Abstract
Background
Thoracic endovascular aortic repair (TEVAR) has become the preferred treatment for chronic type B aortic dissection (CTBAD) at our institution. However, it remains incapable of treating all patients with CTBAD. The present study aims to review our contemporary results with open and endovascular CTBAD repairs since the advent of thoracic endografting.
Methods
The records of all patients undergoing index repair of CTBAD (chronic DeBakey type IIIA, IIIB and repaired type I) at our institution between June 2005 and December 2013, were retrospectively reviewed.
Results
A total of 107 patients underwent CTBAD repair, of whom 70% (n=75) underwent endovascular-based procedures [44 TEVAR, 27 hybrid arch and four hybrid thoracoabdominal aortic aneurysm (TAAA) repair] and 30% (n=32) underwent open procedures (nine open descending and 23 open TAAA). Connective tissue disease (CTD), prior aortic surgery and DeBakey dissection type were strongly associated with the choice of operation. The rates of stroke, paraplegia and operative mortality following endovascular-based repairs were 0%, 0% and 4% (n=3), respectively. Adverse neurologic events were higher following open repair, and rates of stroke, paraplegia, and operative mortality were 16% (n=5), 9% (n=3), and 6% (n=2), respectively. However, 1- and 5-year survival rates were similar for endovascular-based repairs (86% and 65%, respectively), and open repairs (88% and 79%, respectively). Over a median follow-up interval of 34 months, the rate of descending aortic reintervention was 24% (n=18) following endovascular-based repairs and 0% following open repairs (P=0.001). Forty-four percent (n=8) of descending aortic reinterventions were required to treat stent graft complications (five endoleak, two stent graft collapse and one stent graft-induced new entry tear) and the remainder were required to treat metachronous pathology (n=2) or progressive aneurysmal disease related to persistent distal fenestrations (n=8).
Conclusions
Endovascular repair of CTBAD was associated with excellent procedural and survival outcomes, but at the expense of further reinterventions. Open repair remains relevant for patients who are not candidates for endovascular repair and was associated with higher procedural morbidity but similar overall survival and fewer reinterventions.
Keywords: Aortic dissection, aortic surgery, outcomes
Introduction
Of patients with uncomplicated dissection of the descending aorta, 20-40% will ultimately require surgical intervention, usually as a result of aneurysmal degeneration of the false lumen (FL) (1,2). Despite initial skepticism (3), our aortic program was an early adopter of thoracic endovascular aortic repair (TEVAR) for chronic type B aortic dissection (CTBAD) in patients with suitable anatomy, in recognition of the reduced procedural morbidity and mortality with TEVAR compared with open surgery (4) and excellent mid-term outcomes for CTBAD repair (1,2). However, TEVAR remains incapable of treating all CTBAD cases, and a significant proportion of patients require tailored hybrid or open repair due to unsuitable anatomy, prior surgery or connective tissue disease (CTD). The aim of the present study is to present our complete program results with open and endovascular CTBAD repair since the advent of thoracic endografting, with specific focus on procedural morbidity and mortality, survival and requirements for reintervention on the descending aorta.
Methods
Patient population and data collection
This study was approved by the Institutional Review Board of Duke University, and the need for individual patient consent was waived. We retrospectively reviewed the records of all patients at our institution who underwent descending or thoracoabdominal aortic intervention for the treatment of CTBAD (>2 weeks from symptom onset) between June 2005 and December 2013. Patient records were identified from the prospectively maintained Duke Thoracic Aortic Surgery Database (1,2,5,6). The patient cohort included only patients with CTBAD who had previously been managed medically. Patients who had undergone prior interventions for acute type B aortic dissection, or descending or thoracoabdominal aortic interventions at outside institutions were excluded. However, patients treated for residual CTBAD following acute type A aortic dissection repair (repaired DeBakey type I dissection) were included in the analysis. Reinterventions were coded as all subsequent aortic procedures performed following the index descending or thoracoabdominal aortic intervention. Comorbid conditions and postoperative complications were defined using the Society of Thoracic Surgeons definitions (www.sts.org). Aortic arch landing zones were defined using the Ishimaru classification (7). Long-term follow-up and survival were assessed from medical records and the Social Security Death Index.
Patient selection and operative technique
Indications for elective intervention included aneurysmal degeneration with an absolute aortic diameter of ≥5.5 cm, rapid aneurysm enlargement (>5 mm in 6 months) or saccular aneurysm protruding ≥2 cm beyond the aortic wall (1,2). Indications for non-elective intervention included symptomatic aneurysm with impending rupture (n=18), aortoesophageal fistula (n=1), ruptured aneurysm (n=1) or dynamic iliofemoral malperfusion (n=1). Interventions were classified as one of five principal procedures: (I) isolated descending TEVAR with or without left subclavian artery coverage; (II) hybrid arch repair with descending TEVAR; (III) hybrid thoracoabdominal aortic aneurysm (TAAA) repair; (IV) open descending repair; and (V) open TAAA repair. TEVAR, hybrid arch and hybrid TAAA repairs were considered endovascular-based procedures and open descending and open TAAA repairs were considered open procedures.
Our institutional preference is to perform endovascular repair of CTBAD for all non-CTD patients with suitable anatomy (1,2,6,8-10). Our technique for TEVAR in CTBAD has recently been described in detail (11). We recommend isolated descending thoracic aortic TEVAR only for patients with an isolated descending thoracic aneurysm arising adjacent to the primary tear. Specifically, a thoracoabdominal aneurysm should not be present, and we prefer the diameter of the distal descending thoracic aorta at the level of the celiac axis to be ≤42 mm when utilizing TEVAR for CTBAD with aneurysm.
