Abstract
Objective
Multiple sclerosis (MS) is the most common inflammatory disease of the CNS. Experimental autoimmune encephalomyelitis (EAE) is a widely used model for MS. In the present research, our aim was to test the therapeutic efficacy of Calcium (Ca) in an experimental model of MS.
Methods
In this study the experiment was done on C57BL/6 mice. EAE was induced using 200 μg of the MOG35-55 peptide emulsified in CFA and injected subcutaneously on day 0 over two flank areas. In addition, 250 ng of pertussis toxin was injected on days 0 and 2. In the treatment group, 30 mg/kg Ca was administered intraperitoneally four times at regular 48 hour intervals. The mice were sacrificed 21 days after EAE induction and blood samples were taken from their hearts. The brains of mice were removed for histological analysis and their isolated splenocytes were cultured.
Results
Our results showed that treatment with Ca caused a significant reduction in the severity of the EAE. Histological analysis indicated that there was no plaque in brain sections of Ca treated group of mice whereas 4 ± 1 plaques were detected in brain sections of controls. The density of mononuclear infiltration in the CNS of Ca treated mice was lower than in controls. The serum level of Nitric Oxide in the treatment group was lower than in the control group but was not significant. Moreover, the levels of IFN-γ in cell culture supernatant of splenocytes in treated mice were significantly lower than in the control group.
Conclusion
The data indicates that Ca intervention can effectively attenuate EAE progression.
Keywords: MS, CNS, EAE, Calcium, Nitric Oxide
Introduction
Multiple sclerosis (MS) is the most common autoimmune inflammatory disease of the central nervous system (CNS). MS is characterized by immune-mediated demyelination and neurodegeneration of the CNS with lesions predominantly occurring in the white matter.1 Although MS afflicts more than two million people, its etiology remains unresolved and currently there are no adequate therapeutic interventions.2,3 MS is the third major cause of disability in the USA. Unfortunately, the exact statistics of Iranian MS patients are not available, but based on the estimation of the Iranian MS Association; there are approximately 50000 MS patients in Iran, most of whom are living in the Isfahan province.4
The pathogenesis of the disease is characterized by the activation and infiltration of mononuclear cells predominantly antigen-specific CD4+ and CD8+ T cells and B cells in the CNS, reactivation by resident antigen presenting microglial cells, secretion of proinflammatory cytokines/chemokines along with generation of other inflammatory mediators.3 The phagocytes best known to be involved in Experimental autoimmune encephalomyelitis (EAE) are monocyte-derived CD11C+ dendritic cells and macrophages originating from either microglia or monocytes.5
EAE is a widely used model for MS that is initiated and driven by myelin-specific CD4+ T cells producing IFN-γ, TNF-α and IL-17 in the CNS.6 In C57BL/6 mice, EAE is typically induced by immunization with residues 35–55 of myelin oligodendroglial glycoprotein (MOG), a component of the myelin sheath.7,8 The EAE is thought to be a T cell-mediated autoimmune disease, so that, both Th1 and Th17 cells were thought to be responsible for the inflammatory demyelination in MS and EAE, whereas Th2, Foxp3 regulatory T cells and related cytokines have been shown to be important in the resolution stages of the disease.9,10
On the other hand, there is significant evidence that at least part of the pathogenesis of autoimmune demyelinative diseases, such as MS and various other neurodegenerative diseases, may involve the generation of reactive oxygen species (ROS).11 ROS can directly cause demyelination, and they also activate nuclear transcription factor kappa B (NF-κB), thus resulting in the up-regulation of tumor necrosis factor alpha (TNF-α) and inducible nitric oxide synthase (iNOS).12
In living systems, free radicals are constantly generated and they can cause extensive damage to tissue.13 Antioxidants reduce the expression of inflammation related molecules in the CNS such as iNOS and nitrotyrosine; a marker of peroxynitrite reactivity.12
Calcium ions (Ca2+) are essential second messengers in the human body.14 Their widespread roles in biology are paralleled with the immune system. Engagement of several different tyrosine kinase and nontyrosine kinase receptors leads to stimulate Ca2+ influx in many immune cells including macrophages, T cells, B cells, natural killer cells, mast cells, and dendritic cells. In T cells, antigen engagement with T cell receptor (TCR) was initiated by influx of Ca2+. This process activates calcineurin to dephosphorylate NFATc transcription factors. Subsequent nuclear localization and formation of NFATc complexes turn on and off genes required for T cell development, activation, cytokine production, and anergy.15
It has been described that 1000 IU / day vitamin D and 800 mg Ca intake can increase the anti-inflammatory transforming growth factor (TGF)-β1 serum levels.16 The level of TGF-β1 appeared to be low in patients with MS.17 Furthermore, 1,25(OH)2D3 loses its effect when low calcium diets are fed. Hypercalcemia itself suppresses EAE; thus, it is not at all clear whether any vitamin D compound can suppress this disease without hypercalcemia.18
The aim of this study was to determine the therapeutic effect of Ca on the improvement of EAE induced by MOG35-55 in mice and elucidate the involved possible mechanism.
