Abstract
Objective
Nitrous oxide is a common inhalation anesthetic agent in general anesthesia. While it is widely accepted as a safe anesthetic agent, evidence suggests exposure to this gas, leads to hyperhomocysteinemia. The present study aimed to evaluate the effects of single-dose intravenous infusions of vitamin B12, before and after the induction of nitrous oxide anesthesia on homocysteine levels after the surgery.
Methods
This double-blind randomized controlled trial was conducted on 60 patients who were scheduled for elective surgery under general anesthesia, presumably lasting for more than two hours. The subjects were randomly allocated to three groups of 20. For the first group, vitamin B12 solution (1 mg/100 ml normal saline) and 100 ml of normal saline (placebo), were infused before and after the induction of anesthesia, respectively. The second group received placebo and vitamin B12 infusion before and after the induction of anesthesia, respectively. The third group received placebo infusions at both times. Homocysteine levels were measured before and 24 hours after the surgery.
Results
The mean homocysteine and vitamin B12 levels were significantly different within the three groups (p<0.001). In patients who had been infused with vitamin B12 before the surgery, homocysteine levels were significantly lower than the other two groups. In the placebo group, homocysteine levels significantly increased after the surgery.
Conclusion
Nitrous oxide causes hyperhomocysteinemia after general anesthesia. Since vitamin B12 infusion is a safe and inexpensive method to decrease homocysteine levels in these patients, it may be recommended for patients undergoing nitrous oxide anesthesia to be used before induction of anesthesia.
Keywords: Nitrous oxide, Homocysteine, Vitamin B12
Introduction
General anesthesia is the most common type of anesthesia in both elective and emergency surgeries. Every day, a large number of patients referred to clinics and hospitals are operated under general anesthesia. Nitrous oxide is an inhalation anesthetic agent, which is commonly used in general anesthesia. Although nitrous oxide is known to be safe, evidence suggests exposure to this anesthetic agent, either during an operation or constantly in the workplace (e.g., operation room) can possibly cause various complications such as bone marrow suppression, polyneuropathy, postoperative nausea and vomiting, pulmonary hypertension and hyperhomocysteinemia.1,2 It is currently accepted that nitrous oxide inactivates cobalamin (vitamin B12),2,3 through irreversible oxidation of the cobalt atom of vitamin B12.2,4,5 Cobalamin inactivation can last for several days after exposure to nitrous oxide.6 During this period, cobalamin-dependent enzymes, namely methionine synthase are inhibited, the ability of the cells to produce methionine from homocysteine is impaired, and consequently plasma homocysteine level increases.2-7 Since homocysteine has atherogenic properties that can lead to endothelial dysfunction,8-11 its higher plasma levels augment the risk of myocardial infarction, stroke, and dementia in the general population, particularly in individuals with cardiovascular risk factors.12,13 Therefore, the effects of elevated plasma homocysteine levels are very important in cardiac patients.14 Moreover, hyperhomocysteinemia after nitrous oxide exposure can increase cardiovascular complications and postoperative complications (including severe nausea and vomiting, respiratory complications, and wound infection).15 Also, it has been stated that hyperhomocysteinemia is an independent risk factor for coronary artery disease.14
Very few studies have evaluated the effect of vitamin B12 on homocysteine levels after exposure to nitrous oxide during general anesthesia. A previous study suggested that daily intake of B vitamins for at least one week prior to surgery could safely and effectively decrease hyperhomocysteinemia after nitrous oxide anesthesia.16 Despite being very simple, the mentioned method is not applicable for all cases and under all conditions. The only available study on the effects of vitamin B injection on plasma homocysteine levels after nitrous oxide anesthesia revealed the inefficacy of the applied method.7 Considering limited research and existing controversies in this field, the aim of present study was to evaluate the effects of pre and postoperative single-dose intravenous infusion of vitamin B12 on homocysteine levels in patients receiving nitrous oxide anesthesia.
