eight mouse groups (5 mice per group) were prepared (Groups 1–8). O. hannah venom (1.5 LD50) was mixed with NkLN-HuScFv (1:10 or 1:50 w/w in 500 µL of NSS). O. hannah venom (1.5 LD50) was also mixed with irrelevant HuScFv (HuScFv specific to the NS1 protein of influenza A virus), at amounts equal to 1:10 or 1:50 venom:HuScFv). The mixtures were kept at 37 °C for 30 min. Individual mice of Groups 1 and 3 (test groups) were injected i.p. with venom-NkLN-HuScFv mixtures at 1:10 and 1:50, respectively; mice of Groups 2 and 4 received O. hannah venom mixed with influenza virus NS1-specific HuScFv at 1:10 and 1:50, respectively. Mice of Groups 5 and 6 received O. hannah venom in buffer (non-neutralization control) and NkLN-HuScFv alone, respectively. Mice of Groups 7 and 8 received 1.5 LD50 of O. hannah venom mixed with NkLN-HuScFv and irrelevant HuScFv (venom:antibody 1:50) i.p., respectively; ten minutes later, they were given another i.p. dose of NkLN-HuScFv and the irrelevant HuScFv (an antibody amount equal to Groups 3 and 4, respectively). The numbers of dead and alive mice at 48 h post-injection were recorded and the percent of survival of the mice was calculated.
2.8. O. hannah Venom Components Cross-Reactive to NkLN-HuScFv
O. hannah venom was subjected to 2DE, as described above. The separated components were electroblotted onto two pieces of nitrocellulose membranes (NC) The blotted membranes were kept in a Tris-buffered saline solution containing 3% bovine serum albumin (BSA) and 0.2% gelatin (TBST) at 25 °C for 1 h. After blocking, one blotted membrane was probed with the histidine tagged-NkLN-HuScFv and another was reacted with control histidine-tagged HuScFv (specific to influenza virus matrix protein-1) at 25 °C for 1 h and at 4 °C overnight. Separated O. hannah components bound by the HuScFvs were revealed by using the mouse anti-histidine tag antibody, goat anti-mouse immunoglobulin-AP conjugate and BCIP/NBT substrate, respectively. The venom protein spots on the 2DE-gel stained with CBB dye relevant to the spots that appeared on the 2DE-immunoblot membrane probed with NkLN-HuScFv, but that were absent on the membrane probed with the irrelevant HuScFv were excised out and digested with trypsin. Peptides were subjected to protein identification by ESI mass spectrometry using the Ultimate 3000 nano HPLC system (Dionex) coupled to a 4000 Q TRAP mass spectrometer (Applied Biosystems). Tryptic peptides were loaded onto a C18 PepMap100, 3 µm (LC Packings) and separated with a linear gradient of water/acetonitrile/0.1% formic acid (v/v). Spectra were analyzed to identify the proteins of interest using Mascot sequence matching software (Matrix Science) with the Ludwig non-redundant (NR) database.
2.9. Computerized Procedure for Determining the Interactions between NkLN-HuScFv and Long Neurotoxins of N. kaouthia and O. hannah
Interactions between NkLN-HuScFv with N. kaouthia and O. hannah long neurotoxins were elucidated by using computerized simulation. The amino acid sequences of the venom proteins were obtained from the UniProt Protein Knowledgebase [20]. The venom protein sequences were used for searching experimental three-dimensional (3D) structures of the venom components by means of the Protein Model Portal [21] from the Research Collaboratory for Structural Bioinformatics (RCSB) protein data bank [22]. Only the 3D structure of N. kaouthia long neurotoxin was available. Thus, the amino acid sequence of the O. hannah long neurotoxin, as well as the NkLN-HuScFv sequence were subjected to homology modeling [23,24] by the Iterative Threading Assemble Refinement (I-TASSER) server service [25]. The predicted models derived from I-TASSER were subsequently refined by using two server services, i.e., the high-resolution protein structure refinement, ModRefiner [26,27], and the fragment-guided molecular dynamics (FG-MD) simulation [28,29]. The local geometric and physical qualities of the predicted 3D structures were improved to make them come closer to their native state. The refined models were docked according to a fast Fourier transform (FFT)-based program, i.e., PIPER. The antibody-venom component dockings were performed by using the antibody mode available on the automated ClusPro 2.0 protein-protein docking server [30,31,32,33,34]. The largest cluster size, which indicated a region of local minimum energy and a near-native state protein conformation, was chosen for each docking. The protein structure models and the molecular interactions were built and visualized by using PyMOL software (The PyMOL Molecular Graphics System, Version 1.3r1 edu, Schrodinger, LLC, NY, USA).
3. Results
3.1. O. hannah Venom Proteome
The proteins of the O. hannah venom ranged in masses from <10 to 130 kDa (Figure 1). The trypsin digested peptides of proteins in each gel piece of the Figure 1 were subjected to ESI-LC-MS/MS analysis and database search. The O. hannah venom peptides matched with the peptides of the proteins of the database are shown in Table 1. They could be classified into 14 different protein types/families (Table A1), i.e., three finger toxin, phospholipase, cysteine-rich secretory protein (CRiSP), cobra venom factor/complement C3, muscarinic toxin, L-amino acid oxidase, hypothetical protein, low cysteine protein, phosphodiesterase, protease, Vespryn toxin, Kunitz, growth factor activator and others (coagulation factor, endonuclease, 5’-nucleotidase).
Figure 1.

