Abstract
We present ten important specific tips regarding the natural history of Type B aortic dissection, which have a bearing on diagnosis and management decisions.
Keywords: Descending thoracic aorta, aortic dissection, natural history, indications for surgery
Introduction
The natural history of Type B dissections has been difficult to delineate. Despite significant progress in diagnosing and treating patients with Type B dissection, the mortality and morbidity levels are still substantial. Invaluable information on descending aortic dissection has been derived from the International Registry of Aortic Dissection (IRAD) (1).
Aortic rupture is known to occur less frequently in Type B dissections than in Type A dissections. However, aortic ruptures represent the number one cause of death in patients with Type B dissections as they are often fatal (2). Malperfusion is the second leading cause of death from acute Type B aortic dissections, which can cause death or significant morbidity via vascular ischemia of various end organs (2,3).
In this keynote lecture series, we discuss the current understanding of Type B aortic dissection, with an emphasis on criteria for surgical treatment.
Aortic dissection is “the great masquerader”
Severe pain with an abrupt onset is the most common presenting symptom of acute aortic dissection according to the data from IRAD (1), although a small percentage of patients do not experience any pain (4). The classic presentation of acute Type B aortic dissection is characterized by intense, severe interscapular pain, often described by the patients as the worst pain they have ever experienced. The interscapular location, remote from the site of pain in the ubiquitous lumbosacral spine disease, helps to distinguish aortic dissection pain from “ordinary” back pain. The pain associated with Type B aortic dissection often propagates downward toward the lower abdomen as the longitudinal extent of dissection increases (5,6). The pain follows the longitudinal progress of the dissection. However, many patients do not present in this typical fashion, making aortic dissection a serious diagnostic challenge (7). Aortic dissection is difficult to suspect and detect because dissection has “many faces” and is “the great masquerader”. Aortic dissection may cause symptoms that are commonly attributable to other acute conditions. For example, aortic dissection can mimic a heart attack, stroke, or acute abdominal conditions, or a patient may present with an ischemic limb. These vastly different conditions can produce very different symptoms, but can all be explained by compromised arterial inflow to some branch of the aorta due to aortic dissection (Figure 1). Therefore it is very important for the physician to be aware of the many faces that aortic dissection may present, and to consider aortic dissection as a primary cause of any combination of cardiac, neurologic, abdominal, or vascular abnormalities that cannot be otherwise explained (7).
Figure 1.
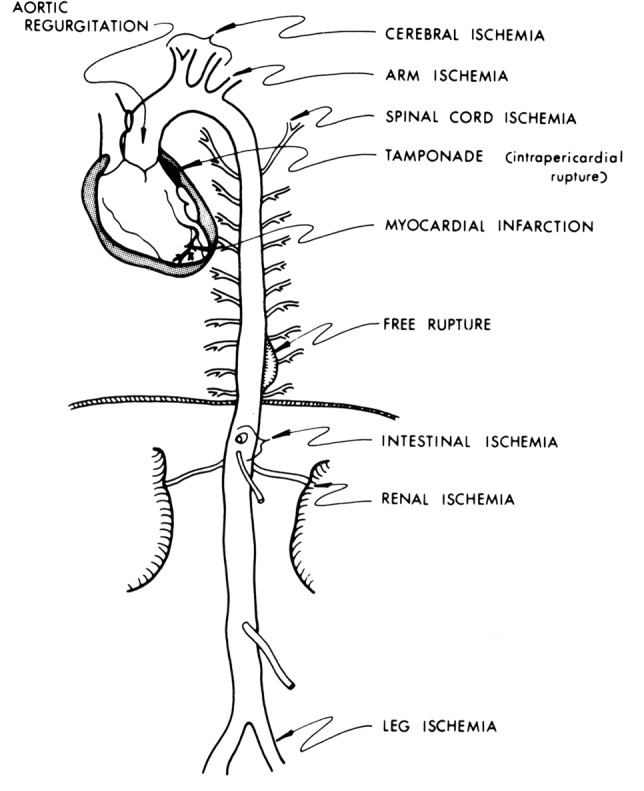
Schematic representation of various conditions that aortic dissection can mimic. [Reprinted with permission from Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Curr Probl Cardiol 2008;33:203-77 (5)].
