Abstract
Objective
Available data on outcomes of combined proximal stent-grafting with distal bare stenting for management of aortic dissection are limited. The objective of this study was to provide a systematic review of outcomes of this approach.
Methods
Studies involving combined proximal stent-grafting with distal bare stenting for management of aortic dissection were systematically searched and reviewed through MEDLINE databases.
Results
A total of four studies were included: 108 patients treated for management of acute (n=54) and chronic (n=54) aortic dissection. The technical success rate was 95.3% (range, 84-100%). The 30-day mortality rate was 2.7% (range from 0% to 5%). The morbidity rate occurring within 30 days was 51.8% (range from 0% to 65%) and included stroke (2.7%), paraplegia (2.7%), retrograde dissection (1.8%), renal failure (14.8%), severe cardiopulmonary complications (5.5%) and bowel ischemia (0.9%). The incidence of type I endoleak was 9.2% (10/108). During follow-up, 5 (4.6%) deaths were related to aortic rupture or aortic repair. Mean re-intervention rate was 12.9%. Two cases (1.9%) of delayed retrograde type A dissection and one case of aortobronchial fistula (0.9%) were reported. The most common delayed complication was thoracic stent-graft migration (4.7%). The rate of device failure was 9.2%. Favorable aortic remodeling was observed: studies reporting midterm follow-up of the true lumen demonstrated a high rate of both false lumen regression and true lumen expansion. At 12 months, complete false lumen thrombosis was observed at the thoracic level in 70.4% and at the abdominal level in 13.5% of patients.
Conclusions
Combined proximal stent-grafting with distal bare stenting appears to be a feasible approach for the management of Type B aortic dissection. Although this approach clearly improved true lumen perfusion and diameter, it failed to completely suppress false lumen patency. However, it should be acknowledged that contemporary data on this approach is limited to small studies with variable results.
Keywords: Thoracic aorta, stent-graft, aortic dissection, bare stent, thoracic endovascular aortic repair (TEVAR), Provisional Extension To Induce Complete Attachment (PETTICOAT)
Introduction
Acute dissection is the most common fatal aortic catastrophe, and the surgical treatment of Stanford type B acute aortic dissection (A-BD) remains a formidable challenge. The standard strategy for uncomplicated A-BD is medical management, with surgical intervention reserved for cases complicated by rupture, malperfusion, intractable pain, uncontrolled hypertension or aneurysmal dilatation.
During the past decade, thoracic endovascular aortic repair (TEVAR) has been increasingly used to treat this condition when intervention has been necessary. The aim is to cover the entry tear to direct aortic flow preferentially into the true lumen. In one review, compared with the 30-day mortality of open repair, endovascular repair of acute complicated type B aortic dissection was associated with a lower mortality (2.8% compared to 29.3%) and therefore is regarded as the surgical therapy of choice (1). Even though it is associated with a lower mortality compared to open surgery, stent-grafting of complicated chronic type B aortic dissection remains controversial due to concerns over durability.
Tsai et al. (2) showed that the natural course of false lumen partial thrombosis in type B aortic dissection has a worse prognosis than that of a completely patent false lumen (3). Complete exclusion of the false lumen could improve the prognosis of this disease. Thus, to promote true lumen expansion and false lumen thrombosis, devices with bare metal stents that extend into the thoracoabdominal aorta have been used in an attempt to induce aortic remodeling. The ultimate aim of this is to prevent aortic aneurysmal evolution or rupture, and decrease the incidence of re-intervention.
The aim of the present study was to provide a systematic review of series that describe the outcome of combined proximal stent-grafting with distal bare stenting for management of aortic dissection.
Methods
Search strategy
A literature search was undertaken to identify all published studies in the past 10 years reporting combined proximal stent-grafting with distal bare stents for the management of aortic dissection. Potentially eligible studies in English were sought through a computerized search of MEDLINE databases from 2002 to September 2012. Key words entered in this search were ‘‘thoracic aorta’’ or ‘‘bare stent’’, ‘‘dissection’’, ‘‘endovascular’’, and ‘‘PETTICOAT’’. Additionally, reference lists of all retrieved articles and reviews were manually screened to further identify potentially relevant studies.
Study selection
Studies were considered for inclusion on the basis of these criteria:
❖ Reporting on combined proximal stent-grafting with distal bare stenting for management of aortic dissection;
❖ Including at least five patients treated with this method;
❖ Reporting on clinical and technical outcomes: technical success, 30-day and overall mortality, renal failure, bowel ischemia, endoleak, aortic rupture, neurological complications, re-intervention rate, retrograde type A dissection, stent-graft migration, cardiac failure, pulmonary distress syndrome or severe lung infection, aortobronchial fistula and device performance.
Studies containing duplicate data were excluded and those with the most recent or detailed data from the same authors were used for analysis.
Data extraction
Data were extracted regarding demographics, co-morbidities, case selection (proportion of acute and chronic dissection, proportion of symptomatic patients, operative details, technical success), and early and midterm outcomes (endoleak, retrograde dissection, aortic rupture, stroke, paraparesis or paraplegia, renal failure, bowel ischemia, severe cardiopulmonary complications, 30-day and midterm mortality, and freedom from re-intervention).
