Abstract
There is increased appreciation by the scientific community that dietary phytochemicals can be potential weapons in the fight against cancer. Emerging data has provided new insights into the molecular and cellular framework needed to establish novel mechanism-based strategies for cancer prevention by selective bioactive food components. The unique chemical composition of the pomegranate fruit, rich in antioxidant tannins and flavonoids has drawn the attention of many investigators. Polyphenol rich fractions derived from the pomegranate fruit have been studied for their potential chemopreventive and/or cancer therapeutic effects in several animal models. Although data from in vitro and in vivo studies look convincing, well designed clinical trials in humans are needed to ascertain whether pomegranate can become part of our armamentarium against cancer. This review summarizes the available literature on the effects of pomegranate against various cancers.
Keywords: Pomegranate extracts, cancer prevention
INTRODUCTION
Despite advances in diagnostic and therapeutic rationales, cancer is still at the forefront of aggressive diseases. The plausible strategies, which may control the occurrence and spread of cancer, thereby reducing the mortality and morbidity associated with the disease include prevention; early diagnosis and intervention; successful treatment of localized cancer and improved management of non-localized cancer [1]. Among these, prevention appears to be the most practical approach for reducing cancer incidence and burden. Chemoprevention, a rapidly evolving field of preventive oncology, focuses on utilizing one or more synthetic and/or naturally occurring bioactive agents to entirely prevent, inhibit, reverse or slow down the progression of carcinogenesis [1]. Epidemiological studies have clearly indicated that a characteristic dietary pattern involving relatively high intake of fruits and vegetables is associated with a significant decrease in the risk of cancer incidence and development [2]. These components of our diet contain an array of phytochemicals such as lycopene, isoflavones and flavonoids in addition to minerals, vitamins and fiber, which are known to prevent disease and promote health [2]. It is recognized that the antioxidants present in fruits and vegetables vary in quantity, structure and functions, and have specific interactions with different types of free radical oxidants that are responsible for inducing oxidative stress in the human body [3].
The pomegranate fruit from the tree Punica granatum, Punicaceae is native to the area of modern day Iran and Iraq, where it has been cultivated since ancient times, and from where it spread to other Asian countries and later to the western world. Pomegranates are cultivated today throughout the world in subtropical and tropical areas in many different microclimatic zones. However, studies suggest that environmental conditions affect the color, taste, and antioxidant capacity of the fruit [4]. Pomegranate has featured virtually in all major religions and has been used for centuries for the management and treatment of diverse ailments. The traditional importance of pomegranate as a medicinal plant is now being reinforced by emerging scientific data that demonstrate that the fruit contains significant anti-oxidant and anti-inflammatory activities and may exhibit anti-carcinogenic properties [2]. Pomegranate is a rich source of hydrolyzable tannins or ellagitannins, catechins, gallocatechins, and anthocyanins. The combination of various types of polyphenols makes the pomegranate antioxidants unique and different from other antioxidants, such as Vitamin A or C, by having a much wider spectrum of action against several and not just one type of free radicals [5].
CHEMICAL CONSTITUENTS OF POMEGRANATE
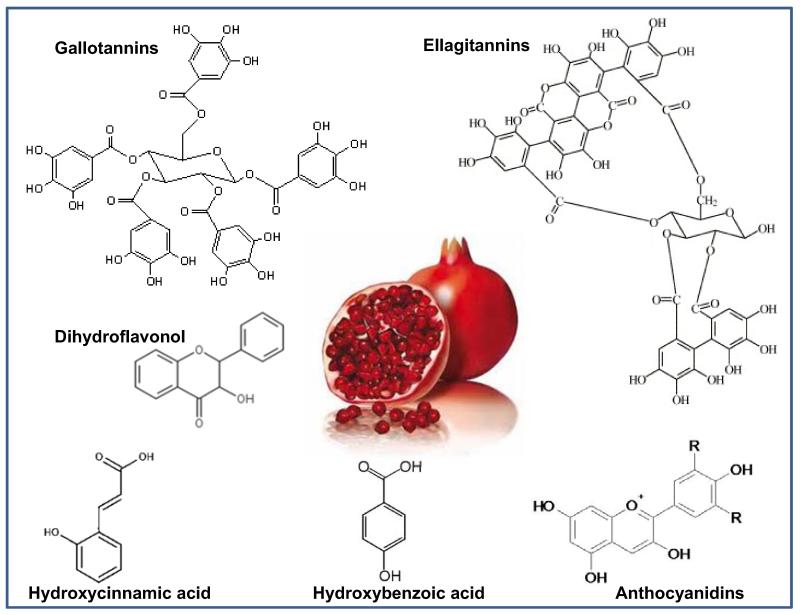
The fruit can be divided into (i) the seeds which constitute ~3% of the fruit weight, (ii) the juice which is roughly 30% and finally (iii) the peel which includes the interior network of membranes present inside the fruit [2]. The seed oil contains conjugated linolenic acid as the predominant fatty acid, with punicic acid (18:3: 9-cis,11-trans,13-cis) being its major isomer. Other components of the oil include sterols, steroids, and cerebrosides [6]. The antioxidant activity of the pomegranate juice is significantly greater than the well-known anti-oxidants red wine and green tea, and is attributed to its polyphenolic content [7]. A number of compounds have been identified in the peel, mesocarp and arils which include anthocyanins, gallotannins, ellagitannins, gallagyl esters, hydroxybenzoic acids, hydroxycinnamic acids and dihydroflavonol (Fig. (1)). Of these, cyanidin–pentoside–hexoside, valoneic acid bilactone, brevifolin carboxylic acid, vanillic acid 4-glucoside and dihydrokaempferol-hexoside have only been reported recently. The ellagitannins are the predominant phenols and the concentration of punicalagin, the typical ellagitannin of pomegranate, ranges from 11 to 20 g/kg in the mesocarp and the peel while the juice contained 4–565 mg/L of the compound [8]. Differences in the phenolic composition have been reported in juices extracted from commercial purposes versus those used in research laboratories. The use of the arils alone or the whole fruit to make juice has an enormous impact on the polyphenol content and consequently the antioxidant capacity of the juice [7, 8]. Furthermore, there is a significant effect of different extracting solvents and temperatures. Extraction with methanol at 60°C has been suggested to be the best method for extracting phenolic compounds while extraction with distilled water yields better results for anthocyanins [9].
Fig. (1).
Chemical constituents of pomegranate.
Since the majority of the phenolic compounds present in pomegranate fruit are thought to be located in the peel and the pericarp, the commercial juices, which are manufactured through a process in which entire fruits are pressed, contain abundant levels of punicalagins, gallic acid, and ellagic acid in contrast to hand-squeezed juices prepared from the arils alone which have only minimal concentrations. The residual solid material obtained after commercial juicing, comprising of the peel, pericarp, and seed tissues, called the pomegranate marc has a substantial amount of polyphenols left in it (20.1%) [10]. The stability of sterilized aqueous extracts prepared from pomegranate marc was evaluated in one study for antioxidant characteristics over a period of 180 d. The results showed that high pH had a negative effect on spectral and antioxidant characteristics. Exposure of the extracts to light resulted in a reduction in clarity and pale color. In contrast, storage at low pH (3.5) in dark packaging retained 67% and 58% of the total soluble phenolic concentration and antioxidant activity, respectively [10]. The ellagitannins, and more specifically punicalagins, present in pomegranate peels, have been reported to possess significant antifungal activity and peel extracts have been suggested as an alternate to the use of synthetic fungicides during storage periods [10]. Treatment of pomegranate fruit with putrescine or spermidine has been found to be effective in maintaining the concentration of ascorbic acid, total phenolic compounds, and anthocyanins [11]. Compared to other foods rich in lignans such as flaxseed or sesame with concentrations of approximately 3000 mg/kg and 400 mg/kg respectively, pomegranate fruits are of minor relevance with regard to dietary lignan uptake. Isolariciresinol is the predominant lignan with concentrations of 45.8 mg/kg in the twigs followed by the peel (10.5 mg/kg) and mesocarp (5.0 mg/kg) [12].
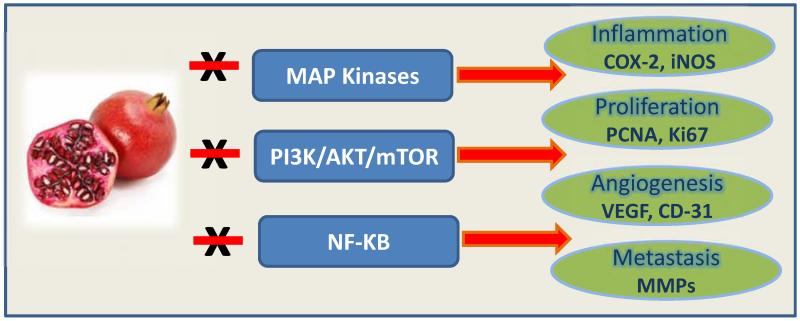
A plethora of evidence indicates that the bioactive compounds from pomegranate derivatives are efficacious in interfering with multiple pivotal pathways implicated in different stages of carcinogenesis [2] (Fig. (2)). Extracts from the pomegranate fruit, seed and peel have been shown to selectively inhibit the growth of prostate and lung cancer cells with no visible toxicity to normal cells [13, 14]. This review summarizes the available data on the cellular and molecular effects of pomegranate derived products from in vitro studies, in vivo preclinical animal model systems and human clinical trials, in selected cancer types (Table 1), in an attempt to elucidate the preventive and therapeutic potential of pomegranate against various cancers.
Fig. (2).
Molecular mechanisms of action of pomegranate derived products.
