Abstract
Although progress has been made in understanding the role of growth factors and their receptors in angiogenesis, little is known about how the Wnt family of growth factors function in the vasculature. Wnts are multifunctional factors that act through the frizzled receptors to regulate proliferation, apoptosis, branching morphogenesis, inductive processes, and cell polarity. All of these processes must occur as developing vascular structures are formed and maintained. Recent evidence has linked the Wnt/Frizzled signaling pathway to proper vascular growth in murine and human retina. Here we review the literature describing the angiogenic functions for Wnt signaling and focus on a newly discovered angiogenic factor, Norrin, which acts through the Wnt receptor, Frizzled4.
Keywords: Wnt, frizzled, norrin, vessel, angiogenesis, retinopathy
INTRODUCTION
Wnts are a family of secreted growth factors that have recently been shown to function in angiogenesis, thanks to discoveries in human vascular disorders in the eye. Blinding diseases in the eye that involve a retinal vascular component include Age Related Macular Degeneration, Glaucoma, Diabetic Retinopathy, Retinopathy of Prematurity, as well as the less common Norrie Disease and Familial Exudative Vitreo-retinopathy (FEVR). Together these diseases account for over fifty percent of global blindness. The hereditary disorders Norrie Disease and FEVR allowed identification of key new angiogenic regulators, including a novel ligand which functions in the Wnt pathway, Norrin [1–3]. Other players implicated in retinal angiogenesis are the Frizzled4 receptor (Fz4) [3] and the co-receptor LRP-5 [4,5], each required for proper development of the vasculature of the eye. Mutations in these genes result in a spectrum of phenotypes, including deafness and mental retardation, but patients are usually identified by the presence of retinal dysplasia resulting from angiogenic defects. This dysplasia can result in either complete blindness or marked visual impairment. In addition to identification of genes involved in retinal angiogenesis that were discovered by hereditary genetic analysis, mouse models were created [6] which, in some cases, recapitulate the human phenotype. Ongoing studies in this area now focus on the molecular and cellular mechanisms by which Wnts function angiogenesis and ultimately may lead to an appreciation of a broader role of Wnt family members in angiogenesis.
WNT SIGNAL TRANSDUCTION
Wnts are a family of secreted glycoproteins that accumulate in the extracellular matrix to activate pathways in adjacent cells. Wnt ligands trigger these pathways by binding an appropriate Frizzled receptor, a member of a family of seven-pass transmembrane proteins [7] of which there are 10 members. A co-receptor, LRP5/6 [8–10] is required to activate the Wnt/β-catenin and Planar Cell Polarity pathways (see below) which can be blocked when LRP5/6 is bound to a secreted regulatory protein, Dickkopf-1 [11–13]. Frizzleds are G-protein-coupled receptors [14,15] and Wnt binding to frizzleds can activate more that one distinct branch of the Wnt signaling cascade. The different branches of the Wnt signaling pathway have been designated the “canonical” signaling pathway, which we refer to as the Wnt/β-catenin signaling pathway, and the “non-canonical” which includes Wnt signaling through Ca2+, Planar Cell Polarity (PCP) and other signaling mechanisms that do not involve β-catenin.
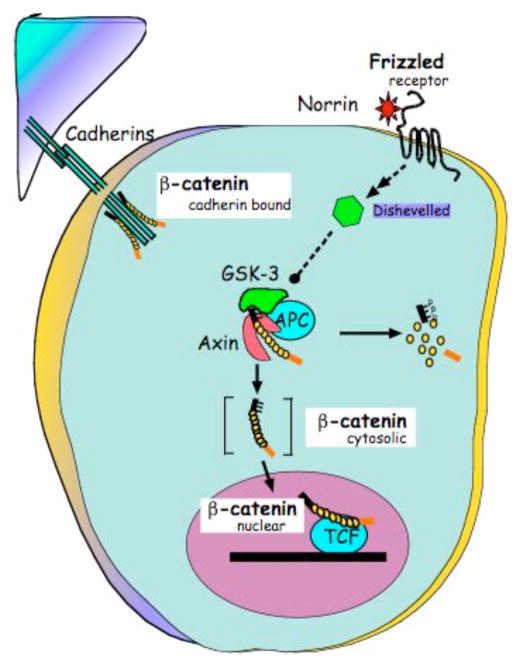
Wnt signaling mediated by the protein β-catenin, schematized in Fig. (1), referred to as Wnt/β-catenin signaling or canonical Wnt signaling, is critical to a diverse array of Wnt functions. Steady-state levels of cytoslic β-catenin are carefully regulated in normal cells by gene products that promote its destruction by phosphorylation and ubiquitin-mediated proteosomal degradation [16,17]. This is a cellular default mechanism that is interrupted, normally by Wnt signaling, and aberrantly, by mutation. The Ser/Thr kinase, glycogen synthase kinase (GSK)-3 is brought into proximity with its target, β-catenin by the tumor suppressor, adenomatous polyposis coli (APC) and axin that, together, function as a scaffold [18]. Phosphorylation of the β-catenin NH2-terminus by GSK-3 is a recognition signal for the E3-ubiquitin ligase β-TrCP [19]. Canonical Wnt signaling elevates the level of cytosolic β-catenin, transiently, by activating Disheveled (Dvl) [20] to block the phosphorylation of β-catenin by GSK-3 [21]. Stabilization of β-catenin promotes its nuclear translocation where it interacts with a family of transcription factors referred to as Lef/Tcf [22]. The complex of β-catenin/Tcf affects gene transcription of large number of characterized Wnt/β-catenin target genes, including vascular endothelial growth factor A (VEGF-A).