Severe morbidity was defined as mortality related to aortic repair, or one of the following non-fatal adverse events occurring within the postoperative hospital period: central nervous system complication (stroke or spinal cord ischemia with permanent deficit), type I endoleak, retrograde type I dissection, acute renal failure (defined as the need to initiate hemodialysis for the first time), cardiac failure, pulmonary distress syndrome or severe lung infection, bowel ischemia, aortobronchial fistula and unplanned return to surgery.
Perioperative severe morbidity rate was defined as severe morbidity occurring within the first 30 postoperative days. Midterm morbidity rate was defined as severe morbidity occurring after the first 30 postoperative days. Data were extracted by two independent analysts (L.C. and B.A.O.).
Results
Search results
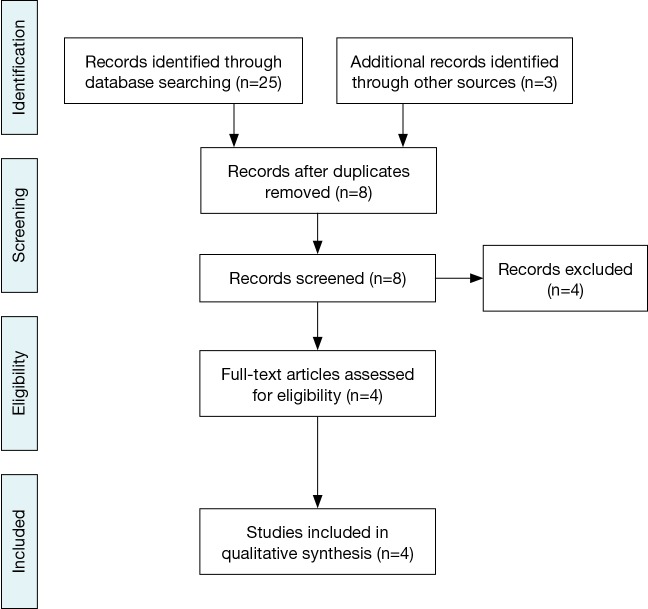
Four studies were selected after literature search, exclusion of duplicate publications and screening for eligibility (Table 1, Figure 1) (3-6).
Table 1. Demographic variables and comorbidities of patients.
| Duration | Type | N | Mean age (years) | Male (%) | Indication (%) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Type A | Type B | Acute dissection | Chronic dissection | ||||||
| Nienaber et al. (4) | – | Retrospective cohort | 12 | 58.7±10 | 83 | 16.6 | 83.4 | 0 | 100 |
| Hofferberth et al. (5) | 2003-2010 | Retrospective cohort | 31 | 57.8±12.5 | 71 | 41.9 | 58.1 | 81 | 19 |
| Lombardi et al. (3) | 2007-2009 | Prospective cohort | 40 | 58±11 | 70 | 0 | 100 | 60 | 40 |
| Melissano et al. (6) | 2005-2011 | Retrospective cohort | 25 | 56±12 | 88 | 0 | 100 | 20 | 80 |
Figure 1.
PRISMA Flowchart of study selection.
Case selection
Patient demographics and presenting features are shown in Table 1. The mean age was 57.5 years and 75.9% were male. The indication for endovascular repair was the presence of features of complicated aortic dissection (type A: 15 or type B: 93; acute: 54 or chronic: 54). The most common co-morbidities were hypertension (87.9%), hyperlipemia (18%), renal failure (15.6%) and cardiac disease (12%).
The most commonly stated indication for intervention was malperfusion due to branch vessel obstruction or true lumen collapse (76/108, 70.3%). Other indications included refractory hypertension (41/108, 37.9%), refractory chest pain (36/108, 33.3%), rapid aortic enlargement (5 mm within 3 months) or transaortic diameter >40 mm (35/108, 32.4%), and periaortic effusion/hematoma (10/108, 9.2%). Acute and chronic dissections could not be separated for the purpose of analysis.
Technical success
The Cook Zénith Dissection device (Cook Medical, Bloomington, IN, USA) was the most commonly deployed graft (96/108, 88.8%). A median of 1.27 stent-grafts (range, 1-3) and 1.27 [1-3] bare stents were used per patient (Table 2). The technical success rate was 95.3% (range, 84-100%), with a median operating time of 141 minutes (range, 40-397 minutes). Supra-aortic branch revascularization was performed in 23.1% of patients (25/108). Adjunctive endovascular procedures were required in 32 patients (29.6%). One patient (0.9%) underwent surgical conversion because a bare-metal strut became lodged in the distal aorta. These outcomes are summarized in Tables 3 and 4.
Table 2. Stent-grafts and bare stents used for treatment and procedure characteristics.
| Stent-graft | Number of SG per patient | Number of bare stents per patient | Adjunctive procedure | Mean procedure time (min) | Median time from dissection to intervention (days) | |
|---|---|---|---|---|---|---|
| Nienaber et al. (4) | • SG: Talent (66.6%), Exluder (16.6%), Vailant (8.3%), Zénith (8.3%) • Bare stent: Sinus (41.6%), Fortress (50%), Z stent (8.4%) |
1.08 [1-2] | 1.08 [1-2] | 0% (0/12) | 70±15 | – |
| Hofferberth et al. (5) | Cook Zénith Dissection | 2.9 [1-5] | – | 61.2% (19/31) • Stent (6/31): Iliac, renal, superior mesenteric arteries; • Ascending aorta open repair (13/31) |
– | 10.4 [1-1,095] |
| Lombardi et al. (3) | Cook Zénith Dissection | 1.27 [1-3] | 1.37 [0-3] | 37.5% (15/40) • Bare stent (9/40): Iliac, renal, superior mesenteric arteries; • Carotid-subclavian bypass (3/40); • Carotid-carotid bypass (3/40) |
163 [40-397] | 20 [0-78] |
| Melissano et al. (6) | Cook Zénith Dissection | 1.32 [1-3] | 1.28 [1-2] | 96% (24/25) • Bare stent: Iliac, renal, superior mesenteric arteries (4/25); • Humeral thrombectomy (1/25); • Carotid-subclavian bypass (2/25); • Carotid-carotid bypass (17/25) |
– | – |
SG, stent-graft.
