Abstract
We report a case of subcutaneous infection in a 67 year-old Cambodian man who presented with a 5-month history of swelling of the right foot. Histopathology was compatible with phaeohyphomycosis and the hyphomycete Phialemoniopsis ocularis was identified by the means of morphological and molecular techniques. The patient responded well to a 6-month oral treatment with voriconazole alone.
Keywords: Phialemoniopsis ocularis, Sarcopodium oculorum, Phaeohyphomycosis, Voriconazole
Highlights
-
•
Subcutaneous phaeohyphomycosis was histopathologically proven in a Cambodian patient.
-
•
The species Phialemoniopsis ocularis was identified by phenotypic and molecular techniques.
-
•
The clinical outcome was satisfactory after a 6-month treatment with oral voriconazole alone.
1. Introduction
Phaeohyphomycoses are fungal infections defined by the presence of pigmented filaments in tissues [1,2]. The clinical presentations of phaeohyphomycosis are mostly superficial, like subcutaneous abscesses or nodules which usually follow a trauma or skin injury [3].
The number of reported phaeohyphomycosis seems to be in increasing over the past decades. According to the published cases, the species involved are diverse, although they mostly belong to the genera Exophiala, Alternaria, Phialophora, Curvularia and Fonsecaea [1]. However, new species have been more recently described [4]: we report here a rare case of a chronic subcutaneous phaeohyphomycosis of the right foot caused by Phialemoniopsis ocularis, formerly known as Sarcopodium oculorum. Since this species is often confused with Phialemonium or Lecythophora species [4], we used phenotypic characteristics and nucleic acid sequences for its definite identification [4,5]. The clinical outcome was satisfactory after a 6-month treatment with oral voriconazole alone.
2. Case
A 67 year-old Cambodian man, living in France for a little over a year, presented with a five month-history of a subcutaneous mass on the outer face of his right first toe (day 0 being the day of hospital admission). In his medical history, the existence of a non-insulin dependent diabetes and asymptomatic chronic infection with hepatitis B virus (HBV) were recorded. There was no known immunodeficiency, and the patient did not remember any trauma. The clinical examination revealed a large painless swelling of the first metatarsophalangeal joint, mimicking a bursitis as shown on MRI (Fig. 1). After discussion in multidisciplinary staff, a skin biopsy was performed on day +14. The presence of a pus collection surrounded by a fibrous shell led to the prescription of antibiotic treatment (amoxicillin – clavulanic acid 1 g/125 mg tid). At day +15, the histopathological analysis of the skin biopsy showed rare pigmented hyphae, with the Hematoxylin and Eosin staining (H&E). Calcofluor brightener (Uvitex 2B Blue®, Ciba-Geigy) and Grocott-Gomori׳s methenamine silver (GMS) staining confirmed the presence of a few septate and dystrophic scattered filaments (Fig. 2).
Fig. 1.
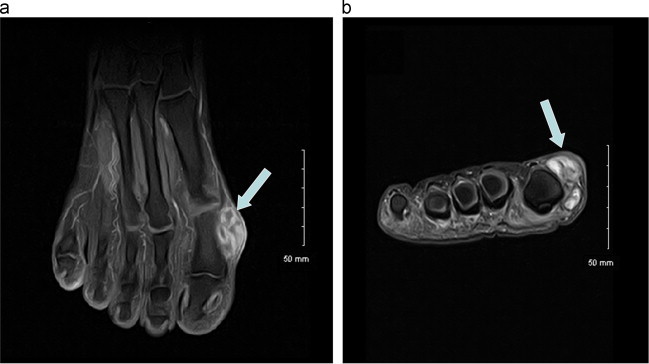
Magnetic Resonance Imaging of the right foot (T1-weighted sequence after Gadolinium-contrast medium injection; (A) in axial plane; (B) in coronal plane). As shown by the light arrows, a subcutaneous mass was highlighted by a hypersignal in the soft parts next to the outer face of the first metatarsophalangeal joint.
Fig. 2.
