The frequencies of CD62L− and FoxP3+ regulatory T cells in peripheral blood are cellular markers of acute graft-versus-host disease in the rat.
Keywords: hematopoietic cell transplantation, donor chimerism, regulatory T cells, biomarkers, animal model
Abstract
GVHD causes extensive morbidity and mortality in patients who receive alloHCT. Predictive and reliable markers for GVHD are currently lacking but required to improve the safety and accessibility of alloHCT. We present an experimental rat model of myeloablative total body irradiation and fully mismatched major and minor histoincompatible, T cell-depleted BMT, followed by delayed infusion of donor lymphocytes. This treatment, in contrast to marrow transplantation alone, resulted in severe aGVHD and 100% lethality within 2–6 weeks. We investigated the reconstitution kinetics and phenotypes of donor leukocyte subpopulations as well as the histopathology of selected organs that may correlate with GVHD, with the goal to find potential disease-related markers. We observed histological changes mainly confined to the skin, with degenerative changes in the basal layer. LNs and spleen showed deranged architecture with markedly increased accumulation of lymphocytes, whereas the gut, liver, and lungs appeared normal. Of the lymphocyte markers tested, donor-derived CD62L+ T cells were markedly decreased in animals suffering from GVHD. Furthermore, we observed peripheral depletion of CD4+CD25hiFoxP3+ Treg, which was in contrast to controls. The relative frequency of these lymphocyte subpopulations in blood may therefore serve as accessible cellular markers of aGVHD. We propose that the animal model presented is instructive for the identification of clinically relevant markers of GVHD, which could improve disease diagnosis and management in alloHCT.
Introduction
alloHCT is an established treatment for hematological malignancies but continues to cause extensive morbidity and mortality in patients who develop GVHD [1]. Despite extensive investigation, current treatment regimens for GVHD are limited, and diagnosis is based mainly on clinical observations and distinct pathologies in target organs [1, 2]. Disease-associated markers for clinical prophylaxis and reliable diagnosis are needed.
Skin, liver, and gut are primary target sites of GVHD, and other organs, such as lungs, spleen, and thymus, can also be involved [1, 3–5]. GVHD is initiated by donor T lymphocyte activation upon transfer to preconditioned recipients of alloHCT. Donor T cells encounter disparate MHC or minor histocompatibility antigens displayed by APCs in the context of MHC molecules. Naïve CD4+ T cell clones, which recognize host alloantigens, respond with activation, proliferation, and differentiation. Naïve CD8+ T cells are also capable of inducing GVHD in response to differences in MHC class I antigens [6]. Stimulated CD4+ T cells release cytokines, which recruit other leukocytes, including CD8+ T cells and NK cells, to peripheral sites of ongoing GVHR [1]. CD4+ T cells mediate helper functions on other immune cells, CD8+ T cells and NK cells are the main effector cell populations in the late stage of GVHD [1]. Together with inflammatory cytokines, these effector cells exert cytotoxicity in aGVHD, which ultimately results in the destruction of host tissue [1]. Early predictive or diagnostic markers of alloimmunity could facilitate timely intervention with immunosuppressive treatment.
NK cells, in addition to their ability to reject allogeneic hematopoietic cells, can destroy infected and malignant leukocytes, including leukemic cells, and thus, have a key role in alloHCT. Inhibition or activation of cytolytic NK cell functions is mediated through binding of “self” or “non-self” MHC class I molecules by NKRs, e.g., killer-cell Ig-like receptors in humans and receptors of the Ly49 gene family in mice and rats [7]. Donor NK cells can eradicate host leukocytes and thereby, facilitate engraftment and prevent leukemic relapse without causing GVHD [8].
Natural Tregs, expressing CD4 and CD25 surface markers and the transcription factor FoxP3, have the ability to suppress activated T cells and are known to alleviate aGVHD [9, 10]. The lack of Tregs has been associated with GVHD in patients and in animal models [11–13].
In this study, we describe a previously established rat model of alloHCT [14] in more detail. DLI caused severe, lethal aGVHD in recipients of MHC-mismatched, T cell-depleted BM. The aim of this investigation was to identify histological and cellular phenotype markers associated with GVHD by studying immune reconstitution following alloHCT and postmortem histopathology of relevant lymphoid and GVHD target organs. We identified the relative frequencies of CD62L+ as well as Tregs in the PB as accessible markers of aGVHD.
MATERIALS AND METHODS
Ethical approval
The institutional veterinarian at the Department of Comparative Medicine, Institute of Basic Medical Sciences, University of Oslo (Norway), has delegated authority from the Norwegian Animal Research Authority and approved the protocols (License Numbers VIT02.02, VIT09.1512, 05.07), which were conducted in compliance with “The Norwegian Regulations on Animal Experimentation” and “The European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes”. Animals used in experiments were frequently monitored for disease symptoms, and those suffering from severe GVHD were killed with CO2 at defined endpoints. Every effort was taken to avoid animals' suffering.
Animals
PVG.7B strain (RT1c haplotype, allelic variant RT7b) rats were obtained from Harlan Laboratories (http://www.harlan.com/) and bred on location. BN (RT1n) rats were purchased from Harlan (The Netherlands) and acclimatized for at least 7 days before experimentation. Rats were housed at the Institute of Basic Medical Sciences, University of Oslo, under controlled conditions with 12:12 h light/dark cycles and access to food and filtered drinking water ad libitum. The animal facilities are subject to routine health monitoring and were screened for common rodent pathogens following Federation of European Laboratory Animal Science Association recommendations [15]. Total body irradiation was performed using a Gammacell 3000 Elan instrument (MDS Nordion, ON, Canada; http://www.mds.nordion.com/). Neuroleptanalgesia was administered by i.m. or s.c. injection of 40 μL/100 g body weight Hypnorm (VetaPharma, UK; http://www.vetapharma.co.uk/ and http://www.hypnorm.co.uk/).
Experimental BMT
Allogeneic BMT was performed as described previously [16]. Male PVG.7B rats served as BM and LN donors and were killed at 10–12 weeks of age. BM cells were flushed from tibiae and femora in Gibco RPMI 1640, supplemented with 2% FBS (Invitrogen, UK; http://www.invitrogen.com/) and subsequently filtered through nylon cell strainers (70 μm mesh; BD Biosciences, Woburn, MA, USA; http://www.bdbiosciences.com/). Mononuclear cells were purified by density gradient centrifugation using Nycoprep 1.077A (Medinor AS, Norway; http://www.medinor.no/). T cells were removed by magnetic separation using OX19 (anti-CD5) and R73 (anti-αβ TCR) supernatant and purified antibodies conjugated to pan-anti-mouse IgG-coated Dynabeads (Invitrogen Dynal AS, Norway). The number of CD3+ T cells was thus reduced more than tenfold to <0.2% of total BM cells (data not shown). For DLI, mesenteric and cervical LNs were removed, processed through cell strainers, washed, and resuspended in PBS. The BM and LN grafts were controlled for cell viability by Trypan blue (Sigma-Aldrich, St. Louis, MO, USA; http://www.sigmaaldrich.com/) exclusion and CD3+ cell content by flow cytometric staining with G4.18 (anti-rat CD3; BD PharMingen, San Diego, CA, USA) mAb before transplantation.
Male BN rats were used as BM recipients at 9 weeks of age and 225–265 g body weight. The rats were subjected to a single dose of 900 cGy whole body irradiation from a 137Cs source at a dose rate of ∼4.1 Gy min−1 prior to BMT. T cell-depleted BM cells (30×106) were administered by i.v. injection in a volume of <1 mL PBS. Fourteen days later, GVHD was invoked by i.v. injection of 7.5 × 106 LN cells containing ∼60% mature T cells (i.e., 5×106 CD3+ T cells; data not shown) in PBS.
The animals were scored by a trained laboratory animals researcher (S.Z.) at least once/week for body weight and GVHD symptoms, comprising activity, kyphosis, skin integrity, fur texture, and weight change (cf. Table 1) using a protocol adapted from Cooke et al. [17]. Rats suffering from irreversible, fatal GVHD with severe weight loss were terminated at defined endpoints (body weight, below 150 g; or cumulative GVHD score, exceeding 7).
