IL-15 promotes Th17 and Th1 responses by its activity on modulating monocytes into IL15-DC in the presence of GM-CSF.
Keywords: human immunology, TLRs, IL-1β, IL-23, inflammation
Abstract
Distinct types of DCs are generated from monocytes using GM-CSF with IL-4 (IL4-DC) or IL-15 (IL15-DC). IL15-DCs are potent inducers of antigen-specific CD8+ T cells, display a phenotype similar to CD14+ cells commonly described in chronically inflamed tissues, and produce high levels of IL-1β and IL-15 in response to TLR4 stimulation. As these cytokines promote Th17 responses, which are also associated with inflammatory diseases, I hypothesized that TLR-primed IL15-DCs favor Th17 activation over IL4-DCs. Compared with IL4-DCs, IL15-DCs stimulated with TLR agonists secreted significantly higher concentrations of the Th17-promoting factors, IL-1β, IL-6, IL-23, and CCL20, and lower levels of the Th1 cytokine, IL-12. In addition, IL15-DCs and not IL4-DCs up-regulated IL-15 on the cell surface in response to TLR agonists. IL15-DCs primed with TLR3 or TLR4 agonists triggered Th17 (IL-17, IL-22, and/or IFN-γ) and Th1 (IFN-γ) responses, whereas IL4-DCs primed with the same TLR agonists activated Th1 (IFN-γ) responses. Secretion of IL-17 and IFN-γ required contact with TLR-primed IL15-DC, and IFN-γ production was mediated by membrane-bound IL-15. These findings identify key differences in monocyte-derived DCs, which impact adaptive immunity, and provide primary evidence that IL-15 promotes Th17 and Th1 responses by skewing monocytes into IL15-DC.
Introduction
Monocytes rapidly infiltrate inflamed tissues and differentiate into functionally distinct subsets of DCs and macrophages in response to cytokines in the microenvironment [1]. Similarly, different types of DCs can be generated in vitro by culturing monocytes with GM-CSF in combination with different cytokines, such as IL-4 (IL4-DC), IL-15 (IL15-DC), IFN-α (IFN-DC), or TNF (TNF-DC) [2–4]. These monocyte-derived DC populations have overlapping and specialized features that can be identified by a unique combination of surface structures and cytokine arrays.
Unlike conventionally derived IL4-DCs, IL15-DCs retain expression of membrane-bound CD14 while up-regulating HLA class II and costimulatory molecules [2–4]. This intermediate phenotype closely resembles the proinflammatory subset of monocyte-derived cells, which has been described in various autoimmune diseases associated with dysregulated IL-15 production [5–7]. In response to TLR4 stimulation, IL15-DCs produced higher levels of IL-1β and IL-15 mRNA than IL4-DCs [3]. This is relevant, as these two cytokines promote activation of Th17 cells, which provide protection from fungi and extracellular bacteria and play a role in the pathogenesis of several autoimmune diseases [8–10].
Increasing evidence indicates that IL15-DCs are more efficient at stimulating antigen-specific CD8+ T cell responses than IL4-DCs [2, 3]. However, little is known regarding the mechanisms responsible for this difference or what effect these DC types have on CD4+ T cell responses. Based on the phenotype of IL15-DCs and the cytokines produced in response to TLR4 stimulation, I tested the hypothesis that TLR-primed IL15-DCs favor Th17 activation over IL4-DCs.
MATERIALS AND METHODS
Cell isolation
PBMCs were isolated by density gradient centrifugation in lymphocyte separation medium (ICN Biomedicals, Costa Mesa, CA, USA) from buffy coats of normal, healthy individuals, according to the manufacturer's instructions. Highly pure (>90%) lymphocytes and monocytes were obtained from healthy donors as above, followed by countercurrent centrifugal elutriation. Cells were viably frozen and stored in liquid nitrogen until used. Upon thawing, cell viability was determined by trypan blue exclusion. Untouched CD4+ T cells (>95% pure) were purified from lymphocytes by magnetic cell separation using the CD4+ T cell isolation kit II, LS columns, and the QuadroMACS separator (Miltenyi Biotec, Germany).
Cell cultures
To generate IL4-DC and IL15-DC, 5 × 105 elutriated monocytes/ml were cultured in cRPMI (RPMI-1640, 10% FBS, 1% L-glutamine, 1% Pen-Strep, and 20 mM Hepes; Invitrogen, Carlsbad, CA, USA), supplemented with 50 ng/ml GM-CSF (CellGenix, Germany) plus 25 ng/ml IL-4 or 100 ng/ml IL-15 (R&D Systems, Minneapolis, MN, USA) in 24-well plates for 3 days. In some experiments, immature DCs were harvested and the cell yield and viability determined by trypan blue exclusion and analyzed by surface and intracellular flow cytometry. TLR agonists Pam3Csk4 (10 μg/ml), poly (I:C) (25 μg/ml), or Escherichia coli 0111:B4 LPS (1 μg/ml; Invivogen, San Diego, CA, USA) were added to DCs for 20 h and culture fluids collected for cytokine and chemokine analysis. A portion of the DCs was harvested and the cell recovery and viability determined by trypan blue exclusion and analyzed by standard flow cytometric methods. Remaining DCs were washed with PBS and incubated with 5 × 105 autologous CD4+ T cells/well in 24-well plates for 48–72 h. Semipermeable transwell inserts (0.4 μm; Sigma-Aldrich, St. Louis, MO, USA) were used to prevent cellular contact between CD4+ T cells and TLR-activated IL15-DCs. To address the bioactivity of IL-15, anti-human IL-15 mAb or the mIgG1 isotype control (both from R&D Systems) was included in TLR-primed DC:T cell cocultures at a final concentration of 10 μg/ml. Alternatively, CD4+ T cells were cultured with ″conditioned″ media from IL15-DC treated with TLR agonists to determine the role of soluble cytokines in an APC-free system.
Secreted cytokine and chemokine analysis
Culture fluids from TLR-stimulated or control DCs were analyzed for human IL-1β, IL-23, IL-27, and IFN-α1 (ELISA; eBioscience, San Diego, CA, USA); CCL20 and CXCL10 (ELISA; R&D Systems); and IL-6, IL-10, and IL-12p70 (Luminex multiplex system; Invitrogen), following the manufacturers' protocols. Supernatants from DC:T cell cocultures were analyzed for IL-4, IL-10, IL-17, IL-21, and IFN-γ (ELISA; eBioscience) and IL-22 (ELISA; R&D Systems) following the manufacturers' protocols.