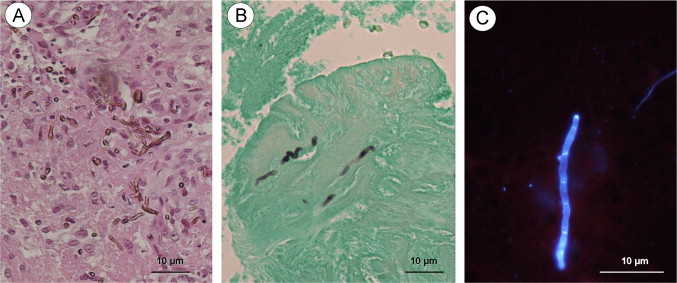
Histological sections of the skin biopsy ((A) H&E staining; (B) GMS staining; (C) Calcofluor fluorescence). The H&E staining showed a few pigmented ramified hyphae within necro-inflammatory granuloma. The GMS impregnation highlighted scattered dark fungal structures on a green background. The affixing of the skin biopsy prepared with the brightener showed few blue-fluorescent filaments with several septa. (For interpretation of references to color in this figure legend, the reader is referred to the web version of this article.)
After four days of incubation at 30 °C and 37 °C on Sabouraud agar supplemented with antibiotics (Sabouraud Dextrose Agar Slants with Chloramphenicol – BBL®, Becton Dickinson, Le-Pont-de-Claix, France), the cultures of the skin biopsy showed downy brown-pigmented colonies. On malt extract agar 2% (MEA Difco®, Becton Dickinson, Detroit MI, USA) at 30 °C, colonies were cottony brown to gray with an uncolored reverse. For identification purposes, the strain was subcultured on oatmeal agar (OA; 30 g oat flakes, 20 g agar, 1 L distilled water), potato dextrose agar (PDA Difco®, Becton Dickinson, Detroit MI, USA) and incubated at 25, 30 and 37 °C. The colonies on OA at 25 °C were smooth at the center, composed mostly by immersed brown mycelia, becoming soft cottony with abundant aerial sporulation at periphery composed by evident short stalked conidial heads. On PDA at 25 °C, colonies were grayish brown, radially folded with sparsely aerial mycelium (Fig. 3A). Hyphae were initially hyaline smooth-walled, becoming brown and slightly verrucose. On both media at 30 °C, cultural characters were similar to those described for 25 °C. The fungus showed a restricted growth at 37 °C after 21 days. On slide cultures (OA) (Figs. 3B–G), conidiophores were short, simples or branched (Figs. 3B–D). Conidia were produced from discrete phialides or from adelophialides both showing distinctive collarettes (Figs. 3E–F). The production of secondary conidia (repeated phialidic germination of the conidia) also occurred (Fig. 3G). The discrete phialides were terminal or lateral and mostly monophialidic, but polyphialides were also observed: they were cylindrical to flask-shaped, and adelophialides were cylindrical or reduced to lateral collarettes on undifferentiated hyphae. Conidia were one-celled, hyaline to subhyaline, smooth-walled, slightly apiculate at base and aggregated in slimy heads. Two types of conidia were present: ellipsoidal to broadly ellipsoidal 5–6×1.5–2 µm2, and allantoid with slightly apiculate base 3–5×1–1.5 µm2. All these characteristics allowed us to identify the isolate as belonging to the genus Phialemoniopsis, despite that conidiomata were not observed.
Fig. 3.
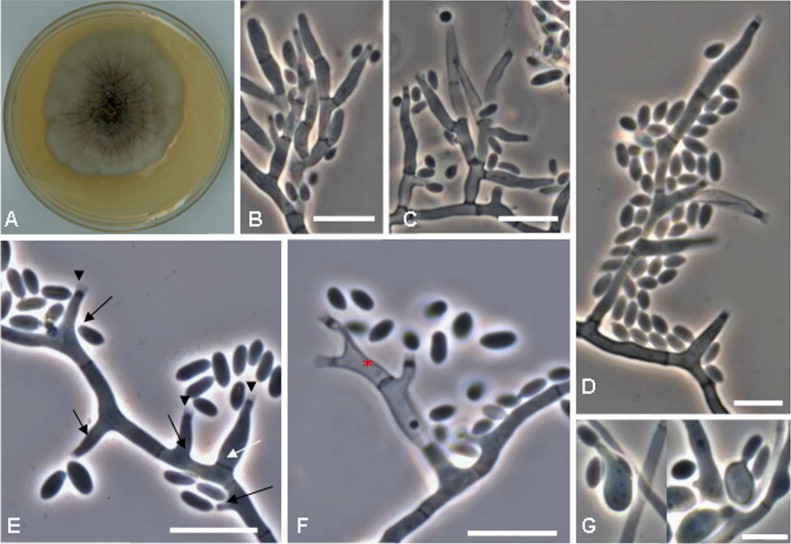
Macro- and microscopical features on lactophenol cotton blue mount of Phialemoniopsis ocularis (CNRMA 12.278) on OA after 21 days of incubation at 25° C ((A) colony on PDA; (B–D) conidiophores, scale bars=10 µm; (E–F) conidiogenous cells and conidia, scale bars=5 µm; (G) phialidic production of secondary conidia, scale bar=5 µm). The black arrowheads show the conspicuous collarettes, the solid black arrows exhibit the adelophialides. The white arrow points to a discrete phialide and the red asterisk to a phialide with two conidiogenic loci (polyphialide). (For interpretation of references to color in this figure legend, the reader is referred to the web version of this article.)