Methods
This double-blind randomized controlled trial was conducted on 60, 18-45-years-old patients with the American Society of Anesthesiologists (ASA) class I and II. The subjects were scheduled for surgery under general anesthesia in Imam Khomeini Hospital (Sari, Iran) and their operations were estimated to take more than two hours. The Ethics Committee of Mazandaran University of Medical Sciences approved this study and it was registered in Iranian clinical trials database (IRCT201209226803N3, http//:www.irct.ir).
The exclusion criteria were pregnancy, diabetes mellitus, emergency surgery, duration of surgery less than two hours, history of anesthesia within the past month, contraindications for nitrous oxide (pneumothorax, mechanical intestinal obstruction, obstruction of the middle ear, laparoscopic surgery, laser surgery, and elevated intracranial pressure), preoperative use of vitamin B12 or folate supplements, contraindications for vitamin B12 (seizure, epilepsy, allergy, and hypersensitivity to vitamin B12), Leber hereditary optic neuropathy disease, vitamin B12 or folate deficiency, megaloblastic anemia, renal failure, hypothyroidism and smoking.
After obtaining institution ethics committee approval and patients’ informed consent, eligible patients were randomly selected using a table of random numbers and were allocated to three groups (20 patients for each group). The first group (group A) was infused with a solution of vitamin B12 (1 mg in 100 ml normal saline) before the surgery and with normal saline with same volume (as placebo) after the discontinuation of nitrous oxide anesthesia. The second group (group B) received infusion of 100 ml of normal saline preoperatively and vitamin B12 (1 mg in 100 ml normal saline) after the discontinuation of nitrous oxide anesthesia. Finally, the third group (group C) received infusions of 100 ml of normal saline at all the mentioned stages. The patients received infusions over a period of 10 minutes.
All subjects were premedicated with 0.02 mg/kg of midazolam and 3 μg/kg of fentanyl. Anesthesia was then induced with propofol (2 mg/kg) and atracurium (0.5 mg/kg) and maintained with Isoflurane 1 minimum alveolar concentration (MAC) and nitrous oxide 60%. During the surgery, atracurium and fentanyl were provided if necessary. All solution and infusion sets were prepared, covered, and labeled as A, B, and C (groups) by an anesthesia technician who was not involved in the study. Afterward, based on a random list, the appropriate solution was delivered to the researcher. Consequently, the anesthesia care team was unaware of solution types.
After the end of anesthesia, in the recovery room, a nurse who was blinded to the groups recorded the presence or absence of nausea and vomiting in the patients. In order to serum vitamin B12 and homocysteine level measurements, blood samples were taken both at the beginning of the study before anesthesia (baseline) and 24 hours after the surgery. Serum homocysteine was measured with an automated biochemistry analyzer (Hitachi 917, Hitachi, Japan, 2008) using homocysteine enzymatic assay (Diazyme, USA). Vitamin B12 serum level was measured by Roche Elecsys immunoassay analyzer (Japan, 2008) with a standard commercial kit (Germany, 2009) and Electrochemiluminescence immunoassay method.
Normality was examined with a Shapiro-Wilk test. The efficacy of vitamin B was assessed by one way analysis of variance (ANOVA). Two-way ANOVA determined whether the groups or genders were different in terms of homocysteine levels. Then Tukey’s test was performed for multiple comparisons. Also chi-square and ANOVA tests were used to compare demographic and clinical characteristics of patients in the three groups. The significance level was set at p<0.05.