Table 1. Summary of the anti-carcinogenic effects of pomegranate-derived products.
| Organ | Study Model | Pomegranate Formulation | Target/ Mechanism(s) | Reference |
|---|---|---|---|---|
| Prostate | ||||
| Monolayer cell cultures |
Cold-pressed or CO2-extracted seed oil, fermented juice polyphenols, and pericarp polyphenols |
Inhibits proliferation and invasion Inhibits secretory phospholipase |
[26, 27] | |
| Standardized extract (POMx, POM Wonderful) containing ellagitannins (37-40%) and ellagic acid (3.4%) but no anthocyanins; Juice (POM Wonderful) containing ellagitannins (1 mg/ml), ellagic acid (0.97 mg/ml) and anthocyanins; ellagitannins and ellagic acid |
Suppresses androgen receptor expression Inhibits androgen-synthesizing enzymes |
[29] | ||
| Acetone extract of pomegranate fruit | Induces apoptosis Increases Bax/ Bcl-2 ratio Increases p21 & p27 Down-regulates cyclins and cdks |
[13] | ||
| POMX prepared from skin and arils minus seeds and standardized to ellagitannins (37%) |
Induces apoptosis & inhibits cell growth Increases JNK phosphorylation Suppresses AKT/mTOR signaling Decreases IGF-1 mRNA levels |
[34] | ||
| Fruit extract (POMX; POM Wonderful) standardized to ellagitannins [punicalagins] (37–40%), and ellagic acid (3.4%); Juice concentrate (POM Wonderful) containing punicalagins (1,561 mg/L), ellagic acid (121 mg/L), anthocyanins (387 mL/L), other hydrolysable tannins (417 mg/L) |
Inhibits NF-κB activity | [30] | ||
| Juice (POM Wonderful) with flavonoids (anthocyanins, catechins, and phenols) constituting 40% of total polyphenols |
Antiproliferative, proapoptotic effects, increase in nitric oxide and reduction in oxidative state in exploratory bioassays |
[37] | ||
| Pomegranate juice; ellagitannins extracted from POMX, urolithins |
Inhibits CYP enzyme activity | [40, 41] | ||
| Pomegranate juice | Up-regulates anti-invasive mi-RNAs (−335,−205,−200, & −126) Down-regulates pro-invasive mi-RNA (−21 and −373) Reduces pro-inflammatory cytokines (IL-6,-12p40,-1β and Rantes) |
[36] | ||
| Athymic nude mice |
Ellagitannin-rich fruit extract (POM Wonderful) standardized to ellagitannins [punicalagin] (37%) and ellagic acid (3.5%); acetone fruit extract |
Inhibits tumor growth & multiplicity Decreases serum PSA levels |
[32, 13] | |
| Fruit extract (POMX; POM Wonderful) standardized to ellagitannins [punicalagins] (37–40%), and ellagic acid (3.4%); juice concentrate (POM Wonderful) containing punicalagins (1,561 mg/L), ellagic acid (121 mg/L), anthocyanins (387 mL/L), and other hydrolysable tannins (417 mg/L) |
Delays emergence of androgen independence Decreases NF-κB activity |
[30] | ||
| TRAMP mice | Acetone extract of pomegranate fruit | Reduces tumor formation Decreases metastasis Increases survival Inhibits IGF-I/AKT/mTOR signaling |
[33] | |
| Human trials | Juice (POM Wonderful) with flavonoids (anthocyanins, catechins, and phenols) constituting 40% of total polyphenols; pomegranate extract (POMX) |
Increases PSA doubling time Disease stabilization |
[37, 38] | |
| Monolayer cell cultures |
Acetone extract of pomegranate fruit | Inhibits UVA-mediated phosporylation of STAT3, AKT, ERK1/2, mTOR & p70S6K Decreases PCNA & Ki-67 expression Up-regulates Bax & Bad Down-regulates Bcl-XL |
[42] | |
| Acetone extract of pomegranate fruit | Inhibits UVB-mediated MAPK phosphorylation; NF-κB/p65 activation | [43] | ||
| Pomegranate extract POMX (POM Wonderful) with 135000 ppm polyphenols with major constituents gallic acid equivalent and ellagitannins |
Protects keratinocytes from UV-B-induced oxidative stress and photo-aging Inhibits UV-B-mediated decrease in cell viability and intracellular GSH content; increase in lipid peroxidation & up-regulation of MMPs −1,−2,−7 and −9 Inhibits MAPKs; c-Jun |
[44] | ||
| Skin | ||||
| Pomegranate fruit extract standardized to ellagitannins [gallic acid & punicalagins] (37.5%) and ellagic acid (2.7%) |
Protects fibroblasts from cell death post UV Decreases NF-κB activity | [45] | ||
| Aqueous extracts of pomegranate juice, peel and seed (POM Wonderful) |
Facilitates skin repair Stimulates type I procollagen synthesis Inhibits MMP-1 production |
[54] | ||
| 3-D EpiDerm | Pomegranate extract POMX (POM Wonderful) with 135000 ppm polyphenols with major constituents gallic acid equivalent and ellagitannins; POMx juice (POM Wonderful) contains anthocyanins, ellagitannins and hydrolyzable tannins; POM seed oil (POM Wonderful) |
Inhibits UVB-induced CPDs & 8-OHdG formation; PCNA expression Increases p21 Inhibits UVB-induced MMPs-1,-2,-3,-7,-8,-9,-11,-12; c-Jun and c-Fos; tropoelastin expression |
[46] | |
| SKH-1 mice | Acetone extract of pomegranate fruit | Inhibits UVB-induced skin edema, hyperplasia, leukocytic infiltration; lipid peroxidation; CPDs & 8-OHdG formation; PCNA, ODC & COX-2 expressions; MAPK phosporylation; NF-κB/p65 activation, phosporylation of c-Jun; MMPs -2,-3,-9 expression Increases p53 and p21 expressions |
[47, 48] | |
| CD-1 mice SKH-1 mice |
Pomegranate seed oil; Acetone extract of pomegranate fruit |
Decreases tumor incidence & multiplicity Inhibits TPA-mediated increase in skin edema and hyperplasia; ODC activity, COX-2 expression; phosphorylation of MAPKs and NF-κB activity |
[52, 31] | |
| Balb/c mice | Pomegranate fruit extract | Delays onset and incidence of tumor Suppresses MAPKs and NF-κB activity |
[53] | |
| Wistar rats | Methanolic extract of dried pomegranate peel | Accelerates wound healing Increases hydroxyproline content |
[55] | |
| Guinea pigs | Methanolic pomegranate peel extract based- ointment |
Accelerates wound healing | [56] | |
| Colon | ||||
| Monolayer cell cultures |
Punicalagin, ellagic acid, standardized pomegranate tannin extract (punicalagin 85%, ellagic acid 1.3% and ellagitannins 12%) and pomegranate juice; POMX (POM Wonderful) |
Induces apoptosis, cell cycle arrest Inhibits growth |
[35, 64] | |
| Pomegranate fruit extract standardized to ellagitannins [punicalagin α and β] (25%) and ellagic acid (3.5%) |
Inhibits Wnt signaling | [67] | ||
| Ellagic acid; urolithins | Inhibits migration and adhesion Inhibits activation of NF-κB & MAPKs Down-regulates COX-2, PGE2, PAI-1 and IL-8 |
[58] | ||
| Pomegranate juice (POM Wonderful) (punicalagin 1.74 g/L), pomegranate tannin extract and punicalagins |
Suppresses TNFα-induced COX-2 expression, AKT & NF-κB activity | [65] | ||
| F344/Ducrj rats | Pomegranate seed oil | Inhibits the incidence and multiplicity of azoxymethane-induced colonic adenocarcinomas Increases PPAR expression |
[68] | |
| TNBS mouse model |
Ellagic acid | Attenuates morphologic alterations associated with cellular injury Maintains glandular architecture Decreases inflammatory cells infiltrate Represses COX-2 and iNOS Inhibits MAPKs and NF-κB signaling |
[59] | |
| Punicic acid | Down-regulates neutrophil hyper-activation Decreases ROS-induced tissue damage |
[60] | ||
| DSS mouse model |
Pomegranate extract standardized to punicalagins (35%), punicalin (13%), ellagic acid glucoside (4.5%) and ellagic acid (8.9%); urolithin A |
Decreases inflammation markers (iNOS, COX-2, PTGES and PGE2) | [61] | |
| Pomegranate flower extract; ellagic acid rich fraction from pomegranate extract |
Attenuates oxidative stress and subsequent colonic inflammation | [63] | ||
| Lung | ||||
| Monolayer cell cultures |
Aqueous extract of pomegranate peel | Anti-oxidant Inhibits myeloperoxidase activity |
[70] | |
| Acetone extract of pomegranate fruit | Decreases cell viability Induces p21 and p27 protein expressions Down-regulates cyclins/cdks Decreases PCNA & Ki67 expression Inhibits MAPKs; PI3K/AKT pathway, NF-κB activity |
[71] | ||
| Athymic nude mice |
Acetone extract of pomegranate fruit | Inhibits tumor growth | [71] | |
| A/J mice | Acetone extract of pomegranate fruit | Decreases tumor incidence & multiplicity Inhibits phosphorylation of MAPKs; PI3K/ AKT/mTOR pathway; NF- κB/p65 activation Inhibits c-Met phosphorylation Decreases Ki-67 & PCNA; iNOS, CD31 & VEGF |
[14] | |
| Breast | ||||
| Monolayer cell cultures |
Urolithins | Inhibits aromatase activity Inhibits proliferation |
[72] | |
| Punicic acid; Cold-pressed or CO2-extracted seed oil, fermented juice polyphenols, and aqueous pericarp extract |
Inhibits proliferation Induces apoptosis dependent on lipid peroxidation and the PKC pathway |
[74, 73] | ||
| Pomegranate fruit extract | Suppresses NF-κB Decreases RhoC and RhoA |
[78] | ||
| Pomegranate seed oil; fermented juice polyphenols |
Down-regulates VEGF and MIF | [79] | ||
| Organ culture | Fermented juice polyphenols | Reduces DMBA-induced lesions | [73, 76] | |
| Blood | ||||
| Monolayer cell cultures |
PSP001polysaccharide from pomegranate rind | Inhibits leukemia cell growth | [80] | |
| Pomegranate juice; pomegranate ellagitannin nanoparticles |
Induces apoptosis; cell cycle arrest | [81, 82] | ||
| Pomegranate ellagitannin nanoparticles | Promotes differentiation in promyelocytic leukemia cells | [82] | ||
BIOAVAILABILITY AND PHARMACOKINETICS
The potential health benefits observed in in vitro and in vivo studies sparked the interest of researchers to examine the bioavailability and bioactivity of compounds present in pomegranate. An in vitro digestion study examining the fate of pomegranate juice showed that pomegranate phenolic compounds are available in increasing amounts during digestion whereas the anthocyanins are largely metabolized to some noncolored forms, oxidized, or degraded into other chemicals [15]. The metabolism of the ellagitannin punicalagin, evaluated in rats showed that punicalagin was transformed by the rat microflora to 6H-dibenzo [b,d]pyran-6-one (urolithin B) derivatives [16]. These studies were expanded upon by conducting trials in humans. Six healthy subjects consumed one litre of pomegranate juice daily, rich in punicalagin isomers, for 5 days. The polyphenols and the in vivo generated metabolites measured in body fluids showed that neither punicalagin nor ellagic acid present in the juice was detected in the plasma or urine. In the plasma, three ellagitannin-derived metabolites, namely 3,8-dihydroxy-6H-dibenzo[b,d]pyran-6-one glucuronide, an unidentified aglycone and hydroxy-6-H-dibenzo[b,d]pyran-6-one glucuronide were found. In the urine, however, these metabolites and their corresponding aglycones became evident 24 h after the consumption of juice [17]. A subsequent study found considerable interindividual differences, identifying “high and low metabolite excretors” which supported the involvement of the human colonic microflora in ellagitannin metabolism. In addition, urolithin B was projected as a reliable biomarker of human exposure to dietary ellagitannins [18]. Studies in the pig model elucidated ellagitannin metabolism further and showed that ellagitannins release ellagic acid in the jejunum, where it is metabolized by the intestinal flora to sequentially yield tetrahydroxy-(urolithin D), trihydroxy-(urolithin C), dihydroxy-(urolithin A), and monohydroxy-(urolithin B) dibenzopyran-6-one metabolites [19]. Comparable findings were reported in mice where the animals were administered polyphenol-rich extract of pomegranate peel [20].
