Abstract
Objective:
To investigate the relationship between fine gradations in body mass index (BMI) and risk of hospitalisation for different types of cardiovascular disease (CVD).
Design, Subjects and Methods:
The 45 and Up Study is a large-scale Australian cohort study initiated in 2006. Self-reported data from 158 546 individuals with no history of CVD were linked prospectively to hospitalisation and mortality data. Hazard ratios (HRs) of incident hospitalisation for specific CVD diagnoses in relation to baseline BMI categories were estimated using Cox regression, adjusting for age, sex, region of residence, income, education, smoking, alcohol intake and health insurance status.
Results:
There were 9594 incident CVD admissions over 583 100 person-years among people with BMI⩾20 kg m−2, including 3096 for ischaemic heart disease (IHD), 1373 for stroke, 411 for peripheral vascular disease (PVD) and 320 for heart failure. The adjusted HR of hospitalisation for all CVD diagnoses combined increased significantly with increasing BMI (P(trend) <0.0001)). The HR of IHD hospitalisation increased by 23% (95% confidence interval (95% CI): 18–27%) per 5 kg m−2 increase in BMI (compared to BMI 20.0–22.49 kg m−2, HR (95% CI) for BMI categories were: 22.5–24.99=1.25 (1.08–1.44); 25–27.49=1.43 (1.24–1.65); 27.5–29.99=1.64 (1.42–1.90); 30–32.49=1.63 (1.39–1.91) and 32.5–50=2.10 (1.79–2.45)). The risk of hospitalisation for heart failure showed a significant, but nonlinear, increase with increasing BMI. No significant increase was seen with above-normal BMI for stroke or PVD. For other specific classifications of CVD, HRs of hospitalisation increased significantly with increasing BMI for: hypertension; angina; acute myocardial infarction; chronic IHD; pulmonary embolism; non-rheumatic aortic valve disorders; atrioventricular and left bundle-branch block; atrial fibrillation and flutter; aortic aneurysm; and phlebitis and thrombophlebitis.
Conclusion:
The risk of hospitalisation for a wide range of CVD subtypes increases with relatively fine increments in BMI. Obesity prevention strategies are likely to benefit from focusing on bringing down the mean BMI at the population level, in addition to targeting those with a high BMI.
Keywords: cardiovascular disease, body mass index, hospitalisation, ischaemic heart disease
Introduction
Obesity is a major public health concern because of its high and increasing prevalence worldwide,1 its serious health consequences2 and the substantial economic burden it places on health-care provision.3 In particular, it is an independent modifiable risk factor for cardiovascular disease (CVD),4, 5 which is the leading cause of morbidity and mortality worldwide. Globally, 23% of ischaemic heart disease (IHD) disability-adjusted life-years were attributable to high body mass index (BMI) in 2010.6
Previous prospective studies have shown that elevated BMI is associated with an increased risk of fatal and non-fatal CVD,7, 8 IHD,9, 10, 11, 12 ischaemic stroke,10 haemorrhagic stroke10 and venous thrombosis.13 However, the relationship of fine incremental changes in BMI to the risk of different types of CVD is unclear, because previous studies have tended to consider BMI in relatively broad categories.8, 9, 11 Furthermore, robust evidence on certain CVD subtypes is lacking, with many studies focusing on fatal CVD outcomes.10, 14 From a population health perspective, studies of the gradient in risk can inform health policy and programs on the consequences of small population differences in BMI and help to identify the likely optimal BMI for cardiovascular health.
This large prospective cohort study aims to investigate the relationship of fine increments of BMI to the risk of hospitalisation for a range of CVD diagnoses in people without a history of previous CVD, including how the relationship varies by age, sex, levels of smoking and a range of other personal characteristics.
Materials and methods
The Sax Institute's 45 and Up Study is a large-scale Australian cohort study of 267 151 men and women aged 45 years and over, randomly sampled from the general population of New South Wales (NSW), Australia. Individuals joined the study by completing a postal questionnaire (distributed from 1 January 2006 to 31 December 2008) and giving informed consent for follow-up through repeated data collection and linkage of their data to population health databases. The study methods are described in detail elsewhere.15
Questionnaire data from study participants have been linked probabilistically to the NSW Admitted Patient Data Collection, which is a complete census of all public and private hospital admissions in NSW. The linked data contain details of admissions in participants from 1 July 2000 to 31 December 2011, including dates of admission and discharge, the primary reason for admission using the International Classification of Diseases 10th revision—Australian, Modification (ICD-10-AM),16 up to 55 additional clinical diagnoses and up to 50 operations or procedures, coded using the Australian Classification of Health Interventions procedure codes.17 Dates of death were ascertained from the date of recruitment up to 31 December 2011 using linkage to the NSW Register of Births, Deaths and Marriages. Death registrations capture all deaths in NSW. Cause of death information was not available at the time of analysis.