As previously described, hybrid arch procedures were performed for patients with inadequate proximal landing zone, including zone 1 coverage with carotid-carotid bypass, zone 0 coverage with complete arch debranching or stented elephant trunk completion following prior total arch replacement (6). Hybrid TAAA procedures with complete visceral debranching were performed for patients with TAAA secondary to CTBAD and deemed poorly suited to conventional repair (9,10); this usually required open infrarenal aorto-bi-iliac Dacron graft replacement in conjunction with visceral debranching, to create adequate distal landing zone given the presence of chronic dissection within the infrarenal aorta +/– iliac arteries (12,13).
Conventional open surgery was reserved for CTBAD patients with CTD (14,15) or anatomy unsuitable for TEVAR or hybrid repair due to inadequate stent graft landing zones. Our institutional preference is to perform open descending and TAAA repairs utilizing cardiopulmonary bypass and deep hypothermia for central nervous system and visceral organ protection (16). Direct aortic cannulation for cardiopulmonary bypass is preferred over femoral arterial cannulation to prevent retrograde embolization of debris from the FL. Preoperative lumbar drains are placed in all open TAAA repairs and open descending thoracic aortic repairs with planned distal anastomosis below the level of T6; drains are managed as previously described (5).
All patients had lifelong aortic surveillance follow-up at the Duke Center for Aortic Disease. Patient follow-up protocols were previously published for patients undergoing endovascular-based (17) and open (18) repairs.
Statistical analysis
Categorical variables were represented as numbers and percentages, and continuous variables were represented as medians and interquartile ranges. Continuous and categorical variables were compared between groups using the Mann-Whitney rank sum test and the Chi-squared test, respectively. Estimates of long-term survival and freedom from reintervention were calculated for all patients using the Kaplan-Meier method. Calculations were performed using STATA 11.1 (StataCorp, College Station, TX, USA).
Results
Demographic, anatomic, and procedural data
Between June 2005 and December 2013, 107 patients at our institution underwent index repair of CTBAD, of whom 75 (70%) underwent endovascular-based procedures (44 TEVAR, 27 hybrid arch, four hybrid TAAA) and 32 (30%) underwent open procedures (nine open descending, 23 open TAAA). The proportion of patients undergoing endovascular repair was equivalent between the first [2005-2009] and second [2010-2013] halves of the study period [42 of 56 (75%) vs. 33 of 51 (65%), P=0.29]. Demographic characteristics are shown in Table 1, and anatomic and procedural characteristics are shown in Table 2. CTD, prior aortic surgery and aortic dissection anatomy were strongly associated with the choice of operation. Ninety percent of CTD patients underwent open repair (the remaining CTD patient was a 77-year-old Marfan patient who underwent zone 0 hybrid arch repair with a Dacron proximal landing zone from prior ascending aortic replacement) compared to 24% of non-CTD patients. Approximately half of the patients in the study had a history of repaired DeBakey type I dissection with residual CTBAD, and only 17% of these patients were candidates for isolated TEVAR repair. Conversely, 60% of patients with isolated descending aortic dissection (DeBakey type IIIa or IIIb) underwent TEVAR as the index procedure. In concert with these findings, patients with a history of prior aortic surgery were less likely to undergo isolated TEVAR intervention and 80% required hybrid or open aortic reconstructions. In total, 80% of patients underwent elective operation and 20% underwent urgent or emergent repair for reasons outlined above.
Table 1. Baseline characteristics.
| Variable | Overall (N=107) | TEVAR (n=44) | Hybrid arch (n=27) | Hybrid TAAA (n=4) | Open descending (n=9) | Open TAAA (n=23) | P value |
|---|---|---|---|---|---|---|---|
| Age [year] | 56 [47,65] | 59 [50,65] | 57 [54,67] | 66 [56,67] | 63 [45,67] | 47 [34,56] | 0.06 |
| Male | 77 (72%) | 28 (64%) | 21 (78%) | 1 (25%) | 8 (89%) | 19 (83%) | 0.08 |
| White race | 53 (50%) | 21 (48%) | 16 (59%) | 2 (50%) | 4 (44%) | 10 (44%) | 0.81 |
| Body mass index [kg/m2] | 27 [23,31] | 30 [26,33] | 25 [23,28] | 27 [22,31] | 28 [24,31] | 24 [21,28] | 0.01 |
| Hypertension | 100 (94%) | 43 (98%) | 24 (89%) | 4 (100%) | 9 (100%) | 20 (87%) | 0.31 |
| Hyperlipidemia | 55 (51%) | 21 (48%) | 14 (52%) | 3 (75%) | 6 (67%) | 11 (48%) | 0.76 |
| Active or recent tobacco use | 64 (60%) | 26 (59%) | 16 (59%) | 2 (50%) | 3 (33%) | 17 (74%) | 0.31 |
| Diabetes | 7 (7%) | 5 (11%) | 1 (4%) | 0 | 0 | 1 (4%) | 0.69 |
| Coronary artery disease | 18 (17%) | 5 (11%) | 5 (19%) | 0 | 2 (22%) | 6 (26%) | 0.53 |
| History of prior stroke | 11 (10%) | 0 | 5 (19%) | 0 | 1 (11%) | 5 (22%) | 0.01 |
| COPD | 18 (17%) | 7 (16%) | 4 (15%) | 1 (25%) | 0 | 6 (26%) | 0.44 |
| Baseline creatinine >1.5 mg/dL | 22 (21%) | 7 (16%) | 11 (41%) | 0 (0%) | 1 (11%) | 3 (13%) | 0.08 |
| Peripheral vascular disease | 11 (10%) | 6 (14%) | 3 (11%) | 0 | 0 | 2 (9%) | 0.88 |
| Connective tissue disease | 10 (9%) | 0 | 1 (4%) | 0 | 0 | 9 (39%) | <0.001 |
| Prior aortic surgery | 60 (56%) | 12 (27%) | 24 (89%) | 3 (75%) | 6 (67%) | 15 (65%) | <0.001 |
Data represented as median (interquartile range) or number (percent). COPD, chronic obstructive pulmonary disease; TAAA, thoracoabdominal aortic aneurysm; TEVAR, thoracic endovascular aortic repair.