Table 3. Summary of technical success and mortality of patients treated by combined proximal stent-grafting with distal bare stenting for management of aortic dissection.
| Mean follow-up (months) | Technical success rate (%) | 1-year survival rate (%) | Overall aortic related mortality | 30-day aortic related mortality |
>30 days aortic related mortality |
||||
|---|---|---|---|---|---|---|---|---|---|
| Rate | Etiology | Rate | Etiology | ||||||
| Nienaber et al. (4) | – | 100 | 91.6 | 8.3% (1/12) | 0% (0/12) | 8.3% (1/12) | Aortic rupture on month 11 | ||
| Hofferberth et al. (5) | 57.3 | 97 | 93.7 | 6.4% (2/31) | 3.2% (1/31) | Stent-graft misdeployment: surgical conversion | 3.3% (1/30) | ||
| Lombardi et al. (3) | – | 100 | 90 | 7.9% (3/38) | 5% (2/40) | • Aortic rupture on day 11; • Sudden death on day 29 |
5% (2/38) | Aortic rupture on day 81 | |
| Melissano et al. (6) | 38±17 | 84 | 100 | 0% (0/25) | 0% (0/25) | 0% (0/25) | |||
Table 4. Summary of 30 days morbidity of patients treated by combined proximal stent-grafting with distal bare stenting for management of aortic dissection.
| Mean follow-up (months) | Overall morbidity | 30-day morbidity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aortic rupture | Type I endoleak | Type II endoleak | Stroke | Paraparesis/Paraplegia | Retrograde dissection | Cardio-pulmonary | Renal failure | Bowel ischemia | |||
| Nienaber et al. (4) | – | 0% (0/12) | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 |
| Hofferberth et al. (5) | 57.3 | 22.5% (7/31) | 0% (0/31) | 3.3% (1/31) | 3.3% (1/31) | 0 | 0 | 0 | 3.3% (1/31) | 12.9% (4/31) | 0 |
| Lombardi et al. (3) | – | 65% (26/40) | 5% (2/40) | 2.5% (1/40) | 7.5% (3/40) | 7.5% (3/40) | 5% (2/40) | 5% (2/40) | 12% (5/40) | 17.5% (7/40) | 2.5% (1/40) |
| Melissano et al. (6) | 38±17 | 52% (13/25) | 0% (0/25) | 16% (4/25) | 12% (3/25) | 0 | 4% (1/25) | 0 | 0 | 20% (5/25) | 0 |
Perioperative outcomes
Perioperative outcomes were those occurring within the first 30 postoperative days and are summarized in Table 4. The overall 30-day mortality was 2.7% (3/108). The early morbidity rate was 51.8% (range, 0-65%). Acute renal failure was the most common early complication (16/108, 14.8%) with six amongst this group requiring dialysis. In studies that reported endoleaks according to subtype, the global incidence of endoleak was 12% (13/108): type I, 5.5% (6/108) and type II, 6.4% (7/108). The incidence of early retrograde dissection was 1.8% (2/108), and periprocedural aortic rupture occurred in 1.8% (2/108). The incidence of neurological complications was 5.4% (6/108), which included two cases each of stroke, paraplegia and paraparesis. The early re-intervention rate was 4.6% and was required for renal artery occlusion, bowel ischemia, type II endoleak or bare stent misdeployment.
Midterm outcomes
Midterm outcomes were defined as those occurring after 30 days and are summarized in Table 5. The all-cause mortality rate was 3.8% (4/105). There were two cases of delayed retrograde type A dissection (1.9%) and one case of aortobronchial fistula (0.9%). The most common delayed complication was stent-graft migration (5/105, 4.7%). The incidence of type I endoleak was 3.8% (4/105) while type II endoleak incidence was not reported. Delayed aortic rupture was reported in 1.9% (2/105). Re-intervention was necessary in 8.5% (4% to 13.3%) of patients for complications such as retrograde type A dissection, type I endoleak, stent-graft migration and aortobronchial fistula (Table 6).