Over the relatively short follow-up period, a small but unknown number of participants are likely to have moved out of the area. Although hospitalisations occurring in neighbouring states would not be captured, these are estimated to make up fewer than 2% of admissions in NSW residents. Hence, follow-up for hospitalisations is considered to be ∼98% complete among those continuing to reside in NSW. Quality assurance data on the data linkage show false-positive and -negative rates of <0.5% and <0.1%, respectively.
Baseline questionnaire data include information on sociodemographic and lifestyle factors, height and body weight, medical and surgical history, functional capacity and physical activity. BMI was calculated from self-reported body weight and height, as the weight in kilograms divided by the height in metres, squared.
Statistical methods
Excluding 374 (0.14%) participants with invalid age and/or date of recruitment, data from 266 777 participants from the 45 and Up Study were linked to data on hospital admissions and deaths. Consistent with established methods,14 people with extreme measures of BMI (<15 kg m−2 or >50 kg m−2) were excluded owing to the increased probability of measurement error. To minimise the impact of reverse causality, participants with a history of cancer and those known to have CVD at baseline were excluded from this study. Reverse causality is a key consideration when assessing the association of BMI with future disease and mortality outcomes, as baseline illness can lead to both weight loss and higher mortality. After excluding people with missing or invalid BMI (n=20 442; 7.7%), self-reported history of cancer other than melanoma and skin cancer (n=28 043; 10.5%), aged below 45 years (n=8), confirmed linkage errors (n=22; 0.01%) and a history of CVD at baseline (defined as self-reported heart disease, stroke or blood clot on the baseline questionnaire; or a hospital admission in the 6 years before entering the study with CVD diagnosis codes in any diagnostic field or CVD-related procedure codes in any procedure code field; see Table 1 footnote; n=59 716; 22%), data on 158 546 participants were available for the main analyses. A sensitivity analysis examined the impact of defining the history of CVD at baseline more narrowly, as hospitalisation for IHD, heart failure, stroke and peripheral vascular disease (PVD) as a primary diagnosis, before baseline.
Table 1. Characteristics of study populationa according to BMI category at baseline.
| Participant characteristic |
BMI (kg m−2) at baseline |
Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Underweight | Normal weight | Overweight | Obese | ||||||
| |
15–18.49 |
18.5–19.99 |
20–22.49 |
22.5–24.99 |
25–27.49 |
27.5–29.99 |
30–32.49 |
32.5–50 |
|
| N | 1867 | 4660 | 20 726 | 35 908 | 36 048 | 26 205 | 15 723 | 17 409 | 158 546 |
| % Out of total | 1.2% | 2.9% | 13.1% | 22.6% | 22.7% | 16.5% | 9.9% | 11.0% | 100% |
| Median age (interquartile range) | 59 (19) | 57 (16) | 57 (15) | 58 (15) | 59 (14) | 58 (13) | 58 (13) | 57 (12) | 58 (14) |
| Male | 22.4 | 19.3 | 28.0 | 42.0 | 52.4 | 54.7 | 48.5 | 37.2 | 43.8 |
| Residing in major cities | 47.0 | 49.7 | 48.4 | 46.7 | 44.8 | 42.5 | 41.6 | 38.8 | 44.5 |
| Tertiary education | 24.0 | 29.9 | 30.9 | 29.7 | 27.1 | 24.5 | 21.8 | 19.1 | 26.4 |
| Household income ⩾$70 000 | 16.5 | 25.7 | 27.5 | 29.7 | 30.8 | 30.4 | 28.2 | 25.4 | 28.9 |
| Current smoker | 18.0 | 11.9 | 9.6 | 7.8 | 7.0 | 7.1 | 7.5 | 8.0 | 8.0 |
| ⩾15 Alcoholic drinks per week | 8.9 | 8.5 | 10.6 | 13.8 | 16.7 | 17.8 | 16.4 | 12.9 | 14.6 |
| Highest physical activity tertile | 40.2 | 39.7 | 40.9 | 38.8 | 36.3 | 33.0 | 30.1 | 24.8 | 35.2 |
| Doctor diagnosed diabetes | 3.2 | 2.4 | 2.5 | 3.6 | 4.7 | 6.7 | 8.3 | 13.7 | 5.8 |
| Current treatment for hypertension | 10.4 | 8.7 | 10.0 | 13.4 | 16.9 | 21.2 | 24.6 | 32.0 | 18.0 |
| Current treatment for hypercholesterolaemia | 6.5 | 5.9 | 6.9 | 9.4 | 11.8 | 14.0 | 14.4 | 14.8 | 11.3 |
| Private health insurance | 56.6 | 64.2 | 67.1 | 68.6 | 69.0 | 67.9 | 66.0 | 61.2 | 67.0 |
| Aspirin treated | 5.9 | 5.3 | 6.4 | 7.7 | 8.8 | 9.8 | 10.3 | 11.3 | 8.7 |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; ICD-10-AM, International Classification of Diseases 10th revision—Australian, Modification.