Methods
C57BL/6 male mice, 8-10 weeks of age were obtained from the Pasture Institute of Iran. Mice were randomly separated into three groups: normal, control and treatment, consisting of 8 mice in each group. All mice were housed in groups of four in cages on a 12-hour light-dark cycle and free access to food and water. All procedures involving animals were performed according to the Animal Ethics guidelines approved by Tehran University of Medical Science.
Initially, anesthesia was done by ether, and each mouse then received a subcutaneous injection of 200 μg synthetic MOG35-55 (MEVGWYRSPFSRVVHLYRNGK;Diapharm Ltd, Russia) solubilized in PBS and mixed with 100 μl of complete Freund's adjuvant (CFA), (Sigma-Aldrich, St.Louis, MO). A 200 μl volume of MOG and CFA was injected subcutanously in two flank areas. Mice were also intraperitoneally (i.p.) injected with 250 ng of pertussis toxin (Sigma-Aldrich, St.Louis, MO). A second, identical injection of pertussis toxin was given after 48 h.19,20
Ca with dose 30 mg/kg was administered i.p. in the treatment group four times at regular 48 hour intervals.21-23 Treatment was carried out 2 days after EAE induction by day 8 post-induction. Control and normal groups received 100 μl PBS. Clinical assessment was performed daily from day 9 post-immunization until day 21. Mice were monitored daily and assessed by clinical score.
The clinical grade was scored as follows: 0=no clinical sign; 1=partial loss of tail tonicity; 2=complete loss of tail tonicity; 3=flaccid tail and abnormal gait; 4=hind leg paralysis; 5=hind leg paralysis with hind body paresis; 6=hind and foreleg paralysis; 7=moribund or death.24
To evaluate by histopathology, mice were sacrificed after 21 days following anesthetization by injection of ketamin and zylasin. Brains were dissected out and fixed with neutral 10% formalin overnight and routinely embedded in paraffin wax. Brain sections (8 μm thick) were stained with hematoxylin and eosin (H&E) that was used to highlight areas of inflammation by darkly staining the nuclei of mononuclear cells. Immune cells stained with H&E were counted in a blinded manner under a light microscope using a 10× magnification with 12 sections examined per animal.25
Total antioxidant capacity was measured according to the FRAP test. In the FRAP test, antioxidants in the sample reduce ferric tripyridyltriazine complex (Fe3+- TPTZ), to a blue colored ferrous form (Fe2+), with an increase in absorbance at 593 nm. The working FRAP reagent was prepared by mixing 10 volumes of 300 mmol/L acetate buffer, pH 3.6, with 1 volume of 10 mmol/L TPTZ (2, 4, 6 Tris(pyridyl)-s-triazine), (Sigma-Aldrich, St Louis, MO) in 40 mmol/L HCl and with 1 volume of 20 mmol/L FeCl3 prepared in deionized water. The working FRAP reagent was then warmed to 37°C and its absorbance against water was read at 593 nm (reagent blank). Subsequently, 30 μl of Serum sample was added to 970 μl of FRAP reagent and the absorbance was monitored within 4 min. The results were expressed as μmol of ferric antioxidant capacity (the FRAP value) and were compared with the standard curve prepared using FeSO4, 7H2O 1 mM in a range of concentrations from 125 to 1000 μM.26