Seeram et al have been keenly involved in delineating the various aspects of pomegranate metabolism and bioactivity. In their initial studies, they showed that upon consumption of 180 ml of pomegranate juice by a healthy volunteer, that contained ellagic acid (25 mg) and punicalagin (318 mg), ellagic acid was detected in the plasma for at least an hour post-ingestion but was rapidly eliminated by four hours [21]. Similar findings were reported after ingestion of a standardized pomegranate extract by volunteers where ellagic acid was bioavailable, with an observed C(max) of 33 ng/mL at 1 h [22]. In another study, 18 healthy subjects were given 180 ml of pomegranate juice concentrate and blood and urine samples were examined. Ellagic acid was detected in the plasma of all subjects with maximum plasma concentrations of 0.06 μmol/L after 1 h. Ellagic acid metabolites, including dimethylellagic acid glucuronide and hydroxy-6H-benzopyran-6-one derivatives (urolithins), were detected in the plasma and urine in conjugated and free forms [23]. This study confirmed previous observations that rapid absorption of ellagitannins occurs after ingestion while urolithin metabolites persist and are excreted in the urine for at least 48 h which may possibly account for the observed effects of chronic administration of pomegranate. The fact that the intestinal microbial transformation of pomegranate ellagitannins may account for systemic antioxidant effects was further underscored by studies that show that the antioxidant activity of urolithins correlates with the number of hydroxyl groups as well as the lipophilicity of the molecule. In this context, urolithins C and D were found to be more potent antioxidants when compared to the parent ellagic acid and punicalagins, while urolithin A was found to have significant anti-inflammatory activity [24].
No difference in bioavailability was found among pomegranate juice, liquid, or powder extract forms of treatment with similar levels of total polyphenols standardized as gallic acid equivalents. Sixteen healthy volunteers sequentially consumed, with a 1-week washout period between treatments, pomegranate juice (8 oz, Wonderful variety), pomegranate polyphenol liquid extract (8 oz), and pomegranate polyphenol powder extract (1,000 mg). Plasma bioavailability, judged based on ellagic acid levels over a 6-hour period, did not show any significant difference, however the time taken to achieve maximum serum concentration was slightly delayed for the powder extract (2.58 h) compared to juice (0.65 h) and liquid extract (0.94 h). Similar levels of urolithin-A glucuronide, were observed in the three groups, reaching levels of approximately 1,000 ng/mL [25]. Taken together, these studies indicate that pomegranate polyphenolic compounds act in multiple ways, with some being absorbed and entering the bloodstream to act directly as antioxidants, and the remainder being digested by the colonic microflora to provide other biologically active substances. In the next section, we will focus on the effects of pomegranate and its derivatives in different cancer models.
ANTI-CANCER EFFECTS OF POMEGRANATE
Pomegranate and Prostate Cancer
The polyphenol-rich fractions, from anatomically discrete sections of the pomegranate fruit, when combined, supra-additively suppressed proliferation and invasion and inhibited secretory phospholipase expression in prostate cancer cells [26]. Initial studies by Albrecht et al had revealed that the juice and oil from pomegranate inhibited proliferation and induced apoptosis in androgen dependent and independent prostate cancer cell lines [27]. Remarkably, pomegranate did not cause cytotoxicity in normal prostate epithelial cells. Furthermore, pomegranate derivatives inhibited the growth of prostate cancer xenografts in nude mice [27]. There is considerable evidence that ellagitannins, abundant in pomegranate, contribute significantly towards its reported biological properties. Ellagic acid, caffeic acid, luteolin, and punicic acid, constituents of pomegranate fruit have been examined for their individual and combined effect on the invasiveness of prostate cancer cells. A supra-additive, possibly synergistic effect in inhibiting in vitro prostate cancer cell invasion across matrigel membranes was observed when these compounds were combined [28]. A consistent suppression of both androgen-synthesizing enzymes and androgen receptor expression was demonstrated with pomegranate treatment. It was inferred that pomegranate exerted its inhibitory effect against prostate cancer through down-regulation of genes involved in androgen synthesis [29]. However, the mechanism of the down-regulation is not fully understood and further studies are needed to determine how the alteration of cell proliferation and apoptosis is related to the expression of androgen synthesizing enzymes and androgen receptor.
Constitutive NF-κB signaling is observed in androgen-independent prostate cancer, and is frequently used as a biochemical indicator for tumor recurrence after surgery. Pomegranate extract inhibited NF-κB signaling, both in in vitro and in vivo prostate cancer models. Induction of apoptosis by pomegranate, in vitro, was shown to be dependent on inhibition of the NF-κB activity. For in vivo studies, SCID mice implanted with LAPC4 prostate cancer cells were used as these are androgen dependent, cease growth on castration, and subsequently regrow after a latency of several weeks as androgen-independent tumors. In addition, LAPC4 cells exhibit constitutive NF-κB activity on emergence of the androgen-independent state. Mice were administered pomegranate extract made from skins of fruit (Wonderful variety) and standardized to ellagitannins, such that it contained 37–40% punicalagins and 3.4% free ellagic acid. Upon castration of animals one week later, pomegranate fed group displayed significantly delayed growth compared with the castrate vehicle control group. Remarkably, tumors in the control group grew despite castration, whereas the pomegranate extract prevented the regrowth observed after castration and was associated with low serum levels and decreased NF-κB activity [30].
We studied the molecular mechanisms involved in the anticancer activity of the pomegranate fruit. Using MALDI-TOF Mass Spectrometry, the pomegranate fruit extracted in our laboratory was found to contain six anthocyanins namely pelargonidin 3-glucoside, cyanidin 3-glucoside, delphinidin 3-glucoside, pelargonidin 3,5-diglucoside, cyaniding 3,5-diglucoside, and delphinidin 3,5-diglucoside; ellagitannins and hydrolysable tannins [31]. We evaluated the anti-proliferative and pro-apoptotic properties of the fruit extract, both in vitro and in vivo. Pomegranate extract inhibited the growth and viability of prostate cancer cells through modulation of the cki-cyclin-cdk network, with up-regulation of p21 and p27 during G1-phase arrest, independent of p53. This correlated with down-regulation of the cyclins D1, D2, and E and cyclin-dependent kinases (cdk) −2, −4, and −6, operative in the G1 phase of the cell cycle [13]. Athymic nude mice implanted with androgen-sensitive prostate cancer cells were administered pomegranate extract (0.1% and 0.2%; wt/vol) in drinking water and tumor growth was compared with the untreated controls. The selection of doses was based on the assumption that a typical healthy individual weighing ~70 kg may be persuaded to drink 250 or 500 ml of pomegranate juice extracted from one or two fruits, respectively. Pomegranate treatment resulted in significant inhibition in tumor growth and volume and was associated with decreased serum Prostate Specific Antigen (PSA) levels [13]. Our data were in agreement with studies done by Seeram et al where they found significant inhibition of LAPC-4 prostate cancer xenograft growth in the SCID mouse model administered an ellagitannin-enriched pomegranate extract orally [32].
For a more comprehensive evaluation of the efficacy of pomegranate against prostate cancer, we used the transgenic TRAMP mouse model. Mice received 0.1 and 0.2% pomegranate fruit extract, equivalent to 250 and 500 ml of pomegranate juice, in drinking water, starting at 6 weeks and examined at 12, 20 and 34 weeks of age. Similar to humans, prostate cancer in TRAMP mice progresses from precursor intraepithelial lesions, to invasive carcinoma that metastasizes to lymph nodes, liver, lungs, and occasionally to the bone. Continuous supplementation of extract to TRAMP mice reduced tumor formation, decreased metastasis and conferred significant survival advantage, over water-fed controls. Tumor burden as analyzed by magnetic resonance imaging and ultrasound imaging was significantly lower in the extract-supplemented mice as was the histological evidence of poorly differentiated adenocarcinomas. In addition, significant inhibition of IGF-I/AKT/mTOR pathways was observed in the prostate tissues and tumors of pomegranate treated animals [33]. Koyama et al have shown that co-treatment of prostate cancer cells with pomegranate extract and IGFBP-3, a protein which decreases during progression of prostate cancer, resulted in synergistic stimulation of apoptosis and additive inhibition of cell growth, associated with increased JNK phosphorylation, and suppression of AKT/mTOR signaling. In contrast, IGF-1 treatment was shown to completely block extract-induced apoptosis in prostate cancer cells. A decrease in IGF-1 mRNA levels in pomegranate treated cells further suggests that pomegranate extract-induced apoptosis in prostate cancer cells involves modulation of the IGF-IGFBP axis [34].
Pomegranate juice has been shown to possess greater bioactivity than its purified polyphenols hinting at the multifactorial effects and chemical synergy of the action of multiple compounds compared to single purified active ingredients [35]. To understand how pomegranate juice inhibits critical cellular processes involved in invasion and metastasis, the effect on micro-RNA (mi-RNA) expression and production of pro-inflammatory cytokines was ascertained using integrated gene expression studies. The expression of genes involved in the cell adhesion machinery such as E-cadherin and intercellular adhesion molecule-1 were stimulated by pomegranate treatment whereas genes that stimulate migration such as hyaluranan-mediated motility receptor and Type I collagen were down-regulated. Studies showed that anti-invasive mi-RNAs such as miR-335, miR-205, miR-200, and miR-126, were up-regulated, whereas pro-invasive mi-RNAs such as miR-21 and miR-373 were down-regulated. Pomegranate juice reduced the levels of secreted pro-inflammatory cytokines/chemokines known to promote tumor growth (IL-6, -12p40, -1β and RANTES) suggesting that the inhibitory effect of pomegranate on prostate cancer cell metastasis is in part mediated through reducing inflammation [36].