Table 1. Assessment of GVHD Symptoms.
| Criteria | Symptoms | ||
|---|---|---|---|
| weight loss | <10% | 10–25% | >25% |
| posture | normal | kyphosis | impaired movement |
| activity | normal | passive | stationary |
| skin integrity | normal | scaling/flaking | lesions/scars |
| fur texture | normal | mild ruffling/poor grooming | alopecia |
| score | 0 | 1 | 2 |
A semiquantitative scoring system was applied to assess GVHD severity. The protocol was adapted from Cooke et al. [17].
Histology
Tissues of interest (liver, lungs, spleen, cervical and mesenteric LNs, thymus, small intestine, Peyer's patches, skin from the abdomen, back, and paws, and lesions, where applicable) were collected at autopsy. Samples were fixed in formalin (PBS with 4% formaldehyde) overnight, dehydrated, and embedded in paraffin. Tissue sections (4 μm) were stained with H&E and safranin and evaluated under light microscopy by an expert pathologist (L.S.).
Immunohistochemical stainings were performed on cryo-preserved tissue samples, which were embedded in Tissue-Tek O.C.T. compound (Sakura, Japan; http://www.sakura.com/) and frozen immediately in a bath of liquid N2 before storage at −80°C. Tissue sections (4 μm) were fixed on glass slides using acetone. Nonspecific binding was blocked using 1.25% BSA (Sigma-Aldrich) before addition of supernatants of OX1, OX8, OX39, 3.2.3, and W3/25 (diluted with an equal volume of BSA solution) for 120 min at ambient temperature. Sections were incubated with Alexa Fluor 546-conjugated goat anti-mouse IgG (Molecular Probes, Invitrogen, Carlsbad, CA, USA) secondary antibody, together with Alexa Fluor 488-conjugated Isolectin B4 (Molecular Probes, Invitrogen) for 60 min at ambient temperature. Between incubations, slides were washed twice in PBS for 5 min, dipped in distilled water and allowed to dry, and finally, mounted in polyvinyl alcohol. Images were captured using a Nikon Eclipse E800 fluorescence microscope (Nikon Instruments, Melville, NY, USA; http://www.nikoninstruments.com/) equipped with Nikon Plan-Fluor objective lenses and a F-VIEW digital camera controlled by AnalySIS 3.2 software (Olympus Soft Imaging Solutions, Germany; http://www.soft-imaging.net/).
Flow cytometry
PB samples of ∼0.5 mL were collected under analgesia from the lateral tail vein using heparinized Hemato-Clad hematocrit tubes (Drummond Scientific, Broomall, PA, USA; http://www.drummondsci.com/). PBMCs were labeled with combinations of fluorochrome-conjugated anti-rat mAb (summarized in Table 2). Peridinin chlorophyll-conjugated Streptavidin (BD PharMingen) was added for secondary staining of biotinylated antibodies. For intracellular staining, buffer solutions from eBioscience (San Diego, CA, USA; http://www.ebioscience.com/) were used for fixation and permeabilization of cells, followed by staining with anti-mouse/rat FoxP3 mAb (eBioscience). Immunostained cells were analyzed on a FACSCalibur flow cytometer using CellQuest software (BD Biosciences, San Jose, CA, USA) and further analyzed by FlowJo software (Tree Star, Ashland, OR, USA; http://www.treestar.com/).
Table 2. Antibodies Used for Flow Cytometric Characterization of T and NK Cell Chimerism.
| Antibody | Clone | Conjugate | Species | Isotype |
|---|---|---|---|---|
| anti-RT7.B | HIS41 [18] | FITC | mouse | IgG1 |
| anti-CD3 | G4.18 [19] | PE | mouse | IgG3, κ |
| anti-CD4 | OX38 | Alexa Fluor 647 | mouse | IgG2a |
| anti-CD4 | W3/25 | FITC | mouse | IgG1 |
| anti-CD8 | OX8 | biotin | mouse | IgG1 |
| anti-CD25 | OX39 | biotin | mouse | IgG1 |
| anti-CD62L | OX85 | biotin | mouse | IgG1 |
| anti-CD134 | OX40 | biotin | mouse | IgG2b |
| anti-FoxP3 | FJK-16s | allophycocyanin | rat | IgG2a, κ |
| anti-NKR-P1A | 3.2.3 [20] | biotin | mouse | IgG1, κ |
| anti-Ly49i2 | STOK2 [21] | biotin | rat | IgG2a |
| anti-Ly49i3/s3/i4/s4 | DAR13 [22] | Alexa Fluor 647 or biotin | mouse | IgG1 |
| anti-Ly49i5/s5 | Fly5 [23] | biotin | mouse | IgG1 |
| anti-Ly49s3 | STOK6 [24] | biotin | rat | IgG2b |
| anti-pan-Ly49a | biotin | |||
| anti-KLRH1 | STOK9 [25] | biotin | rat | IgG2c, κ |
| anti-NKR-P1BPVG | STOK27 [26] | Alexa Fluor 647 or biotin | rat | IgG2a |
A cocktail of STOK2, STOK6, Fly5, and DAR13 antibodies. KLRH1, Killer cell lectin-like receptor H1.
Statistical analysis
Normal distribution of data was assumed and tested using Shapiro-Wilk's test. The probability of significant difference between the groups was tested using a parametric test (Student's t test, unpaired, two-tailed), according to Levene's test of equality of variances. All statistical analyses were performed using PASW Statistics 17.0.2 software (SPSS, Chicago, IL, USA; http://www.spss.com/).
RESULTS
MHC-mismatched BMT and DLI cause pathological changes typical of aGVHD
Clinical BMT across MHC disparities requires extensive T cell depletion of the graft to avoid GVHD and high numbers of hematopoietic cells to overcome the MHC barrier [27]. DLI may be given as therapeutic intervention to improve donor chimerism and to pre-empt or treat neoplastic relapse by way of the graft-versus-tumor effect [28]. With this rat model for alloHCT, we aimed to study the setting of donor-recipient MHC mismatch by using high-intensity conditioning, high doses of T cell-depleted BM, and later treatment with DLI.
Lethally irradiated BN rats were transplanted with 30 × 106 T cell-depleted BM cells from PVG.7B donor rats with full MHC mismatch, and were injected with mature T cells from LNs of the same donor strain 14 days after transplantation to invoke aGVHD. We titrated the dose of DLI required to induce aGVHD in this setup and found that injections of 10 × 106 and 5 × 106 donor T cells reproducibly resulted in lethal aGVHD in all BM recipients (median survival, 16 days and 17 days post-DLI, respectively; n=9 and 8; data not shown). A reduced DLI dose of 1 × 106 T cells also produced 100% mortality but with slower disease kinetics (median survival, 28 days; n=10; data not shown). Doses of 1 × 105 (n=3) or fewer T cells resulted in variable outcomes with lethal aGVHD or symptom-free, long-term survival of individual recipients (unpublished observations). Marrow-transplanted control rats, which did not receive DLI, survived without disease (n=15; data not shown).
The applied transplantation protocol and outcome regarding GVHD severity and survival are summarized in Table 3. Six recipient rats received BMT and a delayed DLI of ∼5 × 106 donor T cells from PVG.7B donors. The recipients developed severe aGVHD and reached the defined endpoints (cf. Materials and Methods) at 17–39 days post-DLI (median survival, 24.5 days; Table 3). aGVHD manifested with rapid weight loss, apathy, severe kyphosis, ruffled fur, alopecia, skin flaking, and occasional skin lesions, as well as infrequent observations of diarrhea and conjunctivitis (mean GVHD score, 7.8 at death). Six control rats, which were transplanted in parallel, received the same treatment but no DLI and did not develop GVHD (survival, >70 days post-DLI; Table 3), except for one rat, which showed GVHD symptoms following BMT (maximal GVHD score, 6 at 21 days) but later improved and stabilized (censored at 35 days; GVHD score, 3; Table 3).