Flow cytometry
Monocytes, IL4-DCs, and IL15-DCs were labeled with the following fluorochrome-conjugated antihuman mAb: CD14, CD83, HLA-DR, and HLA-DQ (BD PharMingen, San Diego, CA, USA); TLR2 (BioLegend, San Diego, CA, USA); TLR4 and TLR8 (Imgenex, San Diego, CA, USA); IL-15 (R&D Systems); HLA-E and TLR3 (eBioscience); or appropriate Ig isotype controls (BD PharMingen)/standard flow cytometry methods. To detect endosomal TLRs, surface antigens were labeled, and then cells were fixed and permeabilized with BD Cytofix/Cytoperm solution and labeled with anti-TLR3 and anti-TLR8 mAb or Ig isotype controls in BD Perm/Wash buffer following the manufacturer's instructions. Purified CD4+ T cells were stained with fluorochrome-conjugated antihuman CD3, CD4, and CD45RO (BD PharMingen); CCR6, IL-1RI, and IL-23R (R&D Systems); and CD161 (eBioscience) mAb or appropriate isotype controls/standard flow cytometry methods. To detect intracellular cytokines, 50 ng/ml PMA and 750 ng/ml ionomycin were added to DC:T cell cocultures for the last 6 h of a 48-h incubation. Monensin (10 ug/ml) was also added to these cocultures for the last 4 h. Cells were labeled with antihuman CD3 and CD4 mAb for surface detection, then fixed and permeabilized with BD Cytofix/Cytoperm solution, and labeled with anti-IFN-γ, anti-IL-17 mAb, or Ig isotype controls in BD Perm/Wash buffer following the manufacturer's instructions. Two hundred thousand live CD3+ cells were acquired on a FACScan flow cytometer and analyzed using CellQuest software (BD PharMingen).
Statistical analyses
Results are displayed as individual data and means ± sd. Statistical significance was determined using two-tailed paired Student's t test. P values <0.05 were considered statistically significant.
RESULTS
Influence of cytokines on TLR expression
Others [2, 3] and I [4] have shown previously that short-term-cultured IL15-DCs display a distinct phenotype from IL4-DCs and produce different cytokines in response to innate stimuli. To determine if this functional disparity is related to TLR expression, we examined TLR expression in immature IL4-DCs and IL15-DCs by flow cytometry. As CD14 is a coreceptor for several TLRs and is differentially expressed by IL15-DCs and IL4-DCs [2–4, 11, 12], it was included in the phenotypic analyses.
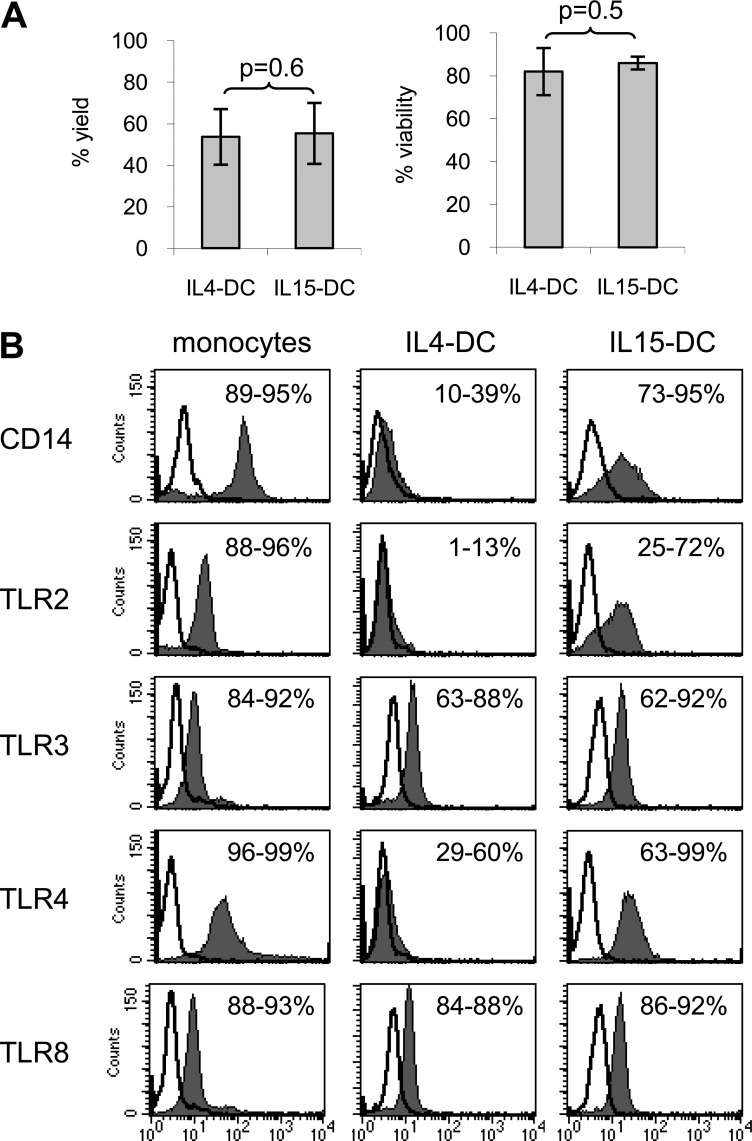
Monocytes were differentiated with GM-CSF plus IL-4 or GM-CSF plus IL-15 for 3 days to generate immature IL4-DCs and IL15-DCs and analyzed by flow cytometry. Despite a similar DC yield and viability between the two monocyte-derived DC types (Fig. 1A), the TLR expression profiles were quite distinct (Fig. 1B). Compared with monocyte precursors, both DC types down-regulated frequencies of CD14, TLR2, and TLR4 on the cell surface, while expressing similar densities of endosomal TLR3 and TLR8. Interestingly, a greater percentage of IL15-DCs expressed TLR2, TLR4, and the CD14 coreceptor compared with IL4-DCs (Fig. 1B).
Figure 1. TLRs are differentially expressed by IL4-DCs and IL15-DCs.
Freshly thawed monocytes were labeled immediately for flow cytometric analysis or cultured with GM-CSF + IL-4 or GM-CSF + IL-15 for 3 days to generate IL4-DCs and IL15-DCs, respectively. (A) DCs were harvested, and the percent yield and viability were calculated by trypan blue exclusion. Data represent the mean ± sd of four independent experiments. (B) Cell populations were labeled with indicated anti-human mAb (filled histograms) or appropriate Ig isotype controls (open histogram overlays) and analyzed by multiparameter flow cytometry. For detection of TLR3 and TLR8, cells were fixed and permeabilized prior to labeling with anti-human mAb (filled histograms) or appropriate Ig isotype controls (open histogram overlays) and analyzed by multiparameter flow cytometry. Numbers indicate the range of percent-positive cells. Histograms from one of four independent experiments are shown.