Table 5. Summary of over 30 days morbidity of patients treated by combined proximal stent-grafting with distal bare stenting for management of aortic dissection.
| Mean follow-up (months) | Overall morbidity | >30 days morbidity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aortic rupture | Type I endoleak | Type II endoleak | Stent-graft migration | Retrograde dissection | Cardio-pulmonary | Renal failure | Aortobronchial fistula | |||
| Nienaber et al. (4) | – | 16.6% (2/12) | 8.3% (1/12) | 8.3% (1/12) | – | 0 | 0 | 0 | 0 | 0 |
| Hofferberth et al. (5) | 57.3 | 16.6% (5/30) | 3.3% (1/30) | 3.3% (1/30) | – | 3.3% (1/30) | 3.3% (1/30) | 0 | 0 | 3.3% (1/30) |
| Lombardi et al. (3) | – | 18.4% (7/38) | 2.6% (1/38) | 2.6% (1/38) | 0 | 5.2% (2/38) | 2.6% (1/38) | 2.6% (1/38) | 2.6% (1/38) | 0 |
| Melissano et al. (6) | 38±17 | 12% (3/25) | 0 | 4% (1/25) | 0 | 8% (2/25) | 0 | 0 | 0 | 0 |
Table 6. Summary of secondary intervention of patients treated by combined proximal stent-grafting with distal bare stenting for management of aortic dissection.
| Mean follow-up (months) | Re-intervention <30 days | Re-intervention >30 days | |||
|---|---|---|---|---|---|
| Nienaber et al. (4) | – | 0% (0/12) | 8.3% (1/12) | Type I endoleak | |
| Hofferberth et al. (5) | 57.3 | 3.2% (1/31) | Bare stent misdeployment | 13.3% (4/30) | • Retrograde type A dissection; • Proximal stent-graft migration; • Aortobronchial fistula; • Type I endoleak |
| Lombardi et al. (3) | – | 7.5% (3/40) | • Liver and gall bladder ischemia • Renal artery stenting • Renal artery stenting |
7.9% (3/38) | • Retrograde type A dissection; • Retrograde type A dissection and stent-graft migration; • Type I endoleak |
| Melissano et al. (6) | 38±17 | 4% (1/25) | Type II endoleak | 4% (1/25) | Stent-graft migration |
Severe morbidity rate
Overall severe morbidity rate was 33.3% (36/108). Perioperative severe morbidity rate was 17.6% (19/108) and midterm severe morbidity rate was 16.2% (17/105).
Device performance
The rate of device failure was 9.2% (10/108). Component separation or device migration necessitating secondary interventions was reported in five patients. One case of focally ruptured and four cases of a stent body misalignment of Cook Zénith Dissection stents were reported.
Aortic remodeling
Rates of complete false lumen thrombosis ranged from 31.2% to 75% at the thoracic level and 3.1% to 25.9% at the abdominal level, as summarized in Table 7. These data were not always complete and the total number of patients in which results were available was low. Studies reporting midterm follow-up of the true lumen demonstrated a high rate of both false lumen regression and true lumen expansion. Nienaber et al. (4) reported an increase in the true lumen size and a concomitant decrease in the false lumen size along the dissected aorta at 12 months with a completely thrombosed thoracic false lumen observed in 75% of patients. The fate of the false lumen at the abdominal aorta level was not reported. Hofferberth et al. (5) reported increased true lumen perfusion and diameter after a mean follow-up of 57.3 months, although perfusion of the abdominal or thoracic aortic false lumen was still observed in 74% of the patients. Melissano et al. (6) noted a significant increase (98%) in true lumen volume at both the thoracic (115%) and abdominal segments (63%) at a mean follow-up of 57.3 months. At midterm follow-up (1 and 2 years), the overall aortic volume tended to decrease compared to preoperative values. The rate of false lumen thrombosis was not reported. The abdominal segment, after initial true lumen expansion, failed to remodel with stable true lumen volume and had a tendency toward enlargement of the overall abdominal aortic volume as a result of abdominal false lumen expansion. Lombardi et al. (3) reported an increase in true lumen size and a concomitant decrease in false lumen size in the dissected aorta at 12 months. A completely thrombosed thoracic false lumen was observed in 31% of patients. Perfusion of thoracic and abdominal aortic false lumen was still present in 68.8% and 96.8% of the patients, respectively.
Table 7. Summary of aortic remodeling of patients treated by combined proximal stent-grafting with distal bare stenting for management of aortic dissection: true lumen (TL) and false lumen (FL).
| Change in TL-FL ratio | Thoracic aorta false lumen thrombosis |
Abdominal aorta false lumen thrombosis |
||||
|---|---|---|---|---|---|---|
| Complete | Partial | Complete | Partial | |||
| Nienaber et al. (4) | At 1 year: Before stenting, TL:FL =4/25=0.16 After stenting, TL:FL =23/11=2.1 |
75% (9/12) | 8.3% (1/12) | – | – | |
| Hofferberth et al. (5) | At 1 year: Before stenting, TL expansion:FL =84/332=0.25 After stenting, TL:FL =216/248=0.87 |
66.6% (18/27) | 33.4% (9/27) | 25.9% (7/27) | 70.3% (19/27) | |
| Melissano et al. (3) | At 2 years: FL reduction: 65% At 2 years: Before stenting, TL:FL =84/332=0.25 After stenting, TL:FL =216/248=0.87 |
– | – | – | – | |
| Lombardi et al. (6) | At 1 year: TL increased (P<0.05) FL decreased (P<0.05) |
31.2% (10/32) | 68.8% (22/32) | 3.1% (1/32) | 81.3% (26/32) | |
Discussion
One of the drawbacks of stent-grafting for complicated thoracic aortic dissection is that despite thrombosing the false lumen adjacent to the stent-graft, the thrombosis of the false lumen is not complete due to retrograde flow through the residual re-entry tear or intimal fenestrations related to branch vessels. This exposes individuals to increased risk of late aneurysmal degeneration and therefore aortic rupture. It has been demonstrated by one group that the natural course of false lumen partial thrombosis in type B aortic dissection has a worse prognosis than that of a completely patent false lumen (2). Therefore, complete exclusion of the false lumen should clearly be the aim wherever possible (2). To promote true lumen expansion and false lumen thrombosis, some authors have proposed the use of bare metal stents in the distal thoracoabdominal aorta. The Provisional Extension To Induce Complete Attachment (PETTICOAT) technique was first reported in 2005 by Mossop et al. (7) and, in 2006, a series of 12 patients was reported (4). This technique eliminates the entry tear and increases the true luminal diameter in the distal aorta through a combination of stent-grafting and bare metal stenting of the visceral and infrarenal segments.