NO was assayed by measuring nitrite as the end product of reaction, which was determined by a colorimeter assay based on the Griess reaction. Griess reagent was prepared by solving 1g sulfanilamide in 100 ml phosphoric acid 5% mixed with 0.1g naphtyl ethylene diamine-HCl (NED) in 100 ml distilled water. Serum sample (100 μl) was mixed with 100 μl of Griess reagent at room temperature for 10 min. Absorbance was measured using microplate reader at 550 nm. Concentration of nitrite was determined by standard curve of sodium nitrite 0.1 molar prepared in distilled water.27,28
Single cell suspensions were prepared from spleens on day 21 post immunization. Splenocytes isolated from mice were treated with NH4Cl to remove red blood cells. Cells were resuspended in PBS and cell count was performed. Then 2×106 cells per well were cultured in 24-well culture plates in RPMI medium supplemented with 10% FCS, 2 mM L-glutamine, 100 μg/ml penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO), in the presence of 20 μg/ml MOG35-55 peptide. Cultures were incubated for 72 hours in 5% CO2 at 37°C. To assay cytokine, cells culture supernatants were collected after stimulation with antigen. IFN-γ was quantified by two-site sandwich ELISA using a commercially available kit (Bender Med. Systems, Austria) on cell culture supernatants. Absorbance was read at 450 nm using a microplate ELISA reader. The concentration of cytokine was estimated from a standard curve generated for each run.
ANOVA was used for statistical comparison of the results. When ANOVA was significant, Fischer’s LSD multiple comparison test was applied. Significance was defined at the 0.05 level. Results were expressed as mean ± S.D.
Results
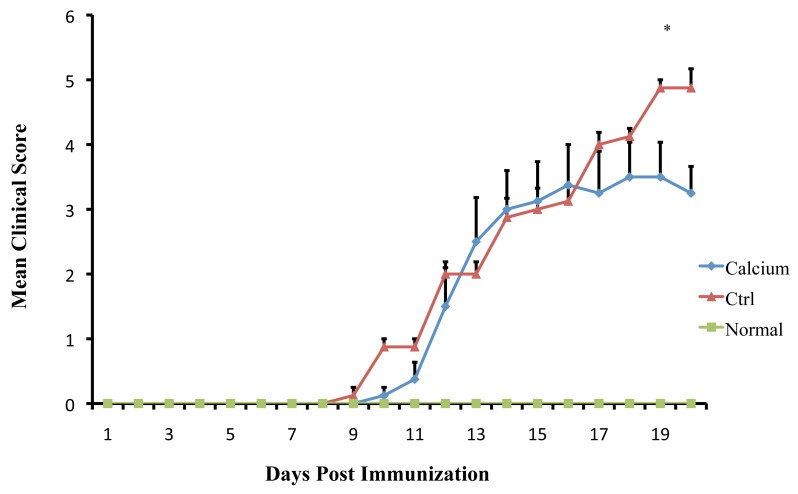
The initial clinical expression of the axonal damage in EAE model is represented by well-defined signs such as weight loss, tail paralysis and hind-limb weakness. All immunized mice with MOG35-55 developed EAE. The clinical assessment indicates that Ca ameliorates the progression of EAE. The clinical course and severity of the disease showed a difference between the treatment and control groups (Fig 1). The mean severity score of disease was significantly higher in control mice (4.87 ± 0.83) than in treated animals (3.5 ± 1.16), (p<0.01). These effects led to significant clinical improvement and delayed disease progression during 21 days of observation, indicating that Ca can ameliorate the progression of EAE.
Figure 1.