The most striking evidence, supporting the beneficial effect of pomegranate juice in prostate cancer was provided by Pantuck et al who reported a prolongation of the PSA doubling time upon consumption of juice, in patients suffering from the disease [37]. A phase II, two-stage clinical trial was conducted in men with prostate cancer with rising PSA, who had undergone primary therapy. The primary end point for the study was effect on PSA variables, such as change in doubling time, while the secondary end points included safety and modulation of biomarkers. Patients with a detectable PSA of greater than 0.2 and less than 5 ng/mL were selected for the study and administered 8 ounces of pomegranate juice daily. Upon conclusion, no serious adverse events were reported and the treatment was well tolerated. The study showed that treatment with pomegranate juice was associated with statistically significant prolongation of PSA doubling time in these patients from a mean of 15 months at baseline to 54 months post treatment. The effect of treatment on prostate cancer cell growth from baseline and post treatment patients’ serum was compared using an in vitro cell culture assay system. A 12% decrease in LnCaP prostate cancer cell proliferation, 17% increase in apoptosis, 23% increase in serum nitric oxide, and significant reductions in oxidative state and sensitivity to oxidation of serum lipids was observed, post treatment [37]. It was proposed that these results be validated with the inclusion of two treatment arms in a dose-response design, as well as the use of a placebo control. A recently concluded single-arm phase II trial examined the effect of two doses of polyphenol-rich pomegranate fruit extract (POMx) in men with recurrent prostate cancer, using changes in PSA doubling time as the primary outcome [38]. This double-blind study randomized men with a rising PSA, without metastases, to receive 1 or 3 g of POMx, stratified by baseline PSA doubling time and Gleason score. After six months of treatment, the median PSA doubling time lengthened from 11.9 months at baseline to 18.5 months, with no significant difference observed between dose groups [38]. The significance of these findings is still not fully understood, reinforcing the need for placebo-controlled studies.
The anti-cancer effect of pomegranate in prostate, colon and other tissues has been attributed to the localization of the bioactive metabolites at higher levels in these organs [32, 35]. González-Sarrías et al assessed whether ellagitanins or their metabolites ellagic acid and urolithins reach the human prostate upon consumption of pomegranate and evaluated the effect on the expression of proliferation biomarkers [39]. Sixty-three patients with BPH or prostate cancer were divided into controls and consumers of walnuts (35 g/day) or pomegranate juice (200 mL/day) for 3 days before surgery. The main metabolite detected was urolithin A glucuronide together with the traces of urolithin B glucuronide and dimethyl ellagic acid. These studies were repeated and the findings corroborated in a parallel rodent study. The fact that metabolites were present in only a small number of prostates was probably due to clearance of the compounds. No apparent changes in the expression of CDKN1A, MKi-67 or c-Myc were found after consumption of the walnuts or pomegranate juice [39]. The prevention of procarcinogen activation mediated through the inhibition of CYP enzyme activity may play an important role in pomegranate juice’s effect on tumor promotion, and progression [40, 41].
Pomegranate and Skin Cancer
Skin cancer is the most common form of cancer in the United States, with more than three million skin cancers diagnosed annually. Each year there are more new cases of skin cancer than the combined incidence of cancers of the breast, prostate, lung and colon. The results of promoting sun safety measures alone to prevent skin cancers have been less than successful and novel strategies are needed for the prevention of skin cancer. To this effect, polyphenol rich dietary compounds are being explored as an alternative approach in the fight against cancer.
Pomegranate and Photocarcinogenesis
The oxidant/antioxidant imbalance induced by ultraviolet (UV) results in the generation of reactive oxygen species (ROS) that cause cellular damage. UV radiation is known to produce a variety of adverse effects that include sunburns, photo-aging, immuno-suppression, photo-dermatoses, DNA mutations which may then lead to cancer [42]. The endogenous antioxidant capacity is a major determinant of the skin response to UV-induced oxidative stress. Pomegranate derivatives have been investigated for possible skin cancer chemopreventive efficacy. Using normal human epidermal keratinocytes monolayer cell cultures, we evaluated the preventive effects of pomegranate extract against UV-A and UV-B radiation. We showed that pomegranate extract inhibited UV-A mediated increase in the phosphorylation of ERK1/2 MAP Kinase, STAT-3 and the AKT/mTOR/p70S6Kinase pro-survival pathway and induced cell cycle arrest in the G-1 phase [42]. Furthermore, UV-B induced phosphorylation of MAP Kinases (MAPKs) and activation and nuclear translocation of NF-κB was inhibited by pomegranate extract treatment [43]. Yet another report from our lab showed that the extract (POMx) protected immortalized human HaCaT keratinocytes from UV-B-induced oxidative stress and photoaging [44]. POMx inhibited UV-B-mediated decrease in cell viability and intracellular glutathione content and prevented increase in lipid peroxidation and up-regulation of MMPs-1, -2, -7 and -9. The phosphorylation of MAPKs and c-Jun was decreased with POMx treatment. In addition to the keratinocytes, the protective effects of pomegranate extract against UV-A and UV-B-induced damage were examined human skin fibroblasts [45]. The extract, at lower doses, protected skin fibroblasts from cell death following UV exposure, presumably related to decreased NF-κB activity. This cytoprotective effect was associated with down-regulation of pro-apoptotic caspase-3, and an increase in G0/G1 phase associated with DNA repair. Interestingly, the study demonstrated that higher polyphenolic concentrations were needed to achieve a significant reduction in UV-induced ROS levels and increase the intracellular antioxidant capacity [45].
Cell culture studies were followed by evaluation of the effects of pomegranate-derived products-juice, extract and oil against UV-B-mediated damage, using the reconstituted human skin (EpiDerm) model [46]. EpiDerm, pretreated with pomegranate-derivatives, was harvested post-UVB exposure, and markers of DNA damage and photoaging were re-assessed. A decrease in UV-B-induced cyclobutane pyrimidine dimers (CPD), and 8-dihydro-2′-deoxyguanosine (8-OHdG) in pomegranate treated skin suggested an augmented DNA repair system. In addition, pomegranate inhibited protein oxidation and decreased the protein expression of proliferating cell nuclear antigen (PCNA). Notably, pomegranate-derived products inhibited UVB-induced increase in the expression of MMPs −1,−2, −3, −7, −9 and −12 and AP-1 constituents, c-Fos and c-Jun. It was observed that all three derivatives possessed similar efficacy in protecting against UVB-induced damage [46].
We extended our in vitro studies to the SKH-1 mouse model. We showed that oral feeding of pomegranate fruit extract to mice afforded substantial protection from the adverse effects of UV-B radiation via modulation in early biomarkers of photo-carcinogenesis. Two studies were designed to ascertain the efficacy of pomegranate against UV-B-mediated adverse effects. In the first study, mice were administered pomegranate fruit extract in drinking water for 14 days, before exposing them to a single dose of UV-B (180 mJ/cm2) irradiation [47]. Pomegranate extract consumption inhibited UV-B-induced edema, hyperplasia and leucocytic infiltration in the murine skin. This was associated with decrease in the expression of the inflammatory marker COX-2 and inhibition of ornithine decarboxylase (ODC) activity, a rate-limiting enzyme in the biosynthesis of polyamines, which play an important role in the regulation of cell transformation and development of cancer. As in the 3-dimensional in vitro Epiderm model, a decrease in hydrogen peroxide generation and lipid peroxidation was observed in the extract-treated group. DNA damage caused by UV-B triggers p53 accumulation, leading to cell cycle arrest allowing more time for the repair or elimination of damaged cells by apoptosis [47]. Pomegranate enhanced UV-B-mediated increases in p53 and p21 and decreased PCNA protein expression in the mouse epidermis. This was accompanied with a marked reduction in the number of CPDs and 8-OHdG positive cells. Pomegranate treatment inhibited UV-B-mediated nuclear translocation of NF-κB, known to be a crucial factor in immuno-inflammatory responses and implicated in photo carcinogenesis. In a subsequent study, we assessed the protective effect of pomegranate, upon multiple UVB exposures [48]. SKH-1 mice fed on 0.2% pomegranate fruit extract were irradiated on alternative days, for a total of seven treatments. Pomegranate effectively inhibited UV-B-induced epidermal hyperplasia and inflammation as evidenced by decreased leucocytic infiltration, protein oxidation and lipid peroxidation, and decreased the expression of MMPs −2,−3 and −9 in murine skin [48].
The polysaccharide fraction isolated from the rind of pomegranate possesses free radical scavenging, anti-glycation, and tyrosinase inhibition properties [49]. In in vitro studies, ellagic acid rich pomegranate extract inhibited tyrosinase activity in mushrooms [50]. Furthermore, continuous oral administration of the extract (100 mg/ml) to brown guinea pigs, for 35 days, was shown to inhibit UV-induced skin pigmentation. This was associated with a decrease in the number of DOPA-positive melanocytes in the epidermis suggesting that decreased skin pigmentation was associated with inhibition of proliferation of melanocytes and melanin synthesis by tyrosinases present in these cells [50]. A double-blind, placebo-controlled human clinical trial was conducted by the same group where women were administered supplements of high and low dose ellagic acid (200 mg/d and 100 mg/d) extracted from pomegranate, for 4 weeks and subjected to ultraviolet irradiation. It was shown that ellagic acid ingested orally had a skin whitening effect even at the lower dose in these subjects. Furthermore it was determined that the inhibitory effect of the extract on UV induced pigmentation in these subjects is possibly through the same mechanism that was elucidated in the aforementioned rodent model [51]. This observation was validated in another human trial where topical and oral administration of pomegranate augmented the protective effect of sunscreens and afforded photoprotection from UVB [2].
Pomegranate and Chemical Carcinogenesis
Pomegranate has been studied in 2-stage mouse skin tumorigenesis model for possible skin cancer chemopreventive efficacy. Skin tumors were initiated in CD-1 mice with an initial topical application of 7,12-dimethylbenzanthracene (DMBA) followed by biweekly promotion using 12-O-tetradecanoylphorbol 13-acetate (TPA) [52]. Tumor incidence and multiplicity were markedly reduced in pomegranate treated versus the untreated control groups. Topical application of 5% pomegranate seed oil, prior to TPA resulted in a 17% decrease in TPA-stimulated ODC activity, hinting at its efficacy against an important event in skin cancer promotion [52]. We used the same protocol to further define the activity of pomegranate against specific molecular targets associated with skin carcinogenesis [31]. Topical application of pomegranate fruit extract (2 mg/animal) prior to TPA, afforded significant protection against TPA-mediated increase in skin edema and hyperplasia and epidermal ODC activity in the SKH-1 mice. This correlated to a decrease in the protein expressions of ODC and COX-2 in the murine skin. Pomegranate treatment resulted in inhibition of TPA-induced phosphorylation of ERK1/2, p38, and JNK1/2 MAPKs, as well as activation of NF-κB. Notably, only 30% of pomegranate treated mice developed tumors as compared to the control where 100% of the mice developed tumors at 16 weeks [31]. A recent study has shown that a 5% extract of pomegranate fruit in combination with diallyl sulfide (DAS) imparts better suppressive activity on skin tumors. Using the DMBA-TPA protocol, it was demonstrated that although the extract and DAS administration alone were effective in delaying the onset and decreasing the tumor incidence, the combination displayed better efficacy, at lower doses. At the molecular level, this inhibition was associated with down-regulation of MAP Kinase and NF-κB activity [53].