These results identify important differences in the phenotype of IL4-DCs and IL15-DCs, which likely impact their ability to sense and respond to danger signals in the microenvironment. IL15-DCs appear better equipped to recognize and respond to ligands of TLR2 or TLR4 compared with IL4-DCs, as they express higher densities of these TLRs together with the coreceptor CD14.
TLR agonists trigger distinct cytokine profiles in IL15-DCs and IL4-DCs
Based on the differential expression of CD14, TLR2, and TLR4 by immature IL4-DCs and IL15-DCs, I predicted that these two DC types would respond differently to agonists of TLR2 and TLR4. To test this hypothesis, immature IL4-DCs and IL15-DCs were incubated overnight with the TLR2/1 agonist Pam3Csk4, the TLR4 agonist LPS from E. coli, or the TLR3 agonist poly (I:C). Cellular responses were then determined by production of cytokines and chemokines thought to mediate CD4+ T cell differentiation.
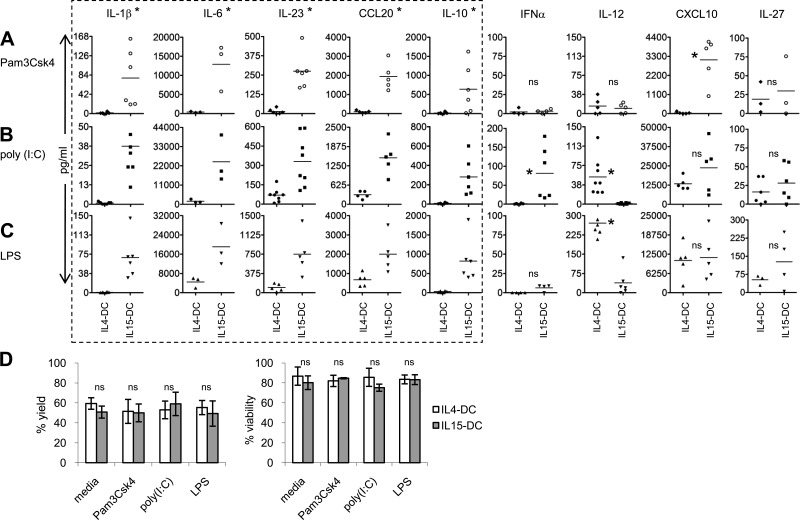
As expected, TLR2 stimulation of IL4-DCs and IL15-DCs resulted in significant differences in cytokine and chemokine production between the two cell types, and IL15-DCs produced higher levels of the Th17-promoting factors IL-1β, IL-6, IL-23, and CCL20, as well as the Th1 chemokine CXCL10 (Fig. 2A). Despite similar expression of TLR3, IL15-DCs secreted significantly more IL-1β, IL-6, IL-23, and CCL20, as well as IFN-α in response to poly (I:C) than did IL4-DCs (Fig. 2B). Conversely, considerably higher concentrations of the Th1-polarizing cytokine IL-12 were detected in culture fluids from TLR3-stimulated IL4-DCs (Fig. 2B). Similar results for all mediators, except for IFN-α, were observed in response to TLR4 stimulation (Fig. 2C). The ability of IL4-DCs, which express lower TLR4 than IL15-DCs and virtually no CD14 (Fig. 1B), to produce IL-12 in response to LPS may be explained by soluble CD14 in the culture media and/or CD14-independent activation of TLR4 [13, 14]. No differences were detected for IL-27, an IL-12 family cytokine reported to promote Th1 and inhibit Th17 responses [15].
Figure 2. TLR agonists induce distinct cytokine profiles from IL4-DCs and IL15-DCs.
IL4-DCs and IL15-DCs were incubated in the presence and absence of (A) 10 μg/ml Pam3Csk4 (TLR2/1), (B) 25 μg/ml poly (I:C) (TLR3), or (C) 1 μg/ml LPS (TLR4) for 20 h. Culture supernatants were collected, and secreted cytokines and chemokines were determined by ELISA kits. Each symbol is the mean of duplicate ELISA values obtained from the different individuals tested after subtracting out levels from media alone. Horizontal bars represent the means in pg/ml from three to eight independent experiments. *P < 0.05, as determined by the paired two-tailed Student's t test. (D) Control and TLR-stimulated DCs were harvested, and the percent yield and viability were determined by trypan blue exclusion. Data represent the mean ± sd of four independent experiments.
In addition to the Th17-promoting mediators, IL15-DCs produced significantly more IL-10 in response to all three TLR agonists compared with IL4-DCs (Fig. 2A–C). This is not surprising, given that IL-4 has been shown to suppress expression of IL-10 in DCs [16]. IL-10 is considered anti-inflammatory, as it inhibits production of IL-2 in T cells and IL-12 in monocyte-derived DCs [17, 18]. In contrast, IL-10 does not affect production of IL-23, IL-15, IL-18, and TNF-α from monocyte-derived DCs [18, 19], which may help explain the disparate IL-12 and IL-23 responses to TLR agonists in IL4-DCs and IL15-DCs (Fig. 2A–C). Furthermore, the striking differences in cytokine profiles are not likely a result of variations in survival and/or expansion rates in the different culture conditions, given that the yield and viability of control and TLR-primed IL4-DCs and IL15-DCs were similar (Fig. 2D).