Fig. 1.
Schematic of the Wnt/β-catenin signaling pathway that responds to Norrin interaction with Frizzled4.
Wnt Frizzled can also stimulate a several different signaling pathways that do not utilize β-catenin as the effector and these have been termed the non-canonical Wnt sigaling pathway. The ability of Wnt/Frizzled signaling to function in Ca2+ signaling has been described [23]. These studies have shown Wnt signal activation can promote the release of intracellular Ca2+ [24] and the activation of Ca2+ -sensitive enzymes such as protein kinase C [25] and Ca2+-calmodulin kinase II (CaMKII) [26]. Wnt signaling may also activate the Ca2+-dependent phosphatase Calcineurin that, in turn activates the transcription factor NFAT [27]. Some branches of Wnt signaling can work in opposition to one another. For instance Ca2+ signaling induced by Wnt may help establish ventral/dorsal polarity in the fertilized Xenopus egg [28] by inhibiting the Wnt/β-catenin signaling. Thus, Wnts and Frizzleds that activate the Ca2+ signaling may not activate the Wnt/β-catenin [26].
The ordered alignment of cells with respect to major body axes is a manifestation of planar cell polarity, originally observed in Drosophila [29]. PCP, in vertebrates, is partly responsible for polarization, elongation, migration and intercalation of cells along the midline during gastrulation [30]; the arrangement of auditory sensory hair cells within the developing ear [31]. Another form of signaling classified as non-canonical Wnt signaling affects planar cell polarity is thought to work via Dishevelled (Dvl), small GTPases RhoA and Rac1, and c-jun N-terminal kinase (JNK) [32].
HUMAN RETINAL DISEASES
The symptoms associated with Norrie Disease were first described by Leopold Heine in 1925, and then characterized by Gordon Norrie in 1927. In 1992 the gene encoding the Norrie Disease Protein (NDP), also referred to as Norrin, was mapped and positionally cloned [1,2]. It was discovered to be a cysteine rich secreted protein with low homology to any other known molecule, but with a conserved cysteine knot motif. Familial exudative vitreoretinopathy (FEVR) was originally considered to be a separate disease from Norrie Disease, possibly based in part on differences in inheritance patterns and phenotype from Norrie Disease. Four loci for FEVR have been mapped and dubbed EVR-1,-2, -3, and -4. Finer mapping allowed gene mutations to be discovered and it became evident that the genes for Norrin [33] (EVR2, alternatively referred to in the literature as NDP, Norrie Disease Pseudoglioma Homolog (NDPH), or Norrin), Frizzled4 [4,33] (EVR1), and LRP5 (EVR4), which maps in proximity to the EVR1 locus [34], each lie within region that cosegregate with the disease phenotype in affected individuals. The gene corresponding to EVR3 is as yet unidentified [34]. The differences in patterns of inheritance are explained by the fact that Norrin is X-linked recessive, Frizzled4 is reported to exhibit both dominant and recessive inheritance patterns, as is LRP-5, though pedigrees with dominant mutations for both Fz4 and LRP-5 are more commonly seen.
Patients with FEVR can present with a range of symptoms including complete blindness due to retinal detachment, persistence of fetal vasculature, and malformations in the retinal vasculature. It is commonly reported that first-degree family members who are asymptomatic often do have retinal defects that are apparent with flourescein angiography [35]. Manifestations unrelated to the eye are also reported, for instance defects in digit formation, or in the case of LRP-5 mutations, bone density abnormalities. Mental retardation is commonly associated with Norrie Disease, although it is unclear whether this is primarily a vascular or a neural defect. It is becoming clear that Norrie Disease and FEVR appear to represent a phenotypic spectrum resulting from lesions in the same pathway.
Osteoporosis pseudoglioma resulting from the LRP-5 mutation has similarities to Norrie Disease and FEVR, but also often involves bone density disorders, thus it seems likely that LRP-5 mutations affect developmental or physiological functions that do not require Norrin and Frizzled4 [36,37]. The term pseudoglioma was invoked to describe the appearance of the detached retina in affected patients.
