Abstract
Monoamine transporters have been implicated in dopamine or serotonin release in response to abused drugs such as methamphetamine or ecstasy (MDMA). In addition, monoamine transporters show substrate-induced inward currents that may modulate excitability and Ca2+ mobilization, which could also contribute to neurotransmitter release. How monoamine transporters modulate Ca2+ permeability is currently unknown. We investigate the functional interaction between the human serotonin transporter (hSERT) and voltage-gated Ca2+ channels (CaV). We introduce an excitable expression system consisting of cultured muscle cells genetically engineered to express hSERT. Both 5HT and S(+)MDMA depolarize these cells and activate the excitation-contraction (EC)-coupling mechanism. However, hSERT substrates fail to activate EC-coupling in CaV1.1-null muscle cells, thus implicating Ca2+ channels. CaV1.3 and CaV2.2 channels are natively expressed in neurons. When these channels are co-expressed with hSERT in HEK293T cells, only cells expressing the lower-threshold L-type CaV1.3 channel show Ca2+ transients evoked by 5HT or S(+)MDMA. In addition, the electrical coupling between hSERT and CaV1.3 takes place at physiological 5HT concentrations. The electrical coupling between monoamine neurotransmitter transporters and Ca2+ channels such as CaV1.3 is a novel mechanism by which endogenous substrates (neurotransmitters) or exogenous substrates (like ecstasy) could modulate Ca2+-driven signals in excitable cells.
Keywords: calcium channels, calcium imaging, L-type calcium channel, N-type calcium channel, monoamine transporters, neurotransmitter transport, skeletal muscle, excitability
1. Introduction
Monoamine neurotransmitter transporters belong to the solute carrier 6 (SLC6) gene family formed by structurally related proteins that use the Na+ driving force to concentrate substrates against their chemical gradient [1]. Their known physiological functions are to limit the neurotransmitter signaling by decreasing its extracellular concentration and to cycle the transmitter molecules back to the presynaptic cells [2]. Small molecules that target monoamine transporters are therapeutically significant; amphetamine and methylphenidate which act on the dopamine transporter (DAT) are used as treatment for attention deficit hyperactivity disorder, whereas serotonin transporter (SERT) inhibitors like fluoxetine or citalopram are effective drugs to treat mood disorders [3, 4]. Drugs that interfere with DAT activity, e.g. cocaine, amphetamine, and certain amphetamine derivatives are stimulants that have high addictive liability [5]. In particular 3,4-methylenedioxy-N-methylamphetamine (MDMA or ecstasy), which is highly abused, share characteristics of both stimulant and empathogen and targets both DAT and SERT [6-8].
Most psychoactive amphetamines that are substrates of monoamine transporters compete with neurotransmitter uptake and are believed to disrupt vesicular neurotransmitter storage and induce efflux of neurotransmitters through the transporter; therefore increasing the extracellular concentration of the neurotransmitter in the brain [9, 10]. Monoamine transporters show significant inward currents when challenged with substrates. These substrate-induced currents are viewed as uncoupled from the actual transport and although they have not been directly implicated in Ca2+ channel activation, they can increase excitability, which presumably further contributes to neurotransmitter release [11].
Voltage-gated Ca2+ channels are well known modulators of cell excitability and the intracellular Ca2+ concentration ([Ca2+]i) in excitable cells [12]. The lower-threshold L-type Ca2+ channel CaV1.3 is involved in dendritic serotonin release from serotonergic neurons [13] and pace-making in dopaminergic neurons [14]. Ca2+ channels in neurons open briefly leading to local events that are not efficiently amplified by intracellular Ca2+ channels from internal stores compared to muscle cells; in addition, neurons express a variety of Ca2+ channels that make the connection between drug-induced currents and Ca2+ channel activation difficult to interpret. To overcome these problems, we have devised a new model in which we express the human SERT (hSERT) in an excitable cell that contains a reliable Ca2+ amplification system that allows us to explore the effect of serotonin (5HT) and S(+)MDMA (the most potent enantiomer of ecstasy) on cytosolic Ca2+ signals. We showed in engineered cultured muscle cells (myotubes) expressing hSERT that both S(+)MDMA and 5HT mobilize Ca2+ evidenced by Ca2+ transients. In addition, hSERT substrates failed to induce Ca2+ signals in CaV1.1-null myotubes, suggesting that hSERT substrate-induced currents depolarize the plasma membrane sufficiently, to the level of voltage-gated Ca2+ channel activation. To test whether the hSERT-mediated depolarization activates voltage-gated Ca2+ channels that are natively expressed in neurons, we co-expressed hSERT either with CaV1.3 or CaV2.2 in HEK293T cells. Both 5HT and S(+)MDMA induce Ca2+ signals in CaV1.3- but not in CaV2.2-expressing cells, suggesting that hSERT-mediated depolarization is within the range of activation of Ca2+ channels that are more sensitive to membrane depolarization. In addition, 5HT showed comparable potency for both, activating the CaV1.3-mediated Ca2+ signal, and for inducing its transport; suggesting that 5HT transport and hSERT-coupling to voltage-gated Ca2+ channels may coexist in the physiological range of 5HT concentration.
In summary we demonstrate that the hSERT-mediated depolarization activates voltage-gated Ca2+ channels, in particular those more sensitive to membrane depolarization. These findings support a mechanism by which hSERT activation may modulate Ca2+ permeability in excitable cells.
2. Results
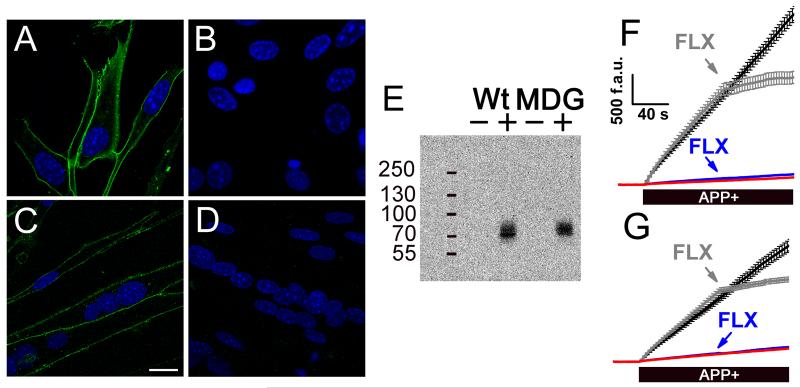
To study whether the membrane depolarization driven by substrate-induced currents of hSERT can couple to the activation of voltage-gated Ca2+ channels, we expressed hSERT in cultured muscle cells (myotubes). Skeletal muscle cells were selected for these experiments because they have the excitation-contraction (EC)-coupling mechanism which is characterized by a tight control of the [Ca2+]i as function of the membrane potential. In addition since EC-coupling is driven by CaV1.1 activation, it is a suitable model to assess voltage-gated Ca2+ channel activation by substrate-induced hSERT currents. The response of EC-coupling in skeletal muscle cells is in the millisecond time scale, thus any depolarization of the membrane potential is almost instantaneously followed by increase in [Ca2+]i [15]. In this study we expressed hSERT in wild type (Wt) and dysgenic (MDG) myotubes to study the depolarization evoked by hSERT substrates. MDG myotubes were used as controls because they do not express CaV1.1 which is the voltage sensor of EC-coupling, therefore they are insensitive to voltage changes [16]. Myotubes do not express hSERT natively, but after transduction with hSERT-IRES-Puromycin retrovirus they expressed this protein as shown in Fig. 1. The hSERT immuno-staining visualized by confocal microscopy showed a plasma membrane localization of the protein in transduced cells (Fig. 1A and 1C). Western blot analysis showed a single band only in transduced cells (Fig. 1E). Moreover, fluoxetine-sensitive APP+ uptake was detected only in myotubes transduced with the hSERT-encoding retrovirus (Fig. 1F and 1G).