SDS-PAGE separated pattern of O. hannah holovenom. M, protein molecular weight standard. Lane 1, SDS-PAGE separated components of the venom stained with Coomassie Brilliant Blue R-250 (CBB) dye. Numbers on the right are the gel pieces containing the O. hannah proteins that were subjected to protein identification by LC-MS/MS. Numbers at the left are protein molecular masses (kDa).
Table 1.
LC-MS/MS Mascot results of in-gel tryptic digestions of O. hannah venom searching against the NCBInr database.
| Gel piece No. | Protein No. | Accession No. | Protein | Protein score | Mass | m/z | z | Peptide score | Sequence |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | gi|391359389 | Zinc | 911 | 69,003 | 650.38 | 2 | 43.19 | NNLLHFSIWR |
| metalloproteinase- | 799.97 | 2 | 64.70 | TNNVIIPCKPTDVK | |||||
| disintegrin-like | 821.39 | 2 | 102.49 | LYCTGGTENPSEGEK | |||||
| ohanin | 861.43 | 2 | 96.53 | ASYSEIEDIGMVDHR | |||||
| 957.01 | 2 | 136.20 | AMCDVLQSVGIVQDYSK | ||||||
| 1,214.54 | 2 | 126.32 | NECDLPEFCIGQSAECPMDR | ||||||
| 810.39 | 3 | 78.10 | LYCTGGTENPSEGEKISSDPCK | ||||||
| 1,220.67 | 2 | 122.89 | NDNAQLLTGVDLNGYTLGSAYLK | ||||||
| 1,222.53 | 2 | 83.89 | NECDLPEFCIGQSAECPMDR | ||||||
| 860.11 | 3 | 74.59 | SPYLVGAAMAHEIGHNLGMEHDTK | ||||||
| 947.44 | 3 | 65.64 | NECDLPEFCIGQSAECPMDRFHK | ||||||
| 1 | 2 | gi|347595788 | L-amino-acid oxidase | 537 | 55,941 | 690.87 | 2 | 62.68 | QTDENAWYLIK |
| 749.50 | 2 | 69.76 | KIVIVGAGISGLTAAK | ||||||
| 762.92 | 2 | 66.38 | EAGHEVVILEASDR | ||||||
| 776.45 | 2 | 64.94 | IILVCTDKFWEK | ||||||
| 1,056.01 | 2 | 117.64 | MSANNPENFGYQLNPNER | ||||||
| 780.07 | 3 | 63.43 | IYFAGEYTAHPHGWIETSMK | ||||||
| 805.42 | 3 | 91.79 | SASQLFDETLDKVTDDCTLQK | ||||||
| 1 | 3 | gi|22654267 | Acidic phospholipase | 510 | 16,432 | 792.41 | 2 | 101.70 | LPACSSIMDSPYVK |
| A2 | 906.40 | 2 | 86.21 | CCQVHDNCYTQAQK | |||||
| 915.89 | 2 | 94.20 | ADNDECAAFICNCDR | ||||||
| 794.71 | 3 | 129.81 | YADYGCYCGAGGSGTPVDKLDR | ||||||
| 872.10 | 3 | 97.84 | VAAHCFAASPYNNNNYNIDTTTR | ||||||
| 1 | 4 | gi|126035663 | Complement- | 235 | 184,237 | 955.61 | 2 | 124.43 | LILNTPLDTQSLLITVR |
| depleting factor | 999.02 | 2 | 68.09 | TDTEEQILVEAHGDNTPK | |||||
| (O. hannah) | 1,065.65 | 2 | 42.31 | AVPFVIVPLQQGLHDIEVR | |||||
| 1 | 5 | gi|82193162 | Long neurotoxin OH-37 | 183 | 9846 | 670.36 | 2 | 91.89 | KLSFGCAATCPK |
| 1,164.04 | 2 | 91.17 | VNPGIDIECCSTDNCNPHPK | ||||||
| 1 | 6 | gi|338855302 | Phosphodiesterase 1 | 160 | 96,311 | 771.08 | 3 | 95.44 | NPAWWGGQPIWHTATYQGLK |
| 1,216.13 | 2 | 65.03 | NEVTSFENIEVYNLMCDLLK | ||||||
| 1 | 7 | gi|82193155 | Short neurotoxin OH-35 | 126 | 9558 | 1,239.13 | 2 | 126.42 | QYTIFGVTPEICADGQNLCYK |
| 1 | 8 | gi|565306229 | Insulin-like growth factor I (O. hannah) | 115 | 12,329 | 936.45 | 2 | 115.04 | GIVEECCFQSCDLVR |
| 1 | 9 | gi|565293365 | Hypothetical protein L345_17517, partial (O. hannah) | 65 | 25,866 | 777.87 | 2 | 64.55 | GIDSSHWNSYCTK |
| 1 | 10 | gi|391359387 | Zinc metalloproteinase-disintegrin-like mikarin | 63 | 18,426 | 796.44 | 2 | 62.61 | TNTPEQDRYLQVK |
| 2 | 11 | gi|544604740 | Ophiophagus venom | 534 | 183,812 | 606.86 | 2 | 43.11 | AVYVLNDKYK |
| factor | 8,867.00 | 2 | 48.71 | YVLPSFEVHLQPSEK | |||||
| 905.04 | 2 | 58.82 | IKLEGDPGAQVGLVAVDK | ||||||
| 955.61 | 2 | 118.78 | LILNTPLDTQSLLITVR | ||||||
| 999.02 | 2 | 104.68 | TDTEEQILVEAHGDNTPK | ||||||
| 1,010.07 | 2 | 94.74 | YLYGEEVEGVAFVLFGVK | ||||||
| 700.05 | 3 | 67.01 | VPVVSEAIHSEGTTLSDGTAK | ||||||
| 810.39 | 3 | 43.35 | LYCTGGTENPSEGEKISSDPCK | ||||||
| 749.51 | 2 | 96.89 | KVVIIGAGISGLTAAK | ||||||
| 3 | 12 | gi|565315338 | 5~-nucleotidase, partial (O. hannah) |
53 | 28,027 | 958.05 | 2 | 53.17 | VLLPSFLAAGGDGYYMLK |
| 4 | 13 | gi|387935404 | Alpha- and beta- | 510 | 28,637 | 573.30 | 2 | 95.85 | GDSGGPLICNR |
| fibrinogenase OHS1 | 812.40 | 2 | 105.35 | IIGGFECNEYEHR | |||||
| 818.40 | 2 | 77.40 | DSCKGDSGGPLICNR | ||||||
| 881.50 | 2 | 102.54 | VMGWGLLTSPEVTFPK | ||||||
| 1,325.22 | 2 | 128.74 | EIQGIVSWGGFPCAQLLEPGVY TK | ||||||
| 4 | 14 | gi|565314693 | Hypothetical protein | 266 | 24,222 | 573.30 | 2 | 95.85 | GDSGGPLICNR |
| L345_07470, partial | 818.40 | 2 | 77.40 | DSCKGDSGGPLICNR | |||||
| (O. hannah) | 1,317.72 | 2 | 92.89 | QIQGVVSWGGFPCAQLLEPGVYT K | |||||
| 4 | 15 | gi|565308117 | hypothetical protein | 206 | 115,185 | 650.38 | 2 | 49.92 | NNLLHFSIWR |
| L345_12124 | 818.40 | 2 | 77.40 | DSCKGDSGGPLICNR | |||||
| (O. hannah) | 871.39 | 2 | 82.22 | NGHSCQNNQGYCFR | |||||
| 957.00 | 2 | 74.28 | AMCDVLQSVGIVQDYSK | ||||||
| 4 | 16 | gi|54035743 | Cobra venom factor | 64 | 184,401 | 841.91 | 2 | 63.74 | VYSYYNLDEKCTK |
| 5 | 17 | gi|565307033 | Hepatocyte growth | 174 | 10,782 | 981.57 | 2 | 104.44 | YSNVVQEALIPIIPDYK |
| factor activator, | 1,161.59 | 2 | 69.52 | FIQPICLPEASMSFPDYYK | |||||
| partial (O. hannah) | 937.51 | 2 | 69.87 | GILDENQWESGLFLPR | |||||
| 5 | 18 | gi|118151738 | Metalloproteinase precursor (Demansia vestigiata) | 62 | 68,267 | 649.30 | 2 | 61.92 | SAECPTDSFQR |
| 5 | 19 | gi|18000318 | Cysteine-rich venom protein (Hydrophis hardwickii) | 58 | 20,109 | 888.95 | 2 | 57.69 | YLYVCQYCPAGNIR |
| 5 | 20 | gi|182705250 | Zinc metalloproteinase/ disintegrin | 53 | 71,170 | 957.00 | 2 | 53.19 | GMCDPKLSVGLVQDYSK |
| 6 | 22 | gi|565292399 | Endonuclease domain- | 234 | 18,064 | 848.51 | 2 | 75.99 | SSTFTLTNIVPQFIK |
| containing 1 protein | 1,055.56 | 2 | 66.13 | GCQQTFAVVGAVPGDTYIAR | |||||
| (O. hannah) | 1,162.10 | 2 | 94.19 | ALQDSQAVLEDYKNLADCNR | |||||
| 888.95 | 2 | 74.76 | YLYVCQYCPAGNIR | ||||||