Pomegranate and Wound Healing
A delay in wound healing might create an environment favoring tumor growth. Aqueous fractions prepared from the pomegranate peel and fermented juice, and lipophilic fractions prepared from pomegranate seeds, were examined in monolayer and human skin organ cultures. Pomegranate seed oil stimulated keratinocyte proliferation in monolayer cultures and resulted in mild thickening of the epidermis, without the loss of ordered differentiation in the skin organ culture. Notably, there was no effect of the seed oil on fibroblast function. In contrast, pomegranate peel extract stimulated procollagen synthesis and inhibited MMP-1 production by dermal fibroblasts, but had no growth-supporting effect on keratinocytes hinting at the differential effect of pomegranate fractions on skin repair [54]. Additional studies on peel and flower extracts of pomegranate were done in animal models, where wound healing activity was assessed by the percent contraction and estimation of skin collagen in terms of the hydroxyproline content [55]. Phenol rich methanolic extract of dried pomegranate peel, formulated as a 10% (wt/wt) water-soluble gel, was used to treat excision wounds on the skin of Wistar rats. Application of the extract resulted in complete healing of wounds within 10 days, associated with increased hydroxyproline content, in contrast to untreated animals where the healing process took 16–18 days [55]. Hayouni et al evaluated a 5% (wt/wt) methanolic extract of pomegranate peel for its wound healing activity in guinea pigs [56]. The ointment applied for 10 consecutive days significantly augmented wound contraction and period of epithelialization. The extract possessed anti-oxidant activity similar to other known anti-oxidants and exhibited significant antibacterial and antifungal activity which presumably aided in its wound healing activity [56]. These studies clearly suggest that pomegranate derivatives possess anti-skin-tumor promoting activity which should be further evaluated in well-designed clinical trials in humans.
Pomegranate and Colon Cancer
Colon cancer is regarded as one of the most preventable forms of cancer and recently there is much emphasis on the importance of dietary modifications in reducing the risk of colon carcinogenesis. Studies indicate that pomegranate polyphenolic ellagitannins and urolithins exert a profound effect on the initiation and promotion stages of colon cancer development. Below, we have summarized the available information on the effects of pomegranate in colonic inflammation and carcinogenesis.
Pomegranate and Colonic Inflammation
Inflammation plays a key role in the development of colon cancer. The anti-inflammatory properties of pomegranate, linked to its cancer protective effect have been attributed to the urolithins, in particular urolithin-A, and ellagic acid, found at relatively high concentrations in the colon. In one study, the colonic fibroblasts were exposed to urolithins and ellagic acid, at concentrations achievable after the consumption of pomegranate, with or without inflammatory cytokines, and the effects on fibroblast migration and monocyte adhesion were determined [57]. There was significant down-regulation of inflammatory markers such as PGE2, PAI-1, and IL-8, as well as other key regulators of cell migration and adhesion. Fibroblast migration and monocyte adhesion was inhibited suggesting that consumption of ellagitanin-containing foods has potential beneficial effects on gut inflammatory diseases [57]. The mechanism of action seems to be via the inhibition of activation of NF-κB and MAPKs, down-regulation of COX-2 and reduction of prostaglandin PGE2 production [58].
In this context, two in vivo models of colitis have predominantly been used to study the anti-inflammatory effects of pomegranate. In the trinitrobenzene sulfonic acid (TNBS) model, intra-colonic administration of TNBS produces inflammatory bowel disease in rats, closely mimicking the human disease. Oral administration of ellagic acid (10 and 20 mg/kg) to these mice diminished the severity of intestinal injuries induced by TNBS. Further, an attenuation of morphologic alterations associated with cellular injury, maintenance of the glandular architecture and decrease of inflammatory cells infiltrate was observed [59]. Ellagic acid treatment repressed COX-2 and iNOS pro-inflammatory proteins expression to basal levels. Correlating to the in vitro data, the protective effect of the ellagic acid was mediated through inhibition of p38, JNK and ERK1/2 MAPKs and NF-κB signaling, with both mechanisms seemingly interconnected [59]. Bousetta et al demonstrated that the conjugated linolenic fatty acid, punicic acid, a major fatty acid of the pomegranate seed oil exerted a strong inhibitory effect on TNFα-induced ROS production by neutrophils, through inhibition of phosphorylation on the priming site Ser345, and of the upstream p38MAPKinase [60]. In the same study, for in vivo experiments, rats were daily gavaged with 0.5 ml of 2% pomegranate seed oil rich in punicic acid, for 10 days, before TNBS treatment. Oral administration of pomegranate seed oil prevented TNBS-induced colitis and lowered ROS-induced tissue damage in rats. The beneficial anti-inflammatory effects of pomegranate seed was attributed to punicic acid-mediated downregulation of neutrophil activation and lipid peroxidation [60].
Acute colitis was induced in male wistar rats through administration of dextran sodium sulfate (DSS) and their diets were supplemented with either the pomegranate extract (250 mg/kg) or the metabolite urolithin-A (15 mg/kg). Treatment with both compounds resulted in a comparable decrease in inflammation markers (iNOS, COX-2, PTGES and PGE2) in the colonic mucosa of these animals and favorably modulated the gut microbiota. Histological studies, however, showed that the extract did not have any significant protective effect on the colonic architecture. Interestingly, the low metabolism exerted by the altered microbiota of DSS-induced rats allowed ellagic acid and even punicalagin from the extract to reach the colon and exert a possible antioxidant action [61]. This finding is consistent with studies by Ogawa et al, who demonstrated that microspheres of ellagic acid reached the colon and exerted an antioxidant effect [62]. Another study, in the same model showed that pomegranate flower extract and its ellagic acid rich fraction administered daily (100 mg/kg and 200 mg/kg), for seven days, markedly attenuated oxidative stress and subsequent colonic inflammation. The observed anti-ulcerative actions of pomegranate were comparable to that of sulphasalazine, a standard drug for the treatment of colitis [63]. Pomegranate peel extract (6 mg/d) administered to mice over a period of 4 weeks counteracted the high fat-induced expression of inflammatory markers both in the colon and the visceral adipose tissue [20].
Pomegranate and Colon Carcinogenesis
The colon cancer chemopreventive properties of pomegranate juice derived ellagitannins and their intestinal bacterial metabolites urolithins have been studied in HT-29 human colon cancer cells [64]. Both ellagitannins and urolithins inhibited CYP1 activity, suppressed cell proliferation and decreased clonogenic efficiency of HT-29 colon cancer cells. Inhibition of cell proliferation was mediated through cell cycle arrest in the G0/G1 and G2/M stages of the cell cycle followed by induction of apoptosis. The study indicated that not only ellagic acid and punicalagins but also other ellagitannins present in pomegranate juice can potentially contribute to colon cancer chemoprevention [64]. Adams et al showed that pomegranate juice significantly suppressed TNFα-induced COX-2 protein expression, AKT activation and NF-κB binding activity in these cells [65]. Interestingly, ellagic acid alone was ineffective in suppressing NF-κB binding activity further suggesting that the interactions between polyphenols such as anthocyanins and flavonols present in the juice may be responsible for the enhanced anti-proliferative activity [65]. This notion has also been validated by Seeram et al who have demonstrated that pomegranate juice possesses higher antioxidant activity than punicalagin and ellagic acid [35].
Cancers of the alimentary canal represent a special case, because the cancerous cells come in direct contact with large amounts of food-related phytochemicals. Ellagic acid was shown to induce apoptosis in colon cancer cells via stimulation of the intrinsic apoptotic pathway [66]. Induction of Fas-independent apoptosis in Caco-2 colon cancer cells was associated with down-regulation of cyclins A and B1 and upregulation of cyclin E and cell-cycle arrest in S phase. Remarkably, normal colon cells were resistant to ellagic acid/punicalagin induced apoptosis [66]. The standardized ellagitanin extracts obtained from pomegranate and berries have been shown to inhibit Wnt signaling, emphasizing further the inhibitory potential of ellagitanin-rich foods against colon carcinogenesis [67]. Administration of linolenic acid rich pomegranate seed oil (0.01, 0.1 and 1%) to rats for 32 days significantly inhibited the incidence and multiplicity of azoxymethane-induced colonic adenocarcinomas, associated with increased expression of peroxisome proliferator-activated receptor gamma protein in the normal mucosa [68]. Though the actual amount of urolithins that accumulate in the colon is not known, it is plausible that continuous consumption of pomegranate juice could provide a sufficient concentration of urolithins to inhibit colon cancer development [64].
Pomegranate and Lung Cancer
There is growing evidence that the connection between inflammation and lung cancer is not coincidental but may indeed be causal. It has been shown that the inflammatory molecules augment the recruitment of macrophages, delay the clearance of neutrophils and cause an increase in ROS [69]. The effect of pomegranate peel extract was evaluated on human neutrophil ROS production, in vitro, and on lipopolysaccharide-induced lung inflammation, in mice [70]. In vitro studies showed that the extract had no effect on superoxide anion generation, suggesting that it does not directly inhibit NADPH oxidase activity and activation pathways, or scavenge superoxide anions. However, the extract inhibited myeloperoxidase activity which may be responsible for the anti-inflammatory effect. In vivo studies showed that the peel extract attenuated lipopolysaccharide-induced lung inflammation in mice [70].
We studied the effect of oral consumption of a human achievable dose of pomegranate fruit extract, on mice implanted with human lung carcinoma A549 xenografts [71]. Cell culture studies showed that pomegranate treatment selectively decreased the viability of A549 cells but had minimal effect on normal bronchial cells. Pomegranate treatment arrested cells in G0-G1 phase of the cell cycle with induction of p21 and p27, decrease in cyclins D1, D2 and E and cdks −2, −4 and −6 protein expressions. This was associated with inhibition of MAPKinase phosphorylation down-regulation of the PI3K/AKT pathway and NF-κB activity, with decrease in the protein expression of proliferation markers Ki-67 and PCNA. Oral administration of pomegranate fruit extract 0.1% and 0.2% (wt/vol) to athymic nude mice implanted with A549 cells resulted in significant inhibition of tumor growth and progression, with greater inhibitory effects observed in animals receiving 0.2% extract [71].