Inciting events for acute aortic dissection—exertion, emotion
A common misconception is that the occurrence of dissection is a random event in a susceptible patient (8). Several studies have shown that aortic dissection follows circadian and diurnal patterns, with a preponderance of instances in the winter months and in the early morning hours, when blood pressure is known to be highest.
In search of factors or forces that might precipitate dissection, our group identified severe exertion and extreme emotion as etiologic factors in the acute onset of aortic dissection in more than two thirds of our patients (including both ascending and descending dissections) (9-11). A dilated aorta can result from destructive processes such as excess proteolysis by matrix metalloproteinases. We hypothesize that extreme emotional distress and severe physical exertion cause an acute increase in blood pressure, which results in the aortic wall exceeding its tensile limit and dissection (Figure 2). A history of extreme emotion or severe exertion should trigger suspicion of acute aortic dissection.
Figure 2.
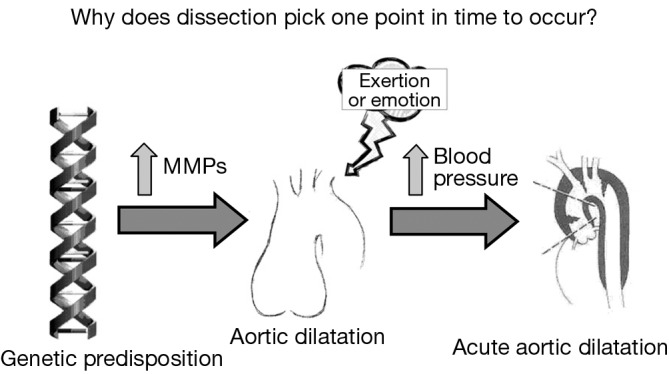
Schematic presentation of possible relationships underlying the instigation of an acute aortic dissection at one particular time. Matrix metalloproteinases [Reprinted with permission from Elefteriades JA. Timing of Acute Aortic Events: How Does Dissection Pick a Date, Time, and Moment to Occur? In: Elefteriades JA. eds. Acute Aortic Disease. New York, NY: Informa Healthcare USA, 2007:169-72. (12)].
Uncomplicated acute Type B dissection is a relatively benign process
Our experience with acute Type B dissections has shown that in the majority of cases, it is a relatively benign disease compared to Type A dissection. The vast majority of patients do well with medical management alone, unless the dissection takes a complicated course. In our published experience of one hundred consecutive patients with acute Type B dissections, we found that the early survival of these patients is high at 91%. Furthermore, 66% of survivors had an uncomplicated course after acute Type B dissection and were successfully treated with anti-impulse therapy alone (Figure 3) (3). It is important to bear in mind this relative benignity when considering widespread routine endovascular therapy of Type B aortic dissection.
Figure 3.
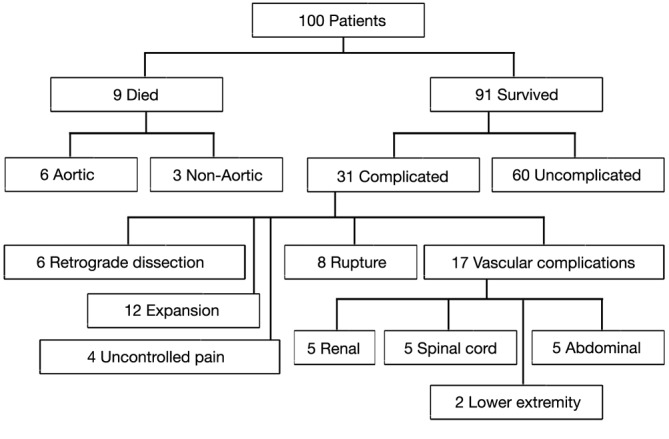
Flow diagram of outcome of 100 consecutive patients with acute descending aortic dissection. [A single patient may have more than one complication.) (Reproduced with permission from Elefteriades JA, Lovoulos CJ, Coady MA, Tellides G, Kopf GS, Rizzo JA. Management of descending aortic dissection. Ann Thorac Surg 1999;67:2002-5. (3)].
