Abstract
Patterns of smoking behavior vary between the sexes. There is evidence that decision-making, which is one of the key “executive functions” necessary for making life-style modifications such as smoking cessation, is relatively lateralized to the right hemisphere in males and left hemisphere in females. In the current study, we examined whether the side of brain lesion has a differential effect on smoking behavior between the sexes. We hypothesized sex differences in smoking cessation based on lesion side. Participants were 49 males and 50 females who were smoking at the time of lesion onset. The outcome variable was abstinence from smoking (quit rate) at least one year post lesion. We found that in patients with left hemisphere damage, quit rates were significantly higher in males than in females; however, in patients with right hemisphere damage, quit rates were not statistically different. The findings support previous cognitive neuroscience literature showing that components of behavior responsible for maintaining addiction tend to be more strongly lateralized in males, whereas in females there is a more bilateral distribution. Our study provides further evidence for differences in lateralization of complex behavior between the sexes, which has significant implications for differences in treatment strategies between the sexes.
Keywords: behavior lateralization, smoking cessation, addiction, gender differences, drug abuse
INTRODUCTION
It is known that males and females differ in both neuroanatomy and behavior. Neuroanatomically, there are not only significant differences in brain size and grey/white matter volumes (see Cahill (2006) for review), but also differences in morphologic and functional lateralization—that is, there are sex-related differences in the structures and functions of the left and right cerebral hemispheres. For example, there is evidence from some structure-specific studies of the amygdala and ventromedial prefrontal cortex (vmPFC), which are involved in social conduct, emotional processing, and decision-making, that the right-hemisphere seems to be critical in males but not females, while the left-hemisphere seems to be critical in females but not males (Cahill, 2003; Tranel & Bechara, 2009; Tranel, Bechara, & Denburg, 2002; Tranel, Damasio, Denburg, & Bechara, 2005).
Behaviorally, it is generally the case that the sexes are alike in most aspects, although moderate to large sex differences do exist in certain domains (Hyde, 2005). There is strong support in the literature for differences in such realms as language and visuospatial abilities (Gur et al., 2010; Voyer, Voyer, & Bryden, 1995). A growing amount of evidence exists for behavioral differences in smoking addiction, which is a behavior that has major health implications. For smokers in an otherwise healthy population, females are less likely to try to quit smoking and more likely to relapse after trying to quit (Perkins, 2001; Piper et al., 2010; Ward, Klesges, Zbikowski, Bliss, & Garvey, 1997; Wetter et al., 1999). Literature is mixed regarding the sex-specific patterns of smoking cessation after diagnoses of a life-threatening illness (e.g., stroke, chronic obstructive pulmonary disease, and cancer) with support for equivalent, higher, and lower quit rates in males than females (Bak et al., 2002; Berg et al., 2013; Redfern, McKevitt, Dundas, Rudd, & Wolfe, 2000; Tashkin et al., 2001; Tseng, Lin, Moody-Thomas, Martin, & Chen, 2012).
The biological and environmental reasons behind sex differences in smoking cessation are multifaceted. These include but are not limited to: 1) greater upregulation of nicotine receptors in the brain; 2) poor responsiveness to nicotine replacement therapy; 3) higher self-reported nicotine withdrawal, 4) increased vulnerability to depression, negative affect, and stress (especially post-cessation); 5) greater sensitivity to non-nicotinic stimuli (e.g., sensory and environmental); and 6) greater concerns for weight gain after smoking cessation in females (see Reynoso, Susabada, and Cepeda-Benito (2005) for review) (Borrelli, Bock, King, Pinto, & Marcus, 1996; Cosgrove et al., 2012; Hatsukami, Skoog, Allen, & Bliss, 1995; Perkins, 1996; Pirie et al., 1992). While the aforementioned sex differences in smoking behavior have been studied extensively, there is a paucity of research investigating whether males and females differ in the broad neural substrates necessary for maintaining smoking addiction.