| 6 | 23 | gi|1584763 | Phospholipase A2 | 137 | 13,447 | 915.89 | 2 | 58.19 | ADNDECAAFICNCDR |
| 794.71 | 3 | 79.24 | YADYGCYCGAGGSGTPVDKLDR | ||||||
| 6 | 24 | gi|565304281 | Endonuclease domain-containing 1 protein, partial (O. hannah) | 126 | 51,035 | 628.36 | 2 | 49.98 | FATLYDKQNR |
| 6 | 26 | gi|565303552 | Phospholipase B-like 1, partial (O. hannah) | 71 | 58,242 | 869.47 | 2 | 73.13 | DLHYATVYWLEAEK |
| 7 | 27 | gi|225547744 | Opharin precursor | 317 | 26,288 | 640.78 | 2 | 43.67 | CPASCFCHNK |
| (O. hannah) | 788.39 | 2 | 78.40 | YKDDFSNCQSLAK | |||||
| 888.95 | 2 | 77.95 | YLYVCQYCPAGNIR | ||||||
| 1,092.02 | 2 | 68.72 | FSCGENLFMSSQPYAWSR | ||||||
| 1,212.51 | 2 | 48.66 | SGPPCGDCPSACDNGLCTNPCK | ||||||
| 8 | 29 | gi|124020977 | PLA2 Hs-1 precursor (Hoplocephalus stephensii) | 61 | 915.88 | 2 | 60.84 | ADNDECKAFICNCDR | |
| 9 | 30 | gi|48428841 | Cysteine-rich venom protein | 123 | 26,229 | 705.39 | 2 | 56.89 | VIQSWYDENKK |
| natrin-2 | 925.46 | 2 | 66.57 | NMLQMEWNSNAAQNAK | |||||
| 9 | 31 | gi|82193154 | Cardiotoxin CTX15 | 104 | 9,346 | 545.84 | 2 | 60.05 | LPSKYDVIR |
| 666.40 | 2 | 43.75 | CLNTPLPLIYK | ||||||
| 738.43 | 2 | 42.00 | FLEQQNQVLQTK | ||||||
| 9 | 32 | gi|32363235 | Thai cobrin | 80 | 12,087 | 757.41 | 3 | 80.32 | ADVTFDSNTAFESLVVSPDKK |
| 9 | 33 | gi|26006829 | Acidic phospholipase | 61 | 15,890 | 847.13 | 3 | 61.15 | VAAICFAGAPYNKENINIDTTTR |
| A2-2 | 792.41 | 2 | 105.12 | LPACSSIMDSPYVK | |||||
| 906.41 | 2 | 77.19 | CCQVHDNCYTQAQK | ||||||
| 915.89 | 2 | 94.44 | ADNDECAAFICNCDR | ||||||
| 794.71 | 3 | 147.85 | YADYGCYCGAGGSGTPVDKLDR | ||||||
| 1,210.54 | 2 | 92.09 | TVTCKADNDECAAFICNCDR | ||||||
| 872.10 | 3 | 101.16 | VAAHCFAASPYNNNNYNIDTTTR | ||||||
| 925.45 | 3 | 109.09 | VAAHCFAASPYNNNNYNIDTTTRC | ||||||
| 10 | 34 | gi|82199673 | Cytotoxin-27 | 251 | 9,305 | 799.38 | 2 | 67.56 | SSADVEVLCCDTNK |
| (ß Cytotoxin) | 1,102.61 | 2 | 115.40 | CLNTPLPLIYTTCPIGQDK | |||||
| 1,138.12 | 2 | 68.32 | KCLNTPLPLIYTTCPIGQDK | ||||||
| 10 | 35 | gi|82175774 | Short neurotoxin | 247 | 8,886 | 560.25 | 2 | 47.13 | YSVCCSTDK |
| OH-46 | 761.34 | 2 | 62.70 | YSVCCSTDKCNK | |||||
| 929.47 | 3 | 137.57 | TTMFFPNHPVLLMGCTYNCPTER | ||||||
| 10 | 36 | gi|123916245 | Cardiotoxin CTX21 | 242 | 9,249 | 978.48 | 2 | 58.44 | SSADVVVVCCDTNKCNK |
| 1,102.61 | 2 | 115.4 | CLNTPLPLIYTTCPIGQDK | ||||||
| 1,138.12 | 2 | 68.32 | KCLNTPLPLIYTTCPIGQDK | ||||||
| 10 | 37 | gi|116242842 | Ohanin | 177 | 21,161 | 749.40 | 2 | 88.66 | FSSSPCVLGSPGFR |
| 1,135.61 | 2 | 88.46 | ADVTFDSNTAFESLVVSPDKK | ||||||
| 1,239.13 | 2 | 140.55 | QYTIFGVTPEICADGQNLCYK | ||||||
| 666.40 | 2 | 59.72 | CLNTPLPLIYK | ||||||
| 799.38 | 2 | 67.56 | SSADVEVLCCDTNK | ||||||
| 1,022.51 | 2 | 64.29 | VSETIEICPDGQNFCFK | ||||||
| 10 | 40 | gi|239977312 | Protease inhibitor TCI | 105 | 8,976 | 998.00 | 2 | 104.67 | AYIPSFYYNPDASACQK |
| 10 | 41 | gi|123913366 | Weak neurotoxin WNTX-34 | 98 | 9,808 | 826.35 | 2 | 97.90 | EIVQCCSTDECNH |
| 10 | 42 | gi|82193157 | Long neurotoxin OH-17 | 88 | 8,032 | 893.90 | 2 | 88.18 | CCSTDNCNPFTPWK |
| 10 | 43 | gi|82193161 | Long neurotoxin OH-55 | 82 | 10,203 | 1,013.59 | 2 | 81.91 | VNLGCAATCPIVKPGVEIK |
| 10 | 44 | gi|128951 | Long neurotoxin-4 | 75 | 8,009 | 941.47 | 2 | 75.32 | IISEACPPGQDLCYMK |
| 10 | 45 | gi|83305935 | Neurotoxin-like protein 1 | 74 | 7,037 | 625.83 | 2 | 74.01 | KDEVIQCCAK |
| 10 | 46 | gi|182705233 | Weak toxin DE-1 | 74 | 9,297 | 650.87 | 2 | 73.67 | VNPPISIICCK |
| 10 | 47 | gi|239938646 | Venom chymotrypsin inhibitor | 52 | 9,130 | 830.42 | 2 | 52.00 | FCELPPEPGLCNAR |
| 10 | 48 | gi|123907650 | Cardiotoxin CTX-23 | 146 | 9,290 | 459.74 | 2 | 33.85 | TCPIGQDK |
| 666.40 | 2 | 58.07 | CLNTPLPLIYK | ||||||
| 978.48 | 2 | 54.36 | SSADVVVVCCDTNKCNK | ||||||
| 10 | 49 | gi|123913365 | Muscarinic toxin-38 | 48 | 9,678 | 756.38 | 2 | 48.13 | TCPDGQNLCYKR |
| 10 | 50 | gi|128944 | Long neurotoxin-2 | 70 | 893.90 | 2 | 69.94 | CCSTDNCNPFPTWK |
3.2. O. hannah Venom Components Cross-Reactive to N. kaouthia Antivenin
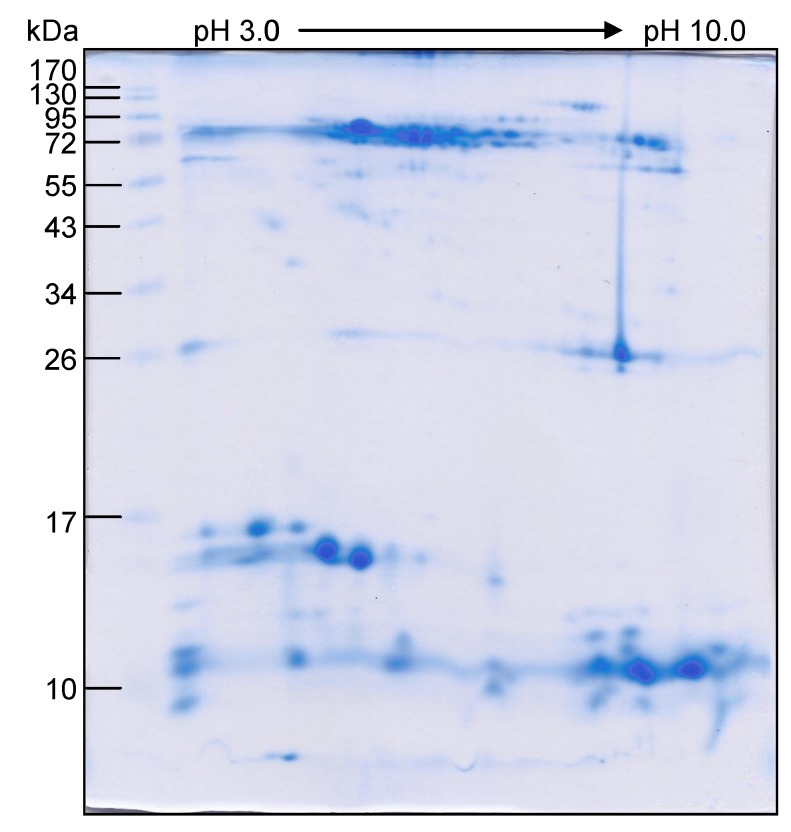
The majority of the venom proteins after 2DE and CBB staining were located in two areas: the ~10–17 and ~26–130 kDa regions (Figure 2). Ten protein spots reacted to the N. kaouthia antivenin, but did not react to the normal horse immunoglobulin (Figure 3). The O. hannah venom peptides generated from the protein spots that matched with the protein sequences of the database are shown in Table 2. They could be classified into six functionally different proteins (Table A2) including: cobra venom factor/complement C3, i.e., O. hannah venom factor precursor protein (Spots 1 and 3); cardiotoxin-like protein (three finger toxin) (Spot 2); cysteine-rich secretory protein (CRiSP), ophanin/opharin/opharin precursor (Spots 5 and 6); weak neurotoxin precursor (three finger toxin) (Spots 7 and 10); long chain neurotoxin (three finger toxin) (Spots 8–10); short chain α-neurotoxin (three finger toxin) (Spots 9 and 10). No protein in the database matched the peptides of spot 4.
Figure 2.
Pattern of O. hannah holovenom proteins after two-dimensional gel electrophoresis (2DE) and CBB staining. Numbers at the left are protein masses in kDa.
Figure 3.
Patterns of O. hannah venom after 2DE at non-linear pH 3.0–10.0 and probed with (a) horse-derived N. kaouthia antivenin and (b) normal horse serum. Encircled spots and numbers are O. hannah proteins that reacted with the antivenin and did not react with the normal horse serum.
Table 2.