Dilatation, when it does occur, affects predominantly the upper descending aorta
There is evidence that in certain patients, the dissected descending aorta dilates in late follow-up (13-15). The growth rates for the descending aorta are slightly greater than for the ascending aorta (Figure 4), and dissected aortas tend to grow slightly faster than aneurysmal aortas (16). However, in our clinical experience we have seen that the dissected descending aorta predominantly dilates in the most proximal portion, and that this process usually takes years to occur. This notion is also supported in the literature (14). This localized, usually indolent pattern of dilatation is also important to bear in mind when considering widespread routine endovascular therapy of Type B aortic dissection.
Figure 4.
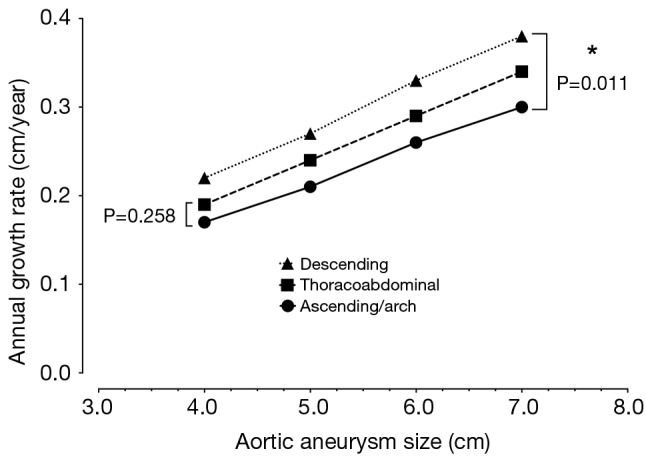
Rates of annual growth of the thoracic aorta.
Type A dissection after Type B dissection occurs, but is quite rare
Intuitively, it would appear reasonable to expect that Type B dissection patients would be innately and commonly prone to Type A dissections. However, the actual rate of occurrence of Type A aortic dissections in patients who have sustained a previous Type B aortic dissection is not well-known. In the present era, many cases of Type A aortic dissection after prior Type B aortic dissection are attributable to retrograde dissection into the aortic arch after thoracic aortic endovascular treatment (17,18). At our institution, we conducted a study of 124 consecutive patients with acute Type B aortic dissection (from 1997 to 2012) in order to determine whether Type A dissections occur naturally in long-term follow-up (19). At a mean radiological follow-up (CT or MRI) of 45.3 months (range 1 to 216 months), only 1.7% of the patients had suffered Type A dissection or a pathologic variant such as intramural hematoma (IMH) or penetrating aortic ulcer (PAU). Among the two patients who developed Type A dissections—one patient developed an ascending dissection 216 months after suffering the Type B dissection, and the second patient developed an IMH one month after the descending dissection (18). Therefore, somewhat surprisingly it appears that Type A aortic dissection is not a frequent natural history event following Type B aortic dissection.
In patients with Marfan syndrome, Type B dissections often do not occur until after ascending aortic replacement
It is well recognized that patients with Marfan syndrome develop Type A aortic dissection. However, it is interesting to note that 16-20% of Marfan patients develop a Type B aortic dissection after the aortic root has been replaced (20,21). One possibility is that preemptive treatment of the dilated aortic root in patients with Marfan syndrome leads to prolonged life expectancy, which in turn increases the likelihood of later adverse events in the descending aorta (20). The other possibility is that replacement of the aortic root itself may trigger downstream aortic events (20), a notion supported by an experimental study that showed increased tension in the wall of the distal aorta after aortic root intervention (22). Predictors for Type B aortic dissection in patients with Marfan syndrome include previous aortic root replacement, larger diameter of the descending aorta, hypertension, aortic regurgitation and impaired aortic compliance (21).
In our clinical experience with 86 patients with Marfan syndrome, 18 patients suffered aortic dissection, out of whom six patients had Type B aortic dissection, five of whom had undergone prior aortic root replacement. The sixth patient had undergone a prior abdominal aortic repair.
Therefore in Marfan’s syndrome, Type B aortic dissection does occur, but with much lower frequency than Type A aortic dissection, and often after prior remote aortic replacement.