Table 3. Transplantation Protocol and Outcome.
| group | BMT + DLI |
BMT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| donor strain | PVG.7B (n = 13) |
|||||||||||
| recipient strain | BN (n = 6) |
BN (n = 6) |
||||||||||
| recipient label | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| transplantation | ||||||||||||
| –14 days post-DLI | 30 × 106 PVG.7B BM cells | 30 × 106 PVG.7B BM cells | ||||||||||
| 0 day post-DLI | 7.5 × 106 PVG.7B LN cells | sham (PBS) | ||||||||||
| survival | [days post-DLI] | [days post-DLI] | ||||||||||
| day of autopsy | 23 | 18 | 17 | 26 | 38 | 39 | 85 | 85 | 85 | 35 | 68 | 68 |
| group median | 24.5 | 85 | ||||||||||
| cause of deatha | ||||||||||||
| G | G | G | G | G | G | S | S | S | C | S | S | |
| body weight | [g] | [g] | ||||||||||
| on the day of BMT | 233 | 238 | 228 | 238 | 237 | 228 | 225 | 234 | 244 | 230 | 235 | 242 |
| group mean (sd) | 234 (5) | 235 (7) | ||||||||||
| on the day of DLI | 240 | 194 | 230 | 232 | 239 | 228 | 225 | 241 | 247 | 227 | 233 | 238 |
| group mean (sd) | 227 (17) | 235 (8) | ||||||||||
| on the day of autopsy | 155 | 144 | 148 | 147 | 170 | 173 | 237 | 260 | 250 | 242 | 254 | 250 |
| group mean (sd) | 156 (12) | 249 (8) | ||||||||||
| cumulative GVHD score [days post-DLI] | ||||||||||||
| 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 1 | 1 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 2 | 1 | 1 |
| 14 | 0 | 4 | 5 | 3 | 2 | 0 | 1 | 1 | 0 | 4 | 0 | 0 |
| 21 | 9 | 3 | 1 | 1 | 0 | 0 | 0 | 6 | 0 | 0 | ||
| 28 | 3 | 4 | 0 | 0 | 0 | 3 | 0 | 0 | ||||
| on the day of autopsy | 9 | 6 | 6 | 9 | 8 | 9 | 0 | 0 | 0 | 3 | 0 | 0 |
G, aGVHD; S, killed; C, censored.
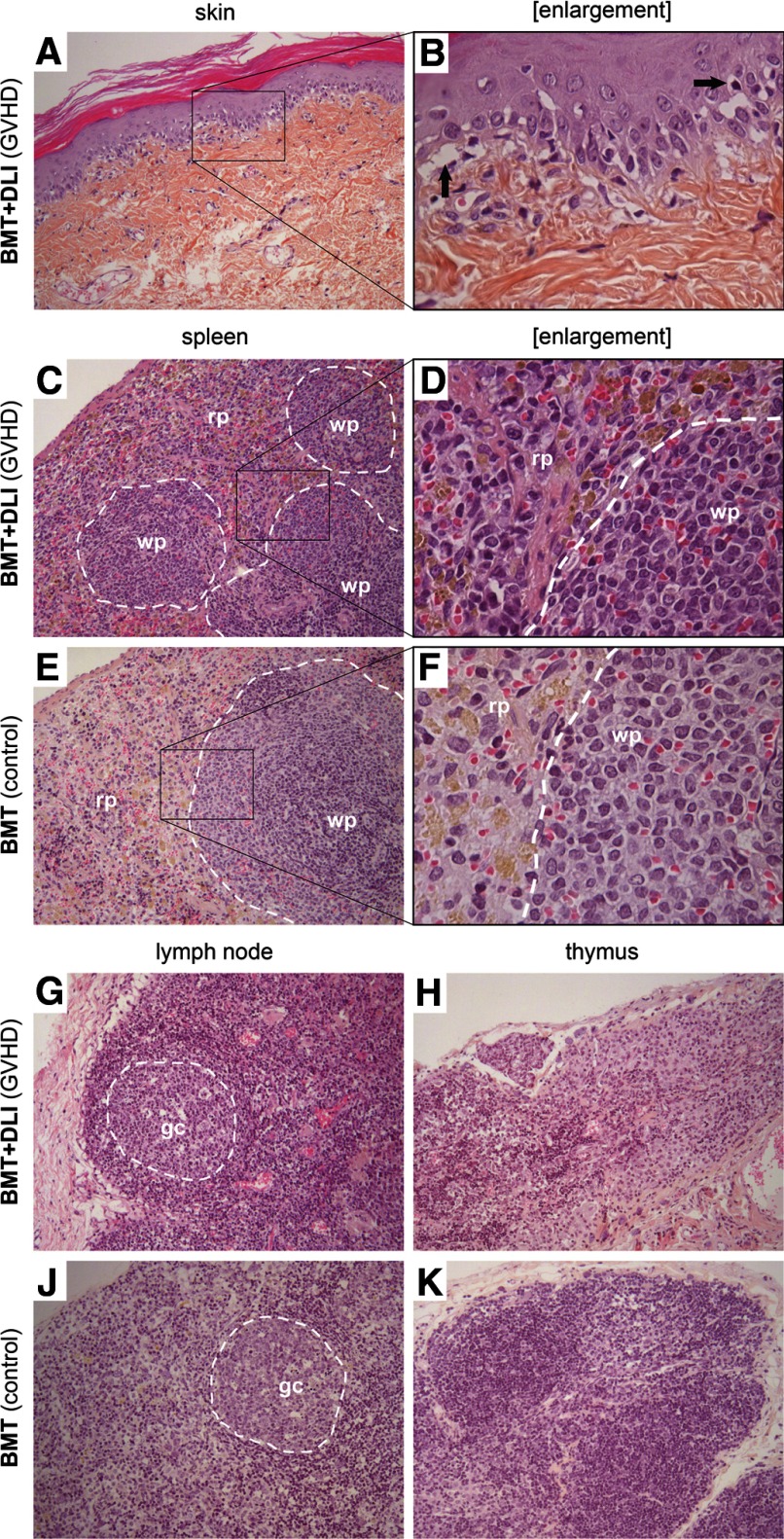
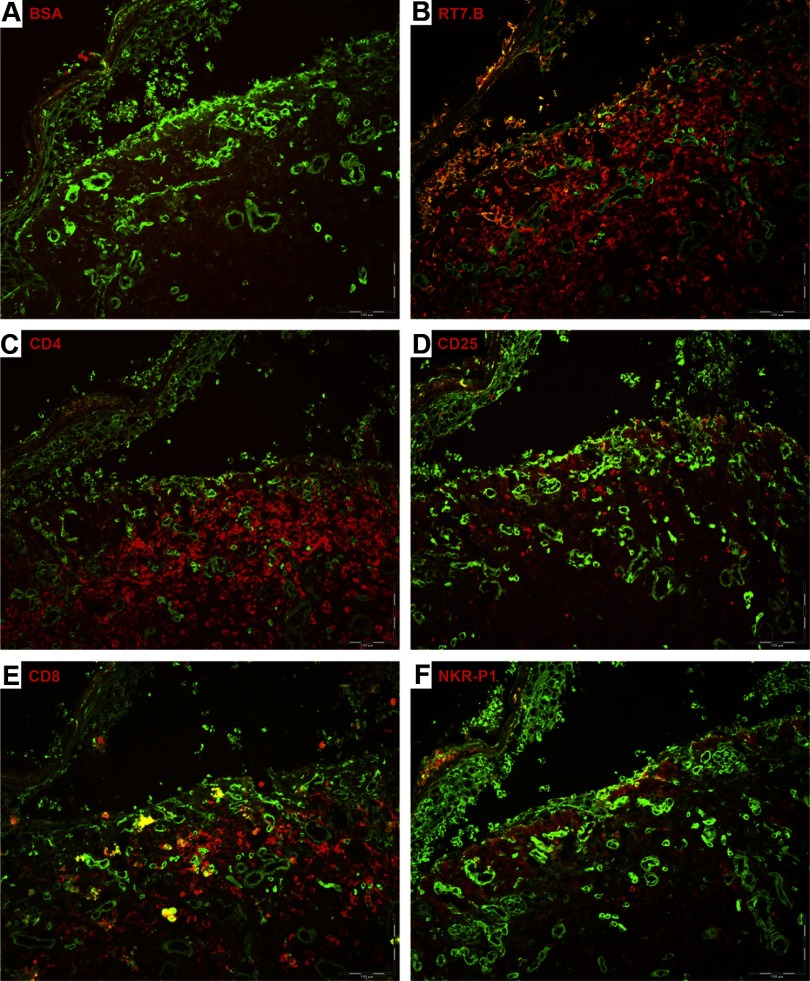
At autopsy, we observed pathological changes of several organs (Fig. 1). Sections from skin showed symptomatic histological changes of GVHD [29] with vacuolar degeneration of epidermal basal cells, focal cleft formation, and occasional single-cell necrosis of keratinocytes (Fig. 1A and B). Occasionally, we observed leukocyte infiltration of the dermis (Fig. 2). The infiltrating cells were CD45+ leukocytes, the majority expressing CD4 and in fewer numbers, CD8 surface markers, whereas NK (NKR-P1+) cells were rarely detected (Fig. 2B–F). Pathological changes were not detected in the liver and lungs macroscopically or microscopically at autopsy. Some of the diseased animals had air in the gut but did not present with significant intestinal histopathology (data not shown).