Table 2. Anatomic and procedural characteristics.
| Variable | Overall (N=107) | TEVAR (n=44) | Hybrid arch (n=27) | Hybrid TAAA (n=4) | Open descending (n=9) | Open TAAA (n=23) | P value |
|---|---|---|---|---|---|---|---|
| Index dissection type | <0.001 | ||||||
| DeBakey type I | 47 (44%) | 8 (18%) | 21 (78%) | 2 (50%) | 5 (56%) | 11 (48%) | |
| DeBakey type IIIa | 5 (5%) | 2 (5%) | 2 (7%) | 0 | 1 (11%) | 0 | |
| DeBakey type IIIb | 55 (51%) | 34 (77%) | 4 (15%) | 2 (50%) | 3 (33%) | 12 (52%) | |
| Time in months from dissection | 31 [7,83] | 17 [4,72] | 40 [14,80] | 56 [50,72] | 43 [22,87] | 31 [9,85] | 0.48 |
| Maximum aortic diameter [cm] | 6 [6,7] | 6 [5,6] | 6 [6,7] | 7 [7,8] | 7 [6,8] | 6 [6,7] | 0.02 |
| Procedure status | 0.35 | ||||||
| Elective | 86 (80%) | 32 (73%) | 25 (93%) | 3 (75%) | 8 (89%) | 18 (78%) | |
| Urgent | 18 (17%) | 11 (25%) | 1 (4%) | 1 (25%) | 1 (11%) | 4 (17%) | |
| Emergent | 3 (3%) | 1 (2%) | 1 (4%) | 0 | 0 | 1 (4%) | |
| Number of endografts implanted | 2 [1,3] | 2 [1,2] | 2 [2,3] | 4 [4,5] | – | – | 0.001 |
| Length of aortic coverage [cm] | 25 [20,32] | 20 [16,29] | 31 [23,39] | 59 [57,63] | – | – | <0.001 |
| Proximal landing zone | <0.001 | ||||||
| Stented elephant trunk | 8 (11%) | 0 | 6 (22%) | 2 (50%) | – | – | |
| Zone 0 | 17 (23%) | 0 | 17 (63%) | 0 | – | – | |
| Zone 1 | 5 (7%) | 0 | 4 (15%) | 1 (25%) | – | – | |
| Zone 2 | 34 (45%) | 33 (75%) | 0 | 1 (25%) | – | – | |
| Zone 3 | 4 (5%) | 4 (9%) | 0 | 0 | – | – | |
| Zone 4 | 7 (9%) | 7 (16%) | 0 | 0 | – | – | |
| Distal landing zone | <0.001 | ||||||
| Above celiac axis | 27 (37%) | 18 (42%) | 9 (33%) | 0 | – | – | |
| To celiac axis | 43 (58%) | 25 (58%) | 18 (67%) | 0 | – | – | |
| Below celiac axis | 4 (5%) | 0 | 0 | 4 (100%) | – | – | |
| Extent of TAAA | 0.44 | ||||||
| Type I | 9 (33%) | – | – | 0 | – | 9 (39%) | |
| Type II | 11 (41%) | – | – | 3 (75%) | – | 8 (35%) | |
| Type III | 6 (22%) | – | – | 1 (25%) | – | 5 (22%) | |
| Type IV | 1 (4%) | – | – | 0 | – | 1 (4%) |
Data represented as median (interquartile range) or number (percent). TAAA, thoracoabdominal aortic aneurysm; TEVAR, thoracic endovascular aortic repair.
Procedural outcomes
Thirty-day in-hospital adverse events are listed in Table 3. The lowest rates of procedural morbidity and mortality were observed with isolated TEVAR, from which no patients experienced stroke, paraplegia or death within the perioperative period. The overall rates of stroke, paraplegia and operative mortality following any endovascular-based repair option were 0%, 0% and 4%, respectively, and the rate of retrograde type A aortic dissection was 4%. In contrast, adverse neurologic events were higher following open aortic repair, with cumulative rates of stroke, paraplegia and operative mortality of 16%, 9% and 6%, respectively. Hospital length of stay was also significantly longer following open repair (median 8 days versus median 4 days for endovascular repair, P=0.001).