It is known that activation of frontal, limbic, paralimbic, striatum, and midbrain regions of both hemispheres occurs in both sexes in response to smoking cues (Due, Huettel, Hall, & Rubin, 2002; Franklin et al., 2007; Wilson, Sayette, Delgado, & Fiez, 2005). A limited number of studies have directly examined sex differences in hemispheric activation in addiction. McClernon, Kozink, and Rose (2008) studied the sexes independently in a smoking cue-reactivity task; they reported cuneus and superior temporal gyrus activation in females and hippocampal and orbitofrontal cortex activation in males. In cocaine-dependent populations, Kilts, Gross, Ely, and Drexler (2004) reported greater activity in the right amygdala in males versus females while viewing cocaine-related images using positron emission tomography (PET) imaging. Also in cocaine-dependent individuals, Potenza et al. (2012) demonstrated sex differences in corticostriatal-limbic hyperactivity during functional magnetic resonance imaging (fMRI), with activity linked to stress cues in females and drug cues in males. In cigarette-dependent populations, one study utilized pseudo-continuous arterial spin-labeled perfusion fMRI to demonstrate greater hippocampus and amygdala activation in males. Although these studies have investigated relationships between sex, brain activation, and drug or stress cues, no studies have examined the direct interaction between sex, brain damage, and smoking behavior. To address this issue, we examined the effect of lesion side on smoking behavior in a group of males and females who were smoking at the time of acute stroke. This was based on the evidence that damage to the insula and/or parts of the BG (especially putamen) leads to sudden and sustained cessation of smoking addiction in a significant number of patients, but the role of sex related differences in smoking behavior was not addressed (Naqvi, Rudrauf, Damasio, & Bechara, 2007).
The goal of the present study was to explore potential sex differences in the neural circuitry of smoking addiction. Disruption of smoking addiction involves a number of factors, including but not limited to urge to smoke, rewarding effects of smoking, and access to cigarettes, and smokers presumably use such factors to make the conscious decision of whether or not to smoke. The main question in this study is what effects sex and laterality of lesion have on smoking behavior, and we are employing the construct of decision-making to frame our hypothesis as to which hemisphere would be more crucial in maintaining smoking behavior. Based on previous evidence that damage to the right hemisphere compromises decision-making in men, we reasoned that in the case of smoking, damage to the right hemisphere in men would hinder one of the key mechanisms of self-control (i.e., decision-making and the “reflective system”), thereby unleashing the intact left-hemisphere “impulsive” system to promote smoking behavior. In contrast, damage to the left hemisphere in men would leave unaltered the key right-hemisphere neural circuit for decision-making and self-control, and thus preserved decision-making capacity would empower the reflective mechanisms of self-control to promote smoking cessation in men. The opposite reasoning would be true for women. Therefore, we hypothesized sex- and hemisphere-related differences in smoking cessation following brain damage, with the specific predictions that: 1) In patients with left hemisphere damage, quit rates will be higher in males than in females; in contrast, (2) In patients with right hemisphere damage, quit rates will be higher in females than in males.
MATERIALS AND METHODS
Participants
We identified 99 lesion patients who smoked at the time of their stroke whom we recruited in a prospective or retrospective manner. The retrospective patients (n = 56) included in this study were drawn from the Patient Registry of the Division of Behavioral Neurology and Cognitive Neuroscience, Department of Neurology, University of Iowa. The prospective patients (n = 43) included in this study were recruited at the time of lesion onset and followed for over one year. The patients in the retrospective group underwent extensive screening and characterization of neuropsychological functioning in connection with their participation in our Patient Registry using standard protocols of the Benton Neuropsychology Laboratory and the Laboratory of Brain Imaging and Cognitive Neuroscience (Tranel, 2007). In keeping with the conditions for enrollment in our Patient Registry, the participants had to be free of a history of mental retardation, learning disability, psychiatric disorder, or substance abuse (other than nicotine). Specific inclusion criteria for this study: patients were over 18 years, were smoking at least one pack per week for more than 2 years at the time of their lesion onset, and had unilateral lesions in neural systems/pathways related to decision-making, emotion, and/or addiction. More specifically, we focused on regions involved in emotional memory, reward-guided behavior, outcome expectancy, reasoning, affect, and other behavior-modifying processes. Such regions include the orbitofrontal cortex, anterior cingulate cortex, lateral and medial prefrontal cortices, amygdala, hippocampus, insula, ventral and dorsal striatum, parietal lobe, and temporal lobe (for reviews see Koob and Volkow (2010); Levine (2009); Pessoa (2008)).
Measurement of smoking behavior
Smoking status at the time of the interview (at least one year post lesion onset) was gathered by a research assistant blind to lesion location and the hypotheses of this study. Patients who reported not smoking in the past month (point prevalence abstinence) were classified as “quitters.” Patients who reported smoking during the past month were classified as “non-quitters.”