Molecular identification was ascertained by PCR amplification and sequencing of the ITS1-5.8S-ITS2 region, the D1-D2 domains of the ribosomal DNA and two fragments of the actin and tubulin genes [6]. The nucleotide sequence European Nucleotide Archive (ENA) accession numbers and the percentage of identity for each locus with the type strain of P. ocularis (IHEM 19077) were HG933293/98.8%, HG933292/100%, HG933291/98.3% and HG933290/99.2% respectively.
Antifungal susceptibility testing was assessed by broth microdilution EUCAST [7] method with some modifications. Briefly, testing of amphotericin B was performed on AM3 medium and MIC endpoints were determined on an automated microplate reader spectrophotometer [6,7]. The minimal inhibitory concentrations (MICs) for amphotericin B, and echinocandins (caspofungin, micafungin, anidulafungin) were high (8 µg/ml) and much lower for azoles (voriconazole (0.25 µg/ml), posaconazole (0.5 µg/ml) and itraconazole (1 µg/ml)).
At day +43, a treatment with voriconazole (V-Fend®, Pfizer, Paris, France) was initiated at a loading dose of 400 mg bid for 24 h, then at 200 mg per os every 12 h. The first clinical assessment after 1.5 month of treatment was satisfactory, except for the dorsal side of the foot, which was still erythematous (day +85). Since drug monitoring previously showed a voriconazole overdosage in blood at day +75 (8 mg/L), the dose was divided by two at day +85. The antifungal treatment was finally stopped after 6 months, at day +246 (Fig. 4).
Fig. 4.
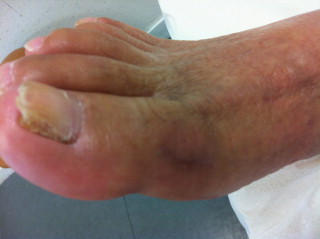
Aspect of the right foot after 6 months of oral antifungal treatment with voriconazole. The initial lesion on the outer part of the hallux had almost disappeared. There was a good healing with no redness or pain. To note, the mycological exploration of the altered nail was not contributive.
3. Discussion
Phaeohyphomycoses include all superficial or deep infections caused by fungi – also named dematiaceous molds – that exhibit a filamentous growth within tissues [3,8], regardless of taxonomic classification or anatomical localization of lesions. One must distinguish them from two other clinical entities: chromoblastomycosis and eumycetoma [2]. Molds responsible for phaeohyphomycosis are cosmopolitan, but are mostly prevalent in the warm and humid climates of (sub-) tropical areas [9]. Although isolated species mainly belong to the genera Exophiala (including ex-Wangiella), Alternaria, Curvularia, Bipolaris, Exserohilum, Phoma, Phialemonium, and Phialophora, it is estimated that more than 70 genera and 130 species of dematiaceous fungi may cause phaeohyphomycosis [1]. Cutaneous and subcutaneous phaeohyphomycosis is usually secondary to direct inoculation, most often in the lower limbs (by trauma or skin penetration by a contaminated plant thorn/splinter) [1,3]. In a chronic and painless course, phaeohyphomycosis begins with the appearance of small papules at the penetration point, and then evolves into a mobile cyst sometimes filled with pus. Ultimately, it results in the formation of nodules in relief or indurated plaques [1,3]. In immunocompromised subjects, phaeohyphomycosis may become opportunistic, and potentially generates a disseminated or invasive infection that is associated with a very poor prognosis [4].