Data are percentage of sample within BMI category, unless indicated otherwise.
Only those without a history of CVD at baseline. A history of CVD at baseline was defined as either self-reported heart disease, stroke or blood clot on the baseline questionnaire or a hospital admission in 6 years before entering the study, with ICD-10-AM diagnosis codes I00–I99, G45 and G46 in any of the 55 diagnostic fields or Australian Health Intervention Classification CVD-related procedure codes (coronary artery bypass angioplasty/stent: 35310, 38306, 35304-00, 30305-00, 38300-00, 38303-00; coronary artery bypass graft: 38497, 38500, 38503, 90201; coronary revascularisation procedures: 38497, 38500, 38503, 90201, 35310, 38306, 35304-00, 30305-00, 38300-00, 38303-00) in any of the 50 procedure code fields.
The main CVD outcome was defined as the first hospitalisation (overnight or day) following recruitment into the 45 and Up Study with a primary diagnosis of CVD at discharge, based on ICD-10-AM three-character codes (I00–I99, G45 and G46). Diagnoses were also grouped as IHD (ICD-10-AM I20–I25), PVD (ICD-10-AM I70–I74), stroke (ICD-10-AM I60–I69, G45, G46) and heart failure (ICD-10-AM I50) consistent with the NSW Health Report 201018 (see Figure 1 footnote), and were also examined individually where at least 50 events were reported. In the analyses of incident CVD hospitalisation since baseline, eligible participants contributed person-years from the date of recruitment until the CVD admission date, date of death or end of follow-up (31 December 2011), whichever was the earliest.
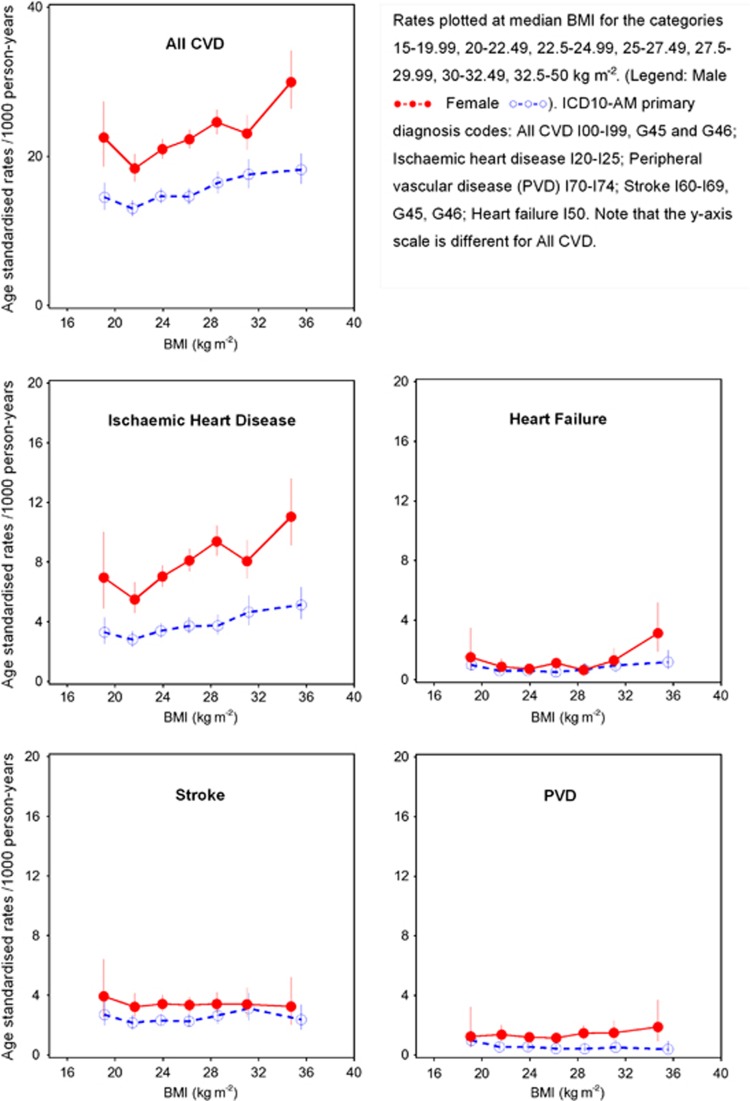
Figure 1.
Age standardised rates per 1000 person-years of all CVD and cause-specific CVD hospitalisation since baseline by BMI categories and sex, directly age-adjusted to 2006 New South Wales population. BMI, body mass index; CVD, cardiovascular disease.