Table 3. 30-day in-hospital adverse events.
| Variable | Overall (N=107) | TEVAR (n=44) | Hybrid arch (n=27) | Hybrid TAAA (n=4) | Open descending (n=9) | Open TAAA (n=23) | P value |
|---|---|---|---|---|---|---|---|
| Stroke (neurologic deficit lasting >72 hours) | 5 (5%) | 0 | 0 | 0 | 1 (11%) | 4 (17%) | 0.01 |
| Permanent paraplegia/paraparesis | 3 (3%) | 0 | 0 | 0 | 0 | 3 (13%) | 0.06 |
| Acute renal failure (Cr >2.0 mg/dL and >2× baseline) | 6 (6%) | 1 (2%) | 2 (7%) | 0 | 0 | 3 (13%) | 0.40 |
| New onset dialysis | 1 (1%) | 0 | 1 (4%) | 0 | 0 | 0 | 0.59 |
| Prolonged ventilation (>24 hours) | 7 (7%) | 1 (2%) | 1 (4%) | 0 | 0 | 5 (22%) | 0.06 |
| Myocardial infarction | 1 (1%) | 1 (2%) | 0 | 0 | 0 | 0 | 0.99 |
| Retrograde type A aortic dissection | 3 (3%) | 2 (5%) | 1 (4%) | 0 | 0 | 0 | 0.81 |
| Death | 5 (5%) | 0 | 3 (11%) | 0 | 1 (11%) | 1 (4%) | 0.11 |
| Postoperative length of stay [days] | 5 [3,8] | 3 [2,5] | 5 [3,6] | 6 [6,8] | 6 [5,6] | 8 [7,12] | 0.01 |
Data represented as median (interquartile range) or number (percent). Cr, creatinine; TAAA, thoracoabdominal aortic aneurysm; TEVAR, thoracic endovascular aortic repair.
To further examine factors associated with poor outcomes in the open repair group, univariate risk factors for major morbidity (stroke, paraplegia/paraparesis or dialysis) or death within 1-year follow-up of operation were assessed (Table 4). In total, 25% (8 of 32) of open repair patients experienced major morbidity or 1-year mortality. The strongest univariate risk factors for poor outcomes were older age and operation within the first half of the study period. Notably, CTD patients did well following open surgery and only 1 of 9 (11%) experienced a major adverse event in the form of a small subdural hematoma secondary to lumbar cerebrospinal fluid drainage that was managed conservatively.
Table 4. Univariate risk factors for major morbidity (stroke, paraplegia/paraparesis, dialysis) or 1-year mortality for patients undergoing open repair.
| Variable | No morbidity/mortality (n=24) | Yes morbidity/mortality (n=8) | P value |
|---|---|---|---|
| Age [year] | 47 [34,60] | 62 [50,66] | 0.05 |
| Age >50 years | 9 (38%) | 6 (75%) | 0.07 |
| Male | 20 (83%) | 7 (88%) | 0.78 |
| White race | 10 (42%) | 4 (50%) | 0.68 |
| Body mass index [kg/m2] | 25 [22,29] | 25 [21,29] | 0.86 |
| Hypertension | 22 (92%) | 7 (88%) | 0.73 |
| Hyperlipidemia | 11 (46%) | 6 (75%) | 0.15 |
| Active or recent tobacco use | 13 (54%) | 7 (88%) | 0.09 |
| Diabetes | 1 (4%) | 0 | 0.56 |
| Coronary artery disease | 6 (25%) | 2 (25%) | 1 |
| History of prior stroke | 4 (17%) | 2 (25%) | 0.60 |
| COPD | 3 (13%) | 3 (38%) | 0.12 |
| Baseline creatinine >1.5 mg/dL | 3 (13%) | 1 (13%) | 1 |
| Peripheral vascular disease | 1 (4%) | 1 (13%) | 0.40 |
| Connective tissue disease | 8 (33%) | 1 (13%) | 0.26 |
| Prior aortic surgery | 16 (67%) | 5 (63%) | 0.83 |
| Index dissection type | 0.54 | ||
| DeBakey type I | 13 (54%) | 3 (38%) | |
| DeBakey type IIIa | 1 (4%) | 0 | |
| DeBakey type IIIb | 10 (42%) | 5 (63%) | |
| Time in months from dissection | 30 [10,64] | 85 [16,142] | 0.26 |
| Maximum aortic diameter (cm) | 6.2 (5.6,7.2) | 6.8 (6.2,8.7) | 0.15 |
| Non-elective operation | 5 (21%) | 1 (13%) | 0.60 |
| Procedure performed | 0.26 | ||
| Open descending | 8 (33%) | 1 (13%) | |
| Open TAAA | 16 (67%) | 7 (88%) | |
| Extent of TAAA | 0.78 | ||
| Type I | 6 (38%) | 3 (43%) | |
| Type II | 6 (38%) | 2 (29%) | |
| Type III | 3 (19%) | 2 (29%) | |
| Type IV | 1 (6%) | 0 | |
| Cardiopulmonary bypass time [min] | 195 [179,227] | 214 [189,247] | 0.32 |
| Cerebral circulatory arrest time [min] | 17 [9,22] | 17 [4,19] | 0.46 |
| Systemic circulatory arrest time [min] | 51 [40,73] | 70 [26,79] | 0.45 |
| Femoral arterial cannulation | 7 (29%) | 4 (50%) | 0.28 |
| Era (years) | 0.04 | ||
| 2005-2009 | 8 (33%) | 6 (75%) | |
| 2010-2013 | 16 (67%) | 2 (25%) |
Data represented as median (interquartile range) or number (percent). COPD, chronic obstructive pulmonary disease; TAAA, thoracoabdominal aortic aneurysm.
Survival
Overall and aorta specific survival for the entire patient cohort is depicted in Figure 1. The overall 1-, 3-, and 5-year survival rates were 87% [95% confidence interval (CI), 80-93%], 77% (95% CI, 68-86%) and 69% (95% CI, 59-80%), respectively, and 5-year aorta specific survival was 92% (95% CI, 87-98%). The aortic deaths in the series (n=9) included six patients who died in the 30-day in-hospital period following the index operation (n=5) or staged reintervention for aortoesophageal fistula (n=1), two patients who died of late ascending aortic complications unrelated to the CTBAD repair, and one patient who experienced a type Ia endoleak following zone 0 hybrid arch repair where operative reintervention was deferred due to advanced dementia and other prohibitive comorbidities.