O. hannah venom components cross-reactive with horse-derived N. kaouthia antivenin.
| Gel piece No. | Accession No. | Protein | Protein score | Mass | m/z | z | Peptide score | Sequence |
|---|---|---|---|---|---|---|---|---|
| 1 | gi|544604740 | O. hannah venom factor | 54 | 183,812 | 428.26 | 2 | 29.96 | VVPEGVQK |
| 710.74 | 3 | 26.31 | AVPFVIVPLQQGLHDIEVR | |||||
| 2 | gi|38049474 | Cardiotoxin-like protein | 187 | 9,818 | 481.73 | 2 | 38.14 | GCIDICPK |
| (O. hannah) | 9,818 | 545.82 | 3 | 27.06 | LPSKYDVIR | |||
| 9,818 | 799.36 | 2 | 65.14 | SSADVEVLCCDTNK | ||||
| 9,818 | 735.39 | 3 | 64.75 | CLNTPLPLIYTTCPIGQDK | ||||
| 3 | gi|387165368 | OVF precursor protein (O. hannah) |
52 | 185,408 | 482.26 | 3 | 27.04 | HFEVGFIQPGSVK |
| 52 | 185,408 | 710.74 | 3 | 25.54 | AVPFVIVPLQQGLHDIEVR | |||
| 4 | No matched protein in the database | |||||||
| 5 | gi|28972961 | Ophanin (O. hannah) | 82 | 27,764 | 567.79 | 2 | 29.55 | SVSPTASNMLK |
| 27,764 | 640.78 | 2 | 54.73 | QSSCQDEWIK | ||||
| 6 | gi|225547744 | Opharin precursor | 46 | 27,200 | 418.73 | 2 | 26.72 | GSIATPYK |
| (O. hannah) | 27,200 | 525.91 | 2 | 20.06 | YKDDFSNCQSLAK | |||
| 7 | gi|82570105 | Weak neurotoxin precursor | 53 | 10,492 | 484.23 | 2 | 28.00 | NGENVCFK |
| (O. hannah) | 10,492 | 551.21 | 3 | 25.30 | EIVQCCSTDECNH | |||
| 8 | gi|82570073 | Long chain neurotoxin precursor (O. hannah) | 25 | 11,155 | 644.28 | 2 | 24.64 | TWCDVFCGSR |
| 9 | gi|82570073 | Long chain neurotoxin | 60 | 11,155 | 644.20 | 2 | 27.10 | TWCDVFCGSR |
| precursor (O. hannah) | 956.40 | 2 | 32.50 | IISETCPPGQDLCYMK | ||||
| gi|51105381 | Long chain neurotoxin | 123 | 8,602 | 603.20 | 2 | 49.40 | IDLGCAATCPK | |
| precursor (O. hannah) | 659.20 | 2 | 50.12 | SWCDVFCTSR | ||||
| 837.30 | 2 | 23.54 | SETCPDGENICYTK | |||||
| gi|51105369 | Long chain neurotoxin | 60 | 10,477 | 606.20 | 2 | 27.95 | LSFGCAATCPK | |
| precursor (O. hannah) | 642.70 | 2 | 32.33 | SWCDAWCGSR | ||||
| 9 | gi|51105375 | Long chain neurotoxin | 38 | 10,538 | 595.70 | 2 | 37.91 | VNLGCAATCPK |
| precursor (O. hannah) | ||||||||
| gi|82570079 | Long chain neurotoxin | 74 | 10,944 | 491.20 | 2 | 41.32 | CYVTPDVK | |
| precursor (O. hannah) | 837.30 | 2 | 32.65 | SETCPDGENICYTK | ||||
| gi|128944 | Long neurotoxin-2 | 72 | 8,602 | 603.20 | 2 | 43.12 | IDLGCAATCPK | |
| (Neurotoxin B) | 937.00 | 3 | 28.64 | TKCYVTPDATSQTCPDGQDICYTK | ||||
| gi|51105373 | Long chain neurotoxin | 77 | 10,830 | 491.20 | 2 | 31.89 | CYVTPDVK | |
| precursor (O. hannah) | 676.00 | 3 | 45.21 | VNLGCAATCPIVKPGVEIK | ||||
| gi|51105391 | Short chain alpha neurotoxin precursor (O. hannah) | 36 | 10,071 | 826.30 | 3 | 35.67 | QYTIFGVTPEICADGQNLCYK | |
| 10 | gi|51105397 | Short chain alpha | 114 | 9,859 | 481.70 | 2 | 29.87 | GCIDICPK |
| neurotoxin precursor | 666.30 | 2 | 36.34 | CLNTPLPLIYK | ||||
| (O. hannah) | 667.20 | 3 | 47.91 | SSADVEVLCCDTNKCNK | ||||
| gi|82570105 | Weak neurotoxin | 46 | 10,492 | 826.20 | 2 | 46.23 | EIVQCCSTDECNH | |
| precursor (O. hannah) | 799.30 | 2 | 41.31 | SSADVEVLCCDTNK | ||||
| 667.20 | 3 | 27.16 | SSADVEVLCCDTNKCNK | |||||
| gi|82570079 | Long chain neurotoxin | 146 | 10,944 | 491.20 | 2 | 38.11 | CYVTPDVK | |
| precursor (O. hannah) | 837.30 | 2 | 36.70 | SETCPDGENICYTK | ||||
| 596.20 | 3 | 27.89 | CCSTDNCNPFTPWK | |||||
| 648.20 | 3 | 43.67 | CCSTDNCNPFTPWKR | |||||
| gi|128944 | Long neurotoxin 2 | 167 | 8,602 | 603.20 | 2 | 27.85 | IDLGCAATCPK | |
| (Neurotoxin B) | 596.20 | 3 | 33.17 | CCSTDNCNPFPTWK | ||||
3.3. Median Lethal Dose (LD50) of O. hannah Venom and the Venom Cross-Neutralization by the Monospecific N. kaouthia Antivenin
The LD50 of the O. hannah venom injected i.p. and i.m. into mice were 1.1 and 0.59 µg/g of body weight, respectively. All mice that received 1.5 LD50 of the O. hannah venom either i.p. or i.m. died within 48 h post-injection. Envenomed mice that received only normal horse serum were all dead within 48 h post-injection also, while mice that received N. kaouthia antivenin alone survived with no detectable adverse effect. The ED50 of the N. kaouthia antivenin mixed with 1.5 LD50 O. hannah venom and injected i.p. to mice was 590 µg per mouse (19.6 µg/g body weight). When used to treat the mice that received 1.5 LD50 of O. hannah venom i.m., the i.v. antivenin ED50 was 404 μg in 60 µL of NSS per mouse or 13.5 μg/g body weight.
3.4. Cross-Neutralization of O. hannah Venom by HuScFv Specific to N. kaouthia Neurotoxin
Mice injected with a mixture of O. hannah venom (1.5 LD50) and NkLN-HuScFv at 1:10 and 1:50 (w/w) (Groups 1 and 2, respectively) had significantly longer death times (111 ± 16.5 and 155 ± 58.7 min, respectively) compared with the mice injected with the venom alone (78 ± 7.3 min) (p = 0.00008 and 0.0004, respectively) (Table 3). The means ± SD of the death times of NkLN-HuScFv treated mice at the venom:HuScFv ratios of 1:10 and 1:50 were also longer than the irrelevant HuScFv neutralization controls (p = 0.01 and 0.04, respectively). Eighty percent of mice that were injected with O. hannah venom (1.5 LD50) mixed with NkLN-HuScFv (venom:HuScFv ratio 1:50 w/w) i.p. and 10 min later received another therapeutic dose of NkLN-HuScFv survived, while all mice that received 1.5 LD50 of the O. hannah venom alone or mixed with irrelevant HuScFv i.p. and followed 10 min later by NSS and the irrelevant HuScFv i.p., respectively, died within 48 h post-injection (Table 3).
Table 3.
Efficacy of HuScFv specific to N. kaouthia long neurotoxin (NkLN-HuScFv) in the cross-neutralization of O. hannah venom.
| Group of mice | Treatment (injected i.p. with) | Ratio of venom : HuScFv (w/w) | Mean ± SD of mouse dead time ( min) | % Survival |
|---|---|---|---|---|
| 1 | Venom-NkLN-HuScFv mixture | 1:10 | 111 ± 16.5 a | 0 |
| 2 | Venom-irrelevant HuScFv mixture | 1:10 | 88 ± 7.2 b | 0 |
| 3 | Venom-NkLN-HuScFv mixture | 1:50 | 155 ± 58.7 a | 0 |
| 4 | Venom-irrelevant HuScFv mixture | 1:50 | 93 ± 6.8 b | 0 |
| 5 | Venom in buffer | NA | 78 ± 7.3 b | 0 |
| 6 | NKLN-HuScFv alone | NA | All mice survived at the end of experiments | 100 |
| 7 | Venom-NkLN-HuScFv mixture and followed 10 min later with NkLN-HuScFv alone | 1:50 | 220 a | 80 |
| 8 | Venom-irrelevant HuScFv mixture and followed 10 min later with irrelevant HuScFv alone | 1:50 | 95 ± 24.4 b | 0 |
Notes: NA, not available. Entries with different superscripts (a versus b) were different significantly at p < 0.05 (t-test). Irrelevant HuScFv were specific to the influenza A virus M1 protein.
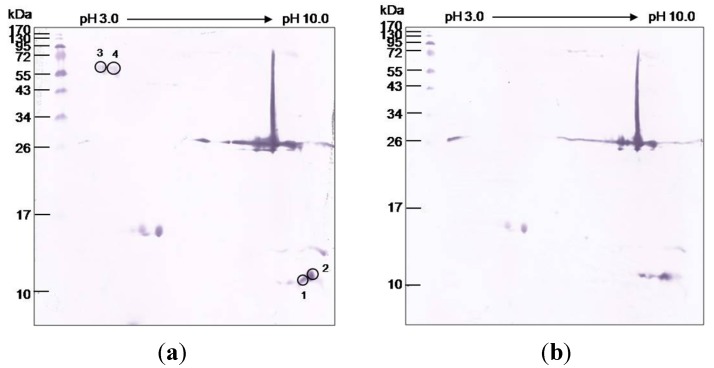
3.5. O. hannah Venom Components Cross-Reactive to NkLN-HuScFv
There were four protein spots (1–4) of the 2DE-O. hannah venom that were bound by the NkLN-HuScFv (Figure 4a), but that were not bound by the irrelevant HuScFv (Figure 4b). The proteins in the spots 1 and 2 were identified as three finger toxins, i.e., short and long chain neurotoxins, while no protein in the database matched with the proteins of the spots 3 and 4.
Figure 4.
O. hannah venom proteins after 2DE and probed with (a) NkLN-HuScFv and (b) irrelevant HuScFv (control). Encircled spots are the O. hannah proteins that reacted with NkLN-HuScFv on the 2DE immunoblot membrane, but did not react with the irrelevant HuScFv (specific to the M1 protein of influenza A virus). Proteins in spots 1 and 2 were identified as O. hannah three finger toxins, i.e., short and long neurotoxins. No proteins in the database matched with the proteins of spots 3 and 4.