Results
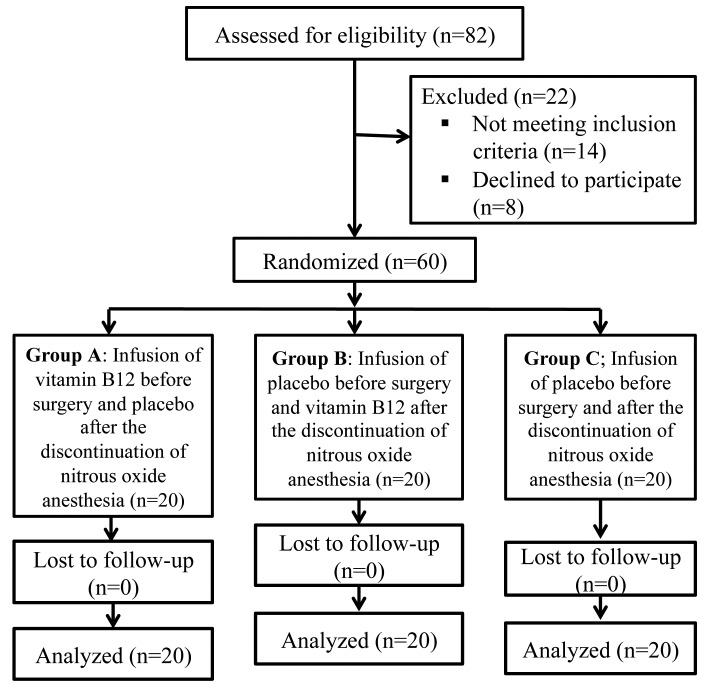
All patients completed the present study and data from all patients were analyzed (Fig. 1). Females constituted 75% (n = 45) of the study population. The mean age of the participants was 38 ± 8 years. There was no statistically significant difference between the three groups with regards to demographic and clinical characteristics (p>0.05). (Table 1)
Figure 1.
Flow chart of the study.
Table 1. Demographic and clinical characteristics of patients in the three groups.
| Variables | Group A | Group B | Group C | p value | |
|---|---|---|---|---|---|
| Sex | Male | 3 (85%) | 7 (65%) | 5 (75%) | 0.34 |
| Female | 17 (15%) | 13 (35%) | 15 (25%) | ||
| Age (year) | 39±6 | 37±7 | 37±8 | 0.42 | |
| Weight (Kg) | 66±11 (Range: 38-85) |
68±9 (Range: 34-88) |
70±10 (Range: 37-90) |
0.37 | |
| Body Mass Index (Kg/m2) | 25±5 (Range: 17–34) |
25±3 (Range: 18-31) |
26±3 (Range: 20±35) |
0.83 | |
| Duration of surgery (minute) | 165±32 | 162±33 | 161±30 | 0.82 | |
Data are expressed as the mean ± standard deviation, or number (%) of cases (only Sex).
Table 2 summarizes homocysteine and vitamin B levels of the three groups before and after the intervention. According to one-way ANOVA, the three groups were significantly different in the mean levels of homocysteine (F [2, 57] = 39.53; p<0.001) and vitamin B12 (F [2, 57] = 56.87; p<0.001) after the intervention. The Tukey's test declared this difference to be between groups A and B and group C. In addition, comparisons between homocysteine levels before and after the intervention revealed a reduction of 5.62 µmol/l in homocysteine level in group A (p<0.001), and an increase of 9.85 µmol/l in group C (p<0.001).
Table 2. Homocysteine (µmol/l) and vitamin B12 (pg/ml) levels before and after the intervention.
| Variable | Group A | Group B | Group C | p | |
|---|---|---|---|---|---|
|
Homocysteine (µmol/l) |
Before | 11.1±6.3 | 9.8±6.1 | 10.2±5.1 | 0.790 |
| After | 5.4±3.9 | 8.2±5.2 | 20.1±6.9 | <0.001 | |
|
Vitamin B12 (pg/ml) |
Before | 262.1±71.1 | 269.9±107.4 | 266±91.5 | 0.960 |
| After | 1219.1±378.5 | 1184.7±442.8 | 212.5±71.4 | <0.001 | |
Data are expressed as the mean ± SD.
There was no statistically significant difference between the three groups in the incidence of postoperative nausea and vomiting (p=0.151).
As Table 3 shows, two-way ANOVA revealed no significant interaction between gender and the three groups after the intervention (F [2, 54] = 0.251; p=0.770). However, the main effect of gender was significant (F [1, 54] = 11.51; p=0.001), i.e., in all groups; homocysteine levels after the intervention were higher in men than in women. The main effect of groups was also significant (F [2, 54] = 31.34; p<0.001). Again, Tukey's test showed that group C was significantly different from groups A and B.