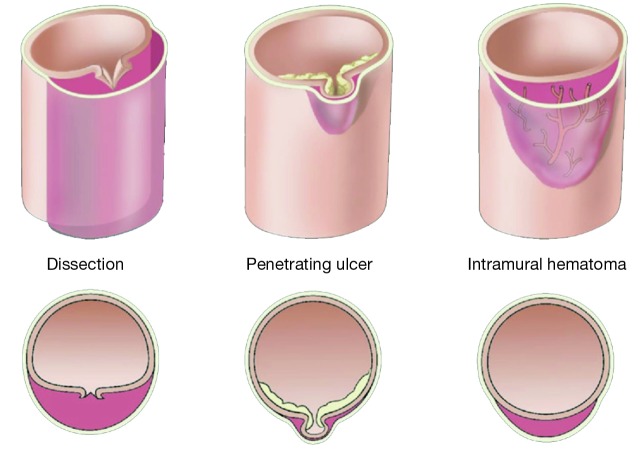
IMH and PAU are variants on a continuum of aortic dissection phenomena (rule of thirds)
IMH and PAU are considered to be pathologic variants of aortic dissection, with an absence of a dissection flap distinguishing it from a classic aortic dissection (Figure 5). We use the rule of “one-thirds” for IMH: one-third of cases remain the same, one-third resolve, and one-third goes on to become typical aortic dissections (with a flap).
Figure 5.
Variant forms of aortic dissection: typical dissection, penetrating aortic ulcer, and intramural hematoma of the aorta. [Reprinted with permission from Elefteriades JA. Thoracic aortic aneurysm: Reading the enemy’s playbook. Curr Probl Cardiol 2008;33:203-77. (5)].
IMH and PAU almost never produce vascular complications due to branch organ occlusion (23). An IMH is a crescentic thickening of the aortic wall, most likely due to rupture of the vaso-vasorum into the media of the aortic wall, without continuous flow communication. Penetrating ulcers of the aorta are ulcerations in the aortic wall thought to be caused by rupture of an atheromatous plaque through the internal elastic lamina, with subsequent localized medial disruption and formation of a blood containing sac within the aortic wall (23). This sac is prone to rupture. IMH and PAU are diseases of advanced age primarily seen in patients over 70.
In our clinical experience, symptomatic IMH and PAU have a high incidence of rupture at initial presentation (38% and 26%, respectively), which is significantly higher than the 8% and 4% incidence of rupture seen in classic Type A and Type B dissection respectively (22). However, it must be noted that incidentally discovered IMH and PAU in asymptomatic patients are much more benign. A recent study from IRAD suggests that IMH may have a slightly more benign course compared with classic Type B dissection in the acute setting and recommends utilizing the “complication-specific” approach to treat these lesions (24). However, we still maintain a low threshold for surgical intervention in patients with IMH and PAU, as our mid-term follow-up has shown substantial late death after hospital discharge (23).
Indications and timing of surgical intervention for acute Type B aortic dissection: the “complication-specific approach”
Early surgery for an acute Type B aortic dissection has traditionally been considered an inappropriate surgery, with a high incidence of anastomotic bleeding (perhaps due to the much lower number of lamellae in the descending aorta) (2,25). This disfavor for early surgery lies in contrast to acute Type A dissections, where surgical intervention is highly favored and usually life-saving. Operative indications and the timing of operations for acute Type B aortic dissection remain individualized and difficult to determine (2).
The most recent interdisciplinary expert consensus document on the management of Type B aortic dissection that reviewed 63 pertinent studies published from 2006 until 2012 found that in acute descending dissection cases, the pooled mortality for medical treatment is 6.4%, 10.2% for thoracic endovascular aortic repair (TEVAR), and 17.5% for open surgery (25). The problem with such studies is that the patients in these groups tend to be very dissimilar, with surgical patients often being the most severely ill at onset of therapy.
Two decades ago, our group proposed the use of a “complication-specific” approach for determining the need for surgical intervention (26). We suggested that the majority of uncomplicated cases of descending aortic dissection could be treated medically by “anti-impulse therapy”—beta-blockade and afterload reduction (Figure 6). Surgical management was recommended for complicated cases of dissection (Figure 7). For each type of complication we recommended a specific surgical technique:
Figure 6.