Mean clinical scores of EAE induced by MOG35-55 in control mice and in mice treated with Ca. All mice were immunized with 200 μg MOG35-55 emulsified in CFA and screened every day for the assessment of clinical signs of EAE. (*p<0.01)
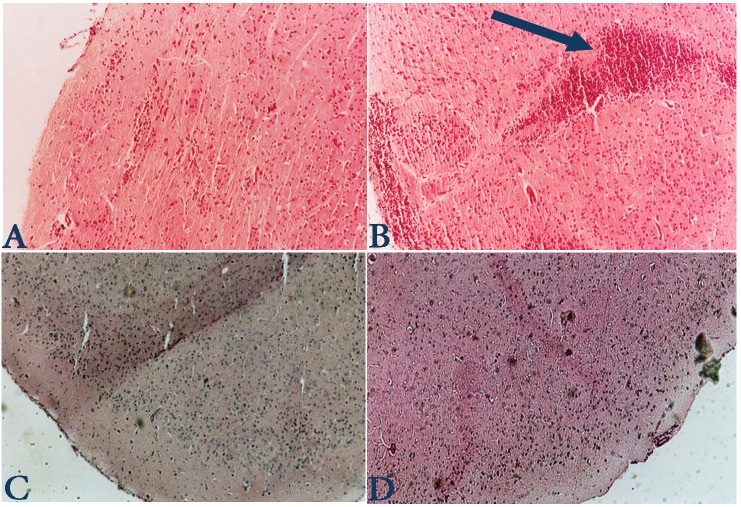
We examined the correlation between the clinical symptoms of EAE with histopathology of CNS in the control and treated mice. Histology analysis indicates that there was no plaque in group of Ca treated mice whereas 4 ± 1 plaques (in each section) were detected in the control group as shown in Fig 2. The density of mononuclear infiltration in the CNS of Ca-treated mice was 500 ± 200, whereas it was 700 ± 185 in the control group, (p<0.001). As shown in Fig 2, the mice treated with Ca displayed a lesser grade of inflammation in the CNS than the control mice. These results indicate that the histopathology of CNS correlates with the clinical severity of EAE in treated and control mice.
Figure 2.
Brain sections from normal (A), control (B) and treated mice (C, D) were stained with H&E. Leukocyte infiltration are less evident in the Ca treated mice in comparison with control mice. In Fig 2B plaque is shown (with arrow) which displays high infiltration of mononuclear cells, whereas in the treatment group no plaque was found.
All mice were sacrificed 21 days after EAE induction and blood samples were taken from their hearts. FRAP reaction was performed on serum sample. Antioxidant capacity was expressed as the FRAP value. The results are shown in Fig. 3. Treated mice showed higher FRAP values (641.86 ± 133.7 μmol) compared to control mice (327.73 ± 11.24 μmol). Statistical analysis showed that antioxidant activity in the treatment group was significantly higher than in the control mice (p<0.001).
Figure 3.
Antioxidant capacity was measured by the FRAP reaction. Data are presented as the mean ± S.D. of duplicate samples from 8 mice. *p<0.05
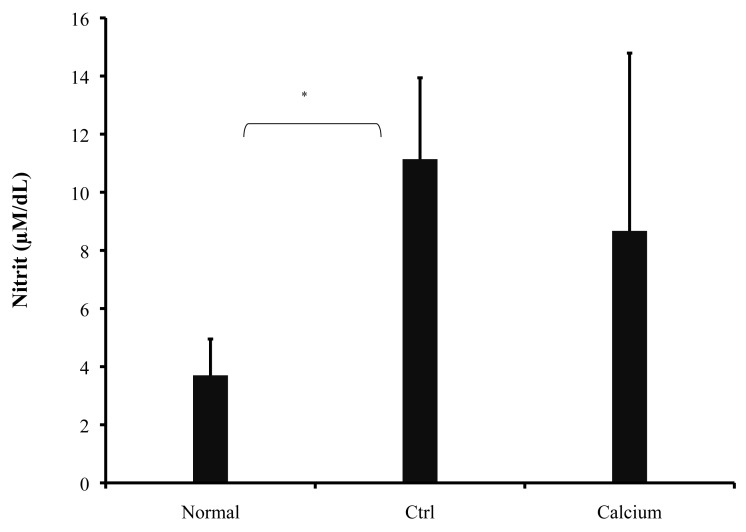
Griess reaction was performed on serum sample. As shown in Fig. 4, NO production was reduced in treated mice but was not significant (8.67 ± 6.12 μM/dL, p>0.05), compared to control mice (11.14 ± 2.8 μM/dL). In the normal group, NO production was 3.7 ± 1.25 μM. Calcium therapy showed a decrease in NO concentration in serum and this was in agreement with clinical findings.