TLR-stimulated IL4-DCs and IL15-DCs differentially up-regulate CD83 and membrane-bound IL-15
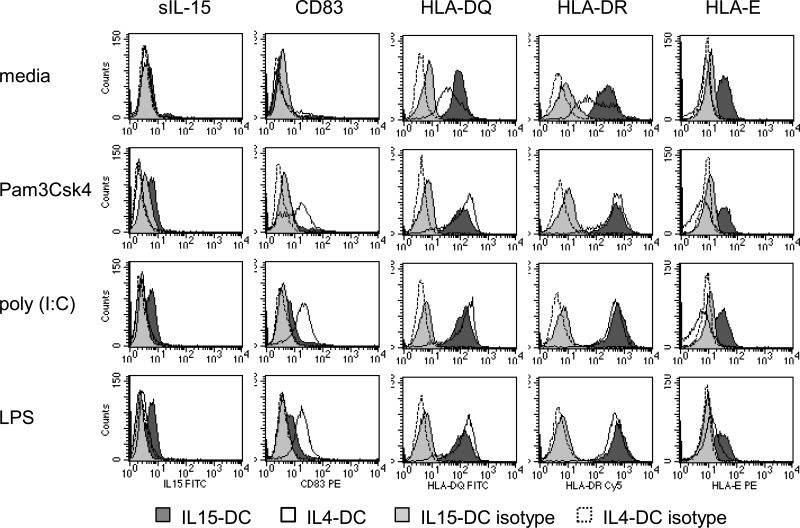
Others have shown [2, 3] that similar to IL4-DCs, IL15-DCs increase expression of CD40, CD80, CD86, and HLA-DR in response to TLR stimulation. Notably, up-regulation of the conventional DC maturation marker, CD83, was impaired significantly in IL15-DCs compared with IL4-DCs activated with TLR agonsists [2, 3]. We recently reported a similar observation using the dietary antigen in celiac disease as the stimulus and in addition, discovered that IL-15 was expressed at the surface of activated IL15-DCs as opposed to CD83 [4]. IL-15 can be expressed on the surface of monocytes and DCs as a membrane-anchored protein and as a form transpresented by its high-affinity receptor, IL-15Rα [20, 21]. The membrane-anchored form activates cells expressing the IL-15R complex, comprised of IL-15Rα, CD122, and CD132, and may also participate in reverse signaling [20, 22]. Transpresentation of IL-15 by IL-15Rα can stimulate leukocytes bearing the low-affinity receptors, CD122 and CD132 [22–24]. Based on these observations, we hypothesized that TLR stimulation would lead to up-regulation of CD83 and IL-15 on the surface of IL4-DCs and IL15-DCs, respectively. Expression of CD83 and IL-15, as well as HLA-DQ, HLA-DR, and HLA-E was evaluated on the surface of IL4-DCs and IL15-DCs stimulated with Pam3Csk4, poly (I:C), or LPS overnight.
As predicted, IL4-DCs up-regulated the maturation marker CD83 and intensities of HLA-DQ and HLA-DR molecules, whereas IL15-DCs expressed membrane-bound IL-15 (sIL-15) and higher intensities of HLA-DQ and HLA-DR molecules in response to TLR stimulation (Fig. 3 and Table 1). The impaired expression of CD83 on IL15-DCs could be related to IL-10 in the culture fluids (Fig. 2A–C), given that this cytokine inhibits expression of costimulatory and maturation markers without affecting IL-15 in monocyte-derived DCs [19, 25]. Alternatively, CD83 may not have been detected on the surface of TLR-primed IL15-DCs, as it was instead produced as the soluble form or because our culture conditions were not optimized to detect CD83 on activated IL15-DCs [26]. HLA-E was constitutively expressed by IL15-DCs and unchanged by TLR stimulation, whereas IL4-DCs lacked expression of HLA-E before and after exposure to TLR agonists, although a slight increase was observed in IL4-DCs from one of the individuals treated with LPS (depicted in Fig. 3).
Figure 3. TLR-stimulated IL4-DCs and IL15-DCs differentially up-regulate CD83 and membrane-bound IL-15.
IL4-DCs and IL15-DCs were incubated in the absence and presence of 10 μg/ml Pam3Csk4 (TLR2/1), 25 μg/ml poly (I:C) (TLR3), or 1 μg/ml LPS (TLR4) for 20 h. Cells were harvested, and surface expression of IL-15 (sIL-15), CD83, HLA-DQ, HLA-DR, and HLA-E was determined by flow cytometry. Filled histograms depict labeling of IL15-DCs for indicated antigens (dark gray) or appropriate isotype controls (light gray). Open histograms depict labeling of IL4-DCs for indicated antigens (solid line) or appropriate isotype controls (dashed line). Data for sIL-15 and CD83 are representative of six different donors tested, and data for HLA molecules are representative of three different donors tested.
Table 1. Phenotype of IL4-DC and IL15-DC Stimulated with TLR Agonists.
| media |
Pam3CSK4 |
poly (I:C) |
LPS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| IL4-DC | IL15-DC | IL4-DC | IL15-DC | IL4-DC | IL15-DC | IL4-DC | IL15-DC | ||
| IL-15 | % | 0–2 | 0–6 | 0–1 | 26–57 | 0–2 | 63–75 | 0–3 | 68–76 |
| MFI | 2–4 | 5–7 | 3–5 | 8–14 | 4–5 | 8–14 | 4–5 | 8–18 | |
| CD83 | % | 2–12 | 0–4 | 47–65 | 0–23 | 72–94 | 0–31 | 88–93 | 28–46 |
| MFI | 18–26 | 16–54 | 71–116 | 24–42 | 73–108 | 15–51 | 81–138 | 12–53 | |
| HLA-DQ | % | 92–94 | 96–98 | 89–95 | 96–97 | 96–97 | 90–97 | 95–97 | 94–96 |
| MFI | 27–56 | 52–96 | 64–158 | 74–124 | 104–169 | 75–138 | 99–163 | 72–137 | |
| HLA-DR | % | 93–95 | 97–98 | 96–99 | 96–99 | 98–99 | 92–98 | 97–99 | 96–98 |
| MFI | 162–246 | 284–468 | 368–528 | 559–792 | 489–517 | 631–976 | 513–550 | 651–1087 | |
| HLA-E | % | 0–1 | 80–81 | 0 | 43–70 | 0–1 | 48–70 | 1–28 | 44–64 |
| MFI | 6–7 | 27–40 | 4–6 | 22–37 | 6–7 | 27–37 | 6–23 | 24–35 | |
MFI, Mean fluorescence intensity.
TLR-primed IL15-DCs favor Th17 activation over IL4-DCs
It is well recognized that membrane-bound and soluble mediators produced by activated DCs impact CD4+ T cell differentiation. Based on the distinct response profiles of IL4-DCs and IL15-DCs depicted in Figs. 2 and 3, it was predicted that IL15-DCs would be better equipped to activate Th17 and memory Th1 responses than IL4-DCs. This is supported by a growing body of evidence demonstrating that IL-1β, IL-6, IL-15, IL-23, and CCL20 are key mediators of Th17 (and memory Th1) responses in humans [7–10, 27–29]. On the other hand, IL4-DCs may be better suited for priming naïve CD4+ T cells than IL15-DCs, as CD83 and IL-12 are critical for polarizing naïve CD4+ T cells into Th1 effectors [30, 31]. Therefore, I tested the hypothesis that TLR-primed IL15-DCs favor Th17 activation over IL4-DCs.