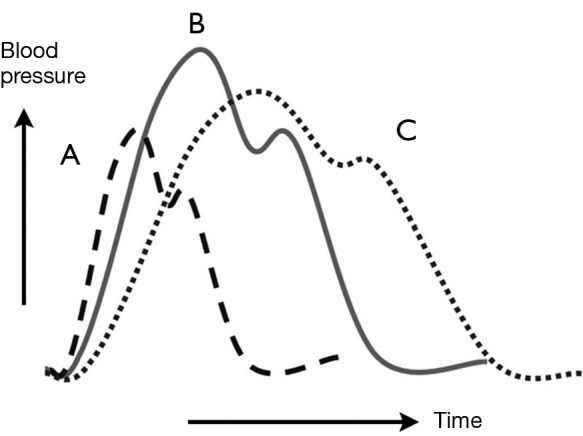
Pharmacologic anti-impulse therapy. Diagram of aortic pressure curves under various conditions. The continuous line (B) represents the baseline state. Administration of a vasodilator agent such as nitroprusside is represented by the dashed curve (A). Beta-blockade administration is represented by the dotted line (C). [Reprinted with permission from Sanz J, Einstein AJ, Fuster V. Acute aortic dissection: Anti-impulse therapy. In: Elefteriades JA. eds. Acute Aortic Disease New York, NY: Informa Healthcare USA, 2007:229-49. (27)].
Figure 7.
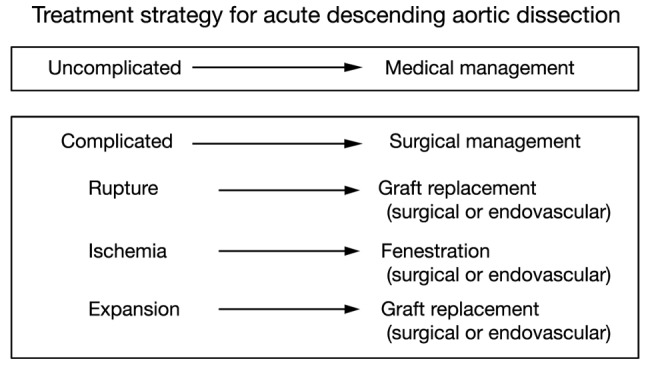
The “complication-specific” approach to acute descending aortic dissection. [Reprinted with permission from Elefteriades JA, Lovoulos CJ, Coady MA, Tellides G, Kopf GS, Rizzo JA. Management of descending aortic dissection. Ann Thorac Surg 1999;67:2002-5; discussion 2014-9. (3)].
❖ In cases of realized aortic rupture, the only appropriate surgical treatment was direct surgical aortic replacement with a tube graft (or, in the present era, a stent graft) to prevent exsanguination;
❖ When the complication was organ ischemia from branch vessel occlusion, we favored the fenestration procedure, which can currently be performed by an endovascular as well as an open surgical approach;
❖ Lastly, if the complication was threatened (impending) rupture manifesting as continued pain, rapid aortic expansion, increasing peri-aortic hematoma, or hemorrhagic pleural effusion, surgical aortic replacement of the aorta was recommended. This can also currently be performed by an endovascular approach (TEVAR).
Establishment of the “complication-specific” approach was helpful, as it spared uncomplicated patients a surgical procedure with a high incidence of postoperative complications (bleeding, paraplegia). Since then, the “complication-specific” approach in treating descending aortic dissection has been incorporated into comprehensive management guidelines (25,28).
Aggressive endovascular intervention for all Type B aortic dissections, with a view toward preventing late sequelae, is currently being evaluated in the INSTEAD and STABLE trials (29,30). It is too early to form conclusions about such therapy.
For late interventions long after Type B aortic dissection, criteria follow those for chronic non-dissected aneurysms—namely attainment of a diameter of 5.5 cm.