In order to compare the results of proximal stent-grafting with distal bare stenting for management of aortic dissection, a systematic review of stent-graft placement without distal bare stenting for management of complicated acute and chronic aortic dissection was performed (Supplementary Tables S1,S2,S3) (8-27). The technical success rates reported for proximal stent-grafting with distal bare stenting for management of aortic dissection were high (95.3%) and were similar to reported success rates of established endovascular techniques using stent-graft without distal bare stenting. The mean 30-day mortality after combined proximal stent-grafting with distal bare stenting for acute and chronic aortic dissection in the present study was 2.7%. This rate of mortality is similar to rates recently reported by several authors on results of TEVAR for acute and chronic dissection. Of note, however, was the rate of severe morbidity. The pooled rate of severe morbidity in this series was 33.3% (36/108). In a meta-analysis describing the results of TEVAR for acute and chronic dissection, a major complication rate of 11.1±1.4% was reported (16). The most critical complications were related to retrograde extension of the dissection into the ascending aorta, neurological complications and aortic rupture. This more extensive approach was associated with a slightly higher rate of dissection into the ascending aorta (3.7% vs. 1.8%), neurological complications (5.5% vs. 3.1%) and aortic rupture (3.7% vs. 2.5%). However, patients treated in this study represent a difficult patient subgroup, with 63.8% of the patients presenting with malperfusion or impending rupture. Eggebrecht et al. (8) reported the most favorable outcomes. Among 12 patients for a malperfusion syndrome, the overall severe morbidity was 16.6%: one patient died from an aortic rupture and another received an additional stent-graft for a type I endoleak. This may be explained by the fact that this group advocated a staged approach to the procedure, allowing recovery from the acute insult of dissection and initial procedure before evaluating the need for extension of the graft using the bare metal components. Persistence of a distal malperfusion syndrome after proximal covered endograft placement is uncommon. Nienaber et al. (4) only reported this issue in 12 patients among a cohort of 100 (12%). This suggests that distal bare stenting could be planned only after evaluation following primary entry tear closure rather than a single stage extensive repair of the thoracoabdominal aorta.
Achieving complete false lumen thrombosis is challenging, and pursuing this goal compounds the risks of multiple procedures, cumulative radiation dose, and contrast exposure. By treating the entire thoracoabdominal aorta, combined proximal stent-grafting with distal bare stenting should limit the number of adjunctive procedures required. The re-intervention rate was 16.6% of patients for severe complications such as occlusion of renal arteries, type I endoleak, retrograde dissection, aortic rupture and aortobronchial fistula.
Hofferberth et al. (5) reported that adjunctive bare metal stenting does not compromise branch vessel perfusion. This statement has to be moderated. Adjunctive intraoperative endovascular procedures to maintain patency of visceral or iliac arteries were required for 19 arteries. When compared with results of a recent study of TEVAR without distal bare stenting for management of complicated aortic dissection (28), the rate of adjunctive endovascular procedures was significantly lower (1% vs. 17.6%) than after bare metal stent deployment in the distal thoracoabdominal aorta. We recently reported an experimental study assessing patency of abdominal branch vessels after bare metal stenting of the thoracoabdominal aorta in a human ex vivo model of type B aortic dissection (Figure 2) (29). This study reported a pressure gradient drop in 25% of the cases in the abdominal branch vessels (i.e., the celiac trunk, the superior mesenteric artery and the renal arteries) after bare metal stenting, which is similar to the 17.6% of adjunctive endovascular procedures reported in clinical practice. Furthermore, a high rate (54.5%) of pressure gradient drop in these branch vessels was reported after bare metal stenting when these arteries were supplied by the false lumen (Figure 3). None of the studies reporting the results of bare metal stenting mentioned the rate of aortic branch vessels supplied by the false lumen before extensive bare stenting, nor their follow-up. However, in clinical practice, 31% of the abdominal branch vessels were reported to be supplied by the false lumen in type B dissection. These findings suggest that bare stent placement, while preventing or removing dynamic malperfusion when the aortic branch vessels are supplied by the true lumen, could on the other hand involve static malperfusion when these arteries are supplied by the false lumen.
Figure 2.
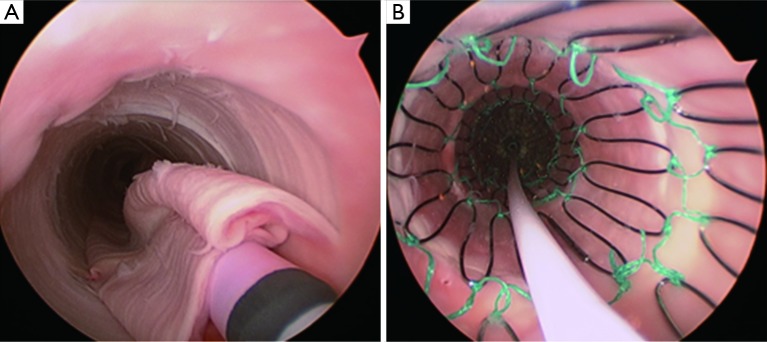
Angioscopy during bare metal stent deployment within the true lumen of a dissected aorta. (A) Sheath of the bare metal stent arising from the true lumen, which is collapsed by the false lumen; (B) After complete deployment of the bare metal stent, the intimal-medial flap is completely re-attached.