The genus Phialemoniopsis has been recently erected to accommodate Phialemoniopsis curvata, formerly named Phialemonium curvatum which was actually found to be phylogenetically far away from the type species of the genus Phialemonium, i.e. P. obovatum [5]. Although it exhibits morphological features similar to Phialemonium (and also to the genus Lecythophora) such as the presence of discrete phialides and adelophialides, the most distinctive character of the Phialemoniopsis species relies in the formation of picnidial or sporodochial-like conidiomata in culture. Although our specimen did not produce conidiomata structures in any agar media tested, groups of short and profusely branched conidiophores were observed on OA, which were similar to those forming in sporodochia. The loss of the ability to form conidiomata, and the production of the intermediate forms, was also noticed by Perdomo et al. [4,5]. Besides, the genus Phialemoniopsis can be also differentiated from Phialemonium by the presence of conspicuous collarettes in the conidiogenous cells, and from Lecythophora because the colonies of the latter are slimy and usually pink or salmon colored. Furthermore, the analysis of the ITS, 28S region sequences and the D1/D2 domains of the ribosomal DNA, and two fragments of the actin and tubulin genes allowed us a reliable identification of the strain as P. ocularis, previously known as S. oculorum [4,5].
In addition to P. curvata and P. ocularis, the genus Phialemoniopsis also encompasses the species P. cornearis and P. pluriloculosa which have all been isolated from plants, decaying vegetables, sewage and water [4,5]. Among the twenties human infections due to the genus Phialemoniopsis, the most were caused by the P. curvata species [4,5]. They corresponded to localized forms, and to deep or disseminated mycoses like arthritis and fungemia respectively [4,10–14]. To our knowledge, only one case of phaeohyphomycosis due to the species P. ocularis was correctly reported in the literature, since Guarro et al. described a corneal ulcer that occurred in a Brazilian boy [15]. The few other cases were retrospectively documented in collection by the means of amplification of the ITS, D1/D2, actin and β-tubulin sequences. These strains had been actually isolated in USA from peritoneal dialysis, left hand sampling and cellulitis aspirate, and formerly identified erroneously as Phialemonium sp. [5].
Few recommendations are available about the treatment of phaeohyphomycosis. Some case series have highlighted the interest of total excision of the lesion, especially when it is single and easily accessible [2,16]. In this specific context, the antifungal drug does not therefore seem essential. In case of incomplete resection or relapse, an adjunctive antifungal medication is thereafter indicated [2]: thus, oral itraconazole is still considered as the standard treatment [1,17]. Besides, some authors attempted its association with terbinafine in Fonsecaea monophora infections, which likely resulted in a synergistic effect [18]. Likewise, intravenous amphotericin B was experienced before an oral relay with a triazole [19]. As demonstrated by our case, voriconazole nowadays appears to be a credible alternative for the management of phaeohyphomycosis [20]. Nevertheless, it is of course difficult to propose an optimal standard treatment for infections due to Phialemoniopsis spp., because of the lack of clinical data. The choice of the antifungal drug should be always based on the MICs assessed in vitro.
Conflict of interest
There are none.
Ethical form
All authors declare no financial conflict of interest concerning this article. No funder has played any decision-making role in this research.
Although the subject presented in this study can not be civilly and physically recognized, he gave us his informed consent for the publication of a scientific article about him.
Acknowledgments
The authors would like to thank all the members of the Centre National de Référence des Mycoses et Antifongiques (CNRMA, Institut Pasteur de Paris – France) for their precious help in the mycological identification.
The authors are very grateful to Kay Mc Carthy for her thorough corrections of the manuscript after its translation into English.