Neuroanatomical classification
Neuroanatomical analysis was based on magnetic resonance (MR) or computerized axial tomography (CT) data (for participants for whom an MR was contraindicated). For those who were enrolled in the Patient Registry, all imaging data were obtained at least three months post lesion onset (chronic epoch), and lesion locations were mapped according to the standard procedures of the University of Iowa Human Neuroanatomy and Neuroimaging Laboratory (Damasio & Frank, 1992; Frank, Damasio, & Grabowski, 1997). Chronic lesion analyses were not available for those who were enrolled specifically for the prospective study. As such, a physician not involved in the study as well as a second rater characterized the lesion location of the prospective participants at lesion onset. These raters were both blind to the study hypotheses and objectives.
Lesion volume
We first determined the extent of the lesion in each region of interest (ROI). The magnitude of a lesion within each ROI was approximated using an ordinal scale (0 = no damage, 1 = 1–25%, 2 = 26–75%, 3 = 76–100%). The detailed method for defining ROIs and determining the extent of a region affected by a lesion is described in Damasio and Damasio (1989). In order to calculate the whole brain damage in each subject, we first determined the brain volume each ROI represented in a standardized brain space. We then applied the ratings of lesion magnitude per ROI to the standardized volume to find the volume of lesion damage per ROI. Manual lesion calculation remains the best representation of lesion volume in our sample of participants with various lesion sizes and locations. These values represent an approximation of lesion size replicable in all participants across raters. These volumes were summed for each hemisphere and for whole brain.
Data analysis
Differences between groups were tested as appropriate with Chi-square (Pearson’s chi square or Fisher’s exact test where applicable), Mann-Whitney U test, or t-test. The main binary dependent variable of interest was whether a patient was classified as being a quitter or a non-quitter after lesion onset. Correlation analysis (Pearson or Spearman where applicable) was used to investigate the effect of demographic and neuropsychological variables on smoking status.
RESULTS
Participant demographic data are presented in Table 1. No statistically significant differences were found between males and females with respect to age (t(97) = 1.95, p = .05, Cohen’s d = 0.39), education (U = 1070.5, p = .66, r = 0.05), IQ (t(28) = 1.21, p = .23, Cohen’s d = 0.44), lesion side (χ2(1, N = 99) = 0.01, p = .93, phi = 0.01), study group (time point retrospective or prospective (χ2(1, N = 99) = 1.77, p = .18, phi = 0.13)), or handedness (χ2(1, N = 94) = 2.00, p = .16, phi = 0.30). There were no significant correlations between smoking status and age, education, or FSIQ (Full Scale IQ; Wechsler’s Adult Intelligence Scale) (r(97) = 0.19, p = .06; r(93) = 0.12, p = .24; and r(28) = 0.33, p = .08). Although the age difference was close to being statistically significant, the males and females were very close in the group means (less than a year and a half) and the difference is of no practical consequence (the majority of patients of both sexes are middle-aged).
Table 1.
Demographics and Neuropsychological Characterization
| Total (n = 99) |
Male (n = 49) |
Female (n = 50) |
p |
|
|---|---|---|---|---|
| Age | 53.0 (11.5) | 52.2 (11.0) | 50.8 (11.7) | .05 |
| Education | 12.6 (2.6) | 13.1 (2.6) | 12.5 (1.7) | .66 |
| FSIQ | 97.8 (12.2) | 100.3 (12.2) | 94.9 (12.0) | .24 |
| Lesion Side | 44 R, 55 L | 22 R, 27 L | 22 R, 28 L | .93 |
| Time point | 56 Re, 43 P | 31 Re, 18 P | 25 Re, 25 P | .18 |
| Handedness | 86 R, 8 L | 42 R, 6 L | 44 R, 2 L | .16 |
Values reported as count or mean (standard deviation). p values as determined by chi-square, independent samples t-test, or Mann-Whitney U test. M, male; F, female; R, right; L, left; B, bilateral; FSIQ, Full-scale Intelligence Quotient as measured by the Wechsler Adult Intelligence Scale III or IV; Re, retrospective; P, prospective.
Lesion volumes (Table 2) were not significantly different between males and females. There were no statistical differences in whole brain, left hemisphere, or right hemisphere damage (U = 584, p = .25, r = −0.13; U = 689, p = 0.97, r = −0.005; and U = 598, p = .29, r = −0.12). In the participant sample as a whole, total lesion volume was not significantly correlated with smoking status (r(73) = 0.15, p = .22). When examining lesion volume in each hemisphere, neither left-sided damage (r(73) = 0.22, p = .05) nor right-sided damage (r(73) = −0.13, p = .29) was correlated with smoking status. There was no significant correlation between smoking status and lesion volume when males and females were considered separately. In males, correlations between lesion volume and smoking status were as follows: whole brain r(31) = 0.08, p = .67; left hemisphere r(31) = 0.26, p = .14; and right hemisphere r(31) = −0.25, p = .16. In females, correlations were as follows: whole brain r(40) = 0.20, p = .20; left hemisphere r(40) = 0.21, p = .19; and right hemisphere r(40) = −0.05, p = .75. In summary, there were no systematic or impressive correlations between lesion volume and smoking status, in either the males or females.