Figure 3.
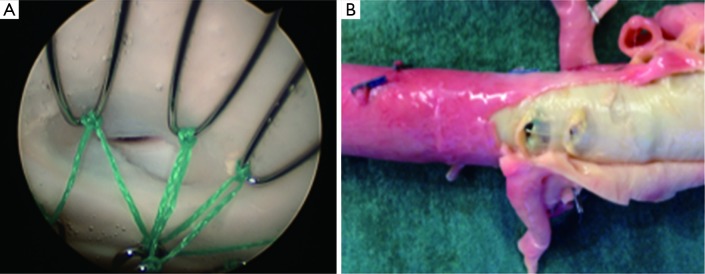
Stenosis of the left renal artery ostia following bare metal stenting, visible on (A) angioscopy, and (B) macroscopically.
Device concerns have also been reported. Bertoglio et al. (30) reported the risk of stent misalignment probably resulting from excessive manipulation of the delivery system or during catheter manipulation during adjunctive or secondary procedure. Melissano et al. (6) reported one focally ruptured bare stent. Lombardi et al. (3) reported component separation or device migration necessitating secondary interventions in two patients. Hofferberth et al. (5) reported a case of the bare stent becoming dislodged in the distal aorta necessitating open surgical conversion leading to the death of the patient.
Combined proximal stent-grafting with distal bare stenting for management of aortic dissection clearly improved true lumen perfusion and diameter, but apparently failed to completely suppress false lumen patency. At 1-year, false lumen patency was still present in 29.6% of the patients at the thoracic level and in 86.5% of the patients at the abdominal level. Data on patients having complete imaging beyond this time period were limited. Dialetto et al. (9) studied aortic remodeling after TEVAR for acute and chronic dissection and, at 1-year, they reported a comparable rate of false lumen patency of 19.4% at the thoracic level. In our experimental study, re-expansion of the true lumen was observed in all cases with complete attachment of the dissection flap after extensive bare metal stenting (29). This may be explained by the fact that, in our model, bare stent placement was performed in a very acute stage of the dissection, without any aortic dilatation or false lumen thrombosis. In the clinical series reported, acute and chronic dissections were mixed and this did not allow separate analysis of outcomes. This suggests that distal bare stenting could be more effective in the acute stage of the dissection without either aneurysmal degeneration or false lumen thrombosis.
This review has several limitations. Although it is the only review examining combined proximal stent-grafting with distal bare stenting for management of aortic dissection, the pooled results are weakened because of lack of standardization in reporting patients’ specific data and end points, and the lack of separate analysis of outcomes for acute and chronic dissections. Furthermore, we specifically focused the review on clinical outcomes. Finally, some small studies were included whereas a larger number of patients are needed to better identify statistically significant differences.
Conclusions
Combined proximal stent-grafting with distal bare stenting for management of aortic dissection clearly improved true lumen perfusion and diameter. However, it failed to completely suppress false lumen patency and carries not negligible risks of severe morbidity. Distal bare stenting could be proposed in case of persistence of a distal malperfusion syndrome after careful evaluation following primary entry tear closure rather than a single stage extensive repair of the thoracoabdominal aorta. Nevertheless, no reliable long-term data exist to assess the durability of combined proximal stent-grafting with distal bare stenting for management of aortic dissection and contemporary conclusions are mainly provided from relatively small case series or retrospective studies. Furthermore, results of management of acute and chronic dissection should be reported separately to allow a more accurate analysis. Prospective trials of combined proximal stent-grafting with distal bare stenting versus stent-grafting without distal bare stenting are needed to assess outcomes of this extensive approach.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
Table S1. Endovascular stent-graft repair without distal bare stenting for management of acute and chronic complicated type B aortic dissection.
| Year | n | Acute | Technical success, % | Retrograde dissection, % | Stroke, % | Paraplegia, % | Renal failure, % | Adjunctive distal reperfusion, % | Aortic rupture, % | 30-day mortality, % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eggebrecht (8) (meta-analysis) | 2006 | 609 | 248 | 98 | 1.9 | 1.9 | 0.8 | N/A | N/A | 2.3 | 5.3 |
| Dialetto (9) | 2005 | 56 | 14 | 100 | 4 | 0 | 0 | N/A | 0 | 1.7 | 10.7 |
| Nethanson (10) | 2005 | 40 | 23 | 95 | N/A | 2.5 | 2.5 | 13 | 0 | 0 | 2.5 |
| Sayer (11) | 2008 | 78 | 38 | 100 | 1.2 | 0 | 2.5 | N/A | 0 | 3.8 | 5.1 |
| Böckler (12) | 2009 | 54 | 24 | 93 | 3.7 | 0 | 0 | N/A | 0 | 0 | 11.1 |
| Kische (13) | 2009 | 180 | 37 | 98.3 | 1.8 | 3.9 | 2.8 | N/A | 2.7 | 4.2 | 5 |
| Younes (14) | 2010 | 23 | 11 | 100 | 0 | 5.5 | 5.5 | 0 | 0 | 0 | 5.5 |
| Parsa (15) | 2010 | 55 | 22 | 100 | 0 | 0 | 2 | 1.8 | 0 | 0 | 2 |
| Yang (16) | 2012 | 61 | 33 | 100 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 6.5 | 6.5 |
| Overall | 1,156 | 450 (38.9%) | 98.3 | 1.8 | 1.9 | 1.2 | N/A | 1 | 2.5 | 5.5 |
Table S2. Endovascular stent-graft repair without distal bare stenting for management of acute complicated type B aortic dissection.