CD4+ T cells were purified from PBMCs of healthy individuals by negative selection and cultured with control or TLR-primed, autologous IL15-DCs or IL4-DCs for 2–3 days. CD4+ T cell purity and the proportion of Th17 cells, which express CCR6, IL-1RI, IL-23R, CD45RO, and CD161, were monitored by flow cytometry [32, 33]. Cell-free supernatants were collected and secreted IL-4, IL-10, IL-17, IL-21, IL-22, and IFN-γ proteins were quantified to determine the types of Th responses supported by TLR-primed IL4-DCs and IL15-DCs.
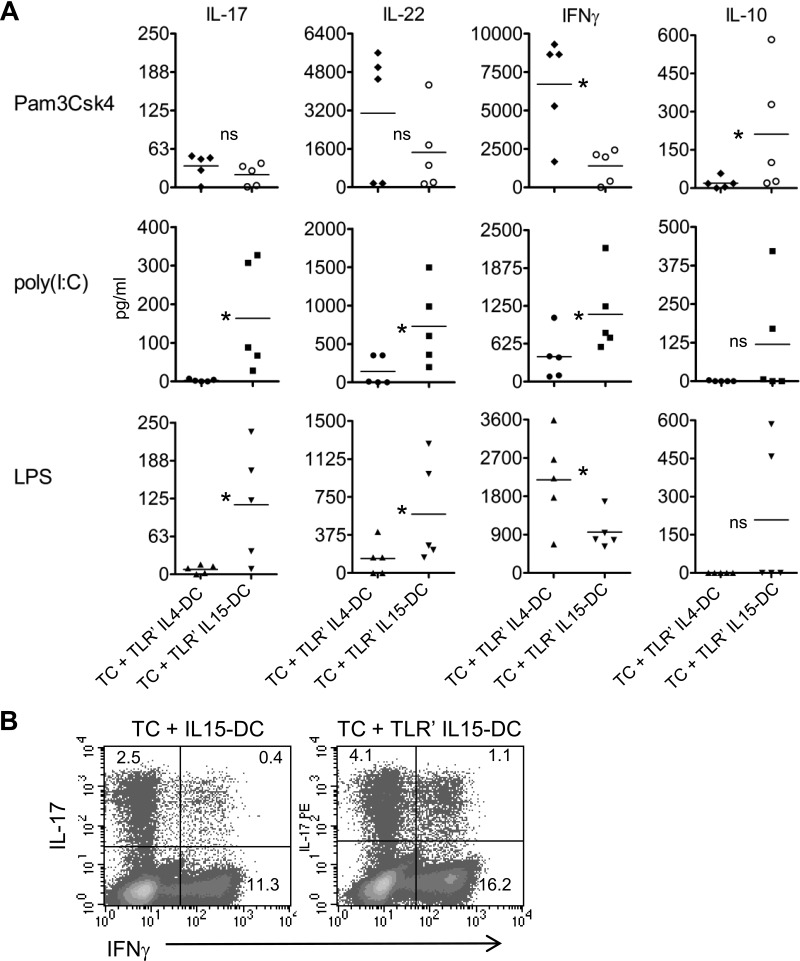
In cocultures with CD4+ T cells, IL15-DCs primed with TLR3 or TLR4 agonists stimulated secretion of IL-17, IL-22, and IFN-γ, a cytokine profile indicative of human Th17 responses [27] (Fig. 4A). This was in sharp contrast to cocultures with TLR3- or TLR4-primed IL4-DCs, which produced the Th1 cytokine IFN-γ in the absence of IL-17 and little IL-22 (Fig. 4A). Intracellular cytokine staining and flow cytometry demonstrated that IL-17+IFN-γ–, IL-17+IFN-γ+, and IL-17–IFN-γ+ CD4+ T cell subsets were increased by TLR-primed IL15-DCs (Fig. 4B).
Figure 4. IL15-DCs primed with TLR3 or TLR4 agonists preferentially induce Th17 responses compared with IL4-DCs.
(A) IL4-DCs and IL15-DCs were primed with the panel of TLR agonists described in Fig. 3 and washed with PBS to remove cytokines and TLR agonists. CD4+ T cells were purified from autologous lymphocytes by negative selection and cultured with unprimed or TLR-primed DC populations in fresh cRPMI for 72 h. Indicated cytokines were measured in cell-free culture fluids by ELISA kits. Each symbol is the mean of duplicate ELISA values obtained from the different individuals tested after subtracting out levels from cocultures with unprimed IL4-DCs and IL-15DCs. Horizontal bars represent the means in pg/ml from five independent experiments. *P < 0.05, as determined by the paired two-tailed Student's t test. (B) Analysis of intracellular cytokine production by purified CD4+ T cells cultured with unprimed or TLR3-primed, autologous IL15-DCs in fresh cRPMI for 48 h following stimulation with PMA and ionomycin for the last 6 h; in the presence of monensin, for the last 4 h. Two hundred thousand CD4+CD3+ cells were gated on, and intracellular detection of FITC-IFN-γ and PE-IL-17 was determined by FACS. Numbers are percent-positive cells, as determined by appropriate intracellular Ig isotype controls. Density plots are from one of five different donors tested in A.
Despite the similar response profile of TLR2/1-primed IL15-DCs, IL-17 responses were replaced with IL-10, together with IL-22 and IFN-γ. The reciprocal secretion of IL-17 and IL-10 may be explained by the transient production of IL-10 in subsets of activated human Th17 cells, newly described by Zielinski et al. [34], or representative of the CCR6+ IL-10-producing, autoreactive memory T cell population, recently identified by Rivino et al. [35]. Providing that IL15-DCs primed with the TLR2/dectin-1 agonist, yeast zymosan, supported IL-17, IL-22, and IFN-γ secretion, similar to TLR3- and TLR4-primed IL15-DCs (data not shown), deviation from IL-17 to IL-10 is not likely a general characteristic of TLR2-primed IL15-DCs and instead, may depend on the TLR2 heterodimer engaged. None of the experimental conditions supported secretion of the Th2 cytokine IL-4 or IL-21, a cytokine associated with T follicular helper and Th17 responses (negative data not shown).
These data provide primary evidence that TLR-activated IL15-DCs preferentially induce Th17 responses compared with IL4-DCs. Interestingly, our findings indicate that IL-10 produced by TLR-primed IL15-DCs (Fig. 2B and C) does not restrain Th17 responses in the presence of IL-1β, IL-6, and IL-23, similar to what has been reported for CD8+ T cells [19]. This intriguing observation may help explain the increased levels of IL-10 in patients with autoimmune diseases associated with chronic activation of Th17 and Th1 responses [7, 36, 37].