Figure 1. Expression of hSERT in cultured skeletal muscle cells.
Myoblasts isolated from wild type (Wt) or dysgenic (MDG) mice were transduced with the hSERT encoding retrovirus. The transduced cells were selected as described in Materials and methods. The cells were differentiated into myotubes and the hSERT expression was evaluated by immunofluorescence visualized by confocal microscopy (scale bar = 20 μm). The hSERT signal is shown in green and the DAPI nuclear staining is shown in blue for Wt-transduced (A), Wt-control (B), MDG-transduced (C), and MDG-control myotubes (D). E, Western blot that shows the expression of hSERT in samples from myotubes transduced with the hSERT-retrovirus (+) or uninfected cells (−). (F and G) The hSERT activity was evaluated measuring the uptake of APP+ by fluorescence microscopy. F, Wt transduced myotubes (black trace) and the effect of fluoxetine (gray trace, FLX). Non-transduced Wt myotubes (red trace) and the effect of fluoxetine (FLX, blue trace) are plotted. G is the same as F but performed in dysgenic (MDG) myotubes. The traces shown are mean ± s.e.m. of n ≥ 25 cells for each condition.
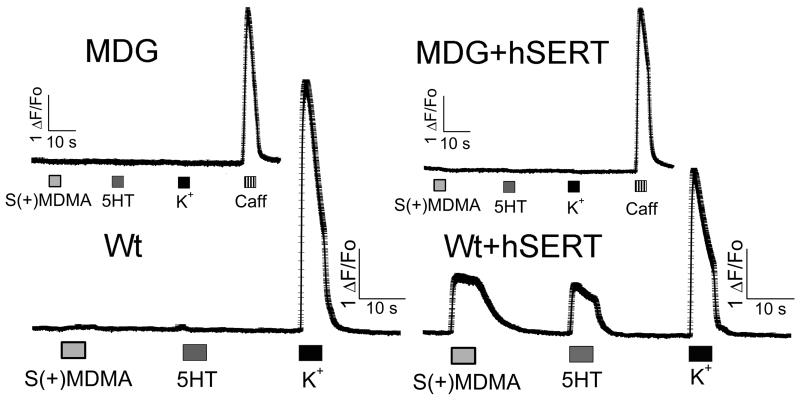
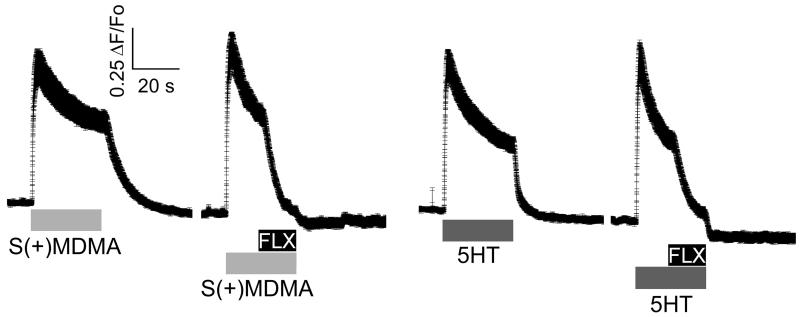
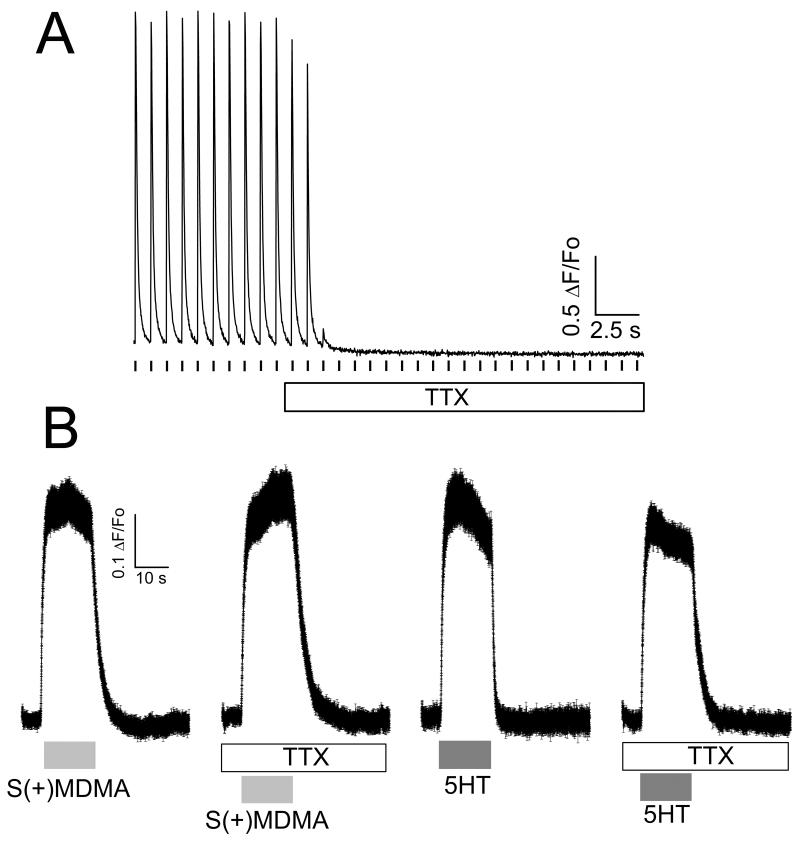
Interestingly, Wt myotubes expressing hSERT showed a fast and reversible increase of [Ca2+]i during exposure to S(+)MDMA, 5HT, or high external K+ (Fig. 2). Conversely, MDG myotubes expressing hSERT did not respond to S(+)MDMA, 5HT or high external K+, but did respond to caffeine, which opens the intracellular Ca2+ channel directly, releasing Ca2+ from internal stores (Fig. 2). The refractoriness of MDG+hSERT myotubes to 5HT or S(+)MDMA strongly suggests that the depolarization, and not any other secondary effect, causes the increase in [Ca2+]i in Wt+hSERT myotubes. Accordingly, [Ca2+]i was unchanged when non-transduced Wt or MDG cells were challenged with 5HT or S(+)MDMA (Fig. 2). Furthermore, the Ca2+ transients induced either by 5HT or by S(+)MDMA were blocked completely by fluoxetine in WT+hSERT myotubes, demonstrating that hSERT-mediated depolarization exceeds the threshold for EC-coupling activation (Fig. 3). In contrast, Ca2+ signals induced by both hSERT substrates are insensitive to tetrodotoxin (TTX, 3μM), whereas the TTX batch used in this study completely blocked action potential-induced Ca2+ transients in myotubes (Fig. 4). Together these data suggest that voltage-gated Na+ channel activity does not contribute in the overall membrane depolarization induced by hSERT currents in myotubes expressing the transporter.
Figure 2. S(+)MDMA and 5HT activate EC-coupling in skeletal muscle cells expressing hSERT.
Differentiated muscle cells in culture (myotubes) were loaded with the permeable Ca2+ sensitive dye Fluo-4AM. The intracellular Ca2+ concentration was monitored at 35°C under constant perfusion by fluorescence microscopy. The acquisition frequency used was 100 Hz. The wild type (Wt) and the Wt myotubes transduced with hSERT retrovirus (Wt+hSERT) were exposed to 10 μM S(+)MDMA, 10 μM 5HT, or high K+ solution (40 mM) as indicated in the figure. The dysgenic (MDG) and the MDG myotubes transduced with hSERT retrovirus (MDG+hSERT) were exposed to the same protocol plus an additional pulse of caffeine 20 mM (Caff). Average traces ± s.e.m. are shown (n ≥ 36).