| Year | n | Technical success, % | Retrograde dissection, % | Stroke, % | Paraplegia, % | Renal failure, % | Adjunctive distal reperfusion, % | Aortic rupture, % | 30-day mortality, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| Conrad (17) | 2009 | 33 | 100 | 9 | 3 | 12 | 0 | 0 | 6 | 12 |
| Khoynezhad (18) | 2009 | 28 | 90 | 3.5 | 3.3 | 0 | 10 | 3.5 | 0 | 11 |
| Shu (19) | 2010 | 45 | 100 | 0 | 0 | 0 | 4.4 | 0 | 0 | 4.4 |
| White (20) | 2011 | 85 | 100 | 0 | 9.4 | 9.4 | 9.4 | 4.7 | 14 | 10.6 |
| Qin (21) | 2012 | 124 | 100 | 2.4 | 0.8 | 0.8 | N/A | 13.7 | 0 | 0 |
| Ehrlich (22) | 2013 | 29 | 100 | 3.4 | 6.8 | 0 | 0 | 20 | 6.8 | 17 |
| Overall | 344 | 99.5 | 2 | 3 | 3.7 | 5.9 | 8.1 | 4.6 | 6.6 |
Table S3. Endovascular stent-graft repair without distal bare stenting for management of chronic complicated type B aortic dissection.
| Year | n | Technical success, % | Retrograde dissection, % | Stroke, % | Paraplegia, % | Renal failure, % | Adjunctive distal reperfusion, % | Aortic rupture, % | 30-day mortality, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| Kim (23) | 2009 | 72 | 97.2 | 0 | 0 | 0 | 1.4 | 0 | 0 | 0 |
| Xu (24) | 2010 | 84 | 91.7 | 0 | 0 | 0 | 4.8 | 0 | 4.8 | 1.2 |
| Czerny (25) | 2010 | 14 | 85.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kang (26) | 2011 | 76 | 96 | 3.9 | 1.3 | 0 | 0 | 0 | 0 | 5 |
| Andacheh (27) | 2012 | 73 | 99 | 5.4 | 1 | 1 | 0 | 0 | 2.7 | 14 |
| Total | 319 | 95.8 | 2.1 | 1.8 | 0.3 | 1.5 | 0 | 1.8 | 4.7 |
References
- 1.Szeto WY, McGarvey M, Pochettino A, et al. Results of a new surgical paradigm: endovascular repair for acute complicated type B aortic dissection. Ann Thorac Surg 2008;86:87-93; discussion 93-4 [DOI] [PubMed] [Google Scholar]
- 2.Tsai TT, Evangelista A, Nienaber CA, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 2007;357:349-59 [DOI] [PubMed] [Google Scholar]
- 3.Lombardi JV, Cambria RP, Nienaber CA, et al. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg 2012;55:629-640.e2. [DOI] [PubMed]
- 4.Nienaber CA, Kische S, Zeller T, et al. Provisional extension to induce complete attachment after stent-graft placement in type B aortic dissection: the PETTICOAT concept. J Endovasc Ther 2006;13:738-46 [DOI] [PubMed] [Google Scholar]
- 5.Hofferberth SC, Foley PT, Newcomb AE, et al. Combined proximal endografting with distal bare-metal stenting for management of aortic dissection. Ann Thorac Surg 2012;93:95-102 [DOI] [PubMed] [Google Scholar]
- 6.Melissano G, Bertoglio L, Rinaldi E, et al. Volume changes in aortic true and false lumen after the “PETTICOAT” procedure for type B aortic dissection. J Vasc Surg 2012;55:641-51 [DOI] [PubMed] [Google Scholar]
- 7.Mossop PJ, McLachlan CS, Amukotuwa SA, et al. Staged endovascular treatment for complicated type B aortic dissection. Nat Clin Pract Cardiovasc Med 2005;2:316-21; quiz 322 [DOI] [PubMed] [Google Scholar]
- 8.Eggebrecht H, Nienaber CA, Neuhäuser M, et al. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J 2006;27:489-98 [DOI] [PubMed] [Google Scholar]
- 9.Dialetto G, Covino FE, Scognamiglio G, et al. Treatment of type B aortic dissection: endoluminal repair or conventional medical therapy? Eur J Cardiothorac Surg 2005;27:826-30 [DOI] [PubMed] [Google Scholar]
- 10.Nathanson DR, Rodriguez-Lopez JA, Ramaiah VG, et al. Endoluminal stent-graft stabilization for thoracic aortic dissection. J Endovasc Ther 2005;12:354-9 [DOI] [PubMed] [Google Scholar]
- 11.Sayer D, Bratby M, Brooks M, et al. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: implications for management. Eur J Vasc Endovasc Surg 2008;36:522-9 [DOI] [PubMed] [Google Scholar]
- 12.Böckler D, Hyhlik-Dürr A, Hakimi M, et al. Type B aortic dissections: treating the many to benefit the few? J Endovasc Ther 2009;16Suppl 1:I80-90 [DOI] [PubMed] [Google Scholar]
- 13.Kische S, Ehrlich MP, Nienaber CA, et al. Endovascular treatment of acute and chronic aortic dissection: midterm results from the Talent Thoracic Retrospective Registry. J Thorac Cardiovasc Surg 2009;138:115-24 [DOI] [PubMed] [Google Scholar]