Table 3. The relation of homocysteine levels (µmol/l) after the intervention with groups and gender.
| Groups | Homocysteine (mean ± SD) |
p (partial Eta squared) | |
|---|---|---|---|
| Group A | Female | 4.4±3.1 | Gender * groups: 0.770 (0.009) |
| Male | 11.2±2.7 | ||
| Group B | Female | 6.8±4.1 | Gender: 0.001 (0.170) |
| Male | 10.8±6.4 | ||
| Group C | Female | 18.7±76.2 | Groups: 0.001 (0.530) |
| Male | 24.2±4.4 | ||
Discussion
The findings of the present study show that vitamin B12 infusion before general anesthesia with nitrous oxide, significantly decreased serum homocysteine levels after surgery. In other words, vitamin B12 infusion before anesthesia could effectively prevent nitrous oxide-induced homocysteine increase. On the other hand, although vitamin B12 infusion after the induction of anesthesia decreased homocysteine levels after the surgery, this difference was not statistically significant. In contrast, in patients who did not receive vitamin B12, nitrous oxide caused significant increase in homocysteine levels after the surgery. In a study on patients undergoing major surgeries, Myles et al.15 observed significantly higher homocysteine levels among patients who were anesthetized with nitrous oxide compared to those under anesthesia without the mentioned gas. They reported the increases of homocysteine levels to be positively related with the duration of exposure to nitrous oxide. They also found a significant relationship between postoperative complications (e.g., cardiovascular complications) and increase in post-operative homocysteine concentrations.
Bander et al.17 showed that nitrous oxide anesthesia significantly increased homocysteine levels and thus increased the incidence of ischemic myocardial infarction after endarterectomy. Nitrous oxide anesthesia has also been suggested to increase serum homocysteine levels and lead to endothelial dysfunction after non-cardiac surgeries.18 Other studies have confirmed the possible association of short exposure to nitrous oxide with homocysteine level increase,19 and higher risk of myocardial infarction.20 Also, many case studies have shown subacute combined degeneration after nitrous oxide anesthesia.21-23
Pichardo et al.24 evaluated children undergoing plastic surgery. Similar to our findings, they reported that nitrous oxide anesthesia for longer than two hours significantly increased serum homocysteine levels during the first 24 hours after the surgery. Furthermore, there was a significant inverse relationship between homocysteine levels and vitamin B12 levels after the surgery, so that patients with higher levels of vitamin B12 had lower serum homocysteine levels. Likewise, on the first postoperative day in the present study, patients who received vitamin B12 infusions had significantly higher serum vitamin B12 and lower serum homocysteine compared to the placebo group.
Nitrous oxide can cause hyperhomocysteinemia by irreversibly oxidizing the cobalt atom of vitamin B12, which is a cofactor for methionine synthase, leading to inhibition of methionine synthase which is involved in the re-methylation of homocysteine to methionine.2 Badner et al.16 evaluated the effect of oral vitamin B during the week before the operation on plasma homocysteine levels after nitrous oxide anesthesia found significant reductions in the patients homocysteine levels. Although this is consistent with our findings, it should be borne in mind that oral consumption of vitamin B one week before surgery may not be feasible in many cases.
Ubbink et al.25 detected the significant effect of vitamin B12 on plasma homocysteine levels in patients with high homocysteine levels. Woodside et al.26 showed the positive significant effect of vitamin B on plasma homocysteine levels in men. In contrast, Rao et al.7 found intravenous injections of vitamin B to be ineffective on hyperhomocysteinemia after nitrous oxide anesthesia. This inconsistency can be explained by the absence of standard techniques of anesthesia and different durations of exposure to nitrous oxide in the mentioned studies. Nevertheless, future studies in this field are warranted.
Conclusion
In the present study, infusions of vitamin B12 before and after general anesthesia reduced homocysteine levels after nitrous oxide anesthesia. However, this reduction was not statistically significant in the latter group. Since elevated serum homocysteine following nitrous oxide anesthesia can increase postoperative complications and morbidity, and hence impose costs on health systems, and also considering the probability of occurrence of neurological degeneration following anesthesia with nitrous oxide in patients with vitamin B12 deficiency, intravenous infusion of vitamin B12 may be recommended as a fast, inexpensive and safe method to prevent hyperhomocysteinemia and retard further neurological degeneration associated with nitrous oxide anesthesia in these patients. Although, further studies in this regard are warranted.