In a follow-up study, we examined the effect of the extract on growth, progression and angiogenesis, in two mouse lung tumor models [14]. Benzo(a)pyrene and N-nitroso-tris-chloroethylurea were used to induce lung tumors in A/J mice. The animals received drinking water supplemented with 0.2% pomegranate fruit extract (w/v) until the termination of the experiment at day 140. Mice supplemented with the extract, exposed to the carcinogens, had statistically significant lower lung tumor multiplicities than mice treated with the carcinogens only. Consistent with the previous studies, tumor studies showed inhibition of MAPKs, PI3K/AKT and NF-κB signaling pathways in the extract treated mice. Decreased phosphorylation of mTOR protein and downstream targets such as p70S6 and 4E-BP1 kinases suggested a suppressive effect of pomegranate on mTOR signaling. This was accompanied with a significant decrease in the expression of markers of proliferation and angiogenesis (c-Met, PCNA, Ki-67, iNOS, CD31 and VEGF). The study demonstrated that pomegranate inhibited lung tumorigenesis through targeting multiple signaling pathways and merits consideration for development as a potential chemopreventive agent against human lung cancer [14].
Pomegranate and Breast Cancer
Estrogen stimulates the proliferation of breast cancer cells and the growth of estrogen-responsive tumors. The aromatase enzyme, which converts androgen to estrogen, plays a key role in breast carcinogenesis. A panel of pomegranate-derived compounds including ellagic acid, gallagic acid, and urolithins A and B (and their acetylated, methylated, and sulfated analogues) were examined for their ability to inhibit aromatase activity. Urolithin B, among these, was found to be the most effective in inhibiting testosterone-induced breast cancer cell proliferation and suppressing aromatase activity [72].
Pomegranate components processed into fermented juice, aqueous pericarp and seed oil extracts blocked endogenous active estrogen biosynthesis and inhibited the steroid-converting enzyme, 17-beta-hydroxysteroid dehydrogenase-1. The seed oil was found to be the most potent followed by fermented juice [73]. In both estrogen sensitive MCF-7 and estrogen resistant MB-MDA-231 cells, the fermented juice polyphenols showed about twice the anti-proliferative effect as fresh juice polyphenols. In the murine mammary gland organ culture, fermented juice polyphenols were effective in inhibiting DMBA-induced cancerous lesion formation [73]. Punicic acid, the omega-5 long chain polyunsaturated fatty acid present in the seed oil inhibited proliferation and induced apoptosis in estrogen sensitive and insensitive breast cancer cell lines, dependent on lipid peroxidation and the PKC pathway [74]. Pomegranate seed linolenic acid isomers were found to modulate estrogen receptor activity in a concentration dependent manner [75]. In effect, both the seed oil and fermented juice polyphenols have been shown to retard oxidation and prostaglandin synthesis, and inhibit breast cancer cell proliferation and invasion, and promote apoptosis [73]. However, in a comparative study where mouse mammary organ cultures were treated with pomegranate fermented juice polyphenols and seed oil before exposure to DMBA, the seed oil was considerably more potent than the juice in causing reduction in the number of DMBA induced lesions [76]. Pomegranate extract in combination with genistein was more effective than the individual treatment in inhibiting growth of breast cancer cells and induction of apoptosis [77]. The decrease in proliferation, invasion, and motility in aggressive breast cancer phenotypes with pomegranate fruit extract treatment is associated with suppressed NF-κB gene expression and a decrease in RhoC and RhoA protein expression [78].
The effect of pomegranate on angiogenic regulation was evaluated by measuring markers of inflammation/angiogenesis in the conditioned media of MCF-7 or MDA-MB-231 human breast cancer cells. A significant down-regulation of VEGF and up-regulation of migration inhibitory factor was observed. These findings were validated in other cell culture systems using human umbilical vein endothelial cells and in myometrial and amniotic fluid fibroblasts. The marked decrease in new blood vessel formation, observed in the chicken chorioallantoic membrane model, in vivo, further demonstrated the anti-angiogenic potential of pomegranate fractions [79].
Pomegranate and Leukemia
Preliminary studies suggest that bioactive actions of pomegranate hold promise against leukemia. The polysaccharide PSP001, isolated from the rind of pomegranate fruit exhibited anti-oxidant activity in addition to growth inhibitory effect on leukemic cell lines [80]. Pomegranate juice induced significant apoptosis in lymphoid and myeloid leukemia cell lines, associated with cell cycle arrest [81]. Self-assembled nanoparticles of partially purified pomegranate ellagitannins (PPE) and gelatin were synthesized using three PPE-to-gelatin mass ratios (1:5, 5:5, and 7:5). The PPE contained (w/w) 16.6% of punicalagin A, 32.5% of punicalagin B, and a small amount of ellagic acid-hexoside and ellagic acid (1%). Only punicalagin anomers were able to bind with gelatin to form nanoparticles, whereas ellagic acid-hexoside or ellagic acid could not. PPE-gelatin nanoparticle suspension was less effective than PPE in inducing the early stage of apoptosis on human promyelocytic leukemia cells HL-60 but had similar effects in inducing late stage of apoptosis and necrosis [82]. There is evidence that pomegranate fruit extract can promote differentiation in human leukemia cells [83]. A case of a 44-year-old Caucasian man with T lymphoblastic leukemia has been described who showed spontaneous remission, with no readily identifiable cause. After the initial diagnosis, while undergoing histological reviews, and receiving no treatment, the patient showed resolution of tumor mass. The patient admitted to regularly drinking pomegranate juice, during this period. However, later there was recurrence of tumor. Whether the pomegranate juice played a role in the patient’s spontaneous remission remains a matter of speculation [84].
FUTURE PROSPECTS AND CONCLUSION
Cancer continues to be one of the leading causes of human death. In this context, the potential beneficial effects of diverse dietary phytochemical agents are being investigated. There is a great deal of interest in the biological activities of pomegranate-derived products, especially with regards to their anticancer properties. In human clinical trials, pomegranate juice has shown promise against prostate cancer. However, the need exists for conducting well designed clinical trials in other cancer models including colon, breast and skin, where substantial in vitro and in vivo data indicates the efficacy of pomegranate against cancer growth and promotion. The use of botanical extract instead of the purified ingredient was responsible for the observed inhibition of multiple targets in the investigative studies, and therefore posits a greater possibility for the enhanced cancer preventive effects. Different methods adopted by various laboratories for studying the biological activities of pomegranate, especially its anti-oxidant effects have made comparative study cumbersome. In addition, extracts were derived from different anatomical constituents of pomegranate such as peel, seed or juice. Therefore, it is vital that a standardized formulation be embraced to be used for subsequent preclinical and clinical studies. One of the major challenges in clinical studies investigating the preventive effect of pomegranate is to show the absence or reduced incidence of a specific disease end point. Such intervention studies have to be long term and will therefore be costly. In addition, new biomarkers have to be identified, developed and verified to analyze the long term disease prevention. Although no major adverse health effects have been reported, so far, by the participants on consumption of pomegranate, little is known of the effects associated with consumption of enriched pomegranate extracts. The combinatory effect of pomegranate with other compounds has been sparsely examined. These studies should be amplified, as the outcomes of the combinations may not only be additive but also synergistic or even antagonistic. It is anticipated that in-depth research into the anticancer activities of naturally occurring compounds may subsequently lead to the development of an effective cocktail for the cure of cancer.
ACKNOWLEDGEMENTS
The original work from the author’s (H. Mukhtar) laboratory outlined in this review was supported by United States Public Health Service Grants R01 CA 78809, R01 CA 101039, P50 DK065303-01, and RO1 CA 120451.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- [1].Mukhtar H, Ahmad N. Cancer chemoprevention: future holds in multiple agents. Toxicol. Appl. Pharmacol. 1999;158(3):207–10. doi: 10.1006/taap.1999.8721. [DOI] [PubMed] [Google Scholar]
- [2].Syed DN, Afaq F, Mukhtar H. Pomegranate derived products for cancer chemoprevention. Semin. Cancer Biol. 2007;17(5):377–85. doi: 10.1016/j.semcancer.2007.05.004. [DOI] [PubMed] [Google Scholar]
- [3].de Kok TM, de Waard P, Wilms LC, van Breda SG. Antioxidative and antigenotoxic properties of vegetables and dietary phytochemicals: the value of genomics biomarkers in molecular epidemiology. Mol. Nutr. Food Res. 2010;54(2):208–17. doi: 10.1002/mnfr.200900288. [DOI] [PubMed] [Google Scholar]
- [4].Schwartz E, Tzulker R, Glazer I, Bar-Ya’akov I, Wiesman Z, Tripler E, Bar-Ilan I, Fromm H, Borochov-Neori H, Holland D, Amir R. Environmental conditions affect the color, taste, and antioxidant capacity of 11 pomegranate accessions’ fruits. J. Agr. Food Chem. 2009;57(19):9197–209. doi: 10.1021/jf901466c. [DOI] [PubMed] [Google Scholar]
- [5].Aviram M, Kaplan M, Rosenblat M, Fuhrman B. Dietary antioxidants and paraoxonases against LDL oxidation and atherosclerosis development. Handbook of Exp. Pharmacol. 2005;(170):263–300. doi: 10.1007/3-540-27661-0_9. [DOI] [PubMed] [Google Scholar]