3.6. Computerized Models of Interactions between N. kaouthia and O. hannah Long Neurotoxins and NkLN-HuScFv
The crystal structure of N. kaouthia long neurotoxin (CBTX) was found in the database (PDB ID: 1CTX), while the 3D structures of the O. hannah long neurotoxin (OH-LNTX28) and NkLN-HuScFv had to be modeled by the I-TASSER service. The modeled scores of the OH-LNTX28 and NkLN-HuScFv were 1.16 and 1.14, while the expected template-modeling (TM) scores were both 0.87 ± 0.07 and the expected similarity (RMSD) scores were 1.2 ± 1.2 and 3.5 ± 2.4 Å, respectively, which indicated the appropriateness of the modeled structures. The details of the interactions between N. kaouthia long neurotoxin (CBTX) and O. hannah long neurotoxin (OH-LNTX28) with NkLN-HuScFv are shown in Table 4 and Figure 5. The alignments of the amino acid sequences of N. kaouthia and O. hannah neurotoxins and the residues of both toxins recognized by NkLN-HuScFv are shown in Figure 5a. The largest clusters obtained from ClusPro 2.0 were 274 and 215 members, respectively. The lowest energies of the representative models of the molecular dockings were −262.9 and −281.2 kcal/mol, respectively. According to the docking outputs, NKLN-HuScFv used the amino acids D55, Y52, Q104, D55/D57, E74 and N28/S31 in the VH domain to interact with six amino acids of the CBTX, including W25, C26, S31 and R36 in loop 2 and K49 and T50 in loop 3 of N. kaouthia long neurotoxin, respectively (Table 4 and Figure 5b,d). The antibody used the VH residues, including Y52, D57, Q104, D57, D54/D55, N28 and S31 of CDRs1-3, to dock on O. hannah mature long neurotoxin (Q2VBP4) residues C26, W29, G31, R33 and K36 in loop 2 and R47 and N49 in loop 3 of the O. hannah long neurotoxin, respectively (Table 4 and Figure 5c,e).
Table 4.
Residues of N. kaouthia and O. hannah long neurotoxins that interacted with NkLN-HuScFv.
| Neurotoxin | NkLN-HuScFv | |
|---|---|---|
| Residue(s) | Domain/Subdomain | |
| CBTX (P01391) | ||
| W25 | D55 | VH/CDR2 |
| C26 | Y52 | VH/CDR2 |
| S31 | Q104 | VH/CDR3 |
| R36 | D55, D57 | VH /CDR2 |
| K49 | E74 | VH/FR3 |
| T50 | N28, S31 | VH/CDR1 |
| OH-LNTX (Q2VBP4) | ||
| C26 | Y52 | VH/CDR2 |
| W29 | D57 | VH/CDR2 |
| G31 | Q104 | VH/CDR3 |
| R33 | D57 | VH/CDR2 |
| K36 | D54, D55 | VH/CDR2 |
| R47 | N28 | VH/CDR1 |
| N49 | S31 | VH/CDR1 |
Figure 5.
(a) Alignments of amino acid sequences of N. kaouthia and O. hannah neurotoxins. The residues in the epitopes recognized by NkLN-HuScFv are contained in the red boxes. (b,c) Illustrations of the computerized molecular dockings between NkLN-HuScFv (green ribbon) with the long neurotoxins (gray ribbons) of N. kaouthia and O. hannah, respectively. (d,e) Space-filling illustrations of the computerized models of the neurotoxins of N. kaouthia and O. hannah, respectively. NkLN-HuScFv interactive amino acids of the toxins are shown in red ball-like structures. NkLN-HuScFv interacted with amino acids W25, C26, S31 and R36 in loop 2 and K49 and T50 in loop 3 of the N. kaouthia long neurotoxin and with the C26, W29, G31, R33 and K36 in loop 2 and R47 and N49 in loop 3 of the O. hannah long neurotoxin.
4. Discussion
In this study, 1DE gel-based proteomic analysis was used to reveal the O. hannah venom proteome. The venom proteins ranged in sizes from <10 to 170 kDa, which conformed to the previous data [35]. The O. hannah venom showed two major protein bands at 10–17 and 72 kDa, which, more or less, similar to the Malaysian O. hannah venom that had predominant bands at 15–20 and 100 kDa [36]. The differences observed in different studies should be due to the different geographical areas of the cobras, as well as the technical details [37].
O. hannah venom components have been studied previously [38,39,40]. By using 2DE and MALDI-ToF mass spectrometry [38], 12 venom proteins were reported, i.e., weak toxin DE-1, cysteine-rich secretory protein, metallothionein-3, metallothionein, hypothetical proteins, putative DNA invertase, ribosomal protein S17P, small heat stress protein class CIII, XYLDLEGF operon transcriptional activator-II, Phe-Met-Arg-Phe amide neuropeptide precursor, cytochrome c-553 precursor and protein similar to the microtubule-associated RP/EB family. Among these proteins, only the weak toxin, DE-1 and cysteine-rich secretory protein were toxic. Seventeen different protein families in the venom gland of Indonesian king cobra (Ophiophagus hannah) were predicted by using a draft genome and deep transcriptome sequencing [39], i.e., three finger toxin, phospholipase-A2 (PLA2), acetylcholine esterase, metalloproteinases, cysteine-rich secretory protein, cobra venom factor (CVF), hyaluronidase (HYA), Kunitz (serine protease inhibitor), lectin, nerve growth factor (NGF), natriuretic peptide (NP), peptidase, phospholipase-B (PLB), vascular endothelial growth factor (VEGF), ohanin, waprin (protease inhibitor) and various other proteins. Recently, pooled samples of king cobra venom from Indonesia, Malaysia, Thailand and two provinces of China were found to contain eight novel PLA2s. Three finger toxins, i.e., OH-55 (long neurotoxin) and OH-27 (beta cardiotoxin), and the Kunitz inhibitor (OH-TCI) were common in all of the five O. hannah venoms. Southeast Asian O. hannah venoms contained higher metalloproteinase, acetylcholine esterase and alkaline phosphatase than the Chinese venoms [41]. In this study, O. hannah venom proteome were revealed by 1 DE and ESI-LC-MS/MS and found that peptides of the venom matched with 14 different protein types/families of the database. Among them, long and short neurotoxins, weak neurotoxin, muscarinic toxin, phospholipase-A2 cardiotoxins, phospholipase-B, zinc metalloproteinases, ohanin, serine protease inhibitor, cobra venom factor (CVF) and cysteine-rich secretory protein (ophanin) proteins were found, which verified the transcriptomic data reported previously [39].
The majority of the venom proteins located at ~10–17 and ~26–130 kDa after 2DE and CBB staining, which reproduced the 1DE data and was relatively similar to the 2DE pattern of O. hannah venom reported previously [36,38]. N. kaouthia antivenin reacted with ten O. hannah proteins, all of which were toxic. N. kaouthia and O. hannah were both elapid snakes and their venoms shared common components [38,41]. The two snake species also have the same geographical and diet predilection [42]. Therefore, the in vivo cross-neutralization of monospecific N. kaouthia antivenin against the O. hannah venom could be expected.
Cross-neutralization of horse-derived monospecific N. kaouthia antivenin of QSMI to O. hannah venom was as expected. The effective dose fifty (ED50) of the N. kaouthia antivenin when mixed with the O. hannah venom (1.5 LD50) and injected i.p. into the mice was 19.6 µg/g body weight, while the ED50 of the antivenin when used to treat the envenomized mice that received the 1.5 LD50 of O. hannah venom i.m. followed by the antivenin intravenously was 13.5 μg/g body weight. Leong et al. [43] reported that the ED50 of the QSMI horse-derived N. kaouthia antivenom when incubated at 37 °C for 30 min with 2.5 LD50 of O. hannah venom and injecting the mixtures into the caudal veins of mice was 2.80–3.35 (average 3.07) mg/mL. Because the amount of the antivenin injected into individual mice in the study [43] was not mentioned and also due to the different LD50 used, the ED50 of the antivenins in the previous and the present studies could not be compared.
The gene sequence coding for HuScFv of clone 8/22/3 [10] that neutralized N. kaouthia neurotoxin and that rescued 100% of the N. kaouthia envenomized mice from lethality was subcloned from pCANTAB5E phagemid vector into pET23b+ plasmid vector in order to improve the HuScFv expression yield. The mice that received intraperitoneally the mixtures of O. hannah venom and NkLN-HuScFv at 1:10 and 1:50 showed significantly longer means ± SD of death times than the non-NkLN-HuScFv controls (p < 0.05). Nevertheless, when another therapeutic dose of the NKLN-HuScFv was given to the mice that received the mixture of 1:50 venom:NkLN-HuScFv, 80% of the mice survived at the end of the experiments, indicating that the specific HuScFv could rescue the mice from O. hannah envenomation in a dose-dependent manner. By the 2DE immunoblotting, NkLN-HuScFv bound to the short and long chain neurotoxins of the O. hannah venom. Thus, the NkLN-HuScFv should mediate the paraspecific protection of the O. hannah envenomed mice by neutralizing these lethal venom components.
The 2DE proteomics and the 2DE western blot analysis used for revealing the components of the O. hannah venom and the components recognized by the antibody, respectively, have some limitations. The 2DE method may not recognize components present in minute amounts in the holovenom [41]. The 2DE immunoblotting provided a qualitative assessment and did not provide the degree of immunoreactivity (avidity/affinity) of each antigen-antibody interaction. Besides, the antibodies could recognize only the denatured venom components in the assay and, thus, could not reveal the binding of conformational epitopes present in the native proteins.