Acknowledgements
The financial support of Research Deputy of Mazandaran University of Medical Sciences is gratefully acknowledged. Also, the authors wish to thank all the study participants for their cooperation.
References
- 1.Krajewski W, Kucharska M, Pilacik B, Fobker M, Stetkiewicz J, Nofer JR, et al. Impaired vitamin B12 metabolic status in healthcare workers occupationally exposed to nitrous oxide. Br J Anaesth 2007. Dec;99(6):812-818 10.1093/bja/aem280 [DOI] [PubMed] [Google Scholar]
- 2.Kalikiri PC, Sachan RG. Nitrous Oxide Induced Elevation of Plasma Homocysteine and Methylmalonic Acid Levels and Their Clinical Implications. JIACM 2005;6(1):48-52 [Google Scholar]
- 3.Deacon R, Lumb M, Perry J, Chanarin I, Minty B, Halsey MJ, et al. Selective inactivation of vitamin B12 in rats by nitrous oxide. Lancet 1978. Nov;2(8098):1023-1024 10.1016/S0140-6736(78)92341-3 [DOI] [PubMed] [Google Scholar]
- 4.Ermens AA, Refsum H, Rupreht J, Spijkers LJ, Guttormsen AB, Lindemans J, et al. Monitoring cobalamin inactivation during nitrous oxide anesthesia by determination of homocysteine and folate in plasma and urine. Clin Pharmacol Ther 1991. Apr;49(4):385-393 10.1038/clpt.1991.45 [DOI] [PubMed] [Google Scholar]
- 5.Horne DW, Holloway RS. Compartmentation of folate metabolism in rat pancreas: nitrous oxide inactivation of methionine synthase leads to accumulation of 5-methyltetrahydrofolate in cytosol. J Nutr 1997. Sep;127(9):1772-1775 [DOI] [PubMed] [Google Scholar]
- 6.Nagele P, Zeugswetter B, Wiener C, Burger H, Hüpfl M, Mittlböck M, et al. Influence of methylenetetrahydrofolate reductase gene polymorphisms on homocysteine concentrations after nitrous oxide anesthesia. Anesthesiology 2008. Jul;109(1):36-43 10.1097/ALN.0b013e318178820b [DOI] [PubMed] [Google Scholar]
- 7.Rao LK, Francis AM, Wilcox U, Miller JP, Nagele P. Pre-operative vitamin B infusion and prevention of nitrous oxide-induced homocysteine increase. Anaesthesia 2010. Jul;65(7):710-715 10.1111/j.1365-2044.2010.06375.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quéré I, Perneger TV, Zittoun J, Bellet H, Gris JC, Daurès JP, et al. Red blood cell methylfolate and plasma homocysteine as risk factors for venous thromboembolism: a matched case-control study. Lancet 2002. Mar;359(9308):747-752 10.1016/S0140-6736(02)07876-5 [DOI] [PubMed] [Google Scholar]
- 9.Hankey GJ, Eikelboom JW, Ho WK, van Bockxmeer FM. Clinical usefulness of plasma homocysteine in vascular disease. Med J Aust 2004. Sep;181(6):314-318 [DOI] [PubMed] [Google Scholar]
- 10.Faraci FM, Lentz SR. Hyperhomocysteinemia, oxidative stress, and cerebral vascular dysfunction. Stroke 2004. Feb;35(2):345-347 10.1161/01.STR.0000115161.10646.67 [DOI] [PubMed] [Google Scholar]
- 11.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 2002. Nov;325(7374):1202-1206 10.1136/bmj.325.7374.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann JF, Sheridan P, McQueen MJ, Held C, Arnold JM, Fodor G, et al. HOPE-2 investigators Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease–results of the renal Hope-2 study. Nephrol Dial Transplant 2008. Feb;23(2):645-653 10.1093/ndt/gfm485 [DOI] [PubMed] [Google Scholar]