Fenestration is highly effective for organ malperfusion in acute aortic dissection
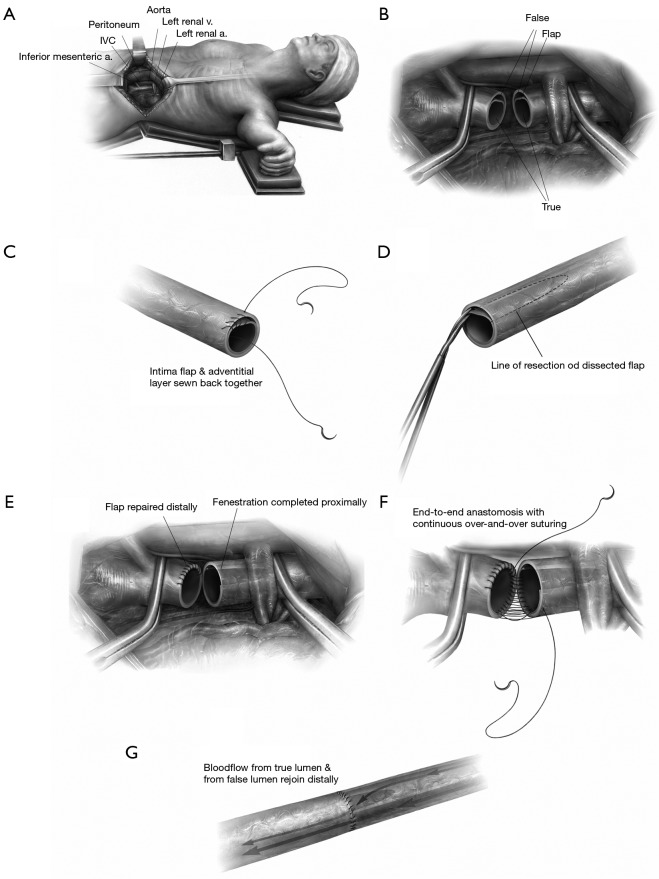
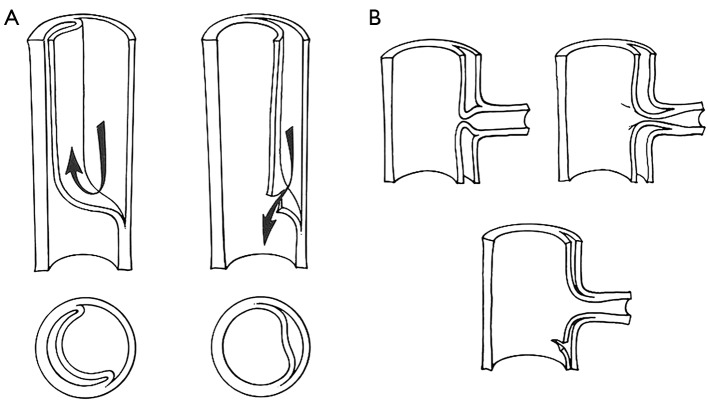
Aortic fenestration is an effective method for decompressing the false lumen of the dissected aorta by creating a window (or “fenestra”) in the intimal layer of the distal part of the aorta (please see the associated surgical technique illustrated in detail in Figure 8) (31). This permits outflow of blood from the false lumen, reduces the intraluminal pressure, and relieves branch vessel obstruction (Figure 9). Over the years, aortic fenestration techniques have proven to provide a safe, effective, and durable treatment alternative to aortic replacement for the specific complication of branch vessel occlusion (33,34). The fenestration must be done early, usually within 24 to 48 hours in order to be effective, before the dissection membrane becomes fixed and the clot becomes organized in the false lumen. The fenestration is effective both above and below the abdominal level at which it is performed.
Figure 8.
Schematic illustration of the surgical fenestration technique: (A) positioning of the patient, incision, exposure of the retroperitoneal space; (B) the abdominal aorta is clamped proximally and distally, and transected, the dissection flap is visualized; (C) dissection in the distal open end of the aorta is repaired by re-approximate the intima to the adventitial layer; (D) fenestration of the intimal flap is performed on the proximal aorta by excising as much intima as possible, in both circumferential and longitudinal extent toward the proximal clamp; (E,F) the fenestrated proximal end is re-anastamosed to the repaired distal end of the aorta; (G) the clamps are removed and blood flow is restored. [Reprinted with permission from Elefteriades JA, Escalon JC. Aortic Fenestration for Dissection. Operative Techniques in Thoracic and Cardiovascular Surgery 2009;14:12-22. (31)]
Figure 9.
Schematic illustration of the benefit of decompressing the false lumen: (A) schematic diagram of aortic dissection, false lumen formation and decompression of the false lumen; (B) decompression of the false lumen restores blood flow to compromised organs. [Reprinted with permission from Crawford ES, Crawford JL, Smith PS. Diseases of the aorta. Baltimore: Williams & Wilkins, 1984. (32)].