Table 2.
Lesion volume in mean mm3 (SD)
| Total (n = 75) |
Female (n = 42) |
Male (n = 33) |
p |
|
|---|---|---|---|---|
| Whole Brain | 26,653 (21,772) | 23,196 (20,988) | 28,780 (22,667) | .25 |
| Left Hemisphere | 13,380 (20,801) | 12,529 (19,606) | 14,463 (22,492) | .97 |
| Right Hemisphere | 12,272 (18,497) | 10,667 (18,059) | 14,316 (19,123) | .29 |
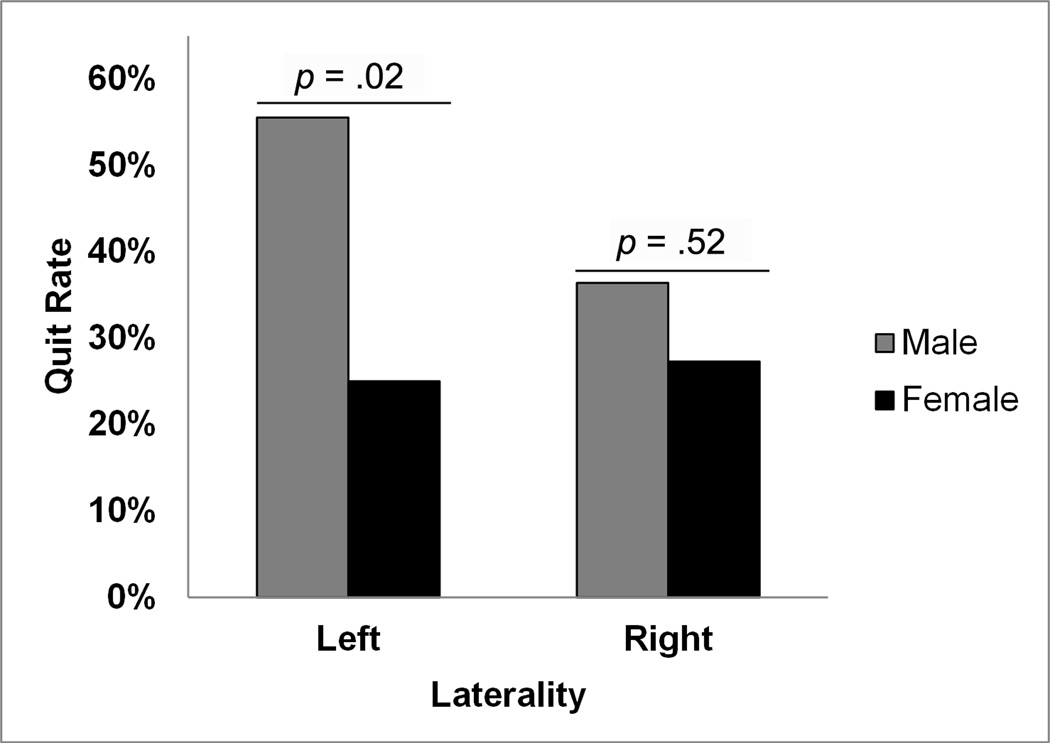
We next explored the effects of sex—within lesion side, as well as the effects of lesion side—within sex, in an omnibus test considering both effects (Figure 1). The result was marginally significant (χ2(1, N = 99) = 6.65, p = .08) with a moderate effect size (Cramer’s V=0.26). For the effects of sex—within lesion side, we compared males and females with lesions to the same side. In examining the participants with left-sided damage alone, lesion volume did not correlate with smoking status (r(36) = 0.27, p = .10). Comparison between sexes revealed a statistically significant difference in those with left-sided lesions, as the male quit rate was over twice that of females (55% versus 25% respectively; χ2(1, n = 55) = 5.35, p = .02). The effect size was moderate at phi = 0.31, while the odds ratio was 3.75 (95% CI [1.20, 11.77]). In examining the participants with right-sided damage alone, lesion volume did not correlate with smoking status (r(35) = 0.06, p = .74). There was no appreciable difference in quit rate between males and females with right-sided lesions (χ2(1, n = 44) = 0.42, p = .52; phi = 0.10; O.R. = 1.52, 95% CI [0.42, 5.47]). To summarize, our patient population demonstrated an effect of sex— within lesion side (male versus female quit rate in those with left-sided lesions; male versus female quit rate in those with right-sided lesions) only for those with left-sided damage.