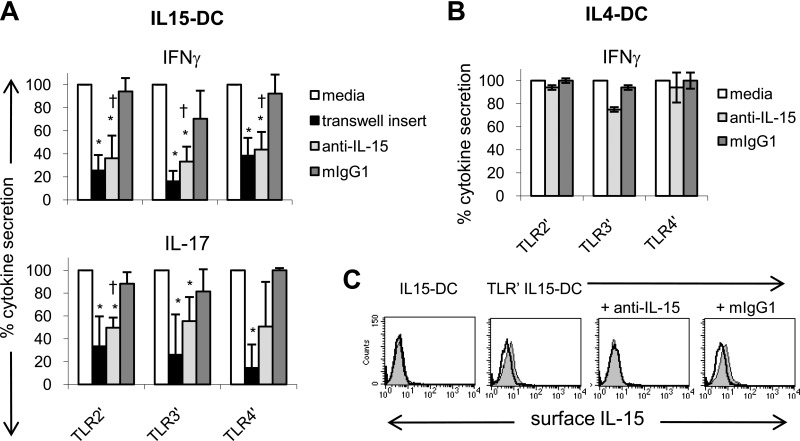
TLR-primed IL15-DCs stimulate IL-17 and IFN-γ secretion by contact- and IL-15-dependent mechanisms
Others [38] and I [4] have reported that IL-15+ APCs activate CD4+ T cells in a contact- and IL-15-dependent manner. Given that membrane-bound IL-15 was detected on TLR-primed IL15-DCs (Fig. 3), and IL-15 was recently shown to activate Th17 cells cultured with allogeneic DCs [10], we investigated the role of membrane-bound IL-15 in our system. A blocking antibody to human IL-15 or the mIgG1 isotype control was added to cocultures containing CD4+ T cells and TLR-primed IL15-DCs. In addition, CD4+ T cells were plated in 0.4 μm semipermeable transwell inserts to prevent T cell contact with activated, autologous IL15-DCs. After 3 days, T cell responses were assessed by measuring secreted IL-17 and IFN-γ proteins in the culture supernatants.
Transwell inserts significantly reduced the levels of IL-17 and IFN-γ in cocultures with TLR-primed, autologous IL15-DCs, demonstrating that contact with IL15-DCs was necessary for secretion of these cytokines (Fig. 5A). In contrast, conditioned media from activated IL15-DCs did not recapitulate the Th17 responses supported by IL15-DCs, providing further evidence that cell-cell interactions are important for induction of these proinflammatory mediators (negative data not shown). Nevertheless, soluble factors such as IL-1β and IL-23 probably enhance IL-17 and IFN-γ secretion, as production of these cytokines was not inhibited completely by transwell inserts (Fig. 5A).
Figure 5. TLR-primed IL15-DCs stimulate IL-17 and IFN-γ secretion by contact- and IL-15-dependent mechanisms.
(A) TLR-primed IL15-DCs were cultured with autologous CD4+ T cells in the presence and absence of 10 μg/ml anti-human IL-15 or mIgG1 for 72 h. In addition, 0.4 μm semipermeable transwell inserts were used to prevent T cells from contacting TLR-primed IL15-DCs. After 3 days, culture fluids were harvested and analyzed for IL-17 and IFN-γ by ELISAs. In each experiment, the cytokine level in cocultures with TLR-primed IL15-DCs (media) was set as 100% and used to calculate the percent cytokine secretion observed with transwell inserts, anti-IL-15, or mIgG1. Data represent the means ± sd of four to five independent experiments. *P < 0.05 between media and transwell or media and anti-IL-15; †P < 0.05 between anti-IL-15 and mIgG1, as determined by the paired two-tailed Student's t test. (B) TLR-primed IL4-DCs were cultured with autologous CD4+ T cells in the presence and absence of 10 μg/ml anti-human IL-15 or mIgG1 for 72 h and culture fluids harvested for IFN-γ ELISA. The percent cytokine secretion observed with and without anti-IL-15 or mIgG1 was calculated as described in A. Data represent the means ± sd of two independent experiments. (C) FACS demonstrating that anti-IL-15 blocks detection of membrane-bound IL-15 on the surface of TLR-primed IL15-DCs, which were incubated with and without antihuman IL-15 or mIgG1 for 1 h and then analyzed for surface expression of IL-15 (filled histograms) by FACS. Unprimed IL15-DCs were included as a negative control. Open histograms depict mIgG1 isotype control staining. One representative experiment is shown.
Antibody blockade of IL-15 on the surface of TLR-primed IL15-DCs (Fig. 5C) inhibited IFN-γ production to the same extent as transwell inserts in all individuals tested (Fig. 5A). Importantly, IFN-γ responses induced by TLR-primed IL4-DCs, which do not express membrane-bound IL-15 (Fig. 3), were unaffected by anti-IL-15 treatment (Fig. 5B). These results strongly suggest that membrane-bound IL-15 mediates the IFN-γ response detected in cocultures with TLR-primed IL15-DCs. In contrast, anti-IL-15 treatment only reduced IL-17 secretion in some experimental conditions, indicating that surface expression of IL-15 may not be required for IL-17 secretion under all circumstances. Thus, separate, although potentially overlapping, contact-dependent mechanisms appear to control the production of IL-17 and IFN-γ in these cocultures. This is not surprising, given that TLR-primed IL15-DCs activated IL-17+IFN-γ–, IL-17+IFN-γ+, and IL-17–IFN-γ+ CD4+ T cell subsets (Fig. 4B).
DISCUSSION
To my knowledge, this is the first study to generate human Th17 responses from PBMCs of healthy individuals using autologous, monocyte-derived cells without addition of antihuman CD3 antibodies or PMA and ionomycin. Although the specificity of the observed Th17 and Th1 responses is currently unknown, we are using this practical approach to explore the following possibilities: TLR-primed IL15-DCs activate Th17 and/or Th1 cells that recognize self or foreign peptides associated with circulating monocytes and/or BSA peptides processed from the culture media [39]; TLR-activated IL15-DCs convert forkhead box p3+ T regulatory cells into Th17 and/or Th1 cells [40]; or IL-15+ APCs stimulate Th17 and Th1 responses independent of TCR specificity. These studies will further our understanding of the ambiguous nature of Th17 cells [8] and offer insight into the mechanisms by which IL-15+ APCs trigger Th17 and Th1 responses in humans. In addition, this experimental method offers a feasible and reproducible method for identifying the intrinsic mechanisms required for surface expression of IL-15 in human monocyte-derived DCs, which are currently not well understood.