References
- 1.Revankar S.G. Phaeohyphomycosis. Infect. Dis. Clin. N. Am. 2006;20:609–620. doi: 10.1016/j.idc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Garnica M., Nucci M., Queiroz-Telles F. Difficult mycoses of the skin: advances in the epidemiology and management of eumycetoma, phaeohyphomycosis and chromoblastomycosis. Curr. Opin. Infect. Dis. 2009;22:559–563. doi: 10.1097/QCO.0b013e328332bbc5. [DOI] [PubMed] [Google Scholar]
- 3.Isa-Isa R., García C., Isa M., Arenas R. Subcutaneous phaeohyphomycosis (mycotic cyst) Clin. Dermatol. 2012;30:425–431. doi: 10.1016/j.clindermatol.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Perdomo H., Sutton D.A., García D., Fothergill A.W., Gené J., Cano J. Molecular and phenotypic characterization of phialemonium and lecythophora isolates from clinical samples. J. Clin. Microbiol. 2011;49:1209–1216. doi: 10.1128/JCM.01979-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perdomo H., García D., Gené J., Cano J., Sutton D.A., Summerbell R. Phialemoniopsis, a new genus of Sordariomycetes, and new species of Phialemonium and Lecythophora. Mycologia. 2013;105:398–421. doi: 10.3852/12-137. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Hermoso D., Hoinard D., Gantier J.-C., Grenouillet F., Dromer F., Dannaoui E. Molecular and phenotypic evaluation of Lichtheimia corymbifera (formerly Absidia corymbifera) complex isolates associated with human mucormycosis: rehabilitation of L. ramosa. J. Clin. Microbiol. 2009;47:3862–3870. doi: 10.1128/JCM.02094-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subcommittee on antifungal susceptibility testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. vol. 14; 2008. p. 982–4. [DOI] [PubMed]
- 8.McGinnis M.R. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J. Am. Acad. Dermatol. 1983;8:1–16. doi: 10.1016/s0190-9622(83)70001-0. [DOI] [PubMed] [Google Scholar]
- 9.Sharma N.L., Mahajan V., Sharma R.C., Sharma A. Subcutaneous pheohyphomycosis in India–a case report and review. Int. J. Dermatol. 2002;41:16–20. doi: 10.1046/j.1365-4362.2002.01337.x. [DOI] [PubMed] [Google Scholar]
- 10.Persy B., Vrelust I., Gadisseur A., Ieven M. Phialemonium curvatum fungaemia in an immunocompromised patient: case report. Acta Clin. Belg. 2011;66:384–386. doi: 10.2143/ACB.66.5.2062593. [DOI] [PubMed] [Google Scholar]
- 11.King D., Pasarell L., Dixon D.M., McGinnis M.R., Merz W.G. A phaeohyphomycotic cyst and peritonitis caused by Phialemonium species and a reevaluation of its taxonomy. J. Clin. Microbiol. 1993;31:1804. doi: 10.1128/jcm.31.7.1804-1810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guarro J., Nucci M., Akiti T., Gené J., Cano J., MDGC Barreiro. Phialemonium fungemia: two documented nosocomial cases. J. Clin. Microbiol. 1999;37:2493. doi: 10.1128/jcm.37.8.2493-2497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao C.Y., Pachucki C., Cali S., Santhiraj M., Krankoski K.L.K., Noble-Wang J.A. Contaminated product water as the source of Phialemonium curvatum bloodstream infection among patients undergoing hemodialysis. Infect Control Hosp Epidemiol Off J Soc Hosp Epidemiol Am. 2009;30:840–847. doi: 10.1086/605324. [DOI] [PubMed] [Google Scholar]
- 14.Rivero M., Hidalgo A., Alastruey-Izquierdo A., Cía M., Torroba L., Rodríguez-Tudela J.L. Infections due to Phialemonium species: case report and review. Med Mycol Off Publ Int Soc Hum Anim Mycol. 2009;47:766–774. doi: 10.3109/13693780902822800. [DOI] [PubMed] [Google Scholar]
- 15.Guarro J., Höfling-Lima A.L., Gené J., De Freitas D., Godoy P., Zorat-Yu M.L. Corneal ulcer caused by the new fungal species Sarcopodium oculorum. J. Clin. Microbiol. 2002;40:3071–3075. doi: 10.1128/JCM.40.8.3071-3075.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa M.M., Galante N.Z., Godoy P., Fischman-Gompertz O., Martelli F., Colombo A.L. Treatment of subcutaneous phaeohyphomycosis and prospective follow-up of 17 kidney transplant recipients. J. Am. Acad. Dermatol. 2009;61:977–985. doi: 10.1016/j.jaad.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Silveira F., Nucci M. Emergence of black moulds in fungal disease: epidemiology and therapy. Curr. Opin. Infect. Dis. 2001;14:679–684. doi: 10.1097/00001432-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J.-M., Xi L.-Y., Zhang H., Xie Z., Sun J.-F., Li X.-Q. Synergistic effects of terbinafine and itraconazole on clinical isolates of Fonsecaea monophora. Eur J Dermatol EJD. 2009;19:451–455. doi: 10.1684/ejd.2009.0728. [DOI] [PubMed] [Google Scholar]
- 19.Baradkar V.P., Mathur M., Kumar S. Phaeohyphomycosis of subcutaneous tissue caused by Phaeoacremonium parasiticum. Indian J. Med. Microbiol. 2009;27:66–69. [PubMed] [Google Scholar]
- 20.Koo S., Klompas M., Marty F.M. Fonsecaea monophora cerebral phaeohyphomycosis: case report of successful surgical excision and voriconazole treatment and review. Med. Mycol. 2010;48:769–774. doi: 10.3109/13693780903471081. [DOI] [PubMed] [Google Scholar]