Figure 1.
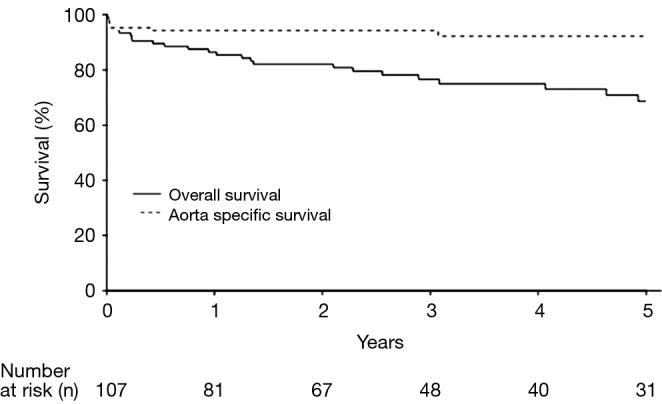
Kaplan-Meier estimates of overall and aorta specific survival for all patients undergoing chronic type B aortic dissection repair.
Overall survival stratified by procedure type is shown in Figure 2. The 1-year survival rates were similar across procedures at 90% (95% CI, 82-100%) for TEVAR, 77% (95% CI, 63-95%) for hybrid arch, 100% (95% CI, 100-100%) for hybrid TAAA, 89% (95% CI, 71-100%) for open descending, and 87% (95% CI, 74-100%) for open TAAA repair. Cumulative 1- and 5-year survival rates were likewise similar between endovascular-based and open procedures and were 86% (78-95%) and 65% (53-80%), respectively, for endovascular-based repairs and 88% (77-100%) and 79% (65-96%), respectively, for open repairs.
Figure 2.
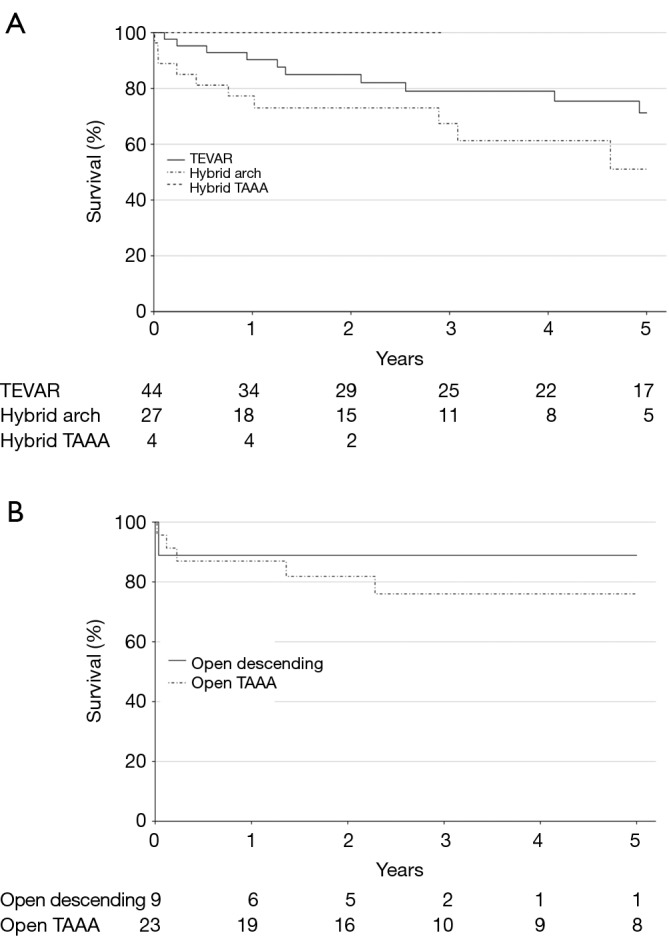
Kaplan-Meier estimates of overall survival stratified by (A) endovascular or (B) open procedure type. TEVAR, thoracic endovascular aortic repair; TAAA, thoracoabdominal aortic aneurysm.
Reinterventions during follow-up
Reinterventions during follow-up are shown in Table 5, stratified by procedure type and location of aortic reintervention. In total, 17% of patients required reintervention for descending aortic pathology and 7% required reintervention for arch pathology, for a total aortic reintervention rate of 23%. Over a median follow-up interval of 34 months, the rate of descending aortic reintervention was 24% (n=18) following endovascular-based repairs and 0% following open repairs (P=0.001). Approximately half of the descending aortic reinterventions (8 of 18; 44%) were required to treat stent graft complications (five endoleak, two stent graft collapse, one stent graft-induced new entry tear) and the remainder were required to treat metachronous pathology (n=2) or progressive aneurysmal disease related to persistent distal fenestrations (n=8). The majority (11 of 18; 61%) of descending aortic reinterventions were able to be addressed via TEVAR alone. However, 9% (7 of 75) of endovascular-based repair patients ultimately required conversion to hybrid arch, hybrid TAAA or open TAAA repair due to type Ia endoleak (n=1), aortoesophageal fistula (n=1) or persistent distal fenestrations leading to continued proximal aneurysm expansion or distal aneurysm disease (n=5). Overall freedom from descending aortic reintervention rates for endovascular patients are shown in Figure 3. At 3-years, freedom from descending aortic reintervention rates were 68% (95% CI, 54-85%) for TEVAR, 82% (95% CI, 67-100%) for hybrid arch and 25% (95% CI, 5-100%) for hybrid TAAA repairs. Reintervention for arch pathology was required in seven patients, including two TEVAR patients who underwent emergent repair of retrograde type A aortic dissection (the remaining retrograde type A aortic dissection patient died prior to operative intervention). All other arch reinterventions entailed treatment of metachronous arch pathology unrelated to the CTBAD repair.