- [6].Kaufman M, Wiesman Z. Pomegranate oil analysis with emphasis on MALDI-TOF/MS triacylglycerol fingerprinting. J. Agr. Food Chem. 2007;55(25):10405–13. doi: 10.1021/jf072741q. [DOI] [PubMed] [Google Scholar]
- [7].Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agr. Food Chem. 2000;48(10):4581–9. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- [8].Guo S, Deng Q, Xiao J, Xie B, Sun Z. Evaluation of antioxidant activity and preventing DNA damage effect of pomegranate extracts by chemiluminescence method. J. Agr. Food Chem. 2007;55(8):3134–40. doi: 10.1021/jf063443g. [DOI] [PubMed] [Google Scholar]
- [9].Rababah TM, Banat F, Rababah A, Ereifej K, Yang W. Optimization of extraction conditions of total phenolics, antioxidant activities, and anthocyanin of oregano, thyme, terebinth, and pomegranate. J. Food Sci. 2010;75(7):C626–32. doi: 10.1111/j.1750-3841.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- [10].Qu W, Breksa AP, Iii, Pan Z, Ma H, McHugh TH. Storage stability of sterilized liquid extracts from pomegranate peel. J. Food Sci. 2012;77(7):C765–72. doi: 10.1111/j.1750-3841.2012.02779.x. [DOI] [PubMed] [Google Scholar]
- [11].Mirdehghan SH, Rahemi M, Serrano M, Guillen F, Martinez-Romero D, Valero D. The application of polyamines by pressure or immersion as a tool to maintain functional properties in stored pomegranate arils. J. Agr. Food Chem. 2007;55(3):755–60. doi: 10.1021/jf062985v. [DOI] [PubMed] [Google Scholar]
- [12].Fischer UA, Jaksch AV, Carle R, Kammerer DR. Determination of lignans in edible and nonedible parts of pomegranate (Punica granatum L.) and products derived therefrom, particularly focusing on the quantitation of isolariciresinol using HPLC-DAD-ESI/MSn. J. Agr. Food Chem. 2012;60(1):283–92. doi: 10.1021/jf203598m. [DOI] [PubMed] [Google Scholar]
- [13].Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci U S A. 2005;102(41):14813–8. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Khan N, Afaq F, Kweon MH, Kim K, Mukhtar H. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res. 2007;67(7):3475–82. doi: 10.1158/0008-5472.CAN-06-3941. [DOI] [PubMed] [Google Scholar]
- [15].Perez-Vicente A, Gil-Izquierdo A, Garcia-Viguera C. In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. J. Agr. Food Chem. 2002;50(8):2308–12. doi: 10.1021/jf0113833. [DOI] [PubMed] [Google Scholar]
- [16].Cerda B, Llorach R, Ceron JJ, Espin JC, Tomas-Barberan FA. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eu. J. Nutr. 2003;42(1):18–28. doi: 10.1007/s00394-003-0396-4. [DOI] [PubMed] [Google Scholar]
- [17].Cerda B, Espin JC, Parra S, Martinez P, Tomas-Barberan FA. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eu. J. Nutr. 2004;43(4):205–20. doi: 10.1007/s00394-004-0461-7. [DOI] [PubMed] [Google Scholar]
- [18].Cerda B, Tomas-Barberan FA, Espin JC. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. J. Agr. Food Chem. 2005;53(2):227–35. doi: 10.1021/jf049144d. [DOI] [PubMed] [Google Scholar]
- [19].Espin JC, Gonzalez-Barrio R, Cerda B, Lopez-Bote C, Rey AI, Tomas-Barberan FA. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J. Agr. Food Chem. 2007;55(25):10476–85. doi: 10.1021/jf0723864. [DOI] [PubMed] [Google Scholar]
- [20].Neyrinck AM, Van Hee VF, Bindels LB, De Backer F, Cani PD, Delzenne NM. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: potential implication of the gut microbiota. Br. J. Nutr. 2012:1–8. doi: 10.1017/S0007114512002206. [DOI] [PubMed] [Google Scholar]
- [21].Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clinica Chimica Acta: Int. J. Clin. Chem. 2004;348(1-2):63–8. doi: 10.1016/j.cccn.2004.04.029. [DOI] [PubMed] [Google Scholar]
- [22].Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, Hingorani L, Derendorf H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum l.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J. Agr. Food Chem. 2006;54(23):8956–61. doi: 10.1021/jf061674h. [DOI] [PubMed] [Google Scholar]
- [23].Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J. Nutr. 2006;136(10):2481–5. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- [24].Bialonska D, Kasimsetty SG, Khan SI, Ferreira D. Urolithins, intestinal microbial metabolites of Pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J. Agr. Food Chem. 2009;57(21):10181–6. doi: 10.1021/jf9025794. [DOI] [PubMed] [Google Scholar]
- [25].Seeram NP, Zhang Y, McKeever R, Henning SM, Lee RP, Suchard MA, Li Z, Chen S, Thames G, Zerlin A, Nguyen M, Wang D, Dreher M, Heber D. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J. Med. Food. 2008;11(2):390–4. doi: 10.1089/jmf.2007.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lansky EP, Jiang W, Mo H, Bravo L, Froom P, Yu W, Harris NM, Neeman I, Campbell MJ. Possible synergistic prostate cancer suppression by anatomically discrete pomegranate fractions. Invest. New Drugs. 2005;23(1):11–20. doi: 10.1023/B:DRUG.0000047101.02178.07. [DOI] [PubMed] [Google Scholar]
- [27].Albrecht M, Jiang W, Kumi-Diaka J, Lansky EP, Gommersall LM, Patel A, Mansel RE, Neeman I, Geldof AA, Campbell MJ. Pomegranate extracts potently suppress proliferation, xenograft growth, and invasion of human prostate cancer cells. J. Med. Food. 2004;7(3):274–83. doi: 10.1089/jmf.2004.7.274. [DOI] [PubMed] [Google Scholar]
- [28].Lansky EP, Harrison G, Froom P, Jiang WG. Pomegranate (Punica granatum) pure chemicals show possible synergistic inhibition of human PC-3 prostate cancer cell invasion across Matrigel. Invest. new drugs. 2005;23(2):121–2. doi: 10.1007/s10637-005-5856-7. [DOI] [PubMed] [Google Scholar]
- [29].Hong MY, Seeram NP, Heber D. Pomegranate polyphenols down-regulate expression of androgen-synthesizing genes in human prostate cancer cells overexpressing the androgen receptor. J. Nutr. Biochem. 2008;19(12):848–55. doi: 10.1016/j.jnutbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rettig MB, Heber D, An J, Seeram NP, Rao JY, Liu H, Klatte T, Belldegrun A, Moro A, Henning SM, Mo D, Aronson WJ, Pantuck A. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-kappaB-dependent mechanism. Mol. Cancer Ther. 2008;7(9):2662–71. doi: 10.1158/1535-7163.MCT-08-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer. J. Int. Du Cancer. 2005;113(3):423–33. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- [32].Seeram NP, Aronson WJ, Zhang Y, Henning SM, Moro A, Lee RP, Sartippour M, Harris DM, Rettig M, Suchard MA, Pantuck AJ, Belldegrun A, Heber D. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J. Agr. Food Chem. 2007;55(19):7732–7. doi: 10.1021/jf071303g. [DOI] [PubMed] [Google Scholar]
- [33].Adhami VM, Siddiqui IA, Syed DN, Lall RK, Mukhtar H. Oral infusion of pomegranate fruit extract inhibits prostate carcinogenesis in the TRAMP model. Carcinogenesis. 2012;33(3):644–51. doi: 10.1093/carcin/bgr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Koyama S, Cobb LJ, Mehta HH, Seeram NP, Heber D, Pantuck AJ, Cohen P. Pomegranate extract induces apoptosis in human prostate cancer cells by modulation of the IGF-IGFBP axis. Growth Hormone & IGF Res. 2010;20(1):55–62. doi: 10.1016/j.ghir.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005;16(6):360–7. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- [36].Wang L, Alcon A, Yuan H, Ho J, Li QJ, Martins-Green M. Cellular and molecular mechanisms of pomegranate juice-induced anti-metastatic effect on prostate cancer cells. Integr. Biol. 2011;3(7):742–54. doi: 10.1039/c0ib00122h. [DOI] [PubMed] [Google Scholar]
- [37].Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ, Seeram N, Liker H, Wang H, Elashoff R, Heber D, Aviram M, Ignarro L, Belldegrun A. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin. Cancer Res. 2006;12(13):4018–26. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- [38].Paller CJ, Ye X, Wozniak PJ, Gillespie BK, Sieber PR, Greengold RH, Stockton BR, Hertzman BL, Efros MD, Roper RP, Liker HR, Carducci MA. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer And Prostatic Diseases. 2012 doi: 10.1038/pcan.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gonzalez-Sarrias A, Gimenez-Bastida JA, Garcia-Conesa MT, Gomez-Sanchez MB, Garcia-Talavera NV, Gil-Izquierdo A, Sanchez-Alvarez C, Fontana-Compiano LO, Morga-Egea JP, Pastor-Quirante FA, Martinez-Diaz F, Tomas-Barberan FA, Espin JC. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol. Nutr. Food Res. 2010;54(3):311–22. doi: 10.1002/mnfr.200900152. [DOI] [PubMed] [Google Scholar]
- [40].Kasimsetty SG, Bialonska D, Reddy MK, Thornton C, Willett KL, Ferreira D. Effects of pomegranate chemical constituents/intestinal microbial metabolites on CYP1B1 in 22Rv1 prostate cancer cells. J. Agr. Food Chem. 2009;57(22):10636–44. doi: 10.1021/jf902716r. [DOI] [PubMed] [Google Scholar]
- [41].Faria A, Monteiro R, Azevedo I, Calhau C. Pomegranate juice effects on cytochrome P450S expression: in vivo studies. J. Med. Food. 2007;10(4):643–9. doi: 10.1089/jmf.2007.403. [DOI] [PubMed] [Google Scholar]
- [42].Syed DN, Malik A, Hadi N, Sarfaraz S, Afaq F, Mukhtar H. Photochemopreventive effect of pomegranate fruit extract on UVA-mediated activation of cellular pathways in normal human epidermal keratinocytes. Photochem. Photobiol. 2006;82(2):398–405. doi: 10.1562/2005-06-23-RA-589. [DOI] [PubMed] [Google Scholar]
- [43].Afaq F, Malik A, Syed D, Maes D, Matsui MS, Mukhtar H. Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappa B in normal human epidermal keratinocytes paragraph sign. Photochem. Photobiol. 2005;81(1):38–45. doi: 10.1562/2004-08-06-RA-264. [DOI] [PubMed] [Google Scholar]
- [44].Zaid MA, Afaq F, Syed DN, Dreher M, Mukhtar H. Inhibition of UVB-mediated oxidative stress and markers of photoaging in immortalized HaCaT keratinocytes by pomegranate polyphenol extract POMx. Photochem. Photobiol. 2007;83(4):882–8. doi: 10.1111/j.1751-1097.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- [45].Pacheco-Palencia LA, Noratto G, Hingorani L, Talcott ST, Mertens-Talcott SU. Protective effects of standardized pomegranate (Punica granatum L.) polyphenolic extract in ultraviolet-irradiated human skin fibroblasts. J. Agr. Food Chem. 2008;56(18):8434–41. doi: 10.1021/jf8005307. [DOI] [PubMed] [Google Scholar]