NORRIN/FRIZZLED/LRP STRUCTURE AND FUNCTION
As mentioned, Norrin was discovered in 1992 by positional cloning as the gene that co-segregates with Norrie Disease [1,2]. Norrin is a 133 amino acid secreted protein with a cysteine knot motif. The Frizzled receptors were long known from drosophila developmental genetics. Frizzled4 is one of ten currently known such receptors for Wnt ligands. The co-receptor LRP-5 was discovered and shown by xenopus axis formation assays to be involved in Wnt signaling [8–10]. LRP-5 is a 1,615 amino acid, single-pass transmembrane protein with four extracellular EGF repeats and intracellular beta-propeller domains [9] LRP-5 is known to participate in Wnt/β-catenin signaling resulting in the stabilization of beta-catenin.
A survey of the mutations in norrin, frizzled4, and LRP-5 found in these vascular disorders shows that lesions are scattered throughout the coding regions of all three genes, and there is little correlation between mutations in any particular region and the manifestation of the disorder. Thus, mutation analysis does not at this point seem to yield any information about structure and function of the gene products in the pathway [38].
Based upon the working knowledge of Wnt signaling and select reports in the literature, Norrin is presumed to bind to Frizzled4 and LRP-5 leading to activation of Wnt/β-catenin signaling (see Fig. 1). The diversity of Wnt signaling pathways that Norrin and Frizzled4 can activate is not fully understood and will be discussed in a later section.
MURINE AND HUMAN RETINAL ANGIOGENESIS
An understanding of the role of Wnt signaling in angiogenesis requires knowledge about angiogenesis in the target tissue where such a role is manifest; that is the retina. The discovery of the connection between Wnt signaling and human retinal vascular disorders was coincident with several studies on the roles of Norrin and Frizzled4 in retinal angiogenesis in the mouse. In some cases, the phenotypes observed in mice with mutations in these genes remarkedly mimicked the phenotypes observed in human patients. Such a comparison played a part in the seminal discovery that Norrin acts as a ligand for Frizzled4 [3].
The mouse retina can serve as a superb model for observing and experimentally perturbing angiogenesis. By virtue of its shape, relative transparency, and the planar organization of vessels, the retina allows observation of a complete vascular system, whereas other vascular beds are arboreal, three dimensional, and embedded in tissues which do not permit observation of the whole vascular system in situ. For these reasons, the retina has become an important model in the study of angiogenesis [39]. The finding that mutations in Norrin and Frizzled4 disrupt murine retinal angiogenesis served to focus interest on the retina both as a model for general angiogenesis, due to the advantages listed above, and as a specific example of the importance of this pathway in angiogenesis during development.
Retinal angiogenesis in the mouse takes place postnatally, from birth to post-natal day 18 (P18). Retinal vessels arise from the central artery, which enters the retina in parallel to the optic nerve at the center of the retina. At P0 a small ring of vessels is apparent in this region, with specialized tip cells, the hallmark of sprouting angiogenesis, extending from the ring. Between P0 and P8, the primary network of vessels grows symmetrically toward the periphery of the retina. This process was well described by the Betsholtz lab [40] as proceeding in a VEGF- and hypoxia-dependent manner. As the developing retina becomes more metabolically active, diffusion becomes insufficient to supply the oxygen needs of the tissue. Astrocytes in the same plane as the developing primary vessels form a network that precedes the angiogenic front. Hypoxia results in stabilization of HIF1-alpha and induction of VEGF in the astrocytes. Retinal vessels migrate and proliferate along the astrocyte scaffolding toward the periphery of the retina until reaching the edge by P8. During this time, remodeling of vessels, arteriovenus specification, recruitment of mural cells for vessel maturation, and pruning of capillary networks, especially surrounding arteries all occur to result in a stable, relatively quiescent, mature primary plexus. The primary plexus lies on the most superficial aspect of the retina, the nerve fiber layer, which is in contact with the vitreous.
Beginning around P7/P8, branching occurs from the primary plexus into the deeper layers of the neural retina. The fully vascularized retina will consist of an arcade of three layers of vessels, the primary plexus, as described, in the nerve fiber layer, and a secondary and tertiary plexus, situated on the inner margin and the outer margin respectively of the inner nuclear plexus (see Fig. 1). By P18, this process is complete.
The human retina is asymmetrical, with a foveal region (the region of greatest concentration of photoreceptors) located nasally to the optic disk, from which arise the central artery and the optic nerve. The human fovea is avascular, and this is a marked difference between the human and mouse retinas, the mouse retina being completely and symmetrically vascularized. The molecular drivers behind this species difference are largely unknown.