Figure 1. Histology of skin and lymphoid organs in rats with aGVHD.
Total body-irradiated BN rats were transplanted with 30 × 106 PVG.7B T cell-depleted BM cells. Samples from the skin and several organs were taken postmortem from rats, which had received DLIs of 5 × 106 donor T cells 14 days after BMT and subsequently developed aGVHD, and control rats, which had not received DLI and did not develop aGVHD. Paraffin sections of the skin (A and B), spleen (C–F), LNs (G and J), and thymus (H and K) are shown (original magnifications: A, C, E, G–K, 100×; B, D, F, 400×). The skin presented typical GVHD-associated pathology with vacuolar degeneration and apoptotic cells in the epidermis as well as focal separation of the epidermis and dermis (B, black arrows). The spleens of GVHD rats presented pathological changes, such as destruction of the typical histological architecture and substantial lymphocyte infiltration in the red (rp) and white pulp (wp), shown in D. LNs also showed pathology as a result of inflammation and cell infiltration (gc, germinal center). The thymus displayed signs of degeneration, which may not be related to the disease. The contrast and brightness of this figure have been adjusted to enhance viewing quality.
Figure 2. Leukocyte infiltration in GVHD skin.
Antibody tissue staining of a skin lesion from a rat transplanted with 30 × 106 PVG.7B BM cells, which developed lethal aGVHD. Epithelial cell-specific marker isolectin (green color) was added to visualize skin morphology. Negative control staining was done with BSA solution and no primary antibody (A). Skin showed typical signs of grade III-IV GVHD with extensive disruption of the dermal-epidermal interface. Substantial infiltration of donor-derived leukocytes (RT7.B; red color) was noted in the dermis (B). The majority of infiltrating cells expressed CD4 and in fewer numbers CD8, whereas NKR-P1+ cells were virtually absent (C, E, and F). CD25 expression was detected on a minor fraction of leukocytes (D).
Several lymphoid organs displayed GVHD-related changes. Spleens of transplanted animals with aGVHD were significantly smaller than those of BMT controls at autopsy. Spleen weight/body weight ratios were on average 0.16% (range, 0.14–0.18%; n=5) and 0.11% (0.07–0.16%; n=7; pooled from two experiments) for BMT and BMT + DLI groups (P=0.015), respectively, compared with nonmanipulated littermate controls (mean, 0.21%; range, 0.20–0.22%; n=3). Histologically, disruption of the normal architecture with depletion of white pulp and a relative increase of mononuclear cells in the red pulp was observed (Fig. 1C and D). LNs were also affected; in particular, mesenteric LNs were small and discolored. An interesting observation was the accumulation of lymphocytes in the cortex and germinal centers of cervical and mesenteric LNs interfering with the normal histological architecture of these tissues (Fig. 1G and J, and data not shown). Several animals presented severe degeneration of the thymus with no clear distinction between cortex and medulla (Fig. 1H).
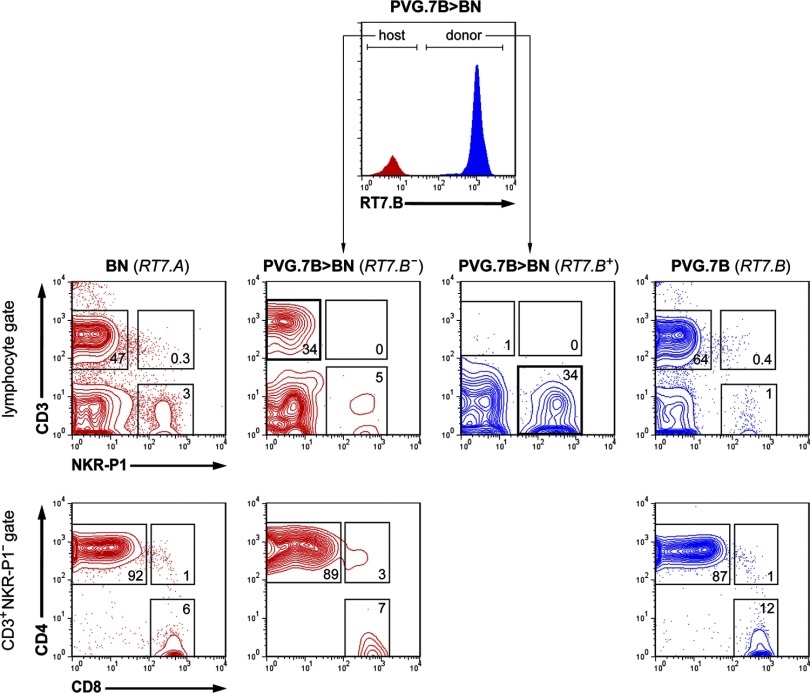
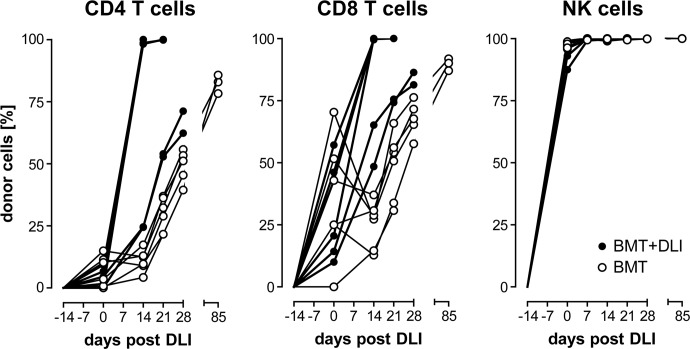
Donor NK cells reconstitute rapidly after alloHCT, whereas donor T cells expand after DLI
To evaluate the reconstitution of important donor lymphocyte populations during ongoing GVHD, we analyzed PB samples weekly starting on the day of DLI. NK cell reconstitution ensued rapidly and completely, as nearly all CD3−NKR-P1+ lymphocytes were derived from the donor (RT7.B+) 14 days after irradiation and BMT (0 day post-DLI; Fig. 3). Donor NK cells were predominant in the PB (25.4–77.9% of total lymphocytes) before DLI was given, and healthy and GVHD-affected rats became stable, complete donor chimeras for NK cells shortly after BMT (Fig. 4), in agreement with previous data following a similar transplantation protocol [14]. NKT (CD3+NKR-P1+) cells were absent from the PB (Fig. 3), suggesting a low capacity of donor BM to produce these cells after conditioning and alloHCT.
Figure 3. PB composition after BMT.