In summary, these findings identify key differences in the phenotype and function of IL15-DCs and IL4-DCs that influence Th cell responses and in addition, offer insight as to the relationship among IL-15, TLRs, and Th17 and Th1 responses, which characterize a number of inflammatory diseases. IL15-DCs promoted contact-dependent Th17 responses and Th1 responses mediated by membrane-bound IL-15, whereas IL4-DCs supported Th1 and Th22 responses, depending on the TLR stimulus used. Based on these novel observations and those of others, we speculate that IL4-DCs are better-suited for priming naïve CD4+ T cells into Th1 effectors in secondary lymphoid tissues, whereas IL15-DCs are superior at activating and maintaining antigen-experienced Th17 and Th1 cells in the periphery. Such considerations should be taken into account for developing DC-based immunotherapies to treat autoimmunity and cancer and prevent infectious disease.
ACKNOWLEDGMENTS
These studies were generously supported by Dr. Dean L. Mann at the University of Maryland Medical Center Division of Immunogenetics. I greatly appreciate the technical assistance provided by Dr. Ferenc Livak at the University of Maryland Greenebaum Cancer Center′s Core Flow Cytometry Facility and Lisa Hester at the University of Maryland School of Medicine's Core Cytokine Laboratory.
Footnotes
- cRPMI
- complete RPMI
- mIgG1
- murine IgG1
- Pam3Csk4
- palmitoyl-3-cysteine-serine-lysine-4
- poly (I:C)
- polyinosinic:polycytidylic acid
- sIL-15
- surface IL-15
- TC
- T cell
AUTHORSHIP
K.M.H. designed, conducted, interpreted, and reported all studies presented in this manuscript.
REFERENCES
- 1. Coquerelle C., Moser M. (2010) DC subsets in positive and negative regulation of immunity. Immunol. Rev. 234, 317–334 [DOI] [PubMed] [Google Scholar]
- 2. Anguille S., Smits E. L., Cools N., Goossens H., Berneman Z. N., Van Tendeloo V. F. (2009) Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties. J. Transl. Med. 7, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dubsky P., Saito H., Leogier M., Dantin C., Connolly J. E., Banchereau J., Palucka A. K. (2007) IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur. J. Immunol. 37, 1678–1690 [DOI] [PubMed] [Google Scholar]
- 4. Harris K. M., Fasano A., Mann D. L. (2010) Monocytes differentiated with IL-15 support Th17 and Th1 responses to wheat gliadin: implications for celiac disease. Clin. Immunol. 135, 430–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McInnes I. B., Gracie J. A. (2004) Interleukin-15: a new cytokine target for the treatment of inflammatory diseases. Curr. Opin. Pharmacol. 4, 392–397 [DOI] [PubMed] [Google Scholar]
- 6. Evans H. G., Gullick N. J., Kelly S., Pitzalis C., Lord G. M., Kirkham B. W., Taams L. S. (2009) In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc. Natl. Acad. Sci. USA 106, 6232–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamada N., Hisamatsu T., Okamoto S., Chinen H., Kobayashi T., Sato T., Sakuraba A., Kitazume M. T., Sugita A., Koganei K., Akagawa K. S., Hibi T. (2008) Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-γ axis. J. Clin. Invest. 118, 2269–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Annunziato F., Romagnani S. (2009) Do studies in humans better depict Th17 cells? Blood 114, 2213–2219 [DOI] [PubMed] [Google Scholar]
- 9. Lee W. W., Kang S. W., Choi J., Lee S. H., Shah K., Eynon E. E., Flavell R. A., Kang I. (2010) Regulating human Th17 cells via differential expression of IL-1 receptor. Blood 115, 530–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mathers A. R., Janelsins B. M., Rubin J. P., Tkacheva O. A., Shufesky W. J., Watkins S. C., Morelli A. E., Larregina A. T. (2009) Differential capability of human cutaneous dendritic cell subsets to initiate Th17 responses. J. Immunol. 182, 921–933 [DOI] [PubMed] [Google Scholar]
- 11. Chun K. H., Seong S. Y. (2010) CD14 but not MD2 transmit signals from DAMP. Int. Immunopharmacol. 10, 98–106 [DOI] [PubMed] [Google Scholar]
- 12. Baumann C. L., Aspalter I. M., Sharif O., Pichlmair A., Bluml S., Grebien F., Bruckner M., Pasierbek P., Aumayr K., Planyavsky M., Bennett K. L., Collinge J., Knapp S., Superti-Furga G. (2010) CD14 is a coreceptor of Toll-like receptors 7 and 9. J. Exp. Med. 207, 2689–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Z., Breider M. A., Carroll R. C., Miller M. S., Bochsler P. N. (1996) Soluble CD14 and lipopolysaccharide-binding protein from bovine serum enable bacterial lipopolysaccharide-mediated cytotoxicity and activation of bovine vascular endothelial cells in vitro. J. Leukoc. Biol. 59, 241–247 [DOI] [PubMed] [Google Scholar]
- 14. Verhasselt V., Buelens C., Willems F., De Groote D., Haeffner-Cavaillon N., Goldman M. (1997) Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J. Immunol. 158, 2919–2925 [PubMed] [Google Scholar]
- 15. Yoshimura T., Takeda A., Hamano S., Miyazaki Y., Kinjyo I., Ishibashi T., Yoshimura A., Yoshida H. (2006) Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J. Immunol. 177, 5377–5385 [DOI] [PubMed] [Google Scholar]
- 16. Yao Y., Li W., Kaplan M. H., Chang C. H. (2005) Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J. Exp. Med. 201, 1899–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Waal Malefyt R., Yssel H., de Vries J. E. (1993) Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J. Immunol. 150, 4754–4765 [PubMed] [Google Scholar]
- 18. Conti L., Cardone M., Varano B., Puddu P., Belardelli F., Gessani S. (2008) Role of the cytokine environment and cytokine receptor expression on the generation of functionally distinct dendritic cells from human monocytes. Eur. J. Immunol. 38, 750–762 [DOI] [PubMed] [Google Scholar]
- 19. Brossart P., Zobywalski A., Grunebach F., Behnke L., Stuhler G., Reichardt V. L., Kanz L., Brugger W. (2000) Tumor necrosis factor α and CD40 ligand antagonize the inhibitory effects of interleukin 10 on T-cell stimulatory capacity of dendritic cells. Cancer Res. 60, 4485–4492 [PubMed] [Google Scholar]