RETINAL ANGIOGENESIS AND NORRIN/FRIZZLED
Mutations in Frizzled4 and Norrin null mice result in strikingly similar phenotypes. This observation from human patients and from mice suggested that these two genes might be working in the same pathway. In 2004, Jeremy Nathan’s lab showed that indeed Norrin and Frizzled4 form a ligand-receptor pair, binding with high specificity and activating canonical Wnt signaling [3]. In both the Norrin and Fz4 null mice, primary retinal angiogenesis is delayed, secondary and tertiary plexus formation is absent, and hyaloid regression is delayed or sometimes absent. LRP-5 null mice are reported to have persistent hyaloid vasculature, similar to the Norrin and Frizzled4 mutants. An effect in the LRP-5 null mice on the retinal vessels has not been reported so it is not yet clear if similar defects exist in LRP-5 mutant mice as in Norrin or Frizzled4 mutant mice.
Null mutations in norrin result in defects in retinal angiogenesis and hyaloid regression [41], observable as early as postnatal day five (P5). Primary superficial vessels form and generally respect the patterning features of radial development and progressive growth from the central artery to the peripheral retina. However angiogenesis is delayed in this plexus and the vessels exhibit abnormalities. Remodeling defects are evident around the vessels as capillary-free zones, capillaries are narrowed, larger vessels are distended, and the attachment of the vessels to the extracellular matrix appears to be disrupted. Vessels are leaky as hemorrhage is evident upon injection of fluorescent dye into the vitreous. A more pronounced defect in Norrin mutant mice is the complete failure of formation of the secondary and tertiary layers of retinal vasculature. Sprouting into the secondary layer begins and then stalls, resulting in what are described as club-like structures of vessels that remain at the sprouting point and fail to progress to form proper vessels, in what should become the secondary and tertiary plexus.
Separate but perhaps related to the retinal angiogenic defect in Norrin mutant mice, is the fact that the hyaloid vasculature in these mice fails to regress, persisting at least until P21, at which point no hyaloid vessels generally remain. Some of the hyaloid vessels are patent, while others are not but fail to disintegrate. Hyaloid regression has been noted to be macrophage dependent, also relying on Wnt signaling, including Wnt-7b [42].
The frizzled4 mutant phenotype is very similar to that of the norrin mutant. Superficial primary vessels are described as tortuous, enlarged, and hemorrhagic, with capillary remodeling defects similar to those seen in the norrin mutant [3]. The density of vessels in the primary layer is reduced as compared to wild type and their formation is delayed, the secondary and tertiary vascular layers are absent, and failure or hyaloid regression is again observed [3].
While a retinal angiogenic phenotype has not been reported for the LRP-5 null mice, the co-receptor has been identified as one of the EVR loci. Several points suggest LRP-5 mutant mice might have a retinal vascular disorder. Frizzled4 and Norrin, EVR-1 and -2 respectively, show similar phenotypes and both are required for retinal angiogenesis. Norrin is known to signal via Wnt/β-catenin, a pathway dependent on LRP 5 or LRP6. Thus, it would be interesting to explore experimentally if LRP-5 were required for retinal vascular patterning.
VASCULAR REGRESSION AND WNT SIGNALING
A transient vasculature of the eye, which includes the hyaloid vasculature, develops after the choroid but before the retinal vessels. These vessels supply the developing retina and lens, and extend in an arboreal fashion from the optic disk, branching through the vitreous, and contact the lens. This network of vessels has several regions that are distinct but continuous: the papillary membrane, the tunica vasculosa lentis, and the hyaloid vessels. We will refer to this system as the hyaloid vasculature, but is should be noted that each set of vessels may show tissue specificity that is not yet apparent. An important aspect of this system is that it is transitory and regresses completely during proper retinal development. In both humans and mice, hyaloid regression is complete by the time retinal angiogenesis is complete. In humans, the hyaloid vessels are completely absent at birth, and in mice, the hyaloid is robust and clearly present from P0 to approximately P5, at which time vessels branches begin to drop out. By P17 only vestigial vessels remain in the wild type mouse, and by P21 no vessels should be present. At this stage, vascularization of the eye is complete.
Hyaloid Vascular Regression Depends on LRP-5 and Wnt7b
Hyaloid regression has been shown to be dependent on macrophage derived Wnt7b signals, requiring the co-receptor LRP-5 [42]. Kato et al. created a mouse strain carrying a truncated form of the LRP-5 protein and, in addition to defects in bone formation, found that hyaloid vessels fail to regress [37]. In these mice it was observed that macrophages are present in the vicinity of the hyaloid vessels, and that apoptosis is observed, but at a lower level than in the wild type mice. Reduced levels of endothelial apoptosis appear to result in vessels maintaining their cohesion and integrity later in development than seen during normal development. In the LRP-5 mutant mice, hyaloid vessels are seen in the adult mice, whereas they are never observed in the adult in the normal case. The observation described above preceded the discovery that hyaloid associated macrophages secrete Wnt7b and thus it has not yet been investigated whether LRP-5 functions in the hyaloid associated macrophages. In LRP-5 mutant mice, the effect of reduced levels of signaling through the co-receptor suggested that Wnt signaling would be involved.