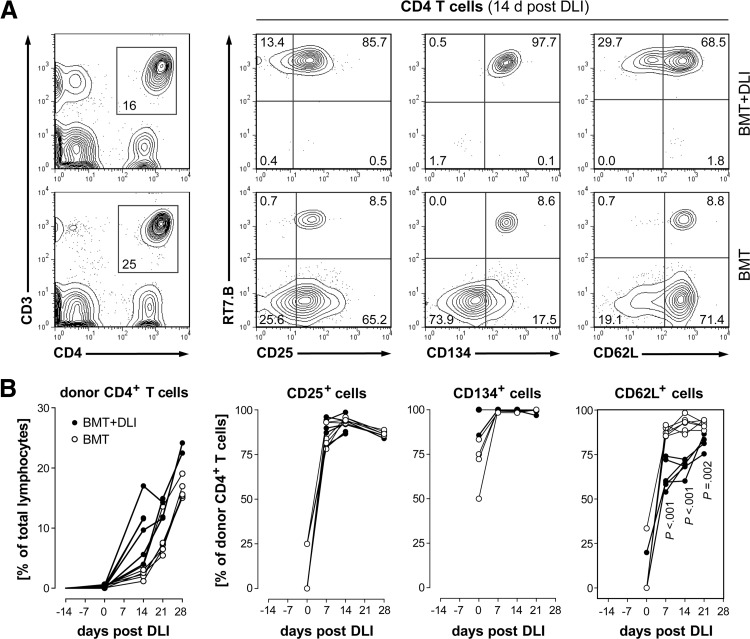
Representative flow cytometric analyses (five-percentile contour plots) of PB lymphocytes (gated on forward- and side-scatter) from normal BN (far left) and PVG.7B rats (far right) are juxtaposed with the composition of host (RT7.B–) and donor (RT7.B+)-derived cells from BN rats 14 days after allogeneic T cell-depleted BMT and prior to DLI (PVG.7B>BN; cf Fig. 1). T cell (CD3+NKR-P1–), NK cell (CD3–NKR-P1+), and NKT cell (CD3+NKR-P1+) populations (middle row) were gated. Only few T cells derived from PVG.7B donor BM cells were present in the PB of transplanted rats, whereas host T cells and donor NK cells (bold squares) were predominant. T cells were further divided into CD4 (CD4+CD8–), CD8 (CD4–CD8+), as well as “double-positive” (CD4+CD8+) subpopulations (bottom row). Host-derived T cells were mostly CD4+CD8–. Numbers indicate the relative frequencies (% of parent) of the respective gates.
Figure 4. Chimerism of lymphocyte populations after DLI.
PB was collected starting on the day of DLI (0 day) from transplanted rats (●; n=6) and control rats, which did not receive DLI (○; n=6). PBMCs were analyzed for CD4 and CD8 T cells as well as NK cells by flow cytometry, as shown in Fig. 3. Donor chimerism—i.e., the relative fraction of donor-derived (RT7.B+) cells—of the respective lymphocyte populations is shown. Donor T cells increased rapidly to full donor chimerism in most rats which received DLI. NK cells were entirely derived from donor BM cells in either group. Symbols represent values from individual animals.
Few circulating CD3+ T cells were found in the circulation prior to DLI, and of those, the majority was derived from the host (RT7.B−; Fig. 3). A minority of peripheral CD4+CD8− (CD4+) T cells (0.0–15.0% of total CD4+ T lymphocytes) was of donor origin (RT7.B+), whereas donor chimerism of CD4−CD8+ (CD8+) T cells was more variable between individual rats (0.0–70.4% of total CD8+ T lymphocytes) prior to DLI (Fig. 4). In most rats, these T cell populations switched to full donor chimerism shortly after receiving DLI. Also, the overall proportion of donor CD4+ and CD8+ T cells increased (4.0–17.1% and 0.8–15.6% of total lymphocytes, respectively) at 14 days post-DLI (Fig. 5A and B, and data not shown). In contrast, the proportion of donor-derived T cells increased at a slower rate in control rats (Fig. 4). The CD4+ and CD8+ T cell populations, respectively, reached ∼80% (78.3–85.7% of total CD4+ T lymphocytes; n=3) and 90% (87.0–91.9% of total CD8+ T lymphocytes; n=3) donor chimerism in the controls when the experiment was terminated.
Figure 5. Developmental kinetics of donor CD4 T cell subpopulations.
(A) Representative flow cytometric analysis of PBMCs taken 14 days post-DLI from rats developing aGVHD (BMT+DLI) and controls (BMT) is shown. CD25, CD134, and CD62L surface markers were expressed differentially on host (RT7.B–) and donor (RT7.B+)-derived CD4+ T cells (CD3+CD4+ lymphocyte gate, left panel). Numbers indicate the relative frequencies of the respective gates and quadrants. (B) The percentage of donor CD4+ T cells in the blood increased rapidly after DLI in rats with GVHD (●; n=6) compared with BMT controls (○; n=6; symbols represent values from individual animals). Donor chimerism of CD4+ T cells expressing CD25 developed similarly in both groups, whereas CD4+CD62L+ donor T cells were less abundant in GVHD. CD134 was expressed on all CD4+ T cells. The high variability at 0 day post-DLI was a result of the low numbers of donor CD4+ T cells present in PB at this time-point (left panel; cf also Fig. 4). Statistical tests indicating significant difference (P<0.05) of group means are shown.
Interestingly, those rats that developed aGVHD early were complete chimeras for donor T cells at 14 days post-DLI, whereas two rats, which acquired GVHD relatively late (GVHD onset after 21 days post-DLI; cf Table 3), displayed consistently lower percentages of donor CD4+ and CD8+ T cells in the PB (Fig. 4).
The frequency of donor CD62+ T cells in PB is altered in aGVHD
To test whether distinct phenotypic subsets could serve as cellular markers of experimental aGVHD, we characterized circulating lymphocytes and measured putative differences during disease onset and progression. Typical expression of CD25 (OX39), CD134 (OX40), and CD62L (OX85) on host- and donor-derived CD4+ T cells 14 days post-DLI is exemplified in Fig. 5A. At this time-point, 100% of CD4+ T cells was donor-derived in most rats which had received DLIs (cf Fig. 4) and made up 10–20% of all PB lymphocytes (Fig. 5B). We observed a rapid expansion of the donor CD4+CD25+ T cell subset with similar kinetics between the treatment and the control group. Virtually all donor CD4+ (Fig. 5B) and CD4− (comprising ∼90% CD8+; data not shown) T cells expressed surface CD134 regardless of DLI. CD62L-positive cells were present initially at similar levels but were decreased significantly in CD4+ and CD4− T cells after DLI compared with controls (Fig. 5B, and data not shown).
Donor Ly49s3+ NK cells are less abundant in PB at late-stage aGVHD
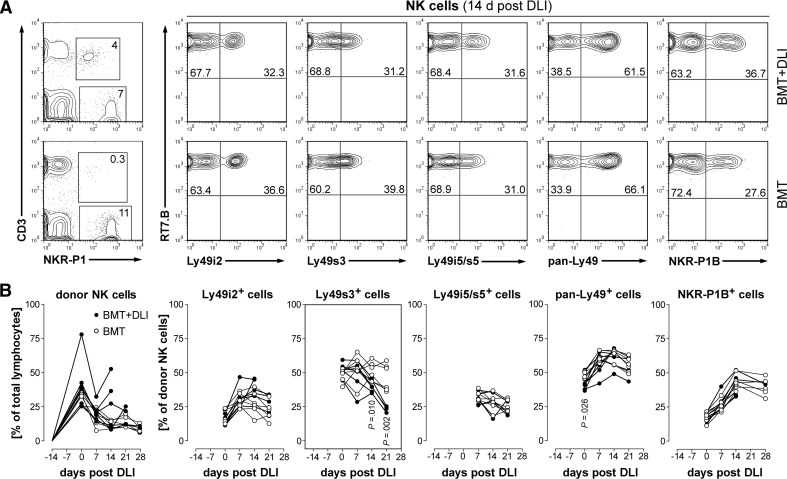
The expression of surface markers, including NKR-P1B and several Ly49Rs, on circulating NK cells is shown in Fig. 6. Although the PB frequencies of donor-derived NK subsets were markedly altered after BMT compared with normal PVG.7B littermates (data not shown), most subsets were not different between BMT controls and rats that developed GVHD (Fig. 6A and B). Notably, Ly49s3+ NK cells were decreased (P≤0.01) at 2 weeks after DLI and beyond.
Figure 6. Developmental kinetics of donor NK cell subpopulations.
(A) Representative flow cytometric analysis of PBMCs taken 14 days post-DLI from rats developing aGVHD (BMT+DLI) and controls (BMT) is shown. NK cells (CD3–NKR-P1+ gate, left panel) were derived entirely from donor PVG.7B (RT7.B+) cells and express several NKRs. Numbers indicate the relative frequencies of the respective gates and quadrants. (B) PB from rats developing GVHD (●; n=6) and BMT controls (○; n=6; symbols represent values from individual animals) was analyzed for donor chimerism of NK cell subsets. The percentage of donor NK cells in the blood was elevated in some rats which received DLI (left panel). The kinetics of donor chimerism of several NK cell subsets was similar in both groups, except the subset expressing Ly49s3, which was reduced in rats with terminal GVHD. Statistical tests indicating significant difference (P<0.05) of group means are shown.