Figure 3. Fluoxetine inhibits the EC-coupling activation induced by hSERT substrates.
Differentiated muscle cells in culture (myotubes) were loaded with the permeable Ca2+ sensitive dye Fluo-4AM. The intracellular Ca2+ concentration was monitored at 35°C under constant perfusion by fluorescence microscopy. The acquisition frequency used was 100 Hz. Ca2+ signal of Wt myotubes expressing hSERT exposed to 10 μM S(+)MDMA, 10 μM 5HT and 1μM fluoxetine as indicated in the figure. The traces shown are the mean ± s.e.m. of n≥30 cells. The peak of the Ca2+ increase induced by S(+)MDMA (0.95 ± 0.102 γF/Fo n = 37) is no different from the 5HT-induced peak (1.0 ± 0.093 γF/Fo n = 36, p > 0.05 t-test).
Figure 4. EC-coupling activation mediated by hSERT substrates does not involve voltage-gated Na+ channels.
(A) Cultured myotubes were loaded with Fluo4-AM and the intracellular Ca2+ concentration was monitored by fluorescence microscopy at room temperature under constant perfusion. The acquisition frequency was 50 Hz. The representative trace shows a myotube electrically paced at 1Hz; the Ca2+ transient was completely blocked by 3 μM TTX. (B). Intracellular Ca2+ concentration was measured by fluorescence microscopy at 35°C under constant perfusion. The acquisition frequency was 100 Hz. After dye loading, hSERT-expressing myotubes were pre-incubated with TTX (3 μM) for 20 min, and TTX was kept in the perfusion solutions during the whole experiment. Control hSERT-myotubes were subjected to the same protocol but in absence of TTX. The traces shown are the mean ± s.e.m. of n≥39 cells. TTX did not affect the amplitude of the Ca2+ signal induced by MDMA (10 μM) or 5HT (10 μM) in myotubes expressing hSERT.
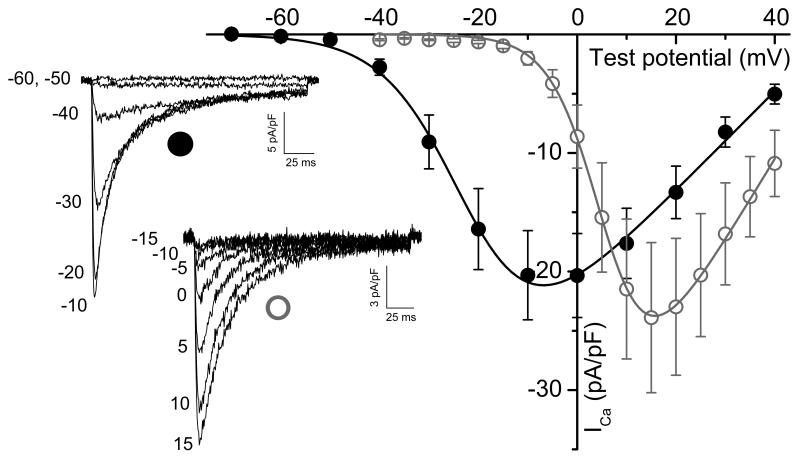
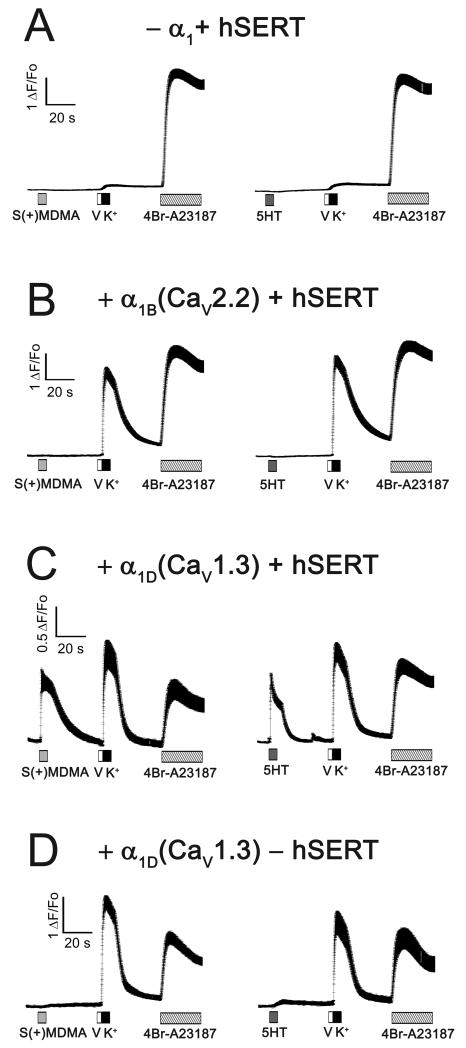
To test the hypothesis that either 5HT- or S(+)MDMA-induced depolarization can activate the lower threshold L-type Ca2+ channel CaV1.3 - or as a control, the high-voltage-activated CaV2.2 Ca2+ channel - were co-expressed with hSERT in HEK293T cells. As shown by others [17] and confirmed here the Ca2+ current (ICa) of CaV1.3 has a ~30 mV left shift in its voltage dependence curve compared to the ICa of CaV2.2 (Fig. 5). Therefore, the activation threshold of ICa is ~−50 mV and ~−15 mV for CaV1.3 and CaV2.2, respectively (Fig. 5). To study the electrical coupling between hSERT and these Ca2+ channels, the co-transfected cells were subjected to intracellular [Ca2+] determination by fluorescence microscopy as described in Materials and methods. When hSERT was expressed without either CaV channel, the cells did not respond to hSERT substrates or to high K+-depolarization (Fig. 6A). In contrast, 5HT and S(+)MDMA evoked fast Ca2+ transients when CaV1.3 (but not CaV2.2) was co-expressed with hSERT: nevertheless, high K+ depolarization evoked Ca2+ transients when either Ca2+ channel was expressed (Fig. 6B and 6C), demonstrating that hSERT-mediated depolarization activates CaV1.3 but not CaV2.2. Finally, cells transfected with CaV1.3 in the absence of hSERT only have a positive response to high K+ depolarization, whereas S(+)MDMA or 5HT are without effect (Fig. 6D).
Figure 5. Voltage-dependence of CaV1.3- and CaV2.2- mediated Ca2+ currents.
HEK293T cells were cotransfected with hSERT-IRES-DsRed, β3, α2γ1 and alternatively CaV1.3 (closed black circles) or CaV2.2 (open gray circles) plasmids. The experiments were done under constant perfusion at 23°C. The currents were evoked by a trend of pulses starting at a holding potential of −70 mV in steps of 10 mV for CaV1.3 or steps of 5 mV for CaV2.2. Representative family of responses for CaV1.3 (closed black circle) or CaV2.2 (open gray circle), and the value of the test potential are shown for each sweep. The current-voltage dependence of the peak current densities were fit according to Eq.1 (Materials and methods) for CaV1.3 (black trace) and CaV2.2 (gray trace). The data are expressed as mean ± s.e.m. The fitting parameters are the following: Gmax, 458 ± 84 and 737 ± 178 (pS/pF); V1/2, −20 ± 1.9 and 7*** ± 0.7 (mV); k, 7.9 ± 0.37, and 5.1*** ± 0.1 (mV) for CaV1.3 (n = 6) and CaV2.2 (n = 8), respectively. *** = p < 0.0001, t-test.
Figure 6. The depolarization induced by hSERT activation is electrically coupled to CaV1.3 opening.