Evaluation of expression of Wnt ligands in the hyaloid associated macrophages led to the finding that Wnt7b is produced by these cells [42]. In the Wnt7b null mutant, and in a PU.1 mouse strain lacking macrophages, the hyaloid vessels also fail to regress [42]. This result was surprising and significant in that it had previously been thought that macrophages were likely to participate in phagocytosis of vessels subsequent to apoptosis, but were not known to supply an apoptotic signal. In the absence of macrophages, Wnt7b, or LRP-5, breakdown of vessels does not occur and the hyaloid vessels remain intact. This is also observed in human disease, including Norrie Disease and FEVR. A similar lack of hyaloid vessel regression can occur in Retinopathy of Prematurity and a condition in humans referred to as Persistence of Fetal Vasculature (PFV). The molecular implications of Norrin, Fz4, and LRP-5 in these human diseases are poorly understood.
Disruption of Wnt-7b in mice leads to perinatal death due to respiratory failure likely from a vascular defect [43]. This occurs after the retinal vasculature defects describes in the previous paragraph. Newborn mutant mice exhibit severe defects in the smooth muscle component of the major pulmonary vessels leading to rupture of major vessels and hemorrhage in the lungs after birth. In this setting, it is speculated that Wnt-7b, which is expressed in airway epithelia, may act on and facilitate survival of pulmonary smooth muscle cells. It is unclear which branch of Wnt signaling is activated by Wnt-7b.
WNT SIGNALING IN ANGIOGENESIS: BEYOND THE RETINA
The discovery that Wnt signaling plays a role in retinal angiogenesis raises the question of whether the role of this pathway is limited to the retina, or whether Wnts are involved more generally in angiogenic signaling in other tissues. Analysis of the Frizzled4 and Norrin mutant mice indicates that there is also a role in female reproductive angiogenesis. It is not yet clear how prevalent the angiogenic activity of Wnts is in angiogenesis in general and future experiments will undoubtedly shed more light on this question.
Hsieh et al. showed that homozygotes that are null for Frizzled4 are infertile, and they observed defects in the formation of the corpora lutea in the ovary, a highly angiogenic tissue [44]. Their group noted that Fz4 −/− mice were infertile and on further investigation determined that Fz4 is expressed in the ovary and was upregulated at specific stages in the ovulatory cycle. In Fz4 mutants, embryo implantation was not seen, though ovulation occurred normally. Histological examination of post-coitus female homozygotes led to the observation that the corpus luteum begins to form, and then becomes disorganized and nonfunctional, coinciding with implantation failure of the embryo and loss of the pregnancy. Because the corpus luteum is vascular and vascular defects had already been noted in the retina of the Fz4 null mice, the ovarian vessels were examined for defects. It was noted that vessels form poorly in the corpus luteum of mutant mice and seem to form early, as compared to wild type. However the vessels in the mutant mice showed early morphological phenotypes indicating that arborization was deficient in the mutant vessels. Expression of VEGF was reduced in the Fz4 −/− ovaries, and the number of cells undergoing apoptosis was increased. The degeneration of the corpus luteum has been established as the primary cause of infertility in this strain, as mating behaviors, response to hormones, and ovulation proceed normally.
The Norrin null mutant mice are almost completely infertile, though the defect is not the same as that seen in Frizzled4 mutant mice. Luhmann et al. reported on the Norrin mutant phenotype and assessed the corpus luteum but found it to be intact and functional [45]. Rather, they reported that in mice null for Norrin, implantation occurs and embryonic development proceeds, but bleeding is observed at the implantation site and the chorioallantois fails to develop, depriving the embryo of placental support. Vessels in the decidua were leaky and morphologically abnormal. The conclusion of the authors is that fetal loss in Norrin null mice is due to failure of differentiation of the decidua and angiogenic defects leading to bleeding and disruption of the maternal-embryonic connection. Expression analysis by in situ hybridization, demonstrated Norrin transcripts in the decidua, the uterus, and in human placenta. Notably, in the highly vascularized regions of the decidua, Norrin expression was detected not in the endothelial cells of the vessels themselves, but in the stromal cells surrounding the vessels.
The failure of Norrin null mice to phenocopy Frizzled4 mutants yields an important clue about the ligand-receptor interactions. The conclusion is drawn that because Norrin null mice do not show defects in the corpus luteum, while Frizzled4 mutants do, that Frizzled4 is likely utilizing a different ligand in this tissue. The opposite case has not been as clearly addressed, as to whether Frizzled4 is the receptor for Norrin in the decidua, given that pregnancy does not progress to the implantation stage in the Frizzled4 null mice. As Norrin is thought to primarily function via Frizzled4 this may suggest that decidual vessels utilize Frizzled4 signaling. Frizzled4 expression has not been reported in the uterus, so this remains an open question.