- 14.Younes HK, Harris PW, Bismuth J, et al. Thoracic endovascular aortic repair for type B aortic dissection. Ann Vasc Surg 2010;24:39-43 [DOI] [PubMed] [Google Scholar]
- 15.Parsa CJ, Schroder JN, Daneshmand MA, et al. Midterm results for endovascular repair of complicated acute and chronic type B aortic dissection. Ann Thorac Surg 2010;89:97-102; discussion 102-4 [DOI] [PubMed] [Google Scholar]
- 16.Yang CP, Hsu CP, Chen WY, et al. Aortic remodeling after endovascular repair with stainless steel-based stent graft in acute and chronic type B aortic dissection. J Vasc Surg 2012;55:1600-10 [DOI] [PubMed] [Google Scholar]
- 17.Conrad MF, Crawford RS, Kwolek CJ, et al. Aortic remodeling after endovascular repair of acute complicated type B aortic dissection. J Vasc Surg 2009;50:510-7 [DOI] [PubMed] [Google Scholar]
- 18.Khoynezhad A, Donayre CE, Omari BO, et al. Midterm results of endovascular treatment of complicated acute type B aortic dissection. J Thorac Cardiovasc Surg 2009;138:625-31 [DOI] [PubMed] [Google Scholar]
- 19.Shu C, He H, Li QM, et al. Endovascular repair of complicated acute type-B aortic dissection with stentgraft: early and mid-term results. Eur J Vasc Endovasc Surg 2011;42:448-53 [DOI] [PubMed] [Google Scholar]
- 20.White RA, Miller DC, Criado FJ, et al. Report on the results of thoracic endovascular aortic repair for acute, complicated, type B aortic dissection at 30 days and 1 year from a multidisciplinary subcommittee of the Society for Vascular Surgery Outcomes Committee. J Vasc Surg 2011;53:1082-90 [DOI] [PubMed] [Google Scholar]
- 21.Qin YL, Deng G, Li TX, et al. Risk factors of incomplete thrombosis in the false lumen after endovascular treatment of extensive acute type B aortic dissection. J Vasc Surg 2012;56:1232-8 [DOI] [PubMed] [Google Scholar]
- 22.Ehrlich MP, Rousseau H, Heijmen R, et al. Midterm results after endovascular treatment of acute, complicated type B aortic dissection: the Talent Thoracic Registry. J Thorac Cardiovasc Surg 2013;145:159-65 [DOI] [PubMed] [Google Scholar]
- 23.Kim U, Hong SJ, Kim J, et al. Intermediate to long-term outcomes of endoluminal stent-graft repair in patients with chronic type B aortic dissection. J Endovasc Ther 2009;16:42-7 [DOI] [PubMed] [Google Scholar]
- 24.Xu SD, Huang FJ, Yang JF, et al. Early and midterm results of thoracic endovascular aortic repair of chronic type B aortic dissection. J Thorac Cardiovasc Surg 2010;139:1548-53 [DOI] [PubMed] [Google Scholar]
- 25.Czerny M, Roedler S, Fakhimi S, et al. Midterm results of thoracic endovascular aortic repair in patients with aneurysms involving the descending aorta originating from chronic type B dissections. Ann Thorac Surg 2010;90:90-4 [DOI] [PubMed] [Google Scholar]
- 26.Kang WC, Greenberg RK, Mastracci TM, et al. Endovascular repair of complicated chronic distal aortic dissections: intermediate outcomes and complications. J Thorac Cardiovasc Surg 2011;142:1074-83 [DOI] [PubMed] [Google Scholar]
- 27.Andacheh ID, Donayre C, Othman F, et al. Patient outcomes and thoracic aortic volume and morphologic changes following thoracic endovascular aortic repair in patients with complicated chronic type B aortic dissection. J Vasc Surg 2012;56:644-50; discussion 650 [DOI] [PubMed] [Google Scholar]
- 28.Yang CP, Hsu CP, Chen WY, et al. Aortic remodeling after endovascular repair with stainless steel-based stent graft in acute and chronic type B aortic dissection. J Vasc Surg 2012;55:1600-10 [DOI] [PubMed] [Google Scholar]
- 29.Faure EM, Canaud L, Cathala P, et al. Assessment of abdominal branch vessel patency after bare-metal stenting of the thoracoabdominal aorta in a human ex vivo model of acute type B aortic dissection. J Vasc Surg. 2014 doi: 10.1016/j.jvs.2013.11.095. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Bertoglio L, Melissano G, Civilini E, et al. Stent misalignment of the Zenith Dissection Endovascular System. J Vasc Surg 2013;57:515-7 [DOI] [PubMed] [Google Scholar]