Partial false lumen thrombosis adversely affects prognosis in Type B aortic dissection
Reports from IRAD showed that a partially thrombosed false lumen in patients with Type B aortic dissection is associated with increased operative mortality (35), while complete thrombosis of the false lumen has beneficial prognostic value. It appears that the aortic growth rate is significantly increased among the aortic segments with a partially thrombosed false lumen compared with patients with complete thrombosis or a patent false lumen (36). Based on these results, the authors recommend that patients with partial thrombosis of the false lumen in Type B dissection may require more intensive follow-up and may benefit from lower thresholds for prophylactic aortic intervention (36).
Conclusions
The natural history of a descending dissection is relatively benign, with the majority of patients doing well with medical therapy unless a complication arises that requires surgical or endovascular intervention.
Video.

Natural history of Type B aortic dissection: ten tips.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903 [DOI] [PubMed] [Google Scholar]
- 2.Gallo A, Davies RR, Coe MP, et al. Indications, timing, and prognosis of operative repair of aortic dissections. Semin Thorac Cardiovasc Surg 2005;17:224-35 [DOI] [PubMed] [Google Scholar]
- 3.Elefteriades JA, Lovoulos CJ, Coady MA, et al. Management of descending aortic dissection. Ann Thorac Surg 1999;67:2002-5; discussion 2014-9. [DOI] [PubMed]
- 4.Tolenaar JL, Hutchison SJ, Montgomery D, et al. Painless Type B Aortic Dissection: Insights From the International Registry of Acute Aortic Dissection. Aorta 2013;1:96-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Current problems in cardiology 2008;33:203-77 [DOI] [PubMed] [Google Scholar]
- 6.Ziganshin BA, Elefteriades JA. Thoracic Aortic Disease. In: Stergiopoulos K, Brown DL. eds. Evidence-Based Cardiology Consult. 1 ed. London: Springer-Verlag, 2014:331-53. [Google Scholar]
- 7.Isselbacher EM. The Symptoms and Signs of Acute Aortic Dissection: Clinical Diagnosis of “The Great Masquerader”. In: Elefteriades JA. eds. Acute Aortic Disease. New York, NY: Informa Healthcare USA, 2007:29-38. [Google Scholar]
- 8.Ziganshin BA, Elefteriades JA. Yale milestones in reading the playbook of thoracic aortic aneurysms. Conn Med 2012;76:589-98 [PubMed] [Google Scholar]
- 9.Elefteriades JA, Hatzaras I, Tranquilli MA, et al. Weight lifting and rupture of silent aortic aneurysms. JAMA 2003;290:2803. [DOI] [PubMed] [Google Scholar]
- 10.Hatzaras I, Tranquilli M, Coady M, et al. Weight lifting and aortic dissection: more evidence for a connection. Cardiology 2007;107:103-6 [DOI] [PubMed] [Google Scholar]
- 11.Hatzaras IS, Bible JE, Koullias GJ, et al. Role of exertion or emotion as inciting events for acute aortic dissection. Am J Cardiol 2007;100:1470-2 [DOI] [PubMed] [Google Scholar]
- 12.Elefteriades JA. Timing of Acute Aortic Events: How Does Dissection Pick a Date, Time, and Moment to Occur? In: Elefteriades JA. eds. Acute Aortic Disease. New York, NY: Informa Healthcare USA, 2007:169-72. [Google Scholar]
- 13.Sueyoshi E, Sakamoto I, Hayashi K, et al. Growth rate of aortic diameter in patients with type B aortic dissection during the chronic phase. Circulation 2004;110:II256-61 [DOI] [PubMed] [Google Scholar]
- 14.Song JM, Kim SD, Kim JH, et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol 2007;50:799-804 [DOI] [PubMed] [Google Scholar]
- 15.Jonker FH, Trimarchi S, Rampoldi V, et al. Aortic expansion after acute type B aortic dissection. Ann Thorac Surg 2012;94:1223-9 [DOI] [PubMed] [Google Scholar]
- 16.Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002;73:17-27; discussion 27-8 [DOI] [PubMed] [Google Scholar]