In mice, targeted disruption of Wnt-2 leads to defects in the placental vasculature marked by reduced capillaries of fetal origin [46]. This defect arises from either increased apoptosis or decreased proliferation of endothelial cells. Other manifestations of the phenotype include pooling of maternal blood, edema, and disruption of the labyrinth zone, possibly due to reduced blood flow to the plancenta. Consistent with this function, Wnt-2 is expressed in fetal vessels of the placenta [46]. Previous studies have defined Wnt-2 as a ligand capable of activating the Wnt/β–catenin signaling cascade [47].
Vascular formation in the mammalian gonad occur in a sex-specific manner, during which endothelial cells migrate from the mesonephros into the gonad to form a coelomic blood vessel. Analysis of Wnt-4 knock-out mice showed that WNT4 represses mesonephric endothelial in the XX gonad, preventing the formation of male-specific coelomic blood vessels [48].
Finally, a compelling case can be made that activated β–catenin signaling occurs in endothelial cells in vivo by following TCF transcriptional activity in whole animals. A β–catenin-activated transgene (BAT) driving expression of nuclear [49]beta-galactosidase reporter (BAT-gal) transgenic mouse strain has been made which expresses the lacZ gene under the control of β–catenin/T cell factor responsive elements. BAT-gal expression identifies a variety of sites of Wnt signaling, like notochord and brain but also identifies endothelial cells (stained with PECAM) as a site of β–catenin/TCF signaling. Thus, based on the analysis of the Frizzled-4 mutant mouse and the BAT-gal mouse, β–catenin signaling functions to regulate endothelial cells in vivo
STUDY OF WNT ANGIOGENIC ACTIVITY IN VITRO
Wnt family members have been implicated in angiogenesis, notably in the developing retina, the placenta, and ovaries. Expression of Wnts has been observed in primary culture human umbilical vein endothelial cells (HUVECs), and human microvascular endothelial cells (HMVECs). Ectopic expression of Wnt family members in cultured endothelial cells has demonstrated that endothelial cells are angiogenically responsive to Wnt signaling. An important caveat in interpreting in vitro data is that the constitution of cells in culture may be changed from the in vivo situation.
Studies using primary endothelial cells have implicated the Wnt/β–catenin pathway as a promoter of endothelial survival and/or proliferation. One study found that microvascular endothelial cells cultured in vitro express Wnt-5a, Wnt-7a, Wnt-10b, and vascular smooth muscle cells express Wnt-5a [50]. Wnts are paracrine factors and may be produced by endothelial, mural, or epithelial cells acting on frizzleds found on endothelial cells. Thus, Wnts could conceivably be expressed from a variety of cellular sources and affect endothelial cells. Endothelial cells grown in vitro express Fz1, Fz3 [50] while vascular smooth muscle cells express Fz3. Our own data demonstrates that Fz-4, Fz-5, Fz-6 are expressed in cultured Human Umbilical Vein Endothelial Cells (HUVEC), see preliminary results. These Frizzleds are expressed in vivo in a variety of mammalian tissues [51]. Thus, multiple Frizzleds are expressed in endothelial cells in vitro, however Frizzleds are not generally expressed in an endothelial-restricted pattern of expression in vivo.
Endothelial cells respond to the activation of the Wnt/β-catenin pathway in vitro, reviewed in [52]. One study reports that expression of Wnt-1, but not Wnt-5a, in HUVEC increases cell proliferation, induces β–catenin stabilization and activates a TCF-responsive transcriptional reporter [50]. Increased degradation of β–catenin has been correlated with endothelial cell survival [53]. We have found that either stabilized β–catenin, Wnt-1 or Wnt-5a can stimulate HUVEC proliferation [54, 55]. Stimulation of endothelial proliferation by β–catenin may also be mediated by PECAM signaling [56]. β–catenin activity is implicated downstream of several distinct signaling pathways that function in endothelial cells [57, 58]. Changes in localization of β-catenin from the membrane to the cytosol, an indicator of Wnt/β-catenin signaling, is found in endothelial cells during neovascularization after experimental induced myocardial infarction [59]. Taken together, these studies implicate the Wnt/β-catenin pathway as a mediator of endothelial cell growth and survival.
SUMMARY/CONCLUSION
The question of the role of Wnt family members in general angiogenesis is one that requires further study for clarification. Aberrations in Wnt expression in tumor cells have been described, however more attention has been given to the proliferative outcomes of expression than to angiogenic effects. Certainly in the retina, there is a quest for drugs that target neovascularization in the eye, particularly as larger segments of the population are being affected by Age Related Macular Degeneration and Diabetic Retinopathy, to name a few. In the case of these diseases, if Fz4 and Norrin turn out to have a limited role, affecting primarily vasculature in the eye, this tissue specificity could possibly be exploited pharmacologically to great advantage.