HEK293T cells were loaded with the permeable Ca2+ sensitive dye Fluo-4AM and the intracellular Ca2+ concentration was measured by fluorescence microscopy at an acquisition rate of 50 Hz. All measurements were done at 35°C under constant perfusion. The tested cells were transfected with the following plasmids: (A) hSERT-IRES-DsRed (without CaV channel); (B) α1B (CaV2.2) and hSERT-IRES-DsRed; (C) α1D (CaV1.3) and hSERT-IRES-DsRed; (D) α1D (CaV1.3) and DsRed (without transporter). In all cases the β3 and α2γ1 plasmids were included in the transfection mix. The transfected cells were identified by their DsRed fluorescence. (A-D) As indicated in each trace, the cells were exposed to 10 μM S(+)MDMA (light gray box) or 10 μM 5HT (dark gray box) for 5 s. After wash the cells were exposed to 200 nM valinomycin (V, white box) for 2 s to increase the K+ permeability and were immediately exposed to 130 mM K+ solution for 5 s (K+, black box) to depolarize the cells toward positive potentials, then after wash the cells were exposed to 3μM 4Br-A23187, a Ca2+ionophore. The traces shown are mean ± s.e.m. of n ≥ 20 cells.
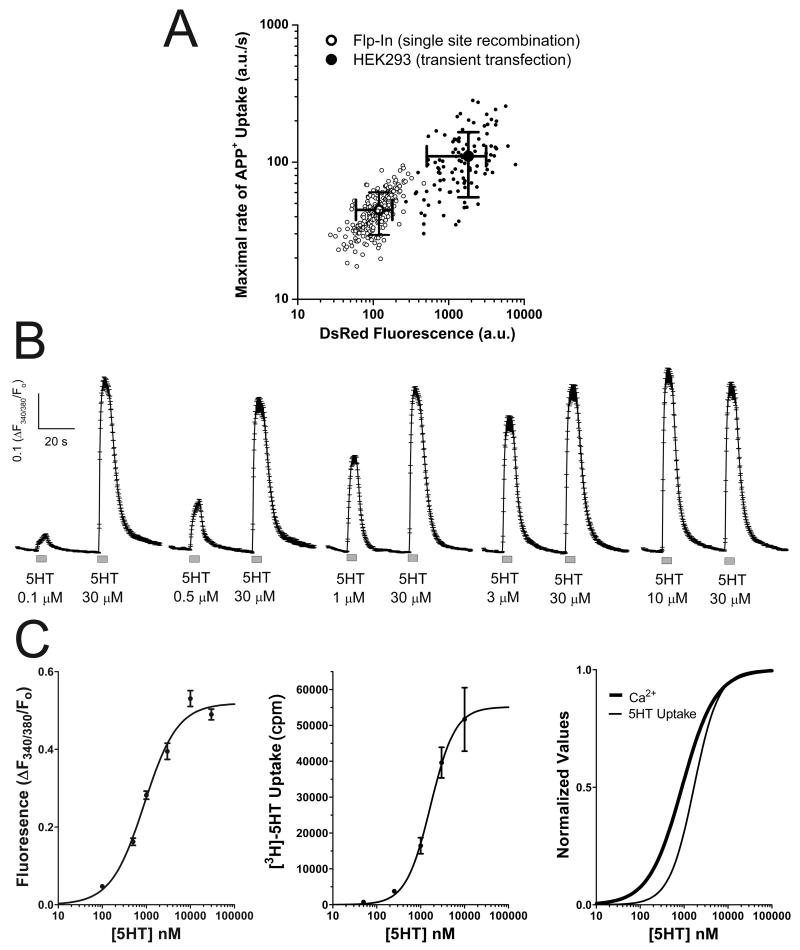
The over-expression of hSERT in transient transfection experiments would lead to non-physiological levels of depolarization upon challenging the cells to hSERT substrates. To address this concern, a stable cell line expressing the hSERT-IRES-DsRED construct was generated using the Flp-In™ T-Rex™ expression system as described in Materials and methods. Since these cells are restricted to a single copy of the gene of interest, the expression level of the inserted gene is lower than in transient tranfection experiments. Using the APP+ assay, the Flp-In cells showed 60% less hSERT maximal activity than HEK cells subjected to transient transfection with the hSERT-IRES-DsRED plasmid, both the transient transfection of HEK cells and the induction of Flp-In cells were carried out 3 days prior to the experiment (Fig. 7A). Similarly the DsRed signal was reduced 93% in Flp-In cells (Fig. 7A). Although hSERT activity is reduced in Flp-In cells, when they were transfected with CaV1.3, 5HT induced Ca2+ signals in a dose-dependent manner (Fig. 7B) whereas untransfected Flp-In cells (no Ca2+ channel but expressing hSERT) are refractory to 5HT treatment (not shown). Interestingly, dose-response experiments for [3H]-5HT uptake done in Flp-In cells showed an EC50 of 1.68 ± 0.38 μM (n = 5, Fig. 7C), whereas the EC50 for the Ca2+ signal induced by 5HT in these cells was 0.90 ± 0.08 μM (n > 57, Fig. 7C) (p < 0.01, t-test). The comparable potency of both actions of 5HT, as a substrate of hSERT, and driving hSERT-mediated depolarization toward CaV1.3 activation, suggests that the coupling between hSERT-mediated depolarization and CaV channel opening is significant in the physiological range of 5HT concentration.
Figure 7. The electrical coupling between hSERT and CaV1.3 take place in the physiological concentration range of 5HT.
(A) Flp-In™ T-Rex™ cells (Flp-In, open circles) expressing the hSERT-IRES-DsRed construct were generated as described in Materials and methods, and HEK293 cells (closed circles) were subjected to transient transfection using the hSERT-IRES-DsRed plasmid. To compare the activity of hSERT among cell types, the maximal rate of APP+ uptake was measured computing the maximum of the first derivative of APP+ signal as function of time. In addition, the DsRed signal was measured for each cell. The maximal rate of APP+ uptake (y axis) and DsRED fluorescence (x axis) is plotted for each individual cell (note that the axis are in logarithmic scale) and the values shown are the mean ± standard deviation. The hSERT activity was 44.8*** ± 15.4 (a.u./s), and 110.4 ± 55.0 (a.u./s) for Flp-In cells (n = 202) and HEK293 cells (n = 107), respectively (*** = p < 0.0001, t-test). The DsRed signal was 119*** ± 60 (a.u.) and 1,821 ± 1,310, for Flp-In cells (n = 202) and HEK293 cells (n = 107), respectively (*** = p < 0.0001, t-test). (B) Flp-In cells were transiently transfected with CaV1.3 as described in Materials and methods, the transfected cells were identified by the EGFP fluorescence and the Ca2+ signal was determined using Fura-2AM. Measurements were done under constant perfusion at 35°C and the acquisition frequency was 3Hz. The cells were exposed to a variable concentration of 5HT followed by 30μM 5HT to record the maximal response. The traces shown are mean ± s.e.m. of n ≥ 57 cells for each condition. (C) The dose-response experiments shown in B were fit to Eq. 2 (Materials and methods) and yield the following fitting parameters for the 5HT- dependent Ca2+ signal: Maximal Response = 0.519 ± 0.013 ([F340/380/Fo]), EC50 = 0.90 ± 0.08 (μM) and Hill Slope = 1.15 ± 0.12 (n > 57). The dose response of [3H]-5HT uptake in Flp-In cells was fit to eq.2 (Materials and methods) and yield de following parameters. Maximal Response = 55,155 ± 5,512 (cpm), EC50 = 1.68 ± 0.38** (μM) and Hill Slope = 1.55 ± 0.43 (n = 5, ** = p < 0.01 vs EC50 of Ca2+ experiments, t-test). The fitted curves of the Ca2+ signal and [3H]-5HT uptake experiments were normalized and superimposed for better comparison.