Incident CVD hospitalisation rates since baseline and 95% confidence intervals were calculated for different levels of BMI (WHO weight classification19 in brackets): 15–18.49 (underweight); 18.5–19.99, 20–22.49 and 22.5–24.99 (normal weight); 25–27.49 and 27.5–29.99 (overweight); 30–32.49 and 32.5–50 kg m−2 (obese); and were age-standardised to the 2006 NSW population, in 5-year age groups, using the direct method.20 Hazard ratios (HR) for specific CVD outcomes and all CVD according to BMI at baseline were estimated using Cox regression modelling, in which the underlying time variable was age. Each outcome of interest was considered as an end point in the corresponding model. The HR and 95% CI are shown initially accounting for age, the underlying time variable and sex (male, female). Models are then presented adjusted for additional covariates (where appropriate) including tobacco smoking (current, past, never), alcohol consumption (0 alcoholic drinks per week, 1–14 alcoholic drinks per week, ⩾15 alcoholic drinks per week), annual pre-tax household income (AU$ <20 000, 20 000–39 999, 40 000–69 999, ⩾70 000), education (<secondary school, secondary school graduation, trade/apprenticeship/certificate/diploma, university graduate), region of residence (major cities, inner regional areas, outer regional/remote areas) and health insurance (private health insurance or no private health insurance). No adjustment was made for physical activity, high blood pressure or high blood cholesterol as these are likely mechanisms in the pathway linking body weight and CVD. Instead, we performed sensitivity analyses adjusting additionally for the level of physical activity, treatment for high cholesterol and treatment for high blood pressure. Missing values for covariates were included in the models as separate categories.
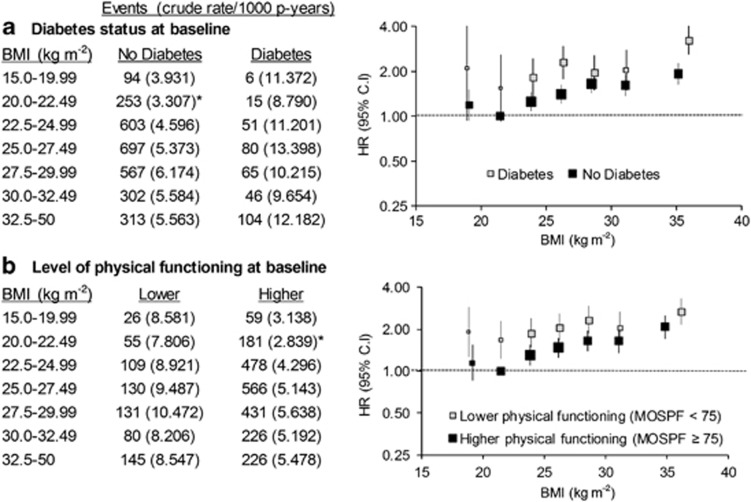
To explore whether the BMI–IHD relationships varied according to diabetes status and whether or not individuals were likely to have subclinical and/or undiagnosed disease at baseline, relative risks of hospital admission for IHD were estimated according to BMI categories in those with and without self-reported diabetes and in individuals with higher and lower levels of physical functioning (based on the 10-item version of the Medical Outcomes Study—Physical Functioning Scale (MOS-PF))21 at baseline; for these analyses a single reference group was used, relating to a BMI of 20–22.49 kg m−2, either no diabetes or high physical functioning. Among people with BMI ⩾20 kg m−2, the relationship of 5 kg m−2 increases in BMI to IHD hospitalisation was also estimated in subgroups of age, sex, smoking, alcohol intake, education, income, private health insurance, tertiles of physical activity (<7, 7–12 and 13 or more weekly sessions, weighted for intensity),22 region of residence, diagnosed diabetes, physical functioning, treatment for hypertension, treatment for high cholesterol and aspirin intake. A physical activity session was defined as mild physical activity (like walking continuously for at least 10 min), moderate physical activity (like gentle swimming, social tennis, vigorous gardening or work around the house) or vigorous physical activity (that makes you breathe harder or puff and pant, like jogging, cycling, aerobics, competitive tennis).
The proportionality assumption was verified by plotting the Schoenfeld residuals against the time variable in each model, with a stratified form or time-dependent form of the model used where covariates displayed non-proportionality of hazards. HRs of CVD hospitalisation were estimated for each BMI category, using BMI category 20–22.49 kg m−2 as the reference group. Tests for trend were performed by modelling median values of the BMI categories as an ordinal variable. The HR associated with each 5 kg m−2 increase in BMI was estimated, restricting the model to participants with BMI ⩾20 kg m−2 and BMI was modelled as a continuous variable. Martingale residual plots were used to investigate the linear functional form of BMI in these models. Where there were concerns, a sensitivity analysis examined whether models restricting the analysis to BMI 20–40 kg m−2 met the linearity assumption. If the restricted models met the linearity assumptions and demonstrated little change in HRs, the initial results were reported. To test for interaction between BMI and other exposures in relation to CVD outcomes, the likelihood ratio test was used to compare the fully adjusted model with and without the interaction term. All statistical tests were two sided, using a significance level of 5%. All analyses were carried out using SAS version 9.3 (SAS Institute, Cary, NC, USA).23
Ethical approval for the study was obtained from the NSW Population and Health Services Research Ethics Committee and the Australian National University Human Research Ethics Committee.