According to the molecular docking results, NkLN-HuScFv used all three CDRs, as well as FR3 of the VH to dock on six residues of N. kaouthia neurotoxin, while the VL domain was refractory. Four neurotoxin residues, i.e., W25, C26, S31 and R 36, are located in the neurotoxin protruded long central loop (loop 2) and the other two, i.e., K49 and T50, are located in loop 3. Both loops are important for high affinity binding of the neurotoxin to the α7 subunit of the neuronal acetylcholine receptor. The results of the 3D structure docking, more or less, conformed to the previous finding, which indicated by means of a phage peptide mimotope search and multiple alignments with the neurotoxin linear sequence that NkLN-HuScFv bound to the 47TVKT50 in loop 3 of the N. kaouthia neurotoxin [10]. Multiple alignments of the N. kaouthia neurotoxin amino acid sequence with neurotoxin sequences of other snakes indicated that the tentative epitopic peptide of the NkLN-HuScFv existed also in the neurotoxins of heterologous snake species, for example, 47TVKP50 and 47KVKP50 of O. hannah neurotoxins (accession No. AAB25587 and P01389, respectively (previous study) and 47RVNP50 (accession No. Q2VBP4 (this study)), 47TVKP50 of Micrurus nigrocinctus (accession No. P80548) and N. melanoleuca (accession No. P01383 and P01388) and 47KVKP50 of N. oxiana (accession No. P01427), N. nivea (accession No. P01390) and Dendroaspis polylepis polylepis (accession No. P01416) [10]. NkLN-HuScFv used all three CDRs of the VH to bind to seven amino acids, i.e., C26, W29, G31, R33 and R36, in loop 2 and R47 and N49 in loop 3 of O. hannah long neurotoxin, which are important also for the acetylcholine receptor binding. Taken altogether, the results indicate that NkLN-HuScFv mediated the neurotoxin neutralization by interfering with the toxin binding to the acetylcholine receptors and, consequently, rescuing the envenomed mice from death.
The HuScFv could be prepared in vitro without animal immunization, and the molecule is a human protein; thus, it should be appropriate and safe for human use. The small antibodies have high solubility, reproducible refolding capacity and thermal stability, which ease the production process. It is envisaged also that a cocktail of the in vitro produced human ScFvs to various toxic venom components shared by different venomous snakes, exemplified by the recombinant NkLN-HuScFv in this study, should contribute to a possible future design of an improved alternative anti-snakebite remedy.
5. Conclusions
By using one-dimensional gel-based proteomics, the O. hannah holovenom was found to consist of 14 different protein types/families. N. kaouthia antivenin produced by the Queen Saovabah Memorial Institute (QSMI), Bangkok, cross-reacted to several functionally different O. hannah venom proteins and could rescue the envenomed mice from lethality. The phage display-derived recombinant N. kaouthia long neurotoxin-specific HuScFv cross-neutralized the O. hannah short and long neurotoxins and was able to extricate the O. hannah envenomed mice from death in a dose escalation manner. The data are useful for snakebite treatment when and where the polyspecific antivenin is not available.
Acknowledgments
The work was financially supported by the DPG5380001 grant of the Thailand Research Fund (TRF) and the National University Research (NRU) project, Office of Commission on Higher Education, Ministry of Education, Thailand.
Appendixes
Table A1.
The orthologous proteins containing peptides matched with O. hannah venom peptides.
| Protein type/family | Protein subtype | Protein name(s) | Function(s) |
|---|---|---|---|
| 1. Three-finger toxin | Long neurotoxin | OH-2, OH-4, OH-17, OH-37,OH-55 and LNTX37 | These three-finger toxins bind to muscular and neuronal nicotinic acetylcholine receptors (α-7-9) and block neuromuscular transmission at the postsynaptic site causing paralysis [44,45]. |
| Short neurotoxin | OH-35, | This neurotoxin binds to muscarinic acetylcholine receptor and blocks synaptic nerve transmission [46]. | |
| OH-46 | This three-finger toxin binds and inhibits the nicotinic acetylcholine receptor [47]. | ||
| Cardiotoxin/Cytotoxin | CTX-15 (OH-84), CTX-21 and CTX-23 | These three-finger toxins are cytotoxic and hemolytic [48]. | |
| CTX-27 (ß Cardiotoxin) | Acts as a beta-blocker by binding to beta-1 and beta-2 adrenergic receptors. It dose-dependently decreases the heart rate. At 100 mg/kg, intraperitoneal injection into mice mediate labored breathing, impaired locomotion, lack of response to external stimuli, and death (after 30 min) [49]. | ||
| Weak neurotoxin | WNTX-34 | This three-finger toxin binds to muscular and neuronal (α-7) nicotinic acetylcholine receptors with low affinity and very low affinity, respectively [46]. | |
| Neurotoxin-like | Neurotoxin-like protein 1 | Unknown | |
| Weak toxin | OH-DE-1 | Binds to the muscular nicotinic acetylcholine receptor causes paralysis by blocking neuromuscular transmission at the postsynaptic site [38,50]. | |
| 2. Phospholipase | OH-PLA2, OH-acidic -1 PLA2 and OH-acidic-2 PLA2 PLA2 Hs-1 precursor |
These PLAs recognize and hydrolyze the sn-2 acyl bonds of phospholipids releasing arachidonic acid and lysophospholipids. They have neurotoxicity, anti-coagulating activity, cardiotoxicity, myonecrotic/myotoxic activity, anti-platelet activity and edema-inducing activity [35,51]. | |
| Phospholipase B-like 1, partial | Unknown | ||
| 3. Cysteine-rich secretory protein (CRiSP) | O. hannah opharin precursor | Binds to voltage-gated calcium channels on smooth muscle and causes high potassium-induced depolarization which weakly blocks smooth muscle contraction [52]. | |
| Cysteine-rich venom protein-2 (Natrin-2) |
This protein acts as an inflammatory modulator that interferes with wound-healing process of the bitten victim by regulating adhesion molecule expression on endothelial cells [53] and also blocks muscle contraction evoked by potassium [54]. | ||
| Cysteine-rich venom protein | Blocks contraction of smooth muscle elicited by high potassium-induced depolarization and target voltage-gated calcium channels (Cav) on smooth muscle [55]. | ||
| 4. Cobra venom factor/ complement C3 | O. hannah complement-depleting factor | Anti-complement activity [56]. | |
| 5. Muscarinic toxin, type A | O. hannah muscarinic toxin-38 | This toxin binds to muscarinic acetylcholine receptor and blocks synaptic nerve transmission [57]. | |
| 6. L- amino acid oxidase | L amino acid oxidase | These enzymes catalyse oxidative deamination of L-amino acid to form α-keto acid, NH4 and H2O2. The enzymatic activity on platelet aggregation is controversial: inhibit platelet aggregation induced by ADP and the thromboxane analog U46619 versus induce platelet aggregation through the hydrogen peroxide formation. The H2O2 also causes edema and human cell apoptosis [58,59,60] | |
| 7. Hypothetical protein | Hypothetical protein L345_17517 Hypothetical protein L345_07470 Hypothetical protein L345_12124 Hypothetical protein L345_15308 |
Unknown function | |
| 8. Low cysteine protein | Thai cobrin | Thai cobrin and ohanin (protein type/family 13, Vespryn) are classified in the same low cysteine protein family [61]. | |
| 9. Phosphodiesterase-1 | Phosphodiesterase-1 | Phosphodiesterase catalyzes the hydrolysis of phosphodiester bonds of double-stranded and single-stranded DNA, rRNA, and tRNAs resulting in 5' mononucleotide formation and subsequent release of free adenosines which are multitoxic [62]. | |
| 10. Proteases | Metalloproteinase | Protobothrops jerdonii disintegrin | It inhibits ADP-induced human platelet aggregation. It binds the receptor GPIIb/GPIIIa on the platelet surface resulting in hemorrhage [63]. |
| Zinc metalloproteinase-disintegrin-like ohanin | Snake venom zinc metalloproteinase that has hemorrhagic activity. Inhibits ADP-, TMVA- and stejnulxin-induced platelet aggregation in a dose-dependent manner (on washed platelet, but not on platelet rich plasm). Also specifically degrades alpha-chain of fibrinogen (FGA) [64] | ||
| Zinc metalloproteinase-disintegrin-like mikarin | Snake venom zinc metalloproteinase that calcium-independently catalyzes the conversion of prothrombin (F2) to alpha-thrombin through the formation of a thrombin intermediate.[65] | ||
| Metalloproteinase-precursor | unknown | ||
| Kallikrein enzyme | OH fibrinogenases -α and -ß | These O. hannah venom serine proteases mediate fibrinogenolysis on both alpha and beta-chains of fibrin and amidolytic activity [66]. | |
| 11. Vespryn toxin | Ohanin | Ohanin is a low cysteine protein. This neurotoxin causes dose-dependent hypolocomotion and hyperalgesia in mice. It may directly act on the central nervous system [61]. | |
| 12. Kunitz | Protease inhibitor |
O. hannah serine protease inhibitor; Protease inhibitor TCI Venom chymotrypsin inhibitor |
Strongly inhibit trypsin and chymotrypsin [67]. |
| 13. Growth factor activator | Hepatocyte growth factor activator, partial Insulin-like growth factor I |
Unknown function | |
| 14. Others | Coagulation factor XII, partial Endonuclease domain-containing 1 5’ nucleotidase, partial (OH) |
Unknown function |
Table A2.