3. Discussion
Most if not all transporters forming the SLC6 gene family show substrate-induced inward ionic currents when challenged near rest. In particular for monoamine neurotransmitter transporters, such as the electroneutral serotonin transporter or the electrogenic dopamine and norepinephrine transporters, the charge measured during substrate flux exceeds by several fold what is predicted by their transport rate and stoichiometry [18-24]. Single channel events have been described in monoamine neurotransmitter transporters, mimicking bona fide ion channels. To see whether the depolarizing currents evoked by hSERT substrates are physiologically relevant in unclamped cells, we expressed hSERT in cultured muscle cells (myotubes). This heterologous expression system has a sensitive built-in machinery to measure membrane depolarization, which is the EC-coupling mechanism. EC-coupling of skeletal muscle cells is based in the physical coupling between the voltage-gated Ca2+ channel (CaV1.1) in the plasma membrane and the ryanodine receptor 1 (RyR1), which is the Ca2+ channel of the sarcoplasmic reticulum [25, 26]. The membrane depolarization induces a conformational change of CaV1.1 that physically opens the RyR1, releasing Ca2+ from internal stores [27]. Thus, MDG myotubes that lack CaV1.1 expression are insensitive to depolarization (Fig. 2). Accordingly, Wt myotubes expressing hSERT activate the EC-coupling machinery upon exposure to 5HT, S(+)MDMA or high K+ external solution (40 mM), whereas these agents have no effect on MDG expressing hSERT myotubes (Fig. 2), suggesting that 5HT and S(+)MDMA depolarize hSERT-expressing myotubes. Although a low density of T-type Ca2+ channels (CaV3.2) can be expressed in differentiated myotubes [28], no increase of cytosolic Ca2+ was detected in MDG myotubes when exposed to K+-depolarization (Fig. 2), suggesting that the T-type channel expression is negligible in our experimental conditions. However, voltage-gated Na+ channel conductance could work as an intermediary between hSERT-mediated depolarization and EC-coupling. This seems not to be the case because TTX did not affect Ca2+ signals mediated by hSERT-mediated depolarization (Fig. 4). Conceivably, the depolarization evoked by hSERT activity is not fast enough to induce coherent activation of voltage-gated Na+ channels and/or the depolarization is too long, and thus Na+ channels become inactivated. Further experiments are needed to address the modulation of voltage-gated Na+ channels by hSERT currents. CaV1.1 is an atypical voltage-gated Ca2+ channel; its Ca2+ current (L-type) is activated at high voltages (threshold ~0 mV) [29], whereas the CaV1.1/RyR1 complex is activated at low voltages (threshold ~−30 mV) [29]. Thus the voltage dependence of the EC-coupling activation approximates more that of a low-voltage-activated Ca2+ channel than that of a high-voltage-activated Ca2+ channel in myotubes. The dose-response curve of the Ca2+ release induced by K+-depolarization in myotubes has been described previously [29, 30]. Using the Goldmann-Hodgkin-Katz equation (Eq. 3 in Materials and methods) we estimate that at rest (4 mM [K+]e), threshold (~15 mM [K+]e), half response (~20 mM [K+]e), or maximal Ca2+ release response (~40 mM [K+]e) in myotubes, the membrane potentials are −60 (known resting membrane potential of myotubes [31]), −43, −38, and −24 mV, respectively. This approximation of the voltage dependency of the Ca2+ release is left shifted ~10 mV compared to patch-clamp recordings reported in myotubes likely due to uncorrected liquid-junction potential [29]. Since 5HT and S(+)MDMA showed Ca2+ release in hSERT-expressing myotubes that is between threshold and half of the maximal K+ response (Fig. 2), the substrate-induced current of hSERT should depolarize the cells to −43 or −38 mV, then taking into account that the resting membrane potential of myotubes is −60 mV, the magnitude of the depolarization induced by these hSERT substrates is about 17 to 22 mV.
Monoamine neurotransmitter transporters coexist with Ca2+ channels, a variety of Cl− and K+ channels, receptors, and other transporters in excitable cells. Since the relative proportions of monoamine transporters to other current inducing membrane proteins are likely to be highly variable among cell types, it is impossible to predict if the substrate-induced current will be sufficient to significantly depolarize native cells. For instance K+ conductance activated by autoreceptors (GIRK channels) [32, 33], TASK channels [34] and Ca2+-activated K+ conductance [35] would oppose and neutralize the depolarization evoked by monoamine transporters current. Case specific studies are required to establish resting membrane potential changes upon monoamine transporter activation. Nevertheless, dopamine-, amphetamine- and methamphetamine-induced DAT current increases action potential firing frequency in dopaminergic neurons from the midbrain [11, 36], demonstrating that the depolarization evoked by DAT activation modulates excitability in native cells. In addition, it has been shown that dopamine induces cytosolic Ca2+ signals in cultured neurons from midbrain, cortex and hippocampus and using pharmacological tools the authors suggest that monoamine transporter-mediated depolarization could activate voltage-gated Ca2+ channels [37].
In the present work, we hypothesize that the depolarization evoked by hSERT activation is sufficient to activate voltage-gated Ca2+ channels. As a proof of concept, we tested this hypothesis in a controlled environment such as the one provided by the heterologous expression systems described in this study. In particular we focused our study in the lower-threshold L-type Ca2+ channel, CaV1.3, because it is expressed in serotonergic and dopaminergic neurons and has been shown to modulate some aspects of their function. SERT is expressed broadly through the cell bodies and along neurite extensions in cultured serotonergic neurons. In addition, SERT is expressed in both serotonergic fibers and cells bodies of the raphe nucleus in the brain [38, 39]. CaV1.3 is also present in the dorsal raphe nucleus and is involved in the AMPA-mediated dendritic serotonin release in serotonergic neurons [13], which is implicated in depression [40, 41]; thus altogether these data suggest that SERT and CaV1.3 may co-localize in the soma and dendrites of serotonergic neurons. In dopaminergic neurons, by means of its voltage dependence characteristics, CaV1.3 drives pacemaking and bursts of electrical activity [14]. In addition, CaV1.3 activity is implicated in selective death of dopaminergic neurons in the substantia nigra in Parkinson’s disease [42]. CaV1.3 has ~ 30 mV left shift in the peak current – voltage curve compared to the high-voltage-activated Ca2+ channel, CaV2.2 (Fig. 5). The threshold of activation that we measured for CaV1.3 is about −50 mV when 5 mM external Ca2+ is used as the charge carrier (Fig. 5), similar to that found by others [17, 43]. Taking into account the sensitivity of CaV1.3 to voltage changes, the estimated 20 mV depolarization achieved by hSERT activation should activate CaV1.3 but not CaV2.2 in physiological conditions. Indeed, when we co-expressed either CaV1.3 or CaV2.2 with hSERT in HEK239T cells, both 5HT and S(+)MDMA evoked clear activation of CaV1.3 (Fig. 6C) but not of CaV2.2 (Fig. 6B) despite that, both types of cells showed convincing Ca transients induced by strong K+-depolarization (Fig. 6B and 6C).