Results
The study population ranged in age from 45 to 103 years, with a median of 57.8 years (interquartile range 13.9 years). The majority of participants (73%) were aged 45–64 years, with 5% aged 80 years or older; just under half the cohort (44%) comprised men. Nearly two-thirds (60%) were overweight or obese (39% and 21%, respectively), with obesity prevalence substantially lower in those aged ⩾80 years (10%). The mean BMI was 26.7 (s.d. 4.8) kg m−2. The proportion of people with diabetes and of those being treated for hypertension and hypercholesterolaemia increased with increasing BMI (excluding underweight) (Table 1).
The median (interquartile range) follow-up time for the cohort was 3.4 (0.6) years for CVD hospitalisation; a total of 9594 incident hospital admissions since baseline with a primary diagnosis of CVD occurred during 583 100 person-years of follow-up among people with BMI⩾20 kg m−2. The age-standardised rates of incident hospitalisation for all CVD combined and IHD were higher in men than women, but for both sexes, increased with increasing BMI (excluding BMI <20 kg m−2, P-value <0.05 for tests for linear trend; Figure 1). However, this pattern was not seen for hospitalisation for PVD and stroke, and for heart failure, rates appeared elevated only in men with BMI 32.5–50 kg m−2 (Figure 1).
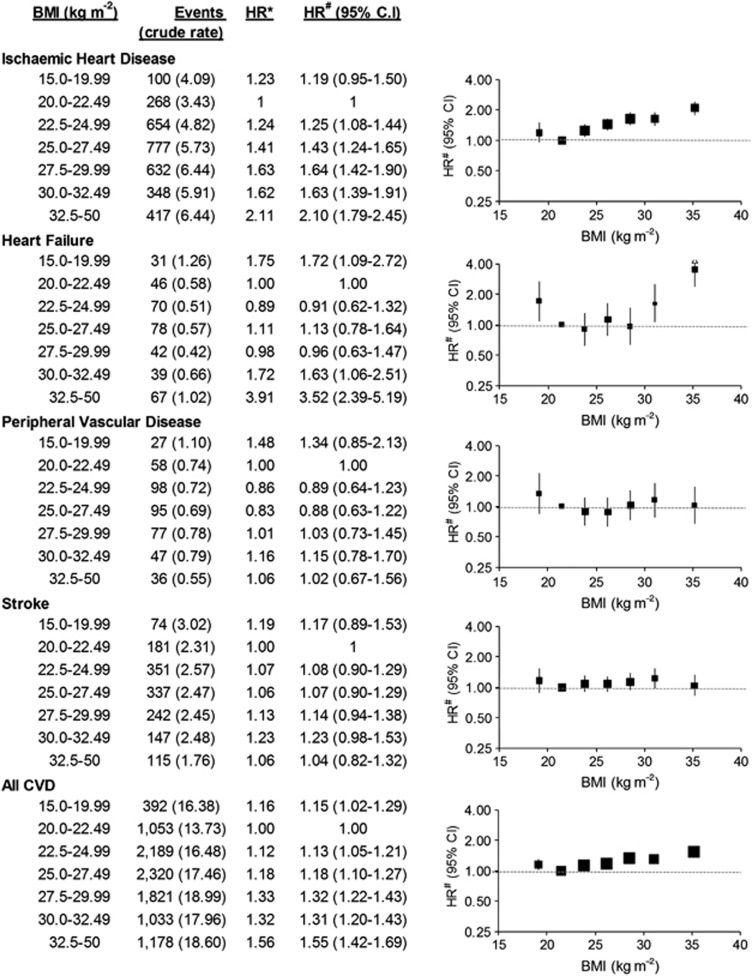
Similar patterns were evident in the adjusted HRs, with HRs of all CVD combined and IHD incident hospitalisation increasing steadily with increasing BMI above 20 kg m−2 (Figure 2; P(trend) <0.0001). BMI showed a J-shaped relation to the risk of heart failure hospitalisation (Figure 2; the HR (95% CI) varying from 0.91 (0.62–1.32) in the 22.5–24.99 kg m−2 category to 3.52 (2.39–5.19) in the 32.5–50 kg m−2 category, relative to BMI 20–22.49 kg m−2). Adjusting additionally for physical activity level, treatment for high cholesterol and treatment for high blood pressure demonstrated little change in HRs (Supplementary Table 1). HRs for stroke or PVD hospitalisation were not significantly elevated at any level of BMI compared with BMI 20.0–22.49 kg m−2. The pattern remained similar when stroke was further divided into ischaemic stroke and haemorrhagic stroke (Supplementary Figure 1). The strong gradient in IHD hospitalisation according to BMI among those without diabetes and among those with high levels of physical functioning at baseline was similar to that observed in the whole cohort (Figure 3). Elevations in CVD risk among individuals with low BMI were seen particularly among those with diabetes and in those with lower levels of physical functioning at baseline.