- 17.Williams JB, Andersen ND, Bhattacharya SD, et al. Retrograde ascending aortic dissection as an early complication of thoracic endovascular aortic repair. J Vasc Surg 2012;55:1255-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Idrees J, Arafat A, Johnston DR, et al. Repair of retrograde ascending dissection after descending stent grafting. J Thorac Cardiovasc Surg 2014;147:151-4 [DOI] [PubMed] [Google Scholar]
- 19.Ziganshin BA, Mukherjee A, Rajakaruna C, et al. How Often Do Type A Dissections Occur in Long-Term Follow-up After Type B Dissection? Abstracts of the American Association of Thoracic Surgery Aortic Symposium 2014:310. [Google Scholar]
- 20.Schoenhoff FS, Jungi S, Czerny M, et al. Acute aortic dissection determines the fate of initially untreated aortic segments in Marfan syndrome. Circulation 2013;127:1569-75 [DOI] [PubMed] [Google Scholar]
- 21.Engelfriet PM, Boersma E, Tijssen JG, et al. Beyond the root: dilatation of the distal aorta in Marfan’s syndrome. Heart 2006;92:1238-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scharfschwerdt M, Sievers HH, Greggersen J, et al. Prosthetic replacement of the ascending aorta increases wall tension in the residual aorta. Ann Thorac Surg 2007;83:954-7 [DOI] [PubMed] [Google Scholar]
- 23.Tittle SL, Lynch RJ, Cole PE, et al. Midterm follow-up of penetrating ulcer and intramural hematoma of the aorta. J Thorac Cardiovasc Surg 2002;123:1051-9 [DOI] [PubMed] [Google Scholar]
- 24.Tolenaar JL, Harris KM, Upchurch GR, Jr, et al. The differences and similarities between intramural hematoma of the descending aorta and acute type B dissection. J Vasc Surg 2013;58:1498-504 [DOI] [PubMed] [Google Scholar]
- 25.Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 2013;61:1661-78 [DOI] [PubMed] [Google Scholar]
- 26.Elefteriades JA, Hartleroad J, Gusberg RJ, et al. Long-term experience with descending aortic dissection: the complication-specific approach. Ann Thorac Surg 1992;53:11-20; discussion 20-1 [DOI] [PubMed] [Google Scholar]
- 27.Sanz J, Einstein AJ, Fuster V. Acute aortic dissection: Anti-impulse therapy. In: Elefteriades JA. eds. Acute Aortic Disease. New York, NY: Informa Healthcare USA, 2007:229-49. [Google Scholar]
- 28.Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg 2008;85:S1-41 [DOI] [PubMed] [Google Scholar]
- 29.Lombardi JV, Cambria RP, Nienaber CA, et al. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg 2012;55:629-40 e2. [DOI] [PubMed]
- 30.Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407-16 [DOI] [PubMed] [Google Scholar]
- 31.Elefteriades JA, Escalon JC. Aortic Fenestration for Dissection. Operative Techniques in Thoracic and Cardiovascular Surgery 2009;14:12-22 [Google Scholar]
- 32.Crawford ES, Crawford JL, Smith PS. Diseases of the aorta: including an atlas of angiographic pathology and surgical technique. Baltimore: Williams & Wilkins, 1984. [Google Scholar]
- 33.Elefteriades JA, Hammond GL, Gusberg RJ, et al. Fenestration revisited. A safe and effective procedure for descending aortic dissection. Arch Surg 1990;125:786-90 [DOI] [PubMed] [Google Scholar]
- 34.Pradhan S, Elefteriades JA, Sumpio BE. Utility of the aortic fenestration technique in the management of acute aortic dissections. Ann Thorac Cardiovasc Surg 2007;13:296-300 [PubMed] [Google Scholar]
- 35.Trimarchi S, Nienaber CA, Rampoldi V, et al. Role and results of surgery in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006;114:I357-64 [DOI] [PubMed] [Google Scholar]
- 36.Trimarchi S, Tolenaar JL, Jonker FH, et al. Importance of false lumen thrombosis in type B aortic dissection prognosis. J Thorac Cardiovasc Surg 2013;145:S208-12 [DOI] [PubMed] [Google Scholar]