References
- 1.Berger W, Meindl A, van de Pol TJ, Cremers FP, Ropers HH, Doerner C, Monaco A, Bergen AA, Lebo R, Warburg M, et al. Nat Genet. 1992;1(3):199–203. doi: 10.1038/ng0692-199. [DOI] [PubMed] [Google Scholar]
- 2.Chen ZY, Hendriks RW, Jobling MA, Powell JF, Breakefield XO, Sims KB, Craig IW. Nat Genet. 1992;1(3):204–8. doi: 10.1038/ng0692-204. [DOI] [PubMed] [Google Scholar]
- 3.Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Cell. 2004;116(6):883–95. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 4.Toomes C, Bottomley HM, Jackson RM, Towns KV, Scott S, Mackey DA, Craig JE, Jiang L, Yang Z, Trembath R, Woodruff G, Gregory-Evans CY, Gregory-Evans K, Parker MJ, Black GC, Downey LM, Zhang K, Inglehearn CF. Am J Hum Genet. 2004;74(4):721–30. doi: 10.1086/383202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiao X, Ventruto V, Trese MT, Shastry BS, Hejtmancik JF. Am J Hum Genet. 2004;75(5):878–84. doi: 10.1086/425080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rehm HL, Zhang DS, Brown MC, Burgess B, Halpin C, Berger W, Morton CC, Corey DP, Chen ZY. J Neurosci. 2002;22(11):4286–92. doi: 10.1523/JNEUROSCI.22-11-04286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. Nature. 1996;382(6588):225–30. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 8.Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. Nature. 2000;407(6803):527–30. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 9.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. Nature. 2000;407(6803):530–5. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 10.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. Nature. 2000;407(6803):535–8. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 11.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. Nature. 2001;411(6835):321–5. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 12.Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Curr Biol. 2001;11(12):951–61. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 13.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Nat Cell Biol. 2001;3(7):683–6. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 14.Liu T, DeCostanzo AJ, Liu X, Wang H, Hallagan S, Moon RT, Malbon CC. Science. 2001;292(5522):1718–22. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- 15.Slusarski DC, Corces VG, Moon RT. Nature. 1997;390(6658):410–3. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- 16.Polakis P. Genes Dev. 2000;14(15):1837–51. [PubMed] [Google Scholar]
- 17.Peifer M, Polakis P. Science. 2000;287(5458):1606–9. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 18.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. Cell. 1997;90(1):181–92. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 19.Latres E, Chiaur DS, Pagano M. Oncogene. 1999;18(4):849–54. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- 20.Klingensmith J, Nusse R, Perrimon N. Genes Dev. 1994;8 (1):118–30. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Yuan H, Weaver CD, Mao J, Farr GH, 3rd, Sussman DJ, Jonkers J, Kimelman D, Wu D. Embo J. 1999;18 (15):4233–40. doi: 10.1093/emboj/18.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Cell. 1997;88(6):789–99. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 23.Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. Trends Genet. 2000;16(7):279–83. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 24.Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Dev Biol. 1997;182(1):114–20. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- 25.Sheldahl LC, Park M, Malbon CC, Moon RT. Curr Biol. 1999;9(13):695–8. doi: 10.1016/s0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- 26.Kuhl M, Sheldahl LC, Malbon CC, Moon RT. J Biol Chem. 2000;275(17):12701–11. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- 27.Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K. Nature. 2002;417(6886):295–9. doi: 10.1038/417295a. [DOI] [PubMed] [Google Scholar]
- 28.Kuhl M, Geis K, Sheldahl LC, Pukrop T, Moon RT, Wedlich D. Mech Dev. 2001;106(1–2):61–76. doi: 10.1016/s0925-4773(01)00416-6. [DOI] [PubMed] [Google Scholar]
- 29.Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL. Development. 1994;120(2):347–60. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- 30.Mlodzik M. Trends Genet. 2002;18(11):564–71. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- 31.Eaton S. Curr Opin Cell Biol. 1997;9(6):860–6. doi: 10.1016/s0955-0674(97)80089-0. [DOI] [PubMed] [Google Scholar]