Natural Tregs are depleted in aGVHD
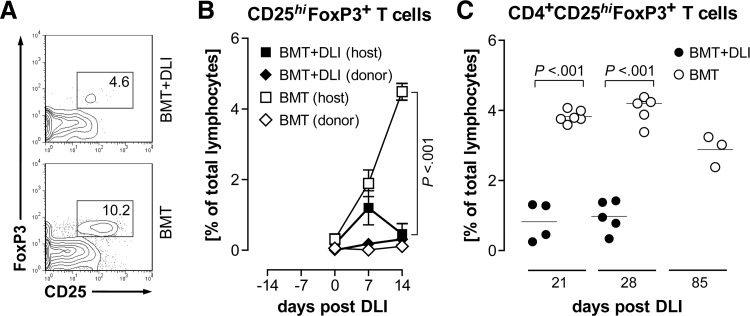
CD4+CD25hiFoxP3+ (“natural”) Tregs are important regulators of aGVHD [9] and have been indicated recently as a practical marker of the disease [30]. We therefore tested whether this cell type was present in normal numbers in the PB of animals suffering from GVHD and found that Tregs were less frequent in rats already at the onset of aGVHD (Fig. 7A and Table 4) compared with BMT controls. Host CD25hiFoxP3+ T cells were reduced significantly (P<0.001) at 14 days after DLI, and the corresponding donor population did not expand in the treatment group or the control group (Fig. 7B). Disregarding graft and host origin, the total frequencies of circulating Tregs were markedly diminished at 21 and 28 days post-DLI (Fig. 7C) at a time when rats were suffering from severe aGVHD. We also determined the numbers of Tregs relative to CD4+ and CD3+ T cells, respectively, to exclude the possibility that this effect was based on overall changes of T cell populations (Table 4). This analysis confirmed that Tregs were reduced significantly within the PB T cell pool.
Figure 7. Tregs are depleted in rats with aGVHD.
(A) Representative flow cytometric analysis of PBMCs taken 28 days post-DLI from a rat with ongoing aGVHD (BMT+DLI) and a control rat (BMT) is shown. CD4+ T lymphocytes (CD3+CD4+-gated) from GVHD rats contained fewer CD25hiFoxP3+ cells. Numbers indicate relative frequencies of the respective gates. (B) CD25hiFoxP3+ T lymphocytes (CD3+-gated) of control rats (empty symbols) were largely derived from the host, whereas these cells were diminished in rats that developed GVHD (filled symbols). Donor (RT7.B+) Tregs (diamond symbols) were lacking in either group. Data are shown as the mean ± sem. (C) The proportion of Tregs was reduced at 21 and 28 days post-DLI, respectively, in rats with GVHD (●) but persisted in BMT controls (○). Symbols represent values from individual animals. Statistical tests for difference of group means are shown.
Table 4. Frequencies of Tregs in PB following BMT.
| PVG.7B > BN |
|||
|---|---|---|---|
| BMT + DLI | BMT | ||
| 14 days post-DLI | [%] | [%] | P value |
| CD25hiFoxP3+/CD3+ | 3.8 (3.0) | 14.8 (2.6) | 0.00028 |
| donor chimerism CD25hiFoxP3+ | 71.5 (40.6) | 2.4 (1.6) | |
| 21 days post-DLI | |||
| Trega/CD3+ | 0.02 (0.01) | 0.11 (0.02) | 0.00006 |
| Treg/CD4+ | 0.04 (0.02) | 0.13 (0.01) | 0.00021 |
| Treg/CD8+ | 0.19 (0.15) | 1.51 (0.44) | 0.00033 |
CD4+CD25hiFoxP3+ T cells. Analysis of Tregs in PB samples from rats, which received DLI (BMT+DLI; n=6 at 14 days and n=4 at 21 days, respectively) and control animals (BMT; n=6) was performed by flow cytometry at the indicated time-points. Tregs were reduced significantly among T cell populations in rats suffering from aGVHD. Results are expressed as mean values and in parentheses the sd. Statistical tests for difference of group means are shown.
DISCUSSION
The search of prognostic biomarkers for GVHD is currently ongoing. A range of GVHD-associated molecules, including a panel of four blood serum biomarkers, has been indicated for disease diagnosis [2, 31]; however, a robust, unique marker for aGVHD has yet to be identified. Animal models have the advantage that unlike human alloHCT patients who receive immunosuppressive treatment, such as steroids and cell-targeted antibodies, experimental GVHD is not influenced by such treatment and thus, better reflects the native disease. We have shown previously that the rat is an adequate experimental model, which resembles the pathology of human skin GVHR [29] and has the potential to identify genes that may predict GVHR and disease [29, 32]. The present study was outlined to characterize processes related to the pathophysiology of aGVHD and to discover disease-associated markers. We rationalized that the use of a relatively high cell dose (5×106 T cells) for DLI to induce rapid and severe aGVHD in a fully MHC-mismatched donor-host combination should facilitate our objective to identify valid GVHD markers. The infusion of donor lymphocytes was delayed to 14 days post-transplant so that the hematopoietic system of the host could be largely replaced by donor-derived cells (this study and unpublished observations) before exposure to donor T cells. This is different to other animal models of alloHCT, where GVHD is commonly induced simultaneously with BMT and might be particularly important regarding the quality of antigen presentation to donor lymphocytes.
In this model, GVHD presented primarily with inflammatory skin, extensive scratching, flaking, occasional lesions, and fur loss. Rapid wasting, together with occasional gas development in the gut, could indicate involvement of the gastrointestinal tract; however, we found no pathology upon histological examination. Other target sites, such as liver and lung epithelium, appeared intact. Consequently, the findings of this study are most relevant for patients with cutaneous GVHD.
It may seem paradoxical that the severe aGVHD associated with rapid and total lethality induced in a MHC-incompatible setting did not result in histological pathology of the primary target organs liver and small intestine. We speculate that accelerated cachexia resulted in death before other organs showed GVHD pathology. Alternatively, in this strain combination, GVHR is specific for the skin but not the gut or the liver. Secondary lymphoid organs, including spleen, as well as cervical and mesenteric LNs, are key sites of allopriming in GVHD pathophysiology and presented defective tissue structures and substantial leukocyte accumulation in moribund recipients.
CD134 is a costimulatory molecule, which we found expressed on all circulating T cells in unperturbed PVG.7B donor rats, as well as after BMT and during GVHD. In our hands, a high proportion of donor CD4+ T cells expressed the activation marker CD25 (IL-2Rα) in transplanted rats, but the reconstitution kinetics of this subset was not different between test and control groups. Taken together, these parameters were not indicative of GVHD in our experimental model and therefore, could not serve as disease-specific markers.
The adhesion molecule CD62L (L-selectin) is a homing factor for naïve T cells and is down-regulated on effector T cells upon activation. It has been shown that the transfer of purified memory CD4+ T cells lacking CD62L does not induce GVHD in lethally irradiated, allogeneic hosts [33, 34] and that ex vivo-expanded Tregs require CD62L to alleviate GVHD [35, 36]. However, the expression and trafficking patterns of different T cell populations in vivo during ongoing GVHD are not well understood [4, 37]. Yamashita et al. [38] reported a significant relative enrichment of CD4+ effector memory (CCR7−CD62low) T cells in the blood of patients with chronic GVHD. Although one must be cautious not to translate results directly from an aGVHD model to the chronic disease form and vice versa, it is possible that effector memory T cells undergo similar regulation in both types of disease. Consistent with this hypothesis, we showed that CD62L expression on circulating donor T cells was reduced after DLI and throughout aGVHD. This finding is likely a result of down-regulation of surface CD62L on T cells when activated in secondary lymphoid organs and their emigration from the blood into peripheral tissues and might in part explain the observed accumulation of leukocytes in the skin, spleen, and LN in disease-affected rats.