Figure 1.
Effects of sex and lesion side on smoking cessation. Smoking cessation is represented by “quit rate,” which is calculated as the number quit divided by the number of total participants in each group. Participants include males with left- (n = 27) and right-sided (n = 22) lesions, and females with left- (n = 28) and right-sided (n = 22) lesions.
With respect to the effect of lesion side within sex, we found that males with left-sided lesions had a higher quit-rate versus those with right-sided lesions (55% and 36%, respectively) although this difference did not reach statistical significance (χ2(1, n = 49) = 1.79, p = .18, phi = 0.19; OR = 2.17; 95% CI [0.69, 7.14]). In the female cohort, both lesion groups had similar quit rates of 27% in women with right-sided lesions and 25% in women with left-sided lesions (χ2(1, n = 50) = 0.03, p = .86, phi = 0.03; OR = 1.13, 95% CI [0.32, 4.00]).
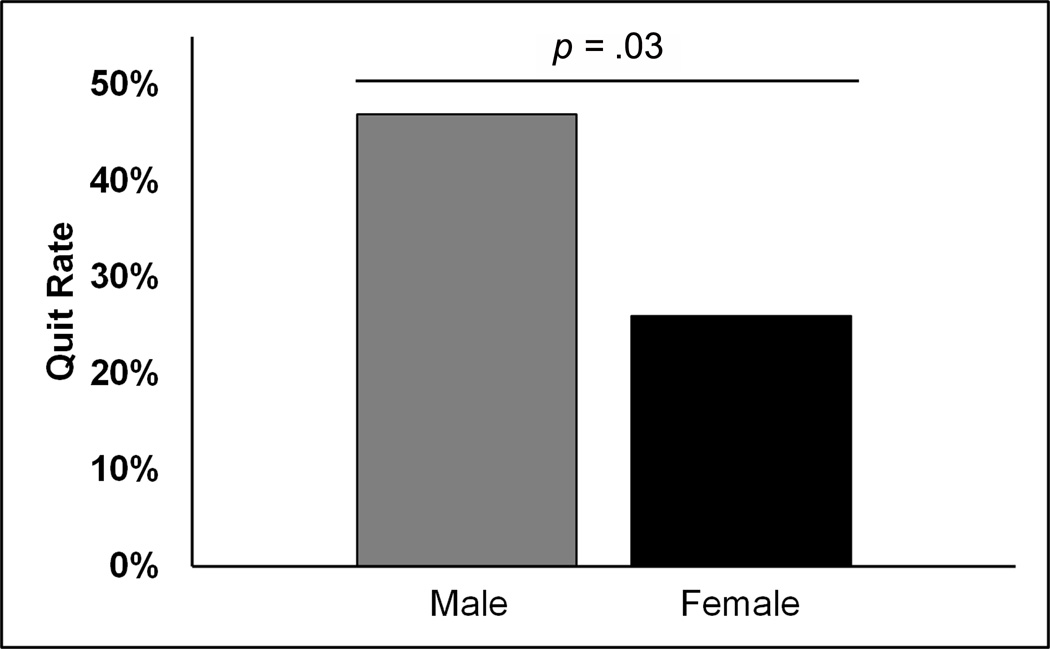
Additionally, we compared point-prevalence smoking cessation between the sexes irrespective of lesion side (Figure 2). Male smokers quit at a significantly higher rate than female smokers, with quit rates of 47% and 26% respectively (χ2(1, N = 99) = 4.70, p = .03, phi= 0.22). The odds ratio (OR) of males quitting was 2.52 (95% CI [1.08, 5.86]). As there were no correlations between total lesion volume, demographics, or neuropsychological characteristics, we did not perform further analyses. Of note, the quit rate of the population as a whole was 36%.
Figure 2.
Sex differences in smoking cessation. Smoking cessation is represented by “quit rate,” which is calculated as the number quit divided by the number of total participants in each group (n= 49 M, 50 F).