- 32.Mlodzik M. Embo J. 1999;18(24):6873–9. doi: 10.1093/emboj/18.24.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robitaille J, MacDonald ML, Kaykas A, Sheldahl LC, Zeisler J, Dube MP, Zhang LH, Singaraja RR, Guernsey DL, Zheng B, Siebert LF, Hoskin-Mott A, Trese MT, Pimstone SN, Shastry BS, Moon RT, Hayden MR, Goldberg YP, Samuels ME. Nat Genet. 2002;32(2):326–30. doi: 10.1038/ng957. [DOI] [PubMed] [Google Scholar]
- 34.Toomes C, Downey LM, Bottomley HM, Mintz-Hittner HA, Inglehearn CF. Br J Ophthalmol. 2005;89(2):194–7. doi: 10.1136/bjo.2004.042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukla D, Singh J, Sudheer G, Soman M, John RK, Ramasamy K, Perumalsamy N. Indian J Ophthalmol. 2003;51 (4):323–8. [PubMed] [Google Scholar]
- 36.Baron R, Rawadi G, Roman-Roman S. Curr Top Dev Biol. 2006;76:103–27. doi: 10.1016/S0070-2153(06)76004-5. [DOI] [PubMed] [Google Scholar]
- 37.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. J Cell Biol. 2002;157(2):303–14. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masckauchan TN, Kitajewski J. Physiology (Bethesda) 2006;21:181–8. doi: 10.1152/physiol.00058.2005. [DOI] [PubMed] [Google Scholar]
- 39.Fruttiger M. Angiogenesis. 2007;10(2):77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- 40.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. J Cell Biol. 2003;161(6):1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luhmann UF, Lin J, Acar N, Lammel S, Feil S, Grimm C, Seeliger MW, Hammes HP, Berger W. Invest Ophthalmol Vis Sci. 2005;46(9):3372–82. doi: 10.1167/iovs.05-0174. [DOI] [PubMed] [Google Scholar]
- 42.Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. Nature. 2005;437(7057):417–21. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shu W, Jiang YQ, Lu MM, Morrisey EE. Development. 2002;129(20):4831–42. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh M, Boerboom D, Shimada M, Lo Y, Parlow AF, Luhmann UF, Berger W, Richards JS. Biol Reprod. 2005;73(6):1135–46. doi: 10.1095/biolreprod.105.042739. [DOI] [PubMed] [Google Scholar]
- 45.Luhmann UF, Meunier D, Shi W, Luttges A, Pfarrer C, Fundele R, Berger W. Genesis. 2005;42(4):253–62. doi: 10.1002/gene.20141. [DOI] [PubMed] [Google Scholar]
- 46.Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Development. 1996;122(11):3343–53. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J. Cell Growth Differ. 1997;8(12):1349–58. [PubMed] [Google Scholar]
- 48.Jeays-Ward K, Dandonneau M, Swain A. Dev Biol. 2004;276(2):431–40. doi: 10.1016/j.ydbio.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 49.Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Proc Natl Acad Sci U S A. 2003;100(6):3299–304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright M, Aikawa M, Szeto W, Papkoff J. Biochem Biophys Res Commun. 1999;263(2):384–8. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Macke JP, Abella BS, Andreasson K, Worley P, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. J Biol Chem. 1996;271(8):4468–76. doi: 10.1074/jbc.271.8.4468. [DOI] [PubMed] [Google Scholar]
- 52.Goodwin AM, D’Amore PA. Angiogenesis. 2002;5(1–2):1–9. doi: 10.1023/a:1021563510866. [DOI] [PubMed] [Google Scholar]
- 53.Wu WB, Peng HC, Huang TF. Exp Cell Res. 2003;286(1):115–27. doi: 10.1016/s0014-4827(03)00105-8. [DOI] [PubMed] [Google Scholar]
- 54.Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Angiogenesis. 2005;8(1):43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- 55.Masckauchan TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, Khoo A, Tycko B, Brown AM, Kitajewski J. Mol Biol Cell. 2006;17(12):5163–72. doi: 10.1091/mbc.E06-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biswas P, Canosa S, Schoenfeld J, Schoenfeld D, Tucker A, Madri JA. Biochem Biophys Res Commun. 2003;303(1):212–8. doi: 10.1016/s0006-291x(03)00313-9. [DOI] [PubMed] [Google Scholar]
- 57.Hanai J, Dhanabal M, Karumanchi SA, Albanese C, Waterman M, Chan B, Ramchandran R, Pestell R, Sukhatme VP. J Biol Chem. 2002;277(19):16464–9. doi: 10.1074/jbc.M112274200. [DOI] [PubMed] [Google Scholar]
- 58.Holnthoner W, Pillinger M, Groger M, Wolff K, Ashton AW, Albanese C, Neumeister P, Pestell RG, Petzelbauer P. J Biol Chem. 2002;277(48):45847–53. doi: 10.1074/jbc.M209354200. [DOI] [PubMed] [Google Scholar]
- 59.Blankesteijn WM, Creemers E, Lutgens E, Cleutjens JP, Daemen MJ, Smits JF. Acta Physiol Scand. 2001;173(1):75–82. doi: 10.1046/j.1365-201X.2001.00887.x. [DOI] [PubMed] [Google Scholar]