Stern et al. [39] recently studied the development of selected NK cell subsets after haploidentical HCT and showed that patients who expressed MHC ligands for inhibitory NKR were at greater risk. In our hands, the reconstitution of donor NK cell subpopulations investigated were not indicative of GVHD, except that the subset expressing surface Ly49s3 (an activating receptor binding to nonclassical MHC class I molecules in BN rats) [22] was reduced significantly in the PB of terminally diseased rats. This could be a result of down-regulation of the receptor in the presence of BN host ligand. Alternatively, cells of this subset, which typically coexpress a variety of Ly49Rs and are more responsive to cytokines [26, 40], might be preferentially recruited to sites of ongoing inflammation and could thus serve as a significant marker for late aGVHD. However, analysis of NKR-P1B and other Ly49 markers did not reflect such changes, and we did not detect NK cells in peripheral sites, e.g., in inflamed skin (Fig. 2). Also, the Ly49s3R gene was not differentially regulated in skin explant or GVHD skin samples derived from the same rat model in a recent microarray study [32].
It should also be noted that the absence of quantitative changes in circulating NK subpopulations does not exclude a role in GVHD, and these cells could have important indirect effects, e.g., through the destruction of host DCs [41].
The observation that Tregs were markedly diminished in rats with GVHD could not be ascribed to a general contraction of the CD3+ or CD4+ T cell pools but represented a specific reduction in line with another report [13]. Shlomchik and colleagues [42] have shown that radiation-resistant, host-derived CD4+CD25+ T cells, which include the Treg type, ameliorated the incidence and severity of de novo chronic GVHD, thus suggesting a key role for this cell population in the control of GVHR. If the in vivo reservoir of host Tregs is depleted during ongoing GVHD [43], the lack of these regulatory cells could be an important factor in the collapse of the host immune defense against alloreactive donor cells. It is not clear from our results whether the relative paucity of Tregs in PB was causal to the onset and/or progression of disease. Nevertheless, our data support the importance of Tregs in regulating alloimmune reactions and their putative use as a biomarker of GVHD [30].
In conclusion, our analysis of leukocyte development in experimental aGVHD identified the following features as putative disease-associated biomarkers: (i) a rapid switch to complete donor T cell chimerism following DLI; (ii) the relative reduction of circulating T cells expressing the homing factor CD62L during the early stage of aGVHD; and (iii) the absence of Tregs from PB. These findings may prove useful to predict clinical GVHD and could serve as possible therapeutic targets in the future.
ACKNOWLEDGMENTS
This study was supported by grant MRTN-CT-2004-512253 (“TRANS-NET” research training network, early-stage researcher grant to S.Z.) of the European Commission's Sixth Framework Program, as well as the Research Council of Norway and Norwegian Cancer Society (grants to B.R.) and Anders Jahre Foundation, Ella and Kristian Nyerrød Foundation, and Henrik Homans Minde Foundation (grants to S.Z.). The authors are grateful to Aaste Aursjø, Johanna Balogh, Linda Manley, Stine Martinsen, Thu Nguyen, Bente Omdal, and Vigdis Wendel for excellent technical assistance; Guttorm Haraldsen for expertise in multicolor immunohistochemical technique and providing reagents; John Torgils Vaage for expert opinion and advice; and Haakon Breien Benestad for critical review of the manuscript. We thank A. Neil Barclay, John C. Hiserodt, and Jaap Kampinga for the kind gifts of hybridoma cell lines.
Footnotes
- aGVHD
- acute graft-versus-host disease
- alloHCT
- allogeneic hematopoietic cell transplantation
- BM
- bone marrow
- BMT
- bone marrow transplantation
- BN
- BN/RijHsd rats
- CD62L
- CD62 ligand
- DLI
- donor lymphocyte infusion
- FoxP3
- forkhead box P3
- GVHD
- graft-versus-host disease
- GVHR
- graft-versus-host reaction
- NKR
- NK receptor
- PB
- peripheral blood
- Treg
- regulatory T cell
AUTHORSHIP
S.Z and B.R. conceived of the study and designed the research. S.Z. performed the experiments, analyzed and interpreted the data, and wrote the manuscript. S.Z., L.S., and B.R. did the histological analysis. L.S., R.D., and B.R. interpreted the data and edited the manuscript. All authors approved the final version of the manuscript.
DISCLOSURES
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare that they have no competing interests.
REFERENCES
- 1. Ferrara J. L., Levine J. E., Reddy P., Holler E. (2009) Graft-versus-host disease. Lancet 373, 1550–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paczesny S., Levine J. E., Braun T. M., Ferrara J. L. (2009) Plasma biomarkers in graft-versus-host disease: a new era? Biol. Blood Marrow Transplant. 15, 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beilhack A., Schulz S., Baker J., Beilhack G. F., Wieland C., Herman E. I., Baker E. M., Cao Y. A., Contag C. H., Negrin R. S. (2005) In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood 106, 1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wysocki C. A., Panoskaltsis-Mortari A., Blazar B. R., Serody J. S. (2005) Leukocyte migration and graft-versus-host disease. Blood 105, 4191–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yanik G., Cooke K. R. (2006) The lung as a target organ of graft-versus-host disease. Semin. Hematol. 43, 42–52 [DOI] [PubMed] [Google Scholar]
- 6. Shlomchik W. D., Couzens M. S., Tang C. B., McNiff J., Robert M. E., Liu J., Shlomchik M. J., Emerson S. G. (1999) Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 285, 412–415 [DOI] [PubMed] [Google Scholar]
- 7. Yokoyama W. M., Riley J. K. (2008) NK cells and their receptors. Reprod. Biomed. Online 16, 173–191 [DOI] [PubMed] [Google Scholar]
- 8. Ruggeri L., Aversa F., Martelli M. F., Velardi A. (2006) Allogeneic hematopoietic transplantation and natural killer cell recognition of missing self. Immunol. Rev. 214, 202–218 [DOI] [PubMed] [Google Scholar]
- 9. Taylor P. A., Lees C. J., Blazar B. R. (2002) The infusion of ex vivo activated and expanded CD4+CD25+ immune regulatory cells inhibits graft-versus-host disease lethality. Blood 99, 3493–3499 [DOI] [PubMed] [Google Scholar]
- 10. Hoffmann P., Ermann J., Edinger M., Fathman C. G., Strober S. (2002) Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 196, 389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miura Y., Thoburn C. J., Bright E. C., Phelps M. L., Shin T., Matsui E. C., Matsui W. H., Arai S., Fuchs E. J., Vogelsang G. B., Jones R. J., Hess A. D. (2004) Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood 104, 2187–2193 [DOI] [PubMed] [Google Scholar]
- 12. Zorn E., Kim H. T., Lee S. J., Floyd B. H., Litsa D., Arumugarajah S., Bellucci R., Alyea E. P., Antin J. H., Soiffer R. J., Ritz J. (2005) Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood 106, 2903–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rieger K., Loddenkemper C., Maul J., Fietz T., Wolff D., Terpe H., Steiner B., Berg E., Miehlke S., Bornhäuser M., Schneider T., Zeitz M., Stein H., Thiel E., Duchmann R., Uharek L. (2006) Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood 107, 1717–1723 [DOI] [PubMed] [Google Scholar]
- 14. Nestvold J. M., Omdal B. K., Dai K. Z., Martens A., Benestad H. B., Vaage J. T., Rolstad B. (2008) A second prophylactic MHC-mismatched bone marrow transplantation protects against rat acute myeloid leukemia (BNML) without lethal graft-versus-host disease. Transplantation 85, 102–111 [DOI] [PubMed] [Google Scholar]
- 15. Nicklas W., Baneux P., Boot R., Decelle T., Deeny A. A., Fumanelli M., Illgen-Wilcke B. (2002) Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab. Anim. 36, 20–42 [DOI] [PubMed] [Google Scholar]