- [46].Afaq F, Zaid MA, Khan N, Dreher M, Mukhtar H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009;18(6):553–61. doi: 10.1111/j.1600-0625.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Afaq F, Khan N, Syed DN, Mukhtar H. Oral feeding of pomegranate fruit extract inhibits early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Photochem. Photobiol. 2010;86(6):1318–26. doi: 10.1111/j.1751-1097.2010.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Khan N, Syed DN, Pal HC, Mukhtar H, Afaq F. Pomegranate Fruit Extract Inhibits UVB-induced Inflammation and Proliferation by Modulating NF-kappaB and MAPK Signaling Pathways in Mouse Skin(dagger) Photochem. Photobiol. 2011 doi: 10.1111/j.1751-1097.2011.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rout S, Banerjee R. Free radical scavenging, anti-glycation and tyrosinase inhibition properties of a polysaccharide fraction isolated from the rind from Punica granatum. Bioresource Technol. 2007;98(16):3159–63. doi: 10.1016/j.biortech.2006.10.011. [DOI] [PubMed] [Google Scholar]
- [50].Yoshimura M, Watanabe Y, Kasai K, Yamakoshi J, Koga T. Inhibitory effect of an ellagic acid-rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation. Biosci. Biotechnol. Biochem. 2005;69(12):2368–73. doi: 10.1271/bbb.69.2368. [DOI] [PubMed] [Google Scholar]
- [51].Kasai K, Yoshimura M, Koga T, Arii M, Kawasaki S. Effects of oral administration of ellagic acid-rich pomegranate extract on ultraviolet-induced pigmentation in the human skin. J. Nutr. Sci. Vitaminol. 2006;52(5):383–8. doi: 10.3177/jnsv.52.383. [DOI] [PubMed] [Google Scholar]
- [52].Hora JJ, Maydew ER, Lansky EP, Dwivedi C. Chemopreventive effects of pomegranate seed oil on skin tumor development in CD1 mice. J. Med. Food. 2003;6(3):157–61. doi: 10.1089/10966200360716553. [DOI] [PubMed] [Google Scholar]
- [53].George J, Singh M, Srivastava AK, Bhui K, Shukla Y. Synergistic growth inhibition of mouse skin tumors by pomegranate fruit extract and diallyl sulfide: evidence for inhibition of activated MAPKs/NF-kappaB and reduced cell proliferation. Food Chem. Toxicol. 2011;49(7):1511–20. doi: 10.1016/j.fct.2011.03.040. [DOI] [PubMed] [Google Scholar]
- [54].Aslam MN, Lansky EP, Varani J. Pomegranate as a cosmeceutical source: pomegranate fractions promote proliferation and procollagen synthesis and inhibit matrix metalloproteinase-1 production in human skin cells. J. Ethnopharmacol. 2006;103(3):311–8. doi: 10.1016/j.jep.2005.07.027. [DOI] [PubMed] [Google Scholar]
- [55].Murthy KN, Reddy VK, Veigas JM, Murthy UD. Study on wound healing activity of Punica granatum peel. J. Med. Food. 2004;7(2):256–9. doi: 10.1089/1096620041224111. [DOI] [PubMed] [Google Scholar]
- [56].Hayouni EA, Miled K, Boubaker S, Bellasfar Z, Abedrabba M, Iwaski H, Oku H, Matsui T, Limam F, Hamdi M. Hydroalcoholic extract based-ointment from Punica granatum L. peels with enhanced in vivo healing potential on dermal wounds. Phytomedicine. 2011;18(11):976–84. doi: 10.1016/j.phymed.2011.02.011. [DOI] [PubMed] [Google Scholar]
- [57].Gimenez-Bastida JA, Larrosa M, Gonzalez-Sarrias A, Tomas-Barberan F, Espin JC, Garcia-Conesa MT. Intestinal Ellagitannin Metabolites Ameliorate Cytokine-Induced Inflammation and Associated Molecular Markers in Human Colon Fibroblasts. J. Agr. Food Chem. 2012 doi: 10.1021/jf300290f. [DOI] [PubMed] [Google Scholar]
- [58].Gonzalez-Sarrias A, Larrosa M, Tomas-Barberan FA, Dolara P, Espin JC. NF-kappaB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br. J. Nutr. 2010;104(4):503–12. doi: 10.1017/S0007114510000826. [DOI] [PubMed] [Google Scholar]
- [59].Rosillo MA, Sanchez-Hidalgo M, Cardeno A, de la Lastra CA. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem. Pharmacol. 2011;82(7):737–45. doi: 10.1016/j.bcp.2011.06.043. [DOI] [PubMed] [Google Scholar]
- [60].Boussetta T, Raad H, Letteron P, Gougerot-Pocidalo MA, Marie JC, Driss F, El-Benna J. Punicic acid a conjugated linolenic acid inhibits TNFalpha-induced neutrophil hyper-activation and protects from experimental colon inflammation in rats. PloS one. 2009;4(7):e6458. doi: 10.1371/journal.pone.0006458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Larrosa M, Gonzalez-Sarrias A, Yanez-Gascon MJ, Selma MV, Azorin-Ortuno M, Toti S, Tomas-Barberan F, Dolara P, Espin JC. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010;21(8):717–25. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- [62].Ogawa Y, Kanatsu K, Iino T, Kato S, Jeong YI, Shibata N, Takada K, Takeuchi K. Protection against dextran sulfate sodium-induced colitis by microspheres of ellagic acid in rats. Life Sci. 2002;71(7):827–39. doi: 10.1016/s0024-3205(02)01737-x. [DOI] [PubMed] [Google Scholar]
- [63].Singh K, Jaggi AS, Singh N. Exploring the ameliorative potential of Punica granatum in dextran sulfate sodium induced ulcerative colitis in mice. Phytotherapy Res. 2009;23(11):1565–74. doi: 10.1002/ptr.2822. [DOI] [PubMed] [Google Scholar]
- [64].Kasimsetty SG, Bialonska D, Reddy MK, Ma G, Khan SI, Ferreira D. Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins. J. Agr. Food Chem. 2010;58(4):2180–7. doi: 10.1021/jf903762h. [DOI] [PubMed] [Google Scholar]
- [65].Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agr. Food Chem. 2006;54(3):980–5. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- [66].Larrosa M, Tomas-Barberan FA, Espin JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J. Nutr. Biochem. 2006;17(9):611–25. doi: 10.1016/j.jnutbio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [67].Sharma M, Li L, Celver J, Killian C, Kovoor A, Seeram NP. Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin A, on Wnt signaling. J. Agr. Food Chem. 2010;58(7):3965–9. doi: 10.1021/jf902857v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kohno H, Suzuki R, Yasui Y, Hosokawa M, Miyashita K, Tanaka T. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 2004;95(6):481–6. doi: 10.1111/j.1349-7006.2004.tb03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cho WC, Kwan CK, Yau S, So PP, Poon PC, Au JS. The role of inflammation in the pathogenesis of lung cancer. Exp. Opin. Therap. Targets. 2011;15(9):1127–37. doi: 10.1517/14728222.2011.599801. [DOI] [PubMed] [Google Scholar]
- [70].Bachoual R, Talmoudi W, Boussetta T, Braut F, El-Benna J. An aqueous pomegranate peel extract inhibits neutrophil myeloperoxidase in vitro and attenuates lung inflammation in mice. Food Chem. Toxicol. 2011;49(6):1224–8. doi: 10.1016/j.fct.2011.02.024. [DOI] [PubMed] [Google Scholar]
- [71].Khan N, Hadi N, Afaq F, Syed DN, Kweon MH, Mukhtar H. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis. 2007;28(1):163–73. doi: 10.1093/carcin/bgl145. [DOI] [PubMed] [Google Scholar]
- [72].Adams LS, Zhang Y, Seeram NP, Heber D, Chen S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev. Res. 2010;3(1):108–13. doi: 10.1158/1940-6207.CAPR-08-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kim ND, Mehta R, Yu W, Neeman I, Livney T, Amichay A, Poirier D, Nicholls P, Kirby A, Jiang W, Mansel R, Ramachandran C, Rabi T, Kaplan B, Lansky E. Chemo-preventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res. Treatment. 2002;71(3):203–17. doi: 10.1023/a:1014405730585. [DOI] [PubMed] [Google Scholar]
- [74].Grossmann ME, Mizuno NK, Schuster T, Cleary MP. Punicic acid is an omega-5 fatty acid capable of inhibiting breast cancer proliferation. Int. J. Oncol. 2010;36(2):421–6. [PubMed] [Google Scholar]
- [75].Tran HN, Bae SY, Song BH, Lee BH, Bae YS, Kim YH, Lansky EP, Newman RA. Pomegranate (Punica granatum) seed linolenic acid isomers: concentration-dependent modulation of estrogen receptor activity. Endocrine Res. 2010;35(1):1–16. doi: 10.3109/07435800903524161. [DOI] [PubMed] [Google Scholar]
- [76].Mehta R, Lansky EP. Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruit extracts in a mouse mammary organ culture. Eu. J. Cancer Prev. 2004;13(4):345–8. doi: 10.1097/01.cej.0000136571.70998.5a. [DOI] [PubMed] [Google Scholar]
- [77].Jeune MA, Kumi-Diaka J, Brown J. Anticancer activities of pomegranate extracts and genistein in human breast cancer cells. J. Med. Food. 2005;8(4):469–75. doi: 10.1089/jmf.2005.8.469. [DOI] [PubMed] [Google Scholar]
- [78].Khan GN, Gorin MA, Rosenthal D, Pan Q, Bao LW, Wu ZF, Newman RA, Pawlus AD, Yang P, Lansky EP, Merajver SD. Pomegranate fruit extract impairs invasion and motility in human breast cancer. Integrat. Cancer Therapies. 2009;8(3):242–53. doi: 10.1177/1534735409341405. [DOI] [PubMed] [Google Scholar]
- [79].Toi M, Bando H, Ramachandran C, Melnick SJ, Imai A, Fife RS, Carr RE, Oikawa T, Lansky EP. Preliminary studies on the anti-angiogenic potential of pomegranate fractions in vitro and in vivo. Angiogenesis. 2003;6(2):121–8. doi: 10.1023/B:AGEN.0000011802.81320.e4. [DOI] [PubMed] [Google Scholar]
- [80].Joseph MM, Aravind SR, Varghese S, Mini S, Sreelekha TT. Evaluation of antioxidant, antitumor and immunomodulatory properties of polysaccharide isolated from fruit rind of Punica granatum. Mol. Med. Rep. 2012;5(2):489–96. doi: 10.3892/mmr.2011.638. [DOI] [PubMed] [Google Scholar]
- [81].Dahlawi H, Jordan-Mahy N, Clench MR, Le Maitre CL. Bioactive actions of pomegranate fruit extracts on leukemia cell lines in vitro hold promise for new therapeutic agents for leukemia. Nutr. Cancer. 2012;64(1):100–10. doi: 10.1080/01635581.2012.630155. [DOI] [PubMed] [Google Scholar]
- [82].Li Z, Percival SS, Bonard S, Gu L. Fabrication of nanoparticles using partially purified pomegranate ellagitannins and gelatin and their apoptotic effects. Mol. Nutr. Food Res. 2011;55(7):1096–103. doi: 10.1002/mnfr.201000528. [DOI] [PubMed] [Google Scholar]
- [83].Kawaii S, Lansky EP. Differentiation-promoting activity of pomegranate (Punica granatum) fruit extracts in HL-60 human promyelocytic leukemia cells. J. Med. Food. 2004;7(1):13–8. doi: 10.1089/109662004322984644. [DOI] [PubMed] [Google Scholar]
- [84].Ceesay MM, Vadher B, Tinwell B, Goderya R, Sawicka E. Spontaneous remission of T lymphoblastic lymphoma. J. Clin. Pathol. 2008;61(8):955–7. doi: 10.1136/jcp.2008.056697. [DOI] [PubMed] [Google Scholar]