Since over-expression of hSERT due to transient transfection could undermine the physiological relevance of our findings, the expression of hSERT was reduced using the Flp-In expression system which inserts a single copy of hSERT cDNA into the cell’s genome by targeted recombination. This procedure decreased hSERT activity by 60% when compared to transiently transfected HEK cells (Fig. 7A). Despite the significant reduction in hSERT expression, the Flp-In cells showed robust coupling between hSERT activity and CaV1.3 opening. Moreover, concentrations as low as 100 nM are sufficient to induce Ca2+ signals implying that a relatively low number of transporters would depolarize the membrane sufficiently to activate CaV1.3 channels (Fig. 7B and 7C). Accordingly, 5HT showed comparable potency for inducing its own transport and for activating CaV1.3-mediated Ca2+ signals, implying that the electrical coupling between hSERT and CaV channels can coexist with 5HT transport in a physiological range of 5HT concentrations.
As mentioned above, one consequence of CaV1.3 activation is the increase of action potential firing in excitable cells such as dopaminergic neurons [14]. Although the participation of CaV1.3 has not been previously implicated, it was suggested that substrate-induced currents mediated by DAT produce bursts of electrical activity in midbrain dopaminergic neurons [11] and Ca2+ currents may be implicated in DA release mechanisms. Therefore, the electrical coupling between a monoamine transporter and CaV1.3 described in the present study supports the hypothesis that monoamine transporter-mediated modulation of cell excitability requires CaV1.3 activation. Further research effort is needed to assess this new hypothesis which is beyond the scope of the present study.
In summary, we demonstrate that hSERT-mediated depolarization activates voltage-gated Ca2+ channels in three independent heterologous expression systems: 1) myotubes engineered to express hSERT undergo ~20 mV depolarization, as estimated by partial activation of the CaV1.1/RyR1 complex when exposed to 5HT or MDMA, 2) in HEK293T cells co-expressing hSERT with voltage-gated Ca2+ channels, the hSERT-mediated depolarization is within the range of activation of CaV1.3 but not CaV2.2, and 3) in Flp-In cells co-expressing hSERT and CaV1.3, despite the lower expression of hSERT, CaV1.3 activation is coupled to hSERT-mediated depolarization. In addition, the 5HT transport and CaV1.3 activation, both mediated by hSERT, occur with similar 5HT potency, suggesting that hSERT/CaV1.3 coupling ensues at physiological concentrations of 5HT.
The trans-activation of Ca2+ channels by the monoamine transporters demonstrated in our models illustrates a new mechanism by which endogenous substrates (neurotransmitters) or artificial substrates (amphetamine-related agents) could modulate excitability and signaling mechanisms directed by the rise of intracellular Ca2+ in excitable cells.
4. Materials and methods
Expression of hSERT and voltage-gated Ca2+ channels in HEK293T cells
The hSERT cDNA (accession number L05568) was subcloned into the bicistronic pIRES2-DsRed expression vector (Clontech) to generate the hSERT-IRES-DsRed plasmid. The CaV2.2 (α1B), CaV1.3 (α1D), β3 and α2γ1 expression plasmids were obtained from Addgene (cat# 26570, 26571, 26574 and 26575, respectively) were kindly provided by Dr. Diane Lipscombe (Department of Neuroscience, Brown University, Providence, Rhode Island, USA). HEK293T cells were cultured on 96 well imaging plates or 12 well plates with 18 mm round glass coverslips inside each well. The culturing media used was Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum. The cells were transfected with the hSERT-pIRES-DsRed plasmid using lipofectamine 2000 (Life Technologies) following the instructions of the manufacturer. When the hSERT-pIRES-DsRed plasmid was co-transfected with Ca2+ channels, Fugene 6 (Promega) was used as transfection reagent. The ratio of DNA used was α1:β3:α2γ1:hSERT-DsRed = 3:3:3:0.4. The transfected cells were identified by the DsRed fluorescent signal.
Generation of hSERT in the Flp-In™ T-Rex™ expression system
To produce stable inducible cell lines using the Flp-In™ T-Rex™ expression system (Invitrogen), the hSERT-IRES-DsRed DNA fragment was subcloned into the pcDNA5/FRT/TO vector to generate the pcDNA5/hSERT-IRES-DsRed/FRT/TO plasmid. The inducible expressing cells were made following the manufacturer’s protocol. Briefly, the Flp-In™ T-rex™ host cells lines are HEK cells with a single FRT recombination site and a Tet repressor gene. These cells were co-transfected with the pcDNA5/hSERT-IRES-DsRed/FRT/TO and the pOG44 plasmids. The latter encodes the Flp recombinase. Clones that have inserted a single copy of the gene of interest into the recombination site acquire a hygromycin resistance. These cells were transiently transfected with the CaV1.3-Ca2+ channel using Fugene 6 (Promega). The ratio of DNA used was α1:β3:α2γ1:EGFP = 3:3:3:0.4. The transfected cells were identified by the EGFP fluorescent signal. hSERT and DsRed were induced adding doxycycline 1μg/mL to the culture media for 3 days.
Permanent expression of hSERT in mouse myoblasts
The hSERT cDNA was inserted in the retroviral expression vector pCMMP-MCS-IRES-Puro (Addgene 36952) that in addition to the inserted cDNA it encodes a puromycin resistance. This plasmid was kindly provided by Dr. Bill Sugden (McArdle Laboratory for Cancer Research, University of Wisconsin, Madison, Wisconsin, USA). The retroviral particles were packaged in HEK293T helper cells as previously described [44]. Wild type (Wt) and dysgenic (MDG) mouse myoblasts were kindly provided by Drs. Paul D. Allen and José R. López (Department of Molecular Biosciences, School of Veterinary Medicine, University of California–Davis, Davis, California, USA). These cells were transduced with the retroviral particles and selected as previously described [44]. Myoblast culture and differentiation to myotubes was done as reported previously [44].
Immunofluorescence
Immunofluorescence was done as previously described [45] using as primary antibody a monoclonal anti-hSERT antibody (mAb Technologies, Stone Mountain, GA) and secondary antibody anti-mouse Alexa Fluor 488 antibody (Invitrogen). The low magnification images were acquired in an epifluorescence microscope (Olympus IX70) equipped with a digital camera (Andor Technology, Belfast, UK) and the proper filter set to detect alexa 488 and DAPI staining. High magnification images were acquired in a confocal microscope (Zeiss LSM 710) using the 63X 1.4NA objective. The confocal images have an optical section of 0.8 μm for both channels.
Western Blot
Total protein extracts were run in SDS-PAGE in duplicates; one gel was subjected to Coomasie blue staining (not shown) and the other was electrically transferred to a PVDF membrane. To detect hSERT expression, the membrane was incubated with anti-hSERT monoclonal mouse antibody and then exposed to a secondary antibody conjugated with horseradish peroxidase. Then the hSERT band was visualized by chemiluminescence using RapidStep ECL Reagent (Calbiochem) and the images were acquired in a digital imaging system (FluorChem E, Protein Simple, Santa Clara, CA).
Measurement of Ca2+ currents
Macroscopic Ca2+ currents (ICa) were measured as previously described [29] with the following modifications: the external solution used was 155 mM tetraethylammonium (TEA)-Cl, 5 mM CaCl2 and 10 mM Hepes, pH 7.4, with TEA-OH. The patch pipette internal solution consisted of 135 mM CsCl, 10 mM Cs2-EGTA, 1 mM CaCl2, 4 mM MgCl2, and 10 mM Hepes, pH 7.4 with CsOH. The whole-cell patch-clamp parameters of the recordings were: cell capacitance = 27 ± 3 pF, access resistance = 5.5 ± 0.5 MΩ, τ = 153 ± 26 μs (n=14). The effective series resistance was compensated and corrected by 80 % using the Axopatch circuit. In these conditions the remaining voltage error is < 1.5 mV. The leak current was subtracted using a −P/6 protocol before each sweep. The pipettes were fire polished, Sylgard coated and had a resistance of ~2.5 MΩ when filled with the internal solution. The liquid-junction potential was not corrected. The recorded signals were acquired at 10 kHz and filtered at 5 kHz.