O. hannah venom components cross-reactive with horse derived N. kaouthia antivenin.
| Spot No(s). | Protein type/family | Protein name |
|---|---|---|
| 1 and 3 | Cobra venom factor/complement C3 | OVF precursor |
| 2 | Three finger toxin | Cardiotoxin-like protein |
| 5 and 6 | Cysteine rich secretory protein (CRiSP) | Ophanin/opharin and opharin precursor |
| 7 and 10 | Three finger toxin which has ancestral ten cysteine arrangements; potent in birds/reptiles [61] | Weak neurotoxin precursor |
| 8, 9 and 10 | Three finger toxin | Long chain neurotoxins |
| 9 and 10 | Three finger toxin | short chain alpha neurotoxin |
| 4 | No match | No match |
Authors Contributions
Wanpen Chaicumpa conceived the project, designed the experiments and served as the corresponding author. Witchuda Danpaiboon did most experiments; Onrapak Reamtong performed LC-MS/MS and protein identification; Yuwaporn Sakolvaree performed 2DE and 2DE immunoblotting; Watee Seesuay performed protein homology modeling and molecular docking; Witchuda Danpaiboon and Fonthip Dong-din-on performed neutralization tests; Wanpen Chaicumpa, Nitat Sookrung, Jeeraphong Thanongsaksrikul, Kanyarat Thueng-in and Potjanee Srimanote supervised Witchuda Danpaiboon; Wanpen Chaicumpa, Witchuda Danpaiboon, Onrapak Reamtong and Watee Seesuay prepared the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization Neglected tropical diseases; snakebites 2011. [(accessed on 23 February 2013)]. Available online: http://www.who.int/neglected_diseases/diseases/snakebites/en/
- 2.Harrison R.A., Hargreaves A., Wagstaff S.C., Faragher B., Lalloo D.G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 2009;3 doi: 10.1371/journal.pntd.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasturiratne A., Wickremasinghe A.R., de Silva N., Gunawardena N.K., Pathmeswaran A., Premaratna R., Savioli L., Lalloo D.G., de Silva J. The global burden of snakebites: A literature analysis and modeling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chippaux J.P. The development and use of immunotherapy in Africa. Toxicon. 1998;36:1503–1506. doi: 10.1016/S0041-0101(98)00140-8. [DOI] [PubMed] [Google Scholar]
- 5.Rojnuckarin P., Suteparak S., Sibunruang S. Diagnosis and management of venomous snakebites in Southeast Asia. Asian Biomed. 2012;6:795–805. [Google Scholar]
- 6.Warrell D.A. WHO/SEARO guidelines for the clinical management of snakebites in the southeast Asian region. Southeast Asian J. Trop. Med. Public Health. 1999;30:1–85. [PubMed] [Google Scholar]
- 7.Theakston R.D., Warrell D.A., Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41:541–557. doi: 10.1016/S0041-0101(02)00393-8. [DOI] [PubMed] [Google Scholar]
- 8.Vejayan J., Tang S.M., Halijah I. The role of conventional two-dimensional electrophoresis (2DE) and its newer applications in the study of snake venoms. In: Heazelwood J.L., Petzold C.J., editors. Biochemistry, Genetics and Molecular Biology, “Proteomic Applications in Biology”. InTech; Kuala Lumpur, Malaysia: 2012. pp. 1–29. ISBN 978-953-307-613-3. [Google Scholar]
- 9.Ahmed S.M., Ahmed M., Nadeem A., Mahajan J., Choudhary A., Pal J. Emergency treatment of a snake bite: Pearls from literature. J. Emerg. Trauma Shock. 2008;1:97–105. doi: 10.4103/0974-2700.43190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkeaw K., Sakolvaree Y., Srimanote P., Tongtawe P., Maneewatch S., Sookrung N., Tungtrongchitr A., Tapchaisri P., Kurazono H., Chaicumpa W. Human monoclonal ScFv neutralize lethal Thai cobra, Naja kaouthia, neurotoxin. J. Proteomics. 2009;72:270–282. doi: 10.1016/j.jprot.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Chavanayarn C., Thanongsaksrikul J., Thueng-in K., Bangphoomi K., Sookrung N., Chaicumpa W. Humanized-single domain antibodies (VH/VHH) that bound specifically to Naja kaouthia phospholipase A2 and neutralized the enzymatic activity. Toxins. 2012;4:554–567. doi: 10.3390/toxins4070554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The National Center for Biotechnology Information (NCBI) Database. [(accessed on 16 March 2014)]; Available online: www. ncbi.nlm.nih.gov/
- 13.NCBI/BLAST Home Page. Standard Protein BLAST Tool. [(accessed on 26 March 2014)]; Available online: http://www.ncbi.nlm.nih.gov/BLAST.
- 14.Vonk F.J., Casewell N.R., Henkel C.V., Heimberg A.M., Jansen H.J., McCleary R.J.R., Kerkkamp H.M.E., Vos R.A., Guerreiro I., Calvete J.J., et al. The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. Proc. Natl. Acad. Sci. USA. 2013;110:20651–20656. doi: 10.1073/pnas.1314702110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakolvaree Y., Maneewatch S., Jiemsap S., Klaysing B., Tongtawe P., Srimanote P., Saengjaruk P., Banyen S., Tapchaisri P., Chongsa-nguan M., et al. Proteome and immunome of pathogenic Leptospira spp. revealed by 2DE and 2DE-immunoblotting with immune serum. Asian Pac. J. Allergy Immunol. 2008;25:53–73. [PubMed] [Google Scholar]
- 16.Meier J., Theakston R.D. Approximate LD50 determinations of snake venoms using eight to ten experimental animals. Toxicon. 1986;24:395–401. doi: 10.1016/0041-0101(86)90199-6. [DOI] [PubMed] [Google Scholar]
- 17.Wisniewski M.S., Hill R.E., Havey J.M., Bogdan G.M., Dart R.C. Australiantigersnake (Notechis scutatus) and mexican coral snake (Micruris species) antivenoms prevent death from United States coral snake (Micrurus fulvius fulvius) venom in a mouse model. J. Toxicol. Clin. Toxicol. 2003;41:7–10. doi: 10.1081/CLT-120018264. [DOI] [PubMed] [Google Scholar]
- 18.Richardson W.H., 3rd, Tanen D.A., Tong T.C., Betten D.P., Carstairs S.D., Williams S.R., Cantrell F.L., Clark R.F. Crotalidae polyvalent immune Fab (ovine) antivenom is effective in the neutralization of South American Viperidae venoms in a murine model. Ann. Emerg. Med. 2005;45:598–602. doi: 10.1016/j.annemergmed.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 19.Reed L.J., Muench H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
- 20. UniPort Home Page Core Data: UniProtKB. [(accessed on 26 March 2014)]. Available online: http://www.uniprot.org.
- 21.Protein Model Portal Home Page. [(accessed on 26 March 2014)]. Available online: http://www.proteinmodelportal.org.
- 22.Research Collaboratory for Structural Bioinformatics Protein Data Bank Home Page. [(accessed on 26 March 2014)]. Available online: http://www.rcsb.org.
- 23.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008;9 doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy A., Kucukural A., Zhang Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protocols. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Zhang’s Research Group Home Page Online Service: I-TASSER. [(accessed on 26 March 2014)]. Available online: http://zhanglab.ccmb.med.umich.edu/I-TASSER/
- 26.Yang Zhang’s Research Group Home Page Online Service: ModRefiner. [(accessed on 26 March 2014)]. Available online: http://zhanglab.ccmb.med.umich.edu/ModRefiner/
- 27.Xu D., Zhang Y. Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys. J. 2011;101:2525–2534. doi: 10.1016/j.bpj.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Zhang’s Research Group Home Page Online Service FG-MD. [(accessed on 26 March 2014)]. Available online: http://zhanglab.ccmb.med.umich.edu/FG-MD/
- 29.Zhang J., Liang Y., Zhang Y. Atomic-level protein structure refinement using fragment-guided molecular dynamics conformation sampling. Structure. 2011;19:1784–1795. doi: 10.1016/j.str.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comeau S.R., Gatchell D.W., Vajda S., Camacho C.J. ClusPro: An automated docking and discrimination method for the prediction of protein complexes. Bioinformatics. 2004;20:45–50. doi: 10.1093/bioinformatics/btg371. [DOI] [PubMed] [Google Scholar]
- 31.Comeau S.R., Gatchell D.W., Vajda S., Camacho C.J. ClusPro: A fully automated algorithm for protein-protein docking. Nucleic Acids Res. 2004;32:W96–W99. doi: 10.1093/nar/gkh354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozakov D., Brenke R., Comeau S.R., Vajda S. PIPER: An FFT-based protein docking program with pairwise potentials. Proteins. 2006;65:392–406. doi: 10.1002/prot.21117. [DOI] [PubMed] [Google Scholar]