Figure 4.
NO production was measured by the Griess reagent. Data are presented as the mean ± S.D. of duplicate samples from 8 mice. *p<0.01
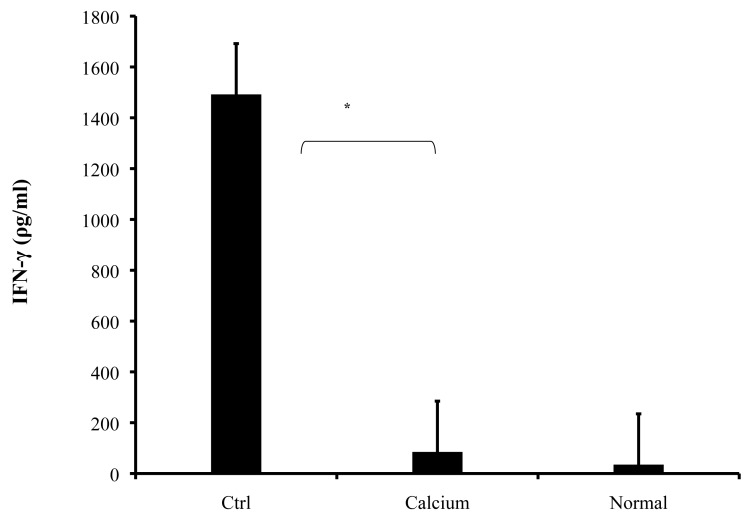
Splenocytes were obtained from mice and cultured for 72 hours in the presence of MOG35-55. The treatment group significantly exhibited lower levels of IFN-γ production in comparison with the diseased group (Fig. 5. 85 ± 57 ρg/ml and 1492 ± 443 ρg/ml respectively, p<0.001).
Figure 5.
Spleen cells were isolated from all mice on day 21 following immunization with MOG35-55 and cultured. The culture supernatants were collected after 72 h and the levels of IFN-γ were measured by ELISA. The values are mean of triplicates, and the error bars represent SD. *p<0.001
Discussion
EAE as an experimental model of MS is, an autoimmune disease of CNS mediated by CD4+ T lymphocytes specific for auto antigens of the myelin sheath, including myelin basic protein, proteolipid protein and myelin oligodendrocyte glycoprotein peptide.28-30 Relapsing/Remitting EAE (RR-EAE) induced by active immunization with the immunodominant epitope of MOG35-55 is characterized by an initial acute phase, followed by a series of remissions and relapses.30 CD4+Th1 cells and their proinflammatory cytokines are suspected to be important in the pathogenesis of MS, and necessary for the induction of RR-EAE.31
The cytokine profile and the nature of cellular infiltrate are markedly different during the clinical course of RR-EAE, so that the over expression of the proinflammatory cytokines, IFN-γ and TNF-α are essential mediators for disease induction. Th2 cell clones specific for encephalitogenic peptides are unable to induce the disease and can inhibit Th1 autoimmune clones, presumably by secreting IL-4, IL-10, and TGF-ß.32
Our findings suggest that Ca is capable of suppressing a pre-activated immune system in the late effector phase leading to disease eruption. The dose-response experiments carried out in the present study demonstrate that Ca has an optimum therapeutic effect at dose 30 mg/kg.