DISCUSSION
In this study, we aimed to elucidate the sex differences in the neural circuitry of smoking addiction. Previous work which failed to find an effect of lesion laterality on smoking cessation did not examine the differences in quit rate in a sex by laterality manner (Naqvi et al., 2007). In fact, when males and females were combined in the current study, we did not see an effect of hemisphere either. This is the main difference between the two studies and the strength of the current study- we aimed to examine laterality by sex rather than within the whole group. Our first hypothesis, which proposed that in patients with left hemisphere damage, quit rates will be higher in males than in females, was supported (Figure 1). While acknowledging the fact that the decision to quit smoking is multifaceted, we believe that left-sided damage allowed the right-sided decision-making circuitry, which is dominant in males, combined with a weakened “impulsive” system on the left side, to reach a higher potency of exerting self-control, thereby enabling men to make the decision to quit smoking. This interpretation may be further elucidated by future studies examining whether other healthy lifestyle changes were also made during this time, as well as including specific probes about reasons for quitting in those who stopped smoking. This finding supports previous findings that components of behavior responsible for maintaining addiction (e.g. decision-making) are lateralized in males (Tranel et al., 2005). Additionally, we observed that males with right-sided lesions had a lower quit rate than males with left-sided lesions; although this difference was not statistically significant, the effect size was moderate.
Our second hypothesis, which proposed that in patients with right hemisphere damage, quit rates will be higher in females than in males, was not supported. We proposed that damage to the right side in women would preserve the decision-making circuitry necessary for making the decision to quit after stroke. Furthermore, females with lesions on either side demonstrated similar quit rates. These findings are not surprising in light of literature supporting bilateral distribution of complex cognitive functions in females. Females show greater bilateral activation and interhemispheric connectivity than males in language functions, assessment of beauty, and spatial processing (Bitan, Lifshitz, Breznitz, & Booth, 2010; Gur et al., 2000; McGlone, 1980; Rilea, 2008). Additionally, recent data from Ingalhalikar et al. (2014) examining diffusion-based structural connectomes suggest that female brains are optimized for interhemispheric (rather than intrahemispheric) communication. Addiction is a multi-dimensional behavior comprised of a number of interrelated networks, and as such, its neural components may be of a more bilateral nature in females. Future studies may explore the effect of bilateral lesions on smoking behavior.
In this sample of patients we found a quit rate of 36% for the population as a whole. This is in line with previous studies examining quit rate post stroke (3 month to 3 years after incident), which reported quit rates ranging from 22–43% (Bak et al., 2002; Ives, Heuschmann, Wolfe, & Redfern, 2008; Redfern et al., 2000; Sauerbeck et al., 2005). In the current study, female smokers as a group were less likely to quit smoking than male smokers following acute stroke. While this study focuses on the biological aspects of smoking behavior, future studies should include contributions of concurrent psychological and environmental factors. One advantage of this study is that we report smoking status at least one year post-stroke. The participants are far from the event itself, and the confounding factors of smoking cessation due to lack of access or initial scare due to a serious health event are minimal.
Limitations of this study should be noted. One such limitation is that data regarding abstinence were based on self-report. Epidemiological reports of socially undesirable behaviors such as cigarette consumption are subject to reporting bias (Fendrich, Mackesy-Amiti, Johnson, Hubbell, & Wislar, 2005); however, self-report has been shown as reliable in numerous primary reports and meta-analyses (Patrick et al., 1994; Wong, Shields, Leatherdale, Malaison, & Hammond, 2012; Yeager & Krosnick, 2010). We note that the participants in our study were not receiving either feedback regarding their smoking status or smoking cessation treatment of any kind from our researchers. Another limitation is that analysis of lesion location is limited to lesion side rather than specific structural location. There are a number of neuroanatomical structures involved in the development and maintenance of addictive behaviors. We noted participants with lesions affecting at least one of the three major neural systems that have been highlighted in recent models of addiction. These systems include: 1) the impulsive system, which is implicated in rewards and learned associations, includes temporal cortex lesions involving the amygdala, and basal ganglia lesions specifically involving the ventral striatum; 2) the reflective system, which is implicated in decision-making, includes the dorsolateral and ventromedial prefrontal cortices; and 3) the somatic system, which is implicated in body-state awareness, includes the insular cortex (Cisler et al., 2013; Kelley & Berridge, 2002; Naqvi et al., 2007; Park et al., 2010; See, Fuchs, Ledford, & McLaughlin, 2003; Tanabe et al., 2009). We did not sample these regions sufficiently in the current dataset to allow definitive conclusions, and this warrants further study. Finally, our interpretations of the roles of lesion laterality are made without the benefit of a bilateral lesion group for comparison. Ideally, future studies could include patients with bilateral damage in order to further explore the contribution of bilaterality to complex cognitive functions—although we would hasten to add that bilateral lesions are exceedingly rare for some of the structures mentioned above, and a functional imaging approach will probably be more feasible for some of these questions.