Figure 2.
Relative risks (95% CI) of CVD admission since baseline, according to BMI categories. Crude rates are per 1000 person-years. HR* adjusted for age and sex only. HR# adjusted for age, sex, region of residence, household income, education, smoking, alcohol intake and health insurance. HR#s are plotted on a log scale at median BMI for the categories and are represented with squares with areas inversely proportional to the variance of the logarithm of the HR, providing an indication of the amount of statistical information available; 95% CIs are indicated by vertical lines. BMI, body mass index; CI, confidence intervals; CVD, cardiovascular disease; HR, hazards ratios.
Figure 3.
Relative risks (95% CI) of hospital admission for ischaemic heart disease in subgroups of (a) diabetes status and (b) physical functioning at baseline, according to BMI categories. Level of physical functioning is based on Medical Outcomes Score—Physical Functioning; <75 (lower functioning) or ⩾75 (higher functioning). *Reference group for HR. HR adjusted for age, sex, region of residence, household income, education, smoking, alcohol intake and health insurance. HRs are plotted on a log scale at median BMI for the categories and are represented with squares with areas inversely proportional to the variance of the logarithm of the HR, providing an indication of the amount of statistical information available; 95% CIs are indicated by vertical lines. BMI, body mass index; CI, confidence intervals; HR, hazards ratios; p-years, person years.
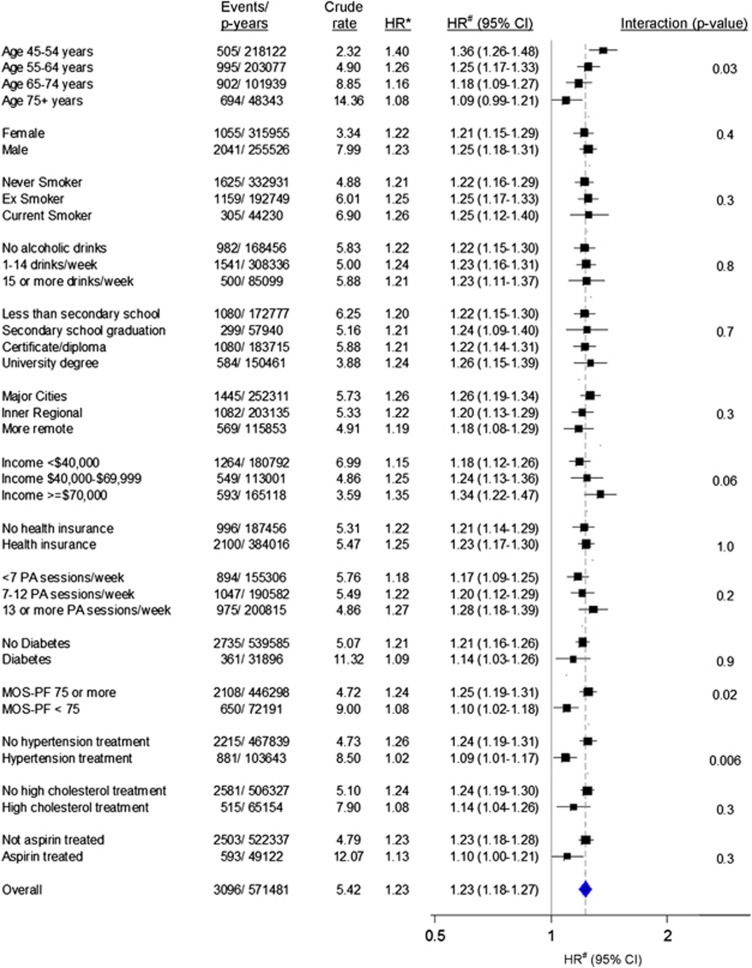
Among people with BMI ⩾20 kg m−2, the HR of IHD hospitalisation per 5 kg m−2 increase in BMI was 1.23 (1.18–1.27) overall and remained significantly elevated in all subgroups of age (except in 75+ years age group), sex, smoking, education, income, private insurance, physical activity, region of residence, diagnosed diabetes, physical functioning, treatment for hypertension, treatment for high cholesterol and aspirin intake (Figure 4). The risk was attenuated significantly with increasing age (P-value (interaction)=0.03); among those treated versus not treated for hypertension (P-value (interaction)=0.006); and among those with lower physical functioning (MOS-PF<75) versus those with higher physical functioning (P-value (interaction)=0.02), but did not vary significantly according to the other factors examined.