The pathogenic processes in EAE are mainly related to the secretion of IFN-γ.33,34 this study showed that treatment with Calcium can inhibit a pro-inflammatory Th1-based cytokine response and attenuate clinical signs in EAE. The results obtained in this research clearly show that Calcium therapy effectively reduces the severity of EAE-associated clinical symptoms and neuroinflammation in the brain. Moreover, Calcium therapy showed an appropriate cytokine response based on decreasing production of the pro-inflammatory Th1 cytokine, IFN-γ.
In addition, the results showed that the level of serum nitric oxide was less than in the control group, because the IFN-γ which can induce NO production might be decreased through decreasing the production of this cytokine.35 On the other hand, enhancement in brain penetration might be explained by the partial disruption of the BBB that occurs in EAE and MS. It should be noted that osmotic disruption of the BBB could be the best way to fully open the barrier for penetration of drugs.36
The current study demonstrates that Ca ameliorates the severity of EAE and attenuates inflammatory cellular infiltration into the CNS. Calcium seems to suppress the activation of macrophages/microglia and decreases production of NO, probably by suppressing the expression of iNOS. Moreover, Ca prevents Th1 differentiation by alleviating the production of IFN-γ. These mechanisms may together indicate the beneficial effect of Ca on EAE and also be applicable to the treatment of MS.
Conclusion
The results of this study suggest that Ca modulates EAE, at least in part, by its antioxidant property, suppressing NO and IFN-γ production. This finding encourages further investigations of Ca and in its role as a potential candidate for developing more efficient therapeutic strategies against multiple sclerosis.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
- 1.Vanheel A, Daniels R, Plaisance S, Baeten K, Hendriks JJ, Leprince P, et al. Identification of protein networks involved in the disease course of experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. PLoS One 2012;7(4):e35544 10.1371/journal.pone.0035544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guglielmetti C, Praet J, Rangarajan JR, Vreys R, De Vocht N, Maes F, et al. Multimodal imaging of subventricular zone neural stem/progenitor cells in the cuprizone mouse model reveals increased neurogenic potential for the olfactory bulb pathway, but no contribution to remyelination of the corpus callosum. Neuroimage 2014. Feb;86:99-110 10.1016/j.neuroimage.2013.07.080 [DOI] [PubMed] [Google Scholar]
- 3.Zaheer S, Wu Y, Bassett J, Yang B, Zaheer A. Glia maturation factor regulation of STAT expression: a novel mechanism in experimental autoimmune encephalomyelitis. Neurochem Res 2007. Dec;32(12):2123-2131 10.1007/s11064-007-9383-0 [DOI] [PubMed] [Google Scholar]
- 4.Farnoush R, Sahebolzamani M, Aliloo L, Rahmani A. Educational, Psycho Mental and Socio economical needs of an Iranian Cohort with Multiple Sclerosis. Oman Med J 2010. Jan;25(1):22-25 10.5001/omj.2010.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy M, Richard JF, Dumas A, Vallières L. CXCL1 can be regulated by IL-6 and promotes granulocyte adhesion to brain capillaries during bacterial toxin exposure and encephalomyelitis. J Neuroinflammation 2012;9:18 10.1186/1742-2094-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey-Bucktrout SL, Caulkins SC, Goings G, Fischer JA, Dzionek A, Miller SD. Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J Immunol 2008. May;180(10):6457-6461 10.4049/jimmunol.180.10.6457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selvaraj RK, Geiger TL. Mitigation of experimental allergic encephalomyelitis by TGF-beta induced Foxp3+ regulatory T lymphocytes through the induction of anergy and infectious tolerance. J Immunol 2008. Mar;180(5):2830-2838 10.4049/jimmunol.180.5.2830 [DOI] [PubMed] [Google Scholar]