Table 5. Reinterventions during follow-up.
| Variable | Overall (N=107) | TEVAR (n=44) | Hybrid arch (n=27) | Hybrid TAAA (n=4) | Open descending (n=9) | Open TAAA (n=23) | P value |
|---|---|---|---|---|---|---|---|
| Duration of follow-up [months] | 34 [13,68] | 44 [16,73] | 27 [9,52] | 23 [17,31] | 28 [9,32] | 34 [16,77] | 0.04 |
| Descending aortic reinterventions | 18 (17%) | 11 (25%) | 4 (15%) | 3 (75%) | 0 | 0 | 0.001 |
| Endovascular reintervention only | 11 (10%) | 6 (14%) | 2 (7%) | 3 (75%) | – | – | |
| Conversion to hybrid arch repair | 1 (1%) | 1 (2%) | 0 | 0 | – | – | |
| Conversion to hybrid TAAA repair | 4 (4%) | 2 (5%) | 2 (7%) | 0 | – | – | |
| Conversion to open TAAA repair | 2 (2%) | 2 (5%) | 0 | 0 | – | – | |
| Indication for descending reintervention | |||||||
| Type IA endoleak | 2 (2%) | 1 (2%) | 1 (4%) | 0 | – | – | |
| Type IB endoleak | 2 (2%) | 1 (2%) | 0 | 1 (25%) | – | – | |
| Type III endoleak | 1 (1%) | 0 | 0 | 1 (25%) | – | – | |
| Stent graft collapse | 2 (2%) | 1 (2%) | 0 | 1 (25%) | – | – | |
| Stent graft-induced new entry tear | 1 (1%) | 0 | 1 (4%) | 0 | – | – | |
| Metachronous saccular aneurysm | 1 (1%) | 1 (2%) | 0 | 0 | – | – | |
| Aortoesophageal fistula | 1 (1%) | 1 (2%) | 0 | 0 | – | – | |
| Persistent FL pressurization via distal fenestrations | 8 (7%) | 6 (14%) | 2 (7%) | 0 | – | – | |
| Reintervention for arch pathology | 7 (7%) | 3 (7%) | 0 | 0 | 0 | 4 (17%) | 0.12 |
| Endovascular | 1 (1%) | 1 (2%) | – | – | – | 0 | |
| Hybrid | 3 (3%) | 0 | – | – | – | 3 (13%) | |
| Open | 3 (3%) | 2 (5%) | – | – | – | 1 (4%) | |
| Total patients requiring any aortic reintervention | 25 (23%) | 14 (32%) | 4 (15%) | 3 (75%) | 0 | 4 (17%) | 0.02 |
Data represented as median (interquartile range) or number (percent). FL, false lumen; TAAA, thoracoabdominal aortic aneurysm; TEVAR, thoracic endovascular aortic repair.
Figure 3.
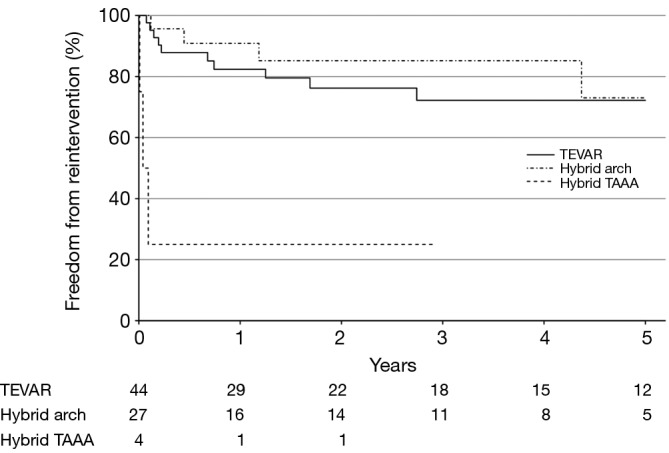
Kaplan-Meier estimates of freedom from descending aortic reintervention stratified by endovascular procedure type. TEVAR, thoracic endovascular aortic repair; TAAA, thoracoabdominal aortic aneurysm.
Discussion
The present study reports the outcomes of CTBAD repair at a single-institution where endovascular repair strategies were preferentially employed. Endovascular-based therapies were associated with low procedural morbidity and mortality rates, but these results were partially tempered by higher rates of descending aortic reintervention during follow-up. Conversely, open repair remained an important therapy for those who were not considered candidates for endovascular repair and was utilized in 30% of patients. Open repair was predictably associated with higher procedural morbidity, but overall survival was similar and no open repair patients required late descending aortic reintervention.