Figure 4.
Relative risk of IHD admission since baseline associated with 5 kg m−2 increase in BMI among those with BMI ⩾20 kg m−2 (n=151 751), in a range of population subgroups. Crude rates are per 1000 person-years. HR* adjusted for age and sex only. HR# adjusted for age, sex, region of residence, household income, education, smoking, alcohol intake and health insurance. HRs are plotted on a log scale and are represented with squares with areas inversely proportional to the variance of the logarithm of the HR, providing an indication of the amount of statistical information available; 95% CIs are indicated by horizontal lines. PA sessions per week: number of physical activity session per week, weighted for intensity. Health insurance includes private health insurance or Department of Veterans Affairs health card. CI, confidence intervals; HR, hazards ratios; MOS-PF, Medical Outcomes Score—Physical Functioning; p-years, person years.
Among participants with a BMI ⩾20 kg m−2, BMI had a linear functional form with all CVD events, including grouped and level 3 ICD-10 codes, except for heart failure (I50) and occlusion and stenosis of precerebral arteries not resulting in cerebral infarction (I65); consequently, I50 and I65 were not analysed in this manner. There was a significant linear trend of increasing HR of hospitalisation for specific ICD-10-AM primary diagnostic codes per 5 kg m−2 increase in BMI for: hypertension; angina pectoris; acute myocardial infarction; chronic IHD; pulmonary embolism; non-rheumatic aortic valve disorders; atrial fibrillation and flutter; aortic aneurysm; stroke not specified as haemorrhage or infarction; phlebitis and thrombophlebitis; and postprocedural disorders of circulatory system (Table 2). No statistically significant trends of increasing risk were observed for: transient cerebral ischaemic attacks, other diseases of the pericardium, non-rheumatic mitral valve disorders, cardiomyopathy, other conduction disorders, paroxysmal tachycardia, subarachnoid haemorrhage, intracerebral haemorrhage, other non-traumatic intracranial haemorrhage, cerebral infarction, other cerebrovascular diseases, other cardiac arrhythmias, atherosclerosis, varicose veins of lower extremities, haemorrhoids or hypotension.
Table 2. HR of incident CVD-related hospital admission since baseline associated with 5 kg m−2 increase in BMI by level-3 ICD-10-AM diagnosis codes with at least 50 events (those with BMI ⩾20 kg m−2 only).
| Events | Crude rate | HRa | HRb (95% CI) | P-valueb | ||
|---|---|---|---|---|---|---|
| G45 | Transient cerebral ischaemic attacks and related syndromes | 543 | 0.942 | 1.00 | 1.00 (0.90–1.10) | 0.9 |
| I10 | Essential (primary) hypertension | 168 | 0.291 | 1.39 | 1.26 (1.08–1.47) | 0.003 |
| I20 | Angina pectoris | 1333 | 2.319 | 1.26 | 1.25 (1.18–1.33) | <0.0001 |
| I21 | Acute myocardial infarction | 1155 | 2.008 | 1.18 | 1.15 (1.08–1.23) | <0.0001 |
| I25 | Chronic ischaemic heart disease | 1167 | 2.028 | 1.26 | 1.29 (1.21–1.37) | <0.0001 |
| I26 | Pulmonary embolism | 330 | 0.572 | 1.40 | 1.39 (1.25–1.55) | <0.0001 |
| I31 | Other diseases of pericardium | 82 | 0.142 | 1.20 | 1.20 (0.94–1.53) | 0.1 |
| I34 | Non-rheumatic mitral valve disorders | 60 | 0.104 | 0.74 | 0.72 (0.51–1.03) | 0.07 |
| I35 | Non-rheumatic aortic valve disorders | 141 | 0.244 | 1.37 | 1.37 (1.14–1.65) | 0.001 |
| I42 | Cardiomyopathy | 66 | 0.114 | 1.33 | 1.28 (1.00–1.65) | 0.05 |
| I44 | Atrioventricular and left bundle-branch block | 129 | 0.223 | 1.27 | 1.27 (1.05–1.55) | 0.02 |
| I45 | Other conduction disorders | 53 | 0.092 | 1.21 | 1.23 (0.90–1.70) | 0.2 |
| I47 | Paroxysmal tachycardia | 254 | 0.440 | 1.10 | 1.11 (0.96–1.27) | 0.2 |
| I48 | Atrial fibrillation and flutter | 1139 | 1.979 | 1.29 | 1.31 (1.23–1.39) | <0.0001 |
| I49 | Other cardiac arrhythmias | 219 | 0.379 | 1.01 | 1.01 (0.85–1.18) | 0.9 |
| I60 | Subarachnoid haemorrhage | 61 | 0.106 | 0.93 | 0.85 (0.63–1.16) | 0.3 |
| I61 | Intracerebral haemorrhage | 109 | 0.189 | 0.81 | 0.83 (0.64–1.07) | 0.2 |
| I62 | Other non-traumatic intracranial haemorrhage | 57 | 0.099 | 1.02 | 0.91 (0.64–1.29) | 0.6 |
| I63 | Cerebral infarction | 367 | 0.636 | 1.02 | 1.03 (0.91–1.16) | 0.7 |
| I64 | Stroke, not specified as haemorrhage or infarction | 202 | 0.350 | 1.10 | 1.22 (1.05–1.43) | 0.01 |
| I67 | Other cerebrovascular diseases | 58 | 0.100 | 1.05 | 1.04 (0.78–1.39) | 0.8 |