- 16. Zinöcker S., Wang M. Y., Gaustad P., Kvalheim G., Rolstad B., Vaage J. T. (2011) Mycoplasma contamination revisited: mesenchymal stromal cells harboring Mycoplasma hyorhinis potently inhibit lymphocyte proliferation in vitro. PLoS ONE 6, e16005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooke K. R., Kobzik L., Martin T. R., Brewer J., Delmonte J. J., Crawford J. M., Ferrara J. L. (1996) An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood 88, 3230–3239 [PubMed] [Google Scholar]
- 18. Kampinga J., Kroese F., Pol G., Opstelten D., Seijen H., Boot J., Roser B., Nieuwenhuis P., Aspinall R. (1990) RT7-defined alloantigens in rats are part of the leucocyte common antigen family. Scand. J. Immunol. 31, 699–710 [DOI] [PubMed] [Google Scholar]
- 19. Nicolls M. R., Aversa G. G., Pearce N. W., Spinelli A., Berger M. F., Gurley K. E., Hall B. M. (1993) Induction of long-term specific tolerance to allografts in rats by therapy with an anti-CD3-like monoclonal antibody. Transplantation 55, 459–468 [DOI] [PubMed] [Google Scholar]
- 20. Chambers W. H., Vujanovic N. L., DeLeo A. B., Olszowy M. W., Herberman R. B., Hiserodt J. C. (1989) Monoclonal antibody to a triggering structure expressed on rat natural killer cells and adherent lymphokine-activated killer cells. J. Exp. Med. 169, 1373–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naper C., Ryan J. C., Nakamura M. C., Lambracht D., Rolstad B., Vaage J. T. (1998) Identification of an inhibitory MHC receptor on alloreactive rat natural killer cells. J. Immunol. 160, 219–224 [PubMed] [Google Scholar]
- 22. Naper C., Hayashi S., Kveberg L., Niemi E. C., Lanier L. L., Vaage J. T., Ryan J. C. (2002) Ly-49s3 is a promiscuous activating rat NK cell receptor for nonclassical MHC class I-encoded target ligands. J. Immunol. 169, 22–30 [DOI] [PubMed] [Google Scholar]
- 23. Naper C., Dai K. Z., Kveberg L., Rolstad B., Niemi E. C., Vaage J. T., Ryan J. C. (2005) Two structurally related rat Ly49 receptors with opposing functions (Ly49 stimulatory receptor 5 and Ly49 inhibitory receptor 5) recognize nonclassical MHC class Ib-encoded target ligands. J. Immunol. 174, 2702–2711 [DOI] [PubMed] [Google Scholar]
- 24. Kveberg L., Dai K. Z., Dissen E., Ryan J. C., Rolstad B., Vaage J. T., Naper C. (2006) Strain-dependent expression of four structurally related rat Ly49 receptors; correlation with NK gene complex haplotype and NK alloreactivity. Immunogenetics 58, 905–916 [DOI] [PubMed] [Google Scholar]
- 25. Naper C., Hayashi S., Løvik G., Kveberg L., Niemi E. C., Rolstad R., Dissen E., Ryan J. C., Vaage J. T. (2002) Characterization of a novel killer cell lectin-like receptor (KLRH1) expressed by alloreactive rat NK cells. J. Immunol. 168, 5147–5154 [DOI] [PubMed] [Google Scholar]
- 26. Kveberg L., Jiménez-Royo P., Naper C., Rolstad B., Butcher G. W., Vaage J. T., Inngjerdingen M. (2010) Two complementary rat NK cell subsets, Ly49s3+ and NKR-P1B+, differ in phenotypic characteristics and responsiveness to cytokines. J. Leukoc. Biol. 88, 87–93 [DOI] [PubMed] [Google Scholar]
- 27. Martelli M. F., Aversa F., Bachar-Lustig E., Velardi A., Reich-Zelicher S., Tabilio A., Gur H., Reisner Y. (2002) Transplants across human leukocyte antigen barriers. Semin. Hematol. 39, 48–56 [DOI] [PubMed] [Google Scholar]
- 28. Kolb H. J. (2008) Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood 112, 4371–4383 [DOI] [PubMed] [Google Scholar]
- 29. Novota P., Sviland L., Zinöcker S., Stocki P., Balavarca Y., Bickeböller H., Rolstad B., Wang X. N., Dickinson A. M., Dressel R. (2008) Correlation of Hsp70–1 and Hsp70–2 gene expression with the degree of graft-versus-host reaction in a rat skin explant model. Transplantation 85, 1809–1816 [DOI] [PubMed] [Google Scholar]
- 30. Magenau J. M., Qin X., Tawara I., Rogers C. E., Kitko C., Schlough M., Bickley D., Braun T. M., Jang P. S., Lowler K. P., Jones D. M., Choi S. W., Reddy P., Mineishi S., Levine J. E., Ferrara J. L., Paczesny S. (2010) Frequency of CD4(+)CD25(hi)FOXP3(+) regulatory T cells has diagnostic and prognostic value as a biomarker for acute graft-versus-host-disease. Biol. Blood Marrow Transplant. 16, 907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paczesny S., Krijanovski O. I., Braun T. M., Choi S. W., Clouthier S. G., Kuick R., Misek D. E., Cooke K. R., Kitko C. L., Weyand A., Bickley D., Jones D., Whitfield J., Reddy P., Levine J. E., Hanash S. M., Ferrara J. L. (2009) A biomarker panel for acute graft-versus-host disease. Blood 113, 273–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Novota P., Zinöcker S., Norden J., Wang X. N., Sviland L., Rolstad B., Dickinson A. M., Walter L., Dressel R. (2011) Expression profiling of major histocompatibility and natural killer complex genes reveals candidates for controlling risk of graft versus host disease. PLoS ONE 6, e16582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anderson B. E., McNiff J., Yan J., Doyle H., Mamula M., Shlomchik M. J., Shlomchik W. D. (2003) Memory CD4+ T cells do not induce graft-versus-host disease. J. Clin. Invest. 112, 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen B. J., Cui X., Sempowski G. D., Liu C., Chao N. J. (2004) Transfer of allogeneic CD62L– memory T cells without graft-versus-host disease. Blood 103, 1534–1541 [DOI] [PubMed] [Google Scholar]
- 35. Taylor P. A., Panoskaltsis-Mortari A., Swedin J. M., Lucas P. J., Gress R. E., Levine B. L., June C. H., Serody J. S., Blazar B. R. (2004) L-Selectinhi but not the L-selectinlo CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood 104, 3804–3812 [DOI] [PubMed] [Google Scholar]
- 36. Ermann J., Hoffmann P., Edinger M., Dutt S., Blankenberg F. G., Higgins J. P., Negrin R. S., Fathman C. G., Strober S. (2005) Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood 105, 2220–2226 [DOI] [PubMed] [Google Scholar]
- 37. Anderson B. E., Taylor P. A., McNiff J. M., Jain D., Demetris A. J., Panoskaltsis-Mortari A., Ager A., Blazar B. R., Shlomchik W. D., Shlomchik M. J. (2008) Effects of donor T-cell trafficking and priming site on graft-versus-host disease induction by naive and memory phenotype CD4 T cells. Blood 111, 5242–5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamashita K., Choi U., Woltz P. C., Foster S. F., Sneller M. C., Hakim F. T., Fowler D. H., Bishop M. R., Pavletic S. Z., Tamari M., Castro K., Barrett A. J., Childs R. W., Illei G. G., Leitman S. F., Malech H. L., Horwitz M. E. (2004) Severe chronic graft-versus-host disease is characterized by a preponderance of CD4+ effector memory cells relative to central memory cells. Blood 103, 3986–3988 [DOI] [PubMed] [Google Scholar]
- 39. Stern M., de Angelis C., Urbani E., Mancusi A., Aversa F., Velardi A., Ruggeri L. (2010) Natural killer-cell KIR repertoire reconstitution after haploidentical SCT. Bone Marrow Transplant. 45, 1607–1610 [DOI] [PubMed] [Google Scholar]
- 40. Kveberg L., Back C. J., Dai K. Z., Inngjerdingen M., Rolstad B., Ryan J. C., Vaage J. T., Naper C. (2006) The novel inhibitory NKR-P1C receptor and Ly49s3 identify two complementary, functionally distinct NK cell subsets in rats. J. Immunol. 176, 4133–4140 [DOI] [PubMed] [Google Scholar]
- 41. Ruggeri L., Capanni M., Urbani E., Perruccio K., Shlomchik W. D., Tosti A., Posati S., Rogaia D., Frassoni F., Aversa F., Martelli M. F., Velardi A. (2002) Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100 [DOI] [PubMed] [Google Scholar]
- 42. Anderson B. E., McNiff J. M., Matte C., Athanasiadis I., Shlomchik W. D., Shlomchik M. J. (2004) Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood 104, 1565–1573 [DOI] [PubMed] [Google Scholar]
- 43. Williams K. M., Hakim F. T., Gress R. E. (2007) T cell immune reconstitution following lymphodepletion. Semin. Immunol. 19, 318–330 [DOI] [PMC free article] [PubMed] [Google Scholar]