Over the last decade, TEVAR has gradually gained popularity for the treatment of CTBAD, given the reduced procedural morbidity as compared to open descending or thoracoabdominal aortic replacement (4). However, the therapeutic rationale of endoluminal therapy for the treatment of chronic FL aneurysms was initially questioned. Critics of TEVAR for CTBAD repair posited that endovascular therapy would fail in most patients due to the presence of uncovered distal fenestrations leading to persistent backfilling and pressurization of the FL. Additionally, the thickened chronic intimal dissection flap was considered poorly amenable to reverse remodeling (3). Despite these early concerns, reports from multiple centers demonstrated lower procedural morbidity and mortality, in addition to successful FL thrombosis, depressurization, reverse remodeling and aneurysm shrinkage in the majority of CTBAD patients treated by TEVAR at mid-term follow-up (1,2,19-26). As a result of the accumulating data, an interdisciplinary expert consensus panel from Western Europe recently recommended TEVAR as the preferred treatment for CTBAD in patients with suitable anatomy, based on a review of nearly 1,100 CTBAD patients treated with TEVAR (8). These recommendations appear to be supported by the present study, where isolated TEVAR for CTBAD was associated with 0% rates of stroke, paraplegia, and operative mortality. These procedural complication rates remained low even after complex hybrid arch and hybrid TAAA repairs were included in the analysis.
Despite the clear short-term benefits, treatment failures and reinterventions are known to be higher in CTBAD patients treated with TEVAR (8,26,27). These findings were confirmed in the present study, where the need for descending aortic reintervention was significantly higher for patients treated with endovascular therapy versus conventional open surgery. However, while reinterventions undoubtedly add expense and mandate close patient surveillance, they did not appear to compromise survival, as only one patient died as a result of staged reintervention for aortoesophageal fistula. Several prior studies of TEVAR have likewise shown no increase in mortality for patients requiring reintervention (24,28). Thus, the high reintervention rate with endovascular therapy may be justified by the low procedural complication rates related to the index procedure. In addition, roughly half of the reinterventions were due to stent graft related complications that will almost certainly decrease in frequency over time as a result of improvements in device design, as well as increased operator experience. Nonetheless, as predicted by TEVAR skeptics, 7% (5 of 75) of CTBAD patients who were treated endovascularly ultimately required conversion to hybrid TAAA or open TAAA repair due to progression of distal aortic pathology or continued FL pressurization via distal fenestrations. These findings suggest that some anatomical dissection configurations may be poorly suited for endovascular repair and further study is required to identify patients who should be directed to primary open repair. The comparative long-term freedom from reintervention and aorta specific survival between equivalent CTBAD patients treated by TEVAR and open surgery also remain to be definitively shown.
Finally, this report highlights the continued importance of open CTBAD repair even in the era of widespread TEVAR adoption, as 30% of patients were deemed unfit for endovascular repair. Compared with endovascular therapy, our results with open surgery were generally similar to other centers and were notable for higher rates of stroke, paraplegia, prolonged ventilation and length of stay, and 25% of patients experienced one or more major adverse events (death within one year, paraplegia, stroke or dialysis) (25,29). However, despite the increased procedural morbidity with open surgery, overall survival appeared excellent and the need for reintervention was low. Further examination of the open repair cohort revealed that younger patients and those with CTD generally did well following surgery, justifying the preferred use of open repair in these patients. Conversely, elderly patients and those with more comorbidities did worse with open repair, highlighting the rationale for endovascular therapy in these patients whenever possible. In addition, our data suggests an improvement in outcomes with surgeon and institutional experience, as only 11% (2 of 18) of open repair patients experienced a major adverse event in the second half of the study period versus 43% (6 of 14) in the first half of the study period. Thus, further refinements to operative technique and attention to institutional results will ideally continue to improve outcomes with open repair and lead to results that parallel other noted high-volume centers specializing in open descending/TAAA aortic repair.
Limitations
Patients who underwent open or endovascular CTBAD repair were not equivalent, and the choice of operation was carefully selected based on patient characteristics and aortic anatomy. Hence, conclusions regarding differences in outcomes between groups should be made with caution and with the understanding that the patient groups were not intended to be similar and outcomes were not risk-adjusted. In addition, the present report is further limited by the observational study design and the constraints of sample size, which limits the comparison of findings between procedures as well as with other published reports. Lastly, the operations were performed by three principal surgeons using standardized techniques and results may not be generalizable to other practitioners in other settings.