- 8.Horstmann L, Schmid H, Heinen AP, Kurschus FC, Dick HB, Joachim SC. Inflammatory demyelination induces glia alterations and ganglion cell loss in the retina of an experimental autoimmune encephalomyelitis model. J Neuroinflammation 2013;10(1):120 10.1186/1742-2094-10-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furlan R, Kurne A, Bergami A, Brambilla E, Maucci R, Gasparini L, et al. A nitric oxide releasing derivative of flurbiprofen inhibits experimental autoimmune encephalomyelitis. J Neuroimmunol 2004. May;150(1-2):10-19 10.1016/j.jneuroim.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 10.Li X, Li TT, Zhang XH, Hou LF, Yang XQ, Zhu FH, et al. Artemisinin analogue SM934 ameliorates murine experimental autoimmune encephalomyelitis through enhancing the expansion and functions of regulatory T cell. PLoS One 2013;8(8):e74108 10.1371/journal.pone.0074108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu F, Selak M, O’Connor J, Croul S, Lorenzana C, Butunoi C, et al. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci 2000. Aug;177(2):95-103 10.1016/S0022-510X(00)00343-9 [DOI] [PubMed] [Google Scholar]
- 12.Moriya M, Nakatsuji Y, Miyamoto K, Okuno T, Kinoshita M, Kumanogoh A, et al. Edaravone, a free radical scavenger, ameliorates experimental autoimmune encephalomyelitis. Neurosci Lett 2008. Aug;440(3):323-326 10.1016/j.neulet.2008.05.110 [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Khan S, Lakshman K, Jayaveera KN, Sheshadri Shekar D, Narayan Swamy VB, et al. Lakshman K. Jayaveera KN. Sheshadri Shekar D. Narayan Swamy VB. Velumurga C In Vitro α-Amylase Inhibition and Antioxidant Activities of Methanolic Extract of Amaranthus Caudatus Linn. Oman Med J 2011. May;26(3):166-170 10.5001/omj.2011.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baird GS, Rainey PM, Wener M, Chandler W. Reducing routine ionized calcium measurement. Clin Chem 2009. Mar;55(3):533-540 10.1373/clinchem.2008.116707 [DOI] [PubMed] [Google Scholar]
- 15.Radermacher AN, Crabtree GR. Monster protein controls calcium entry and fights infection. Immunity 2008. Jan;28(1):13-14 10.1016/j.immuni.2007.12.009 [DOI] [PubMed] [Google Scholar]
- 16.Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol 2003. Jan;134(1-2):128-132 10.1016/S0165-5728(02)00396-X [DOI] [PubMed] [Google Scholar]
- 17.Andjelkovic Z, Vojinovic J, Pejnovic N, Popovic M, Dujic A, Mitrovic D, et al. Disease modifying and immunomodulatory effects of high dose 1 alpha (OH) D3 in rheumatoid arthritis patients. Clin Exp Rheumatol 1999. Jul-Aug;17(4):453-456 [PubMed] [Google Scholar]
- 18.Wang Y, Marling SJ, Zhu JG, Severson KS, DeLuca HF. Development of experimental autoimmune encephalomyelitis (EAE) in mice requires vitamin D and the vitamin D receptor. Proc Natl Acad Sci U S A 2012. May;109(22):8501-8504 10.1073/pnas.1206054109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peiris M, Monteith GR, Roberts-Thomson SJ, Cabot PJ. A model of experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice for the characterisation of intervention therapies. J Neurosci Methods 2007. Jul;163(2):245-254 10.1016/j.jneumeth.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 20.Jiang HR, Al Rasebi Z, Mensah-Brown E, Shahin A, Xu D, Goodyear CS, et al. Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J Immunol 2009. Jan;182(2):1167-1173 10.4049/jimmunol.182.2.1167 [DOI] [PubMed] [Google Scholar]