- 20. Neely G. G., Epelman S., Ma L. L., Colarusso P., Howlett C. J., Amankwah E. K., McIntyre A. C., Robbins S. M., Mody C. H. (2004) Monocyte surface-bound IL-15 can function as an activating receptor and participate in reverse signaling. J. Immunol. 172, 4225–4234 [DOI] [PubMed] [Google Scholar]
- 21. Mortier E., Woo T., Advincula R., Gozalo S., Ma A. (2008) IL-15Rα chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J. Exp. Med. 205, 1213–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giron-Michel J., Giuliani M., Fogli M., Brouty-Boye D., Ferrini S., Baychelier F., Eid P., Lebousse-Kerdiles C., Durali D., Biassoni R., Charpentier B., Vasquez A., Chouaib S., Caignard A., Moretta L., Azzarone B. (2005) Membrane-bound and soluble IL-15/IL-15Rα complexes display differential signaling and functions on human hematopoietic progenitors. Blood 106, 2302–2310 [DOI] [PubMed] [Google Scholar]
- 23. Stonier S. W., Ma L. J., Castillo E. F., Schluns K. S. (2008) Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 transpresentation. Blood 112, 4546–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mortier E., Quemener A., Vusio P., Lorenzen I., Boublik Y., Grotzinger J., Plet A., Jacques Y. (2006) Soluble interleukin-15 receptor α (IL-15R α)-sushi as a selective and potent agonist of IL-15 action through IL-15R β/γ. Hyperagonist IL-15 × IL-15R α fusion proteins. J. Biol. Chem. 281, 1612–1619 [DOI] [PubMed] [Google Scholar]
- 25. Corinti S., Albanesi C., la Sala A., Pastore S., Girolomoni G. (2001) Regulatory activity of autocrine IL-10 on dendritic cell functions. J. Immunol. 166, 4312–4318 [DOI] [PubMed] [Google Scholar]
- 26. Hock B. D., Kato M., McKenzie J. L., Hart D. N. (2001) A soluble form of CD83 is released from activated dendritic cells and B lymphocytes, and is detectable in normal human sera. Int. Immunol. 13, 959–967 [DOI] [PubMed] [Google Scholar]
- 27. Boniface K., Blumenschein W. M., Brovont-Porth K., McGeachy M. J., Basham B., Desai B., Pierce R., McClanahan T. K., Sadekova S., de Waal Malefyt R. (2010) Human Th17 cells comprise heterogeneous subsets including IFN-γ-producing cells with distinct properties from the Th1 lineage. J. Immunol. 185, 679–687 [DOI] [PubMed] [Google Scholar]
- 28. Oppmann B., Lesley R., Blom B., Timans J. C., Xu Y., Hunte B., Vega F., Yu N., Wang J., Singh K., et al. (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13, 715–725 [DOI] [PubMed] [Google Scholar]
- 29. Depaolo R. W., Abadie V., Tang F., Fehlner-Peach H., Hall J. A., Wang W., Marietta E. V., Kasarda D. D., Waldmann T. A., Murray J. A., Semrad C., Kupfer S. S., Belkaid Y., Guandalini S., Jabri B. (2011) Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature 471, 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu C. Y., Demeure C., Kiniwa M., Gately M., Delespesse G. (1993) IL-12 induces the production of IFN-γ by neonatal human CD4 T cells. J. Immunol. 151, 1938–1949 [PubMed] [Google Scholar]
- 31. Prechtel A. T., Turza N. M., Theodoridis A. A., Steinkasserer A. (2007) CD83 knockdown in monocyte-derived dendritic cells by small interfering RNA leads to a diminished T cell stimulation. J. Immunol. 178, 5454–5464 [DOI] [PubMed] [Google Scholar]
- 32. Acosta-Rodriguez E. V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. (2007) Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8, 639–646 [DOI] [PubMed] [Google Scholar]
- 33. Kleinschek M. A., Boniface K., Sadekova S., Grein J., Murphy E. E., Turner S. P., Raskin L., Desai B., Faubion W. A., de Waal Malefyt R., Pierce R. H., McClanahan T., Kastelein R. A. (2009) Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 206, 525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zielinski C. E., Lanzavecchia A., Sallusto F. (2010) Human Th17 subsets that differ for IL-10 production, pathogen specificity and priming requirements. In DC2010: Forum on Vaccine Science, Lugano, Switzerland [Google Scholar]
- 35. Rivino L., Gruarin P., Haringer B., Steinfelder S., Lozza L., Steckel B., Weick A., Sugliano E., Jarrossay D., Kühl A. A., Loddenkemper C., Abrignani S., Sallusto F., Lanzavecchia A., Geginat J. (2010) CCR6 is expressed on an IL-10-producing, autoreactive memory T cell population with context-dependent regulatory function. J. Exp. Med. 207, 565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al-Janadi M., al-Dalaan A., al-Balla S., al-Humaidi M., Raziuddin S. (1996) Interleukin-10 (IL-10) secretion in systemic lupus erythematosus and rheumatoid arthritis: IL-10-dependent CD4+CD45RO+ T cell-B cell antibody synthesis. J. Clin. Immunol. 16, 198–207 [DOI] [PubMed] [Google Scholar]
- 37. García de Tena J., Manzano L., Leal J. C., San Antonio E., Sualdea V., Alvarez-Mon M. (2006) Distinctive pattern of cytokine production and adhesion molecule expression in peripheral blood memory CD4+ T cells from patients with active Crohn′s disease. J. Clin. Immunol. 26, 233–242 [DOI] [PubMed] [Google Scholar]
- 38. Benito-Miguel M., Garcia-Carmona Y., Balsa A., Perez de Ayala C., Cobo-Ibanez T., Martin-Mola E., Miranda-Carus M. E. (2009) A dual action of rheumatoid arthritis synovial fibroblast IL-15 expression on the equilibrium between CD4+CD25+ regulatory T cells and CD4+CD25– responder T cells. J. Immunol. 183, 8268–8279 [DOI] [PubMed] [Google Scholar]
- 39. Restani P., Ballabio C., Cattaneo A., Isoardi P., Terracciano L., Fiocchi A. (2004) Characterization of bovine serum albumin epitopes and their role in allergic reactions. Allergy 59 (Suppl. 78), 21–24 [DOI] [PubMed] [Google Scholar]
- 40. Koenen H. J., Smeets R. L., Vink P. M., van Rijssen E., Boots A. M., Joosten I. (2008) Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 112, 2340–2352 [DOI] [PubMed] [Google Scholar]