In summary, this study provides evidence for differences in lateralization of complex behavior, namely smoking behavior, between the sexes. Smoking behavior following brain injury requires a decision-making process that takes into account the value of reward and the magnitude of risk. We have examined whether this behavior is driven by different hemispheres in men and women, regardless of the reasoning behind that behavior. In men, right-sided lesions yielded deficits in decision-making regarding real-world smoking behavior change post stroke; in contrast, left-sided lesions allowed for changes in smoking behavior manifest in an increased rate of smoking cessation after a life-threatening event. In women, damage to either side produced similar effects in behavior change post stroke, suggesting a more bilateral distribution for the neural networks responsible for maintenance of smoking behavior after a life-threatening event. These findings suggest that therapeutic strategies for smoking cessation aimed at neuromodulation, such as deep brain stimulation (DBS) or transcranial magnetic stimulation (TMS), may involve sex-specific targeting in which one takes a lateralized approach in men but a more bilateral focus in women.
Acknowledgements
This work was supported by the National Institute on Drug Abuse under Grants [R01 DA023051, R01 DA022549]; and the National Institute of Neurological Disorders and Stroke under Grant [P50 NS19632].
Footnotes
Authorship: All authors of the paper have fulfilled the criteria for authorship.
Declaration of Interest: Authors have no competing interests to declare.
REFERENCES
- Bak S, Sindrup SH, Alslev T, Kristensen O, Christensen K, Gaist D. Cessation of smoking after first-ever stroke: a follow-up study. Stroke. 2002;33(9):2263–2269. doi: 10.1161/01.str.0000027210.50936.d0. [DOI] [PubMed] [Google Scholar]
- Berg CJ, Thomas AN, Mertens AC, Schauer GL, Pinsker EA, Ahluwalia JS, Khuri FR. Correlates of continued smoking versus cessation among survivors of smoking-related cancers. Psycho-Oncology. 2013;22(4):799–806. doi: 10.1002/pon.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Lifshitz A, Breznitz Z, Booth JR. Bidirectional connectivity between hemispheres occurs at multiple levels in language processing but depends on sex. The Journal of Neuroscience. 2010;30(35):11576–11585. doi: 10.1523/JNEUROSCI.1245-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli B, Bock B, King T, Pinto B, Marcus BH. The impact of depression on smoking cessation in women. American Journal of Preventive Medicine. 1996;12(5):378–387. [PubMed] [Google Scholar]
- Cahill L. Sex-related influences on the neurobiology of emotionally influenced memory. Annals of the New York Academy of Sciences. 2003;985:163–173. doi: 10.1111/j.1749-6632.2003.tb07080.x. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;7(6):477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, Andrew James G, Kilts CD. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Research. 2013;213(1):39–46. doi: 10.1016/j.pscychresns.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Esterlis I, McKee SA, Bois F, Seibyl JP, Mazure CM, O'Malley SS. Sex differences in availability of beta2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Archives of General Psychiatry. 2012;69(4):418–427. doi: 10.1001/archgenpsychiatry.2011.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Damasio AR. Lesion Analysis in Neuropsychology. New York: Oxford University Press; 1989. [Google Scholar]
- Damasio H, Frank R. Three-dimensional in vivo mapping of brain lesions in humans. Archives of Neurology. 1992;49(2):137–143. doi: 10.1001/archneur.1992.00530260037016. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159(6):954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Fendrich M, Mackesy-Amiti ME, Johnson TP, Hubbell A, Wislar JS. Tobacco-reporting validity in an epidemiological drug-use survey. Addictive Behaviors. 2005;30(1):175–181. doi: 10.1016/j.addbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage. 1997;5(1):13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32(11):2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Gur RC, Alsop D, Glahn D, Petty R, Swanson CL, Maldjian JA, Gur RE. An fMRI study of sex differences in regional activation to a verbal and a spatial task. Brain and Language. 2000;74(2):157–170. doi: 10.1006/brln.2000.2325. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. The Journal of Neuroscience Methods. 2010;187(2):254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Skoog K, Allen S, Bliss B. Gender and the effects of different doses of nicotine gum on tobacco withdrawal symptoms. Experimental and Clinical Psychopharmacology. 1995;3(2):163–173. [Google Scholar]