- 33.Brenke R., Hall D.R., Chuang G.Y., Comeau S.R., Bohnuud T., Beglov D., Schueler-Furman O., Vajda S., Kozakov D. Application of asymmetric statistical potentials to antibody-protein docking. Bioinformatics. 2012;28:2608–2614. doi: 10.1093/bioinformatics/bts493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozakov D., Beglov D., Bohnuud T., Mottarella S., Xia B., Hall D.R., Vajda S. How good is automated protein docking? Prot. Struct. Funct. Bioinforma. 2013;81:2159–2166. doi: 10.1002/prot.24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang M.Z., Gopalakrishnakone P., Chung C.M., Kini R.M. Complete amino acid sequence of an acidic, cardiotoxic phospholipase A2 from the venom of Ophiophagus hannah (king cobra): A novel cobra venom enzyme with “pancreatic loop”. Arch. Biochem. Biophys. 1997;338:150–156. doi: 10.1006/abbi.1996.9814. [DOI] [PubMed] [Google Scholar]
- 36.Vejayan J., Shin Y.L., Ponnudurai G., Ambu S., Ibrahim I. Protein profile analysis of Malaysian snake venoms by two-dimensional gel electrophoresis. J. Venom. Anim. Toxins.Trop. Dis. 2010;16:623–630. doi: 10.1590/S1678-91992010000400013. [DOI] [Google Scholar]
- 37.Wej J.E., Lu Q.M., Lin Y., Li D.S., Xiong Y.L., Wang W.Y. Alpha-neurotoxins of Naja atra and Naja kaouthia snakes in different regions. Acta Biochim. Biophy. Sin. 2003;35:683–688. [PubMed] [Google Scholar]
- 38.Nawarak J., Sinchaikul S., Wu C.Y., Liau M.Y., Phutrakul S., Chen S.T. Proteomics of snake venoms from Elapidae and Viperidae families by multidimensional chromatographic methods. Electrophoresis. 2003;24:2838–2854. doi: 10.1002/elps.200305552. [DOI] [PubMed] [Google Scholar]
- 39.Vonk F.J. Ph.D. Thesis. Faculty of Science, Leiden University; Leiden, The Netherlands: Sep 6, 2012. Snake evolution and prospecting of snake venom. [Google Scholar]
- 40.Chang H.C., Tsai T.S., Tsai I.H. Functional proteomic approach to discover geographic variations of king cobra venoms from Southeast Asia and China. J. Proteomics. 2013;26:141–153. doi: 10.1016/j.jprot.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Kulkeaw K., Chaicumpa W., Sakolvaree Y., Tongtawe P., Tapchaisri P. Proteome and immunome of the venom of the Thai cobra, Naja kaouthia. Toxicon. 2007;49:1026–1041. doi: 10.1016/j.toxicon.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Chanhome L., Cox M.J., Vasaruchapong T., Chaiyabutr N., Sitprija V. Characterization of venomous snakes of Thailand. Asian Biomedicin. 2011;5:311–328. [Google Scholar]
- 43.Leong P.K., Sim S.M., Fung S.Y., Sumana K., Sitprija V., Tan N.H. Cross neutralization of Afro-Asian cobra and Asian krait venoms by a Thai polyvalent snake antivenom (Neuro Polyvalent Snake Antivenom) PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng S.S., Kumar T.K., Jayaraman G., Chang C.C., Yu C. Solution structure of toxin B, a long neurotoxin from the venom of the king cobra (Ophiophagus hannah) J. Biol. Chem. 1997;272:7817–7823. doi: 10.1074/jbc.272.12.7817. [DOI] [PubMed] [Google Scholar]
- 45.Chang L.S., Liou J.C., Lin S.R., Huang H.B. Purification and characterization of a neurotoxin from the venom of Ophiophagus hannah (king cobra) Biochem. Biophys. Res. Commun. 2002;294:574–578. doi: 10.1016/S0006-291X(02)00518-1. [DOI] [PubMed] [Google Scholar]
- 46.Li J., Zhang H., Liu J., Xu K. Novelgenes encoding six kinds of three finger toxins in Ophiophagus hannah (king cobra) and function characterization of two recombinant long-chain neurotoxins. Biochem. J. 2006;398:233–242. doi: 10.1042/BJ20060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He Y.Y., Lee W.H., Zhang Y. Cloning and purification of alpha-neurotoxins from king cobra (Ophiophagus hannah) Toxicon. 2004;44:295–303. doi: 10.1016/j.toxicon.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H.L., Xu S.J., Wang Q.Y., Song S.Y., Shu Y.Y., Lin Z.J. Structure of a cardiotoxic phospholipase A(2) from Ophiophagus hannah with the “pancreatic loop”. J. Struct. Biol. 2002;138:207–215. doi: 10.1016/S1047-8477(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 49.Rajagopalan N., Pung Y.F., Zhu Y.Z., Wong P.T., Kumar P.P., Kini R.M. Beta-cardiotoxin: A new three-finger toxin from Ophiophagus hannah (king cobra) venom with beta-blocker activity. FASEB J. 2007;21:3685–3695. doi: 10.1096/fj.07-8658com. [DOI] [PubMed] [Google Scholar]
- 50.Joubert F.J. Snake venoms. The amino-acid sequence of polypeptide DE-1 from Ophiophagus hannah (king cobra) venom. Hoppe Seylers Z. Physiol. Chem. 1977;358:565–574. doi: 10.1515/bchm2.1977.358.1.565. [DOI] [PubMed] [Google Scholar]
- 51.Tsai H.I. Phospholipase A2 of Asian snake venoms. J. Toxicol. Toxin Rev. 1997;16:79–114. doi: 10.3109/15569549709016450. [DOI] [Google Scholar]
- 52.Yamazaki Y., Hyodo F., Morita T. Wide distribution of cysteine-rich secretory proteins in snake venoms: Isolation and cloning of novel snake venom cysteine-rich secretory proteins. Arch. Biochem. Biophys. 2003;412:133–141. doi: 10.1016/S0003-9861(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 53.Wang L.Y., Kuo H.J., Lee C.H. Cobra CRISP functions as an inflammatory modulator via a novel Zn2+- and heparan sulfate-dependent transcriptional regulation of endothelial cell adhesion molecules. J. Biol. Chem. 2010;285:37872–37883. doi: 10.1074/jbc.M110.146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J., Shen B., Guo M., Lou X., Duan Y., Cheng X.P., Teng M., Niu L., Liu Q., Huang Q., et al. lockingeffect and crystalstructure of natrintoxin, a cysteine-richsecretoryprotein from Naja atra venom that targets the BKCa channel. Biochemistry. 2005;44:10145–10152. doi: 10.1021/bi050614m. [DOI] [PubMed] [Google Scholar]
- 55.Yamazaki Y., Koike H., Sugiyama Y., Motoyoshi K., Wada T., Hishinuma S., Mita M., Morita T. Cloning and characterization of novel snake venom proteins that block smooth muscle contraction. Eur. J. Biochem. 2002;269:2708–2715. doi: 10.1046/j.1432-1033.2002.02940.x. [DOI] [PubMed] [Google Scholar]
- 56.Zeng L., Sun Q.Y., Jin Y., Zhang Y., Lee W.H., Zhang Y. Molecularcloning and characterization of a complement-depletingfactor from kingcobra, Ophiophagus hannah. Toxicon. 2012;60:290–301. doi: 10.1016/j.toxicon.2012.04.344. [DOI] [PubMed] [Google Scholar]
- 57.Jerusalinsky D., Kornisiuk E., Alfaro P. Muscarinictoxins: Novelpharmacologicaltools for the muscariniccholinergic system. Toxicon. 2000;38:747–761. doi: 10.1016/S0041-0101(99)00196-8. [DOI] [PubMed] [Google Scholar]
- 58.Jin Y., Lee W.H., Zeng L., Zhang Y. Molecular characterization of L-amino acid oxidase from king cobra venom. Toxicon. 2007;50:479–489. doi: 10.1016/j.toxicon.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 59.Li Z.Y., Yu T.F., Lian E.C. Purification and characterization of L-amino acid oxidase from king cobra (Ophiophagus hannah) venom and its effects on human platelet aggregation. Toxicon. 1994;32:1349–1358. doi: 10.1016/0041-0101(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 60.Tan N.H., Fung S.Y. Snake venom L-amino acid oxidases and their potential biomedical applications. Malaysian J. Biochem. Mol. Biol. 2008;16:1–10. [Google Scholar]
- 61.Pung Y.F., Wong P.T., Kumar P.P., Hodgson W.C., Kini R.M. Ohanin, a novel protein from king cobra venom, induces hypolocomotion and hyperalgesia in mice. J. Biol. Chem. 2005;280:13137–13147. doi: 10.1074/jbc.M414137200. [DOI] [PubMed] [Google Scholar]
- 62.Rokyta R.D., Wray P.K., Lemmon R.A., Lemmon E.M., Caudle S.B. A high-throughput venom-gland transcriptome for the eastern diamond back rattle snake (Crotalus adamanteus) and evidence for pervasive positive selection across toxin classes. Toxicon. 2000;38:747–761. doi: 10.1016/S0041-0101(99)00196-8. [DOI] [PubMed] [Google Scholar]
- 63.Chen R.Q., Jin Y., Wu J.B., Zhou X.D., Lu Q.M., Wang W.Y., Xiong Y.L. A new protein structure of P-II class snake venom metalloproteinases: It comprises metalloproteinase and disintegrin domains. Biochem. Biophys. Res. Commun. 2003;310:182–187. doi: 10.1016/j.bbrc.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 64.Guo X.X., Zeng L., Lee W.H., Zhang Y., Jin Y. Isolation and cloning of a metalloproteinase from king cobra snake venom. Toxicon. 2007;49:954–965. doi: 10.1016/j.toxicon.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Gao R., Manjunatha K.R., Gopalakrishnakone P. A novel prothrombin activator from the venom of Micropechis ikaheka: Isolation and characterization. Arch. Biochem. Biophys. 2002;408:87–92. doi: 10.1016/S0003-9861(02)00447-2. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y., Lee W.H., Xiong Y.L., Wang W.Y., Zu S.W. Characterization of OhS1, an arginine/lysine amidase from the venom of king cobra (Ophiophagus hannah) Toxicon. 1994;32:615–623. doi: 10.1016/0041-0101(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 67.He Y.Y., Liu S.B., Lee W.H. Isolation, expression and characterization of a novel dual serine protease inhibitor, OH-TCI, from king cobra venom. Peptides. 2008;29:1692–1699. doi: 10.1016/j.peptides.2008.05.025. [DOI] [PubMed] [Google Scholar]