- 13.Abdollahi A, Shoar TS. Hyperhomocysteinemia in HIV-Infected Individuals: Correlation of a Frequent Prothrombotic Factor with CD4+ Cell Count. Oman Med J 2012. May;27(3):224-227 10.5001/omj.2012.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antoniades C, Antonopoulos AS, Tousoulis D, Marinou K, Stefanadis C. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. Eur Heart J 2009. Jan;30(1):6-15 10.1093/eurheartj/ehn515 [DOI] [PubMed] [Google Scholar]
- 15.Myles PS, Chan MT, Leslie K, Peyton P, Paech M, Forbes A. Effect of nitrous oxide on plasma homocysteine and folate in patients undergoing major surgery. Br J Anaesth 2008. Jun;100(6):780-786 10.1093/bja/aen085 [DOI] [PubMed] [Google Scholar]
- 16.Badner NH, Freeman D, Spence JD. Preoperative oral B vitamins prevent nitrous oxide-induced postoperative plasma homocysteine increases. Anesth Analg 2001. Dec;93(6):1507-1510 10.1097/00000539-200112000-00034 [DOI] [PubMed] [Google Scholar]
- 17.Badner NH, Beattie WS, Freeman D, Spence JD. Nitrous oxide-induced increased homocysteine concentrations are associated with increased postoperative myocardial ischemia in patients undergoing carotid endarterectomy. Anesth Analg 2000. Nov;91(5):1073-1079 [DOI] [PubMed] [Google Scholar]
- 18.Myles PS, Chan MT, Kaye DM, McIlroy DR, Lau CW, Symons JA, et al. Effect of nitrous oxide anesthesia on plasma homocysteine and endothelial function. Anesthesiology 2008. Oct;109(4):657-663 10.1097/ALN.0b013e31818629db [DOI] [PubMed] [Google Scholar]
- 19.Badner NH, Drader K, Freeman D, Spence JD. The use of intraoperative nitrous oxide leads to postoperative increases in plasma homocysteine. Anesth Analg 1998. Sep;87(3):711-713 [DOI] [PubMed] [Google Scholar]
- 20.Leslie K, Myles PS, Chan MT, Forbes A, Paech MJ, Peyton P, et al. Nitrous oxide and long-term morbidity and mortality in the ENIGMA trial. Anesth Analg 2011. Feb;112(2):387-393 10.1213/ANE.0b013e3181f7e2c4 [DOI] [PubMed] [Google Scholar]
- 21.Lin RJ, Chen HF, Chang YC, Su JJ. Subacute combined degeneration caused by nitrous oxide intoxication: case reports. Acta Neurol Taiwan 2011. Jun;20(2):129-137 [PubMed] [Google Scholar]
- 22.Renard D, Dutray A, Remy A, Castelnovo G, Labauge P. Subacute combined degeneration of the spinal cord caused by nitrous oxide anaesthesia. Neurol Sci 2009. Feb;30(1):75-76 10.1007/s10072-009-0013-2 [DOI] [PubMed] [Google Scholar]
- 23.Flippo TS, Holder WD., Jr Neurologic degeneration associated with nitrous oxide anesthesia in patients with vitamin B12 deficiency. Arch Surg 1993. Dec;128(12):1391-1395 10.1001/archsurg.1993.01420240099018 [DOI] [PubMed] [Google Scholar]
- 24.Pichardo D, Luginbuehl IA, Shakur Y, Wales PW, El-Sohemy A, O’Connor DL. Effect of nitrous oxide exposure during surgery on the homocysteine concentrations of children. Anesthesiology 2012. Jul;117(1):15-21 10.1097/ALN.0b013e318259a8cc [DOI] [PubMed] [Google Scholar]
- 25.Ubbink JB, Vermaak WJ, van der Merwe A, Becker PJ, Delport R, Potgieter HC. Vitamin requirements for the treatment of hyperhomocysteinemia in humans. J Nutr 1994. Oct;124(10):1927-1933 [DOI] [PubMed] [Google Scholar]
- 26.Woodside JV, Yarnell JW, McMaster D, Young IS, Harmon DL, McCrum EE, et al. Effect of B-group vitamins and antioxidant vitamins on hyperhomocysteinemia: a double-blind, randomized, factorial-design, controlled trial. Am J Clin Nutr 1998. May;67(5):858-866 [DOI] [PubMed] [Google Scholar]