Conclusions
Endovascular repair of CTBAD was associated with excellent procedural and survival outcomes but at the expense of more reinterventions, mandating close aortic surveillance by an experienced aortic center. Open CTBAD repair remains important for patients who are not candidates for endovascular repair and was associated with higher procedural morbidity but similar overall survival and fewer reinterventions. Aortic referral centers that seek to treat large numbers of dissection patients should maintain expertise and strive to optimize institutional outcomes with both open and endovascular techniques.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Parsa CJ, Schroder JN, Daneshmand MA, et al. Midterm results for endovascular repair of complicated acute and chronic type B aortic dissection. Ann Thorac Surg 2010;89:97-102; discussion 102-4 [DOI] [PubMed] [Google Scholar]
- 2.Parsa CJ, Williams JB, Bhattacharya SD, et al. Midterm results with thoracic endovascular aortic repair for chronic type B aortic dissection with associated aneurysm. J Thorac Cardiovasc Surg 2011;141:322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg 2008;85:S1-41 [DOI] [PubMed] [Google Scholar]
- 4.Coady MA, Ikonomidis JS, Cheung AT, et al. Surgical management of descending thoracic aortic disease: open and endovascular approaches: a scientific statement from the American Heart Association. Circulation 2010;121:2780-804 [DOI] [PubMed] [Google Scholar]
- 5.Hanna JM, Andersen ND, Aziz H, et al. Results with selective preoperative lumbar drain placement for thoracic endovascular aortic repair. Ann Thorac Surg 2013;95:1968-74; discussion 1974-5. [DOI] [PubMed]
- 6.Andersen ND, Williams JB, Hanna JM, et al. Results with an algorithmic approach to hybrid repair of the aortic arch. J Vasc Surg 2013;57:655-67; discussion 666-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishimaru S.Endografting of the aortic arch. J Endovasc Ther 2004;11Suppl 2:II62-71 [DOI] [PubMed] [Google Scholar]
- 8.Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 2013;61:1661-78 [DOI] [PubMed] [Google Scholar]
- 9.Hughes GC, Andersen ND, Hanna JM, et al. Thoracoabdominal aortic aneurysm: hybrid repair outcomes. Ann Cardiothorac Surg 2012;1:311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes GC, Barfield ME, Shah AA, et al. Staged total abdominal debranching and thoracic endovascular aortic repair for thoracoabdominal aneurysm. J Vasc Surg 2012;56:621-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes GC, Andersen ND, McCann RL. Endovascular Repair of Chronic Type B Aortic Dissection with Aneurysmal Degeneration. Op Tech Thorac Cardiovasc Surg 2013;18:101-16 [Google Scholar]
- 12.Shah AA, Bhattacharya SD, McCann RL, et al. Pan-aortic hybrid treatment of mega-aorta syndrome. J Vasc Surg 2011;53:1398-401 [DOI] [PubMed] [Google Scholar]
- 13.Ganapathi AM, Andersen ND, Hanna JM, et al. Comparison of attachment site endoleak rates in Dacron versus native aorta landing zones after thoracic endovascular aortic repair. J Vasc Surg 2014;59:921-9 [DOI] [PubMed] [Google Scholar]
- 14.Rajagopal K, Rogers JG, Lodge AJ, et al. Two-stage total cardioaortic replacement for end-stage heart and aortic disease in Marfan syndrome: case report and review of the literature. J Heart Lung Transplant 2009;28:958-63 [DOI] [PubMed] [Google Scholar]
- 15.Hughes GC. Aggressive aortic replacement for Loeys-Dietz syndrome. Tex Heart Inst J 2011;38:663-6 [PMC free article] [PubMed] [Google Scholar]
- 16.Parsa CJ, Hughes GC. Surgical options to contend with thoracic aortic pathology. Semin Roentgenol 2009;44:29-51 [DOI] [PubMed] [Google Scholar]
- 17.Hughes GC, Daneshmand MA, Swaminathan M, et al. “Real world” thoracic endografting: results with the Gore TAG device 2 years after U.S. FDA approval. Ann Thorac Surg 2008;86:1530-7; discussion 1537-8 [DOI] [PubMed] [Google Scholar]
- 18.Ganapathi AM, Englum BR, Hanna JM, et al. Frailty and risk in proximal aortic surgery. J Thorac Cardiovasc Surg 2014;147:186-191.e1. [DOI] [PMC free article] [PubMed]
- 19.Andacheh ID, Donayre C, Othman F, et al. Patient outcomes and thoracic aortic volume and morphologic changes following thoracic endovascular aortic repair in patients with complicated chronic type B aortic dissection. J Vasc Surg 2012;56:644-50; discussion 650 [DOI] [PubMed] [Google Scholar]
- 20.Lee M, Lee do Y, Kim MD, et al. Outcomes of endovascular management for complicated chronic type B aortic dissection: effect of the extent of stent graft coverage and anatomic properties of aortic dissection. J Vasc Interv Radiol 2013;24:1451-60 [DOI] [PubMed] [Google Scholar]
- 21.Mani K, Clough RE, Lyons OT, et al. Predictors of outcome after endovascular repair for chronic type B dissection. Eur J Vasc Endovasc Surg 2012;43:386-91 [DOI] [PubMed] [Google Scholar]
- 22.Nathan DP, Woo EY, Fairman RM, et al. Stent grafting for aneurysmal degeneration of chronic descending thoracic aortic dissections. J Vasc Surg 2012;55:963-7 [DOI] [PubMed] [Google Scholar]
- 23.Qing KX, Yiu WK, Cheng SW. A morphologic study of chronic type B aortic dissections and aneurysms after thoracic endovascular stent grafting. J Vasc Surg 2012;55:1268-75; discussion 1275-6 [DOI] [PubMed] [Google Scholar]
- 24.Scali ST, Feezor RJ, Chang CK, et al. Efficacy of thoracic endovascular stent repair for chronic type B aortic dissection with aneurysmal degeneration. J Vasc Surg 2013;58:10-7.e1. [DOI] [PMC free article] [PubMed]
- 25.Leshnower BG, Szeto WY, Pochettino A, et al. Thoracic endografting reduces morbidity and remodels the thoracic aorta in DeBakey III aneurysms. Ann Thorac Surg 2013;95:914-21 [DOI] [PubMed] [Google Scholar]
- 26.Kang WC, Greenberg RK, Mastracci TM, et al. Endovascular repair of complicated chronic distal aortic dissections: intermediate outcomes and complications. J Thorac Cardiovasc Surg 2011;142:1074-83 [DOI] [PubMed] [Google Scholar]
- 27.Thrumurthy SG, Karthikesalingam A, Patterson BO, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg 2011;42:632-47 [DOI] [PubMed] [Google Scholar]
- 28.Shah AA, Barfield ME, Andersen ND, et al. Results of thoracic endovascular aortic repair 6 years after United States Food and Drug Administration approval. Ann Thorac Surg 2012;94:1394-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zoli S, Etz CD, Roder F, et al. Long-term survival after open repair of chronic distal aortic dissection. Ann Thorac Surg 2010;89:1458-66 [DOI] [PubMed] [Google Scholar]