- 21.Goldberg P, Fleming MC, Picard EH. Multiple sclerosis: decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypotheses 1986. Oct;21(2):193-200 10.1016/0306-9877(86)90010-1 [DOI] [PubMed] [Google Scholar]
- 22.Bak M, Serdaroglu E, Guclu R. Prophylactic calcium and vitamin D treatments in steroid-treated children with nephrotic syndrome. Pediatr Nephrol 2006. Mar;21(3):350-354 10.1007/s00467-005-2118-z [DOI] [PubMed] [Google Scholar]
- 23.Merki-Feld GS, Neff M, Keller PJ. A 2-year prospective study on the effects of depot medroxyprogesterone acetate on bone mass-response to estrogen and calcium therapy in individual users. Contraception 2003. Feb;67(2):79-86 10.1016/S0010-7824(02)00460-2 [DOI] [PubMed] [Google Scholar]
- 24.Skundric DS, Zakarian V, Dai R, Lisak RP, Tse HY, James J. Distinct immune regulation of the response to H-2b restricted epitope of MOG causes relapsing-remitting EAE in H-2b/s mice. J Neuroimmunol 2003. Mar;136(1-2):34-45 10.1016/S0165-5728(03)00005-5 [DOI] [PubMed] [Google Scholar]
- 25.Natarajan C, Muthian G, Barak Y, Evans RM, Bright JJ. Peroxisome proliferator-activated receptor-gamma-deficient heterozygous mice develop an exacerbated neural antigen-induced Th1 response and experimental allergic encephalomyelitis. J Immunol 2003. Dec;171(11):5743-5750 10.4049/jimmunol.171.11.5743 [DOI] [PubMed] [Google Scholar]
- 26.Nencini C, Cavallo F, Capasso A, Franchi GG, Giorgio G, Micheli L. Evaluation of antioxidative properties of Allium species growing wild in Italy. Phytother Res 2007. Sep;21(9):874-878 10.1002/ptr.2168 [DOI] [PubMed] [Google Scholar]
- 27.Phizackerley PJ, Al-Dabbagh SA. The estimation of nitrate and nitrite in saliva and urine. Anal Biochem 1983. May;131(1):242-245 10.1016/0003-2697(83)90161-6 [DOI] [PubMed] [Google Scholar]
- 28.Kayhan B, Aharoni R, Arnon R. Glatiramer acetate (Copaxone) regulates nitric oxide and related cytokine secretion in experimental autoimmune encephalomyelitis. Immunol Lett 2003. Sep;88(3):185-192 10.1016/S0165-2478(03)00085-3 [DOI] [PubMed] [Google Scholar]
- 29.Tuohy VK, Lu Z, Sobel RA, Laursen RA, Lees MB. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J Immunol 1989. Mar;142(5):1523-1527 [PubMed] [Google Scholar]
- 30.Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol 1995. Jul;25(7):1951-1959 10.1002/eji.1830250723 [DOI] [PubMed] [Google Scholar]
- 31.Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol Scand 1988. Oct;78(4):318-323 10.1111/j.1600-0404.1988.tb03663.x [DOI] [PubMed] [Google Scholar]
- 32.Miller A, al-Sabbagh A, Santos LM, Das MP, Weiner HL. Epitopes of myelin basic protein that trigger TGF-beta release after oral tolerization are distinct from encephalitogenic epitopes and mediate epitope-driven bystander suppression. J Immunol 1993. Dec;151(12):7307-7315 [PubMed] [Google Scholar]
- 33.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science (New York, NY. 1994 Aug 26;265(5176):1237-40. [DOI] [PubMed]
- 34.Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, et al. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med 1994. Nov;180(5):1961-1966 10.1084/jem.180.5.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao BG, Ma CG, Xu LY, Link H, Lu CZ. IL-12/IFN-gamma/NO axis plays critical role in development of Th1-mediated experimental autoimmune encephalomyelitis. Mol Immunol 2008. Feb;45(4):1191-1196 10.1016/j.molimm.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 36.Kizelsztein P, Ovadia H, Garbuzenko O, Sigal A, Barenholz Y. Pegylated nanoliposomes remote-loaded with the antioxidant tempamine ameliorate experimental autoimmune encephalomyelitis. J Neuroimmunol 2009. Aug;213(1-2):20-25 10.1016/j.jneuroim.2009.05.019 [DOI] [PubMed] [Google Scholar]