The voltage dependence of the ICa was fit to the following equation:
| (Eq.1) |
Where Gmax is the maximal conductance, V is the test potential, V1/2 is the potential at which G=1/2 Gmax, k represents a slope parameter and Vr is the reversal potential.
Measurements of intracellular Ca2+
The Ca2+ sensitive dyes Fluo-4AM and Fura-2AM (Life Technologies) were dissolved in DMSO pluronic F-127 20% and then were diluted in imaging solution (IS: 130 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes, 10 glucose, pH 7.4, in mM) to get a final concentration of 5.5 μM. The cells were loaded for 12 min with Fluo-4AM or 25 min with Fura-2AM all at 37°C. Then the cells were washed twice with IS and placed on the stage of an epifluorescence microscope. All the compounds used were dissolved in IS and when high K+ solution was used equimolar amount of NaCl was substituted by KCl. 5HT was purchased from Sigma and S(+)MDMA hydrochloride was a gift from the National Institute on Drug Abuse NIDA (NIH). The setup consists of a Olympus IX70 microscope equipped with a Polycrome V (Till Photonics, Gräfelfing, Germany) as a light source, a Luca S digital camera (Andor Technology, Belfast, UK), and an automatic perfusion system (AutoMate Scientific, Berkeley, CA). The imaging system was controlled by the Live Acquisition Software from Till Photonics. The measurements were done under constant perfusion at RT (23°C) or at 35°C using a ThermoClamp-1 heater (AutoMate Scientific, Berkeley, CA). The objective used was an Olympus 20X 0.8NA Oil. Measurements were done using an excitation wavelength of 490/10 nm, a dichroic mirror 505LP and an emission wavelength of 535/50 nm for Fluo-4. The Fura-2 signal was acquired switching the excitation wavelength between 340/10 nm and 380/10 nm at 6Hz, the dichroic mirror used was LP490 and the emission wavelength was 510/40 nm. The transfected cells were identified by visualization of DsRed (excitation 550/15 nm, dichroic mirror 570LP and emission 620/60 nm) or EGFP fluorescence (excitation 490/10 nm, dichroic mirror 505LP, emission 535/50 nm). The reported values are the fluorescence values divided by the basal level of each cell and reported as γF/Fo for Fluo-4 and γF(340/380)/Fo for Fura-2. All signals were background subtracted. The Ca2+ signal was acquired at 50 or 100 Hz as indicated in the figure legends for Fluo-4 or 3Hz for Fura-2. Electrical stimulation was delivered by 2 platinum wires positioned close to the cells using a micromanipulator; square 0.5 ms pulses were applied at 1 Hz using a Grass S48 stimulator.
The dose-response relationships for Ca2+ and [3H]-5HT uptake experiments were fit by the equation:
| (Eq.2) |
Where X is the concentration of 5HT or S(+)MDMA applied, Y(X) is the response measured, Ymax is the maximal response, EC50 is the concentration that produces half-maximal response, and n is the Hill slope parameter.
To estimate the depolarization evoked by external [K+] the Goldmann-Hodgkin-Katz equation was used:
| (Eq.3) |
Where Em is the membrane potential; Pion permeability of the ion; [ion]e and [ion]i are the extracellular and intracellular concentration of the ion, respectively; R is the ideal gas constant, T is the temperature, F is the Faraday’s constant. The parameters used in the equation are as follows: relative permeability (K+ = 100; Na+ = 5 and Cl− = 10). In contrast to adult fibers, cultured myotubes have low Cl− permeability at rest because of their underdeveloped T-tubule system [46, 47], external and internal concentration in mM (K+ variable, 105; Na+ 130, 7 and Cl− 130, 20, respectively) at 35°C.
APP+ uptake assay
4-(4-(dimethylamino)phenyl)-1-methylpyridinium (APP+), a fluorescent substrate of hSERT, was used to monitor hSERT activity by fluorescence microscopy as previously described [48]. Cells grown on imaging plates were placed on the stage of the fluorescence microscope describe above. The wavelengths used to detect the APP+ signal were 460/10 nm for excitation and 535/50 nm for emission.
[3H]-5HT uptake
Flp-In cells expressing hSERT were counted and 2×106 cells were exposed to different concentrations of 5HT where 3% of a given concentration consisted of [3H]-5HT. The 5HT uptake was carried out for 10 min at 37°C in IS (see composition above). Non-specific uptake was measured adding 5 μM fluoxetine to the radioactive cocktail. Then the cells were centrifuged, washed once with cold 1X PBS, centrifuged again, cell pellets were resuspended in Ecoscint H (National Diagnostics, Atlanta, GA, USA), and the radioactivity counted using a liquid scintillation counter. The mean ± s.e.m. of 5 independent experiments done in duplicates are reported.
Statistics
All data are presented as mean ± s.e.m. unless indicated in the figure legend. Comparisons were made by unpaired, two-tailed t-test, with p< 0.05 considered significant.
Highlights.
S(+)MDMA (ecstasy) and 5HT (serotonin) induce Ca2+ mobilization in cultured muscle cells expressing hSERT.
hSERT-mediated depolarization activates CaV1.3 but not CaV2.2 in HEK293 cells.
The electrical coupling between hSERT and CaV1.3 takes place at physiological concentrations of 5HT.
hSERT-mediated depolarization activates voltage-gated calcium channels.