- Hyde JS. The gender similarities hypothesis. American Psychologist. 2005;60(6):581–592. doi: 10.1037/0003-066X.60.6.581. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Verma R. Sex differences in the structural connectome of the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(2):823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives SP, Heuschmann PU, Wolfe CD, Redfern J. Patterns of smoking cessation in the first 3 years after stroke: the South London Stroke Register. European Journal of Cardiovascular Prevention and Rehabilitation. 2008;15(3):329–335. doi: 10.1097/HJR.0b013e3282f37a58. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. The Journal of Neuroscience. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. American Journal of Psychiatry. 2004;161(2):233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DS. Brain pathways for cognitive-emotional decision making in the human animal. Neural Networks. 2009;22(3):286–293. doi: 10.1016/j.neunet.2009.03.003. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33(9):2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- McGlone J. Sex differences in human brain asymmetry: a critical survey. Behavioral and Brain Sciences. 1980;3:215–227. [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the Insula Disrupts Addiction to Cigarette Smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, Heinz A. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. The Journal of Neuroscience. 2010;30(22):7749–7753. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. American Journal of Public Health. 1994;84(7):1086–1093. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine versus non-nicotine reinforcement as determinants of tobacco smoking. Experimental and Clinical Psychopharmacology. 1996;4:166–177. [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15(5):391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9(2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, Loh WY. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine & Tobacco Research. 2010;12(6):647–657. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirie PL, McBride CM, Hellerstedt W, Jeffery RW, Hatsukami D, Allen S, Lando H. Smoking cessation in women concerned about weight. American Journal of Public Health. 1992;82(9):1238–1243. doi: 10.2105/ajph.82.9.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. American Journal of Psychiatry. 2012;169(4):406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern J, McKevitt C, Dundas R, Rudd AG, Wolfe CDA. Behavioral Risk Factor Prevalence and Lifestyle Change After Stroke : A Prospective Study. Stroke. 2000;31(8):1877–1881. doi: 10.1161/01.str.31.8.1877. [DOI] [PubMed] [Google Scholar]
- Reynoso J, Susabada A, Cepeda-Benito A. Gender Differences in Smoking Cessation. Journal of Psychopathology and Behavioral Assessment. 2005;27(3):227–234. [Google Scholar]
- Rilea SL. A lateralization of function approach to sex differences in spatial ability: a reexamination. Brain and Cognition. 2008;67(2):168–182. doi: 10.1016/j.bandc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Sauerbeck LR, Khoury JC, Woo D, Kissela BM, Moomaw CJ, Broderick JP. Smoking cessation after stroke: education and its effect on behavior. The Journal of Neuroscience Nursing. 2005;37(6):316–319. 325. [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Annals of the New York Academy of Sciences. 2003;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biological Psychiatry. 2009;65(2):160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashkin D, Kanner R, Bailey W, Buist S, Anderson P, Nides M, Jamerson B. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet. 2001;357(9268):1571–1575. doi: 10.1016/s0140-6736(00)04724-3. [DOI] [PubMed] [Google Scholar]
- Tranel D. Theories of clinical neuropsychology and brain-behavior relationships: Luria and beyond. In: Morgan JE, Ricker JH, editors. Textbook of clinical neuropsychology. New York: Taylor and Francis; 2007. pp. 27–39. [Google Scholar]
- Tranel D, Bechara A. Sex-related functional asymmetry of the amygdala: preliminary evidence using a case-matched lesion approach. Neurocase. 2009;15(3):217–234. doi: 10.1080/13554790902775492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38(4):589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128(Pt 12):2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Tseng TS, Lin HY, Moody-Thomas S, Martin M, Chen T. Who tended to continue smoking after cancer diagnosis: the national health and nutrition examination survey 1999-2008. BMC Public Health. 2012;12:784. doi: 10.1186/1471-2458-12-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychological Bulletin. 1995;117(2):250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Ward KD, Klesges RC, Zbikowski SM, Bliss RE, Garvey AJ. Gender differences in the outcome of an unaided smoking cessation attempt. Addictive Behaviors. 1997;22(4):521–533. doi: 10.1016/s0306-4603(96)00063-9. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology. 1999;67(4):555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine & Tobacco Research. 2005;7(4):637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SL, Shields M, Leatherdale S, Malaison E, Hammond D. Assessment of validity of self-reported smoking status. Health Reports. 2012;23(1):47–53. [PubMed] [Google Scholar]
- Yeager DS, Krosnick JA. The validity of self-reported nicotine product use in the 2001-2008 National Health and Nutrition Examination Survey. Medical Care. 2010;48(12):1128–1132. doi: 10.1097/MLR.0b013e3181ef9948. [DOI] [PubMed] [Google Scholar]