Acknowledgements
We would like to thank Dr. Paul D. Allen and Dr. José R. López (Department of Molecular Biosciences, School of Veterinary Medicine, University of California–Davis, Davis, California, USA) for providing the mouse myoblast used in this study. Confocal microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility supported, in part, with funding from NIH-NINDS Center core grant (5P30NS047463). This work was supported by National Institutes of Health Grants R01 DA033930 (L.J.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- 1.Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- 2.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 3.Wilens TE, Morrison NR, Prince J. An update on the pharmacotherapy of attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2011;11:1443–65. doi: 10.1586/ern.11.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandrioli R, Mercolini L, Saracino MA, Raggi MA. Selective serotonin reuptake inhibitors (SSRIs): therapeutic drug monitoring and pharmacological interactions. Curr Med Chem. 2012;19:1846–63. doi: 10.2174/092986712800099749. [DOI] [PubMed] [Google Scholar]
- 5.Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Steele TD, McCann UD, Ricaurte GA. 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”): pharmacology and toxicology in animals and humans. Addiction. 1994;89:539–51. doi: 10.1111/j.1360-0443.1994.tb03330.x. [DOI] [PubMed] [Google Scholar]
- 7.Bedi G, Hyman D, de Wit H. Is ecstasy an “empathogen”? Effects of +/−3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol Psychiatry. 2010;68:1134–40. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scahill L, Anderson GM. Is ecstasy an empathogen? Biol Psychiatry. 2010;68:1082–3. doi: 10.1016/j.biopsych.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–33. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–98. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 11.Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci. 2002;5:971–8. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- 12.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colgan LA, Cavolo SL, Commons KG, Levitan ES. Action potential-independent and pharmacologically unique vesicular serotonin release from dendrites. J Neurosci. 2012;32:15737–46. doi: 10.1523/JNEUROSCI.0020-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putzier I, Kullmann PH, Horn JP, Levitan ES. Cav1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. J Neurosci. 2009;29:15414–9. doi: 10.1523/JNEUROSCI.4742-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia J, Beam KG. Measurement of calcium transients and slow calcium current in myotubes. J Gen Physiol. 1994;103:107–23. doi: 10.1085/jgp.103.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell JA, Petherbridge L, Flucher BE. Formation of triads without the dihydropyridine receptor alpha subunits in cell lines from dysgenic skeletal muscle. J Cell Biol. 1996;134:375–87. doi: 10.1083/jcb.134.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helton TD, Xu W, Lipscombe D. Neuronal L-type calcium channels open quickly and are inhibited slowly. J Neurosci. 2005;25:10247–51. doi: 10.1523/JNEUROSCI.1089-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mager S, Min C, Henry DJ, Chavkin C, Hoffman BJ, Davidson N, Lester HA. Conducting states of a mammalian serotonin transporter. Neuron. 1994;12:845–59. doi: 10.1016/0896-6273(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 19.Sonders MS, Amara SG. Channels in transporters. Curr Opin Neurobiol. 1996;6:294–302. doi: 10.1016/s0959-4388(96)80111-5. [DOI] [PubMed] [Google Scholar]
- 20.Carvelli L, McDonald PW, Blakely RD, Defelice LJ. Dopamine transporters depolarize neurons by a channel mechanism. Proc Natl Acad Sci U S A. 2004;101:16046–51. doi: 10.1073/pnas.0403299101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin F, Lester HA, Mager S. Single-channel currents produced by the serotonin transporter and analysis of a mutation affecting ion permeation. Biophys J. 1996;71:3126–35. doi: 10.1016/S0006-3495(96)79506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeFelice LJ, Goswami T. Transporters as channels. Annu Rev Physiol. 2007;69:87–112. doi: 10.1146/annurev.physiol.69.031905.164816. [DOI] [PubMed] [Google Scholar]
- 23.Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci. 1997;17:960–74. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galli A, Blakely RD, DeFelice LJ. Norepinephrine transporters have channel modes of conduction. Proc Natl Acad Sci U S A. 1996;93:8671–6. doi: 10.1073/pnas.93.16.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takekura H, Bennett L, Tanabe T, Beam KG, Franzini-Armstrong C. Restoration of junctional tetrads in dysgenic myotubes by dihydropyridine receptor cDNA. Biophys J. 1994;67:793–803. doi: 10.1016/S0006-3495(94)80539-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paolini C, Protasi F, Franzini-Armstrong C. The relative position of RyR feet and DHPR tetrads in skeletal muscle. J Mol Biol. 2004;342:145–53. doi: 10.1016/j.jmb.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Tanabe T, Beam KG, Adams BA, Niidome T, Numa S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature. 1990;346:567–9. doi: 10.1038/346567a0. [DOI] [PubMed] [Google Scholar]
- 28.Bidaud I, Monteil A, Nargeot J, Lory P. Properties and role of voltage-dependent calcium channels during mouse skeletal muscle differentiation. J Muscle Res Cell Motil. 2006;27:75–81. doi: 10.1007/s10974-006-9058-5. [DOI] [PubMed] [Google Scholar]
- 29.Eltit JM, Szpyt J, Li H, Allen PD, Perez CF. Reduced gain of excitation-contraction coupling in triadin-null myotubes is mediated by the disruption of FKBP12/RyR1 interaction. Cell Calcium. 2011;49:128–35. doi: 10.1016/j.ceca.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eltit JM, Bannister RA, Moua O, Altamirano F, Hopkins PM, Pessah IN, Molinski TF, Lopez JR, Beam KG, Allen PD. Malignant hyperthermia susceptibility arising from altered resting coupling between the skeletal muscle L-type Ca2+ channel and the type 1 ryanodine receptor. Proc Natl Acad Sci U S A. 2012;109:7923–8. doi: 10.1073/pnas.1119207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eltit JM, Yang T, Li H, Molinski TF, Pessah IN, Allen PD, Lopez JR. RyR1-mediated Ca2+ leak and Ca2+ entry determine resting intracellular Ca2+ in skeletal myotubes. J Biol Chem. 2010;285:13781–7. doi: 10.1074/jbc.M110.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–68. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–46. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA. Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J Neurosci. 2002;22:1256–65. doi: 10.1523/JNEUROSCI.22-04-01256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deignan J, Lujan R, Bond C, Riegel A, Watanabe M, Williams JT, Maylie J, Adelman JP. SK2 and SK3 expression differentially affect firing frequency and precision in dopamine neurons. Neuroscience. 2012;217:67–76. doi: 10.1016/j.neuroscience.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Branch SY, Beckstead MJ. Methamphetamine produces bidirectional, concentration-dependent effects on dopamine neuron excitability and dopamine-mediated synaptic currents. J Neurophysiol. 2012;108:802–9. doi: 10.1152/jn.00094.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaarmann A, Gandhi S, Gourine AV, Abramov AY. Novel pathway for an old neurotransmitter: dopamine-induced neuronal calcium signalling via receptor-independent mechanisms. Cell Calcium. 2010;48:176–82. doi: 10.1016/j.ceca.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Lau T, Horschitz S, Berger S, Bartsch D, Schloss P. Antidepressant-induced internalization of the serotonin transporter in serotonergic neurons. FASEB J. 2008;22:1702–14. doi: 10.1096/fj.07-095471. [DOI] [PubMed] [Google Scholar]
- 39.Sukiasyan N, Hultborn H, Zhang M. Distribution of calcium channel Ca(V)1.3 immunoreactivity in the rat spinal cord and brain stem. Neuroscience. 2009;159:217–35. doi: 10.1016/j.neuroscience.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Busquet P, Nguyen NK, Schmid E, Tanimoto N, Seeliger MW, Ben-Yosef T, Mizuno F, Akopian A, Striessnig J, Singewald N. CaV1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int J Neuropsychopharmacol. 2010;13:499–513. doi: 10.1017/S1461145709990368. [DOI] [PubMed] [Google Scholar]
- 41.Casamassima F, Hay AC, Benedetti A, Lattanzi L, Cassano GB, Perlis RH. L-type calcium channels and psychiatric disorders: A brief review. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1373–90. doi: 10.1002/ajmg.b.31122. [DOI] [PubMed] [Google Scholar]
- 42.Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–6. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 43.Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92:2633–41. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- 44.Li H, Ding X, Lopez JR, Takeshima H, Ma J, Allen PD, Eltit JM. Impaired Orai1-mediated resting Ca2+ entry reduces the cytosolic [Ca2+] and sarcoplasmic reticulum Ca2+ loading in quiescent junctophilin 1 knock-out myotubes. J Biol Chem. 2010;285:39171–9. doi: 10.1074/jbc.M110.149690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eltit JM, Li H, Ward CW, Molinski T, Pessah IN, Allen PD, Lopez JR. Orthograde dihydropyridine receptor signal regulates ryanodine receptor passive leak. Proc Natl Acad Sci U S A. 2011;108:7046–51. doi: 10.1073/pnas.1018380108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fahlke C, Zachar E, Rudel R. Single-channel recordings of chloride currents in cultured human skeletal muscle. Pflugers Arch. 1992;421:108–16. doi: 10.1007/BF00374816. [DOI] [PubMed] [Google Scholar]
- 47.Dulhunty AF. Distribution of potassium and chloride permeability over the surface and T-tubule membranes of mammalian skeletal muscle. J Membr Biol. 1979;45:293–310. doi: 10.1007/BF01869290. [DOI] [PubMed] [Google Scholar]
- 48.Solis E, Jr., Zdravkovic I, Tomlinson ID, Noskov SY, Rosenthal SJ, De Felice LJ. 4-(4-(dimethylamino)phenyl)-1-methylpyridinium (APP+) is a fluorescent substrate for the human serotonin transporter. J Biol Chem. 2012;287:8852–63. doi: 10.1074/jbc.M111.267757. [DOI] [PMC free article] [PubMed] [Google Scholar]