| I70 | Atherosclerosis | 226 | 0.392 | 0.93 | 0.92 (0.78–1.09) | 0.3 |
| I71 | Aortic aneurysm and dissection | 131 | 0.227 | 1.43 | 1.41 (1.16–1.71) | 0.001 |
| I80 | Phlebitis and thrombophlebitis | 232 | 0.402 | 1.40 | 1.42 (1.25–1.61) | <0.0001 |
| I83 | Varicose veins of lower extremities | 574 | 0.996 | 1.04 | 1.06 (0.97–1.15) | 0.2 |
| I84 | Haemorrhoids | 1708 | 2.975 | 0.98 | 0.98 (0.93–1.04) | 0.5 |
| I95 | Hypotension | 188 | 0.326 | 1.18 | 1.16 (0.98–1.36) | 0.1 |
| I97 | Postprocedural disorders of circulatory system, not elsewhere classified | 78 | 0.135 | 1.60 | 1.51 (1.23–1.85) | <0.0001 |
Abbreviations: BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; ICD-10-AM, International Classification of Diseases 10th revision—Australian, Modification.
Crude rates are per 1000 person-years.
Although there were 90 incident hospitalisations for I65 (occlusion and stenosis of precerebral arteries, not resulting in cerebral infarction) and 342 incident hospitalisations for heart failure, they are not included here owing to concerns about the linear functional form of BMI in the model, with BMI as a continuous variable. Heart failure has been addressed in detail in Figure 2.
HR adjusted for age and sex only.
HR adjusted for age, sex, region of residence, household income, education, smoking, alcohol intake and health insurance.
Sensitivity analyses defining the history of CVD at baseline using a more restricted set of primary CVD diagnosis codes showed little change in the HRs (n=181 868; 13 400 incident CVD hospitalisations since baseline; Supplementary Table 2).
Discussion
This large population-based prospective cohort study provides the most comprehensive evidence to date for an association between fine gradations of increasing BMI and the risk of hospitalisation for different types of CVD. The lowest age-standardised rates of CVD and IHD hospitalisation were seen in the BMI category of 20–22.49 kg m−2, which is consistent with previous studies on IHD incidence12 and mortality (in people with and without previous CVD).8, 10 The risk of hospitalisation for all CVD combined rose gradually with increasing above-normal BMI, with IHD comprising a major component of all CVD.
The risk of IHD hospitalisation more than doubled, and that for heart failure tripled, among those with BMI 32.5–50 kg m−2, compared with those with BMI 20–22.49 kg m−2, and each 5 kg m−2 increase in BMI was associated with a 20% or greater increase in risk of hospitalisation with a primary diagnosis of hypertension; angina pectoris; chronic IHD; pulmonary embolism; atrial fibrillation and flutter; non-rheumatic aortic valve disorders; atrioventricular and left bundle-branch block; paroxysmal tachycardia; aortic aneurysm; phlebitis and thrombophlebitis; and a 15% increase in risk of hospitalisation for a primary diagnosis of acute myocardial infarction. The continuous positive associations between baseline BMI and the risks of IHD hospitalisation were greatest among younger middle-aged adults (45–54 years), with each 5 kg m−2 higher BMI above 20 kg m−2 associated with a 36% higher risk. HRs were not as high in older age groups up to age 75 years, but were still substantial (18–25%) and the higher absolute risk of CVD with increasing age should also be considered. The attenuation in the BMI-associated risk of IHD events with increasing age seen here has been noted previously.10, 14 This age-related pattern has been postulated to be due to several factors including the pathophysiology of ageing, age-related issues in disease detection and diagnosis, comorbidity, competing risks, selective survival and ceiling effects.24 No large or systematic variation in the BMI-associated risks of IHD were seen according to a range of other population attributes, including sex, smoking status, alcohol consumption, physical activity, education and income. Risks were significantly attenuated in those receiving treatment for hypertension, which indicated the potentially positive influence of medical interventions. Although the number of statistical tests conducted should be borne in mind, this could also be consistent with hypertension being one of the causal mechanisms in the BMI–CVD relationship.14 The reason for the significant attenuation in the BMI–IHD relationship in people with moderate-to-severe functional impairment is unclear.
Our results lend strong support to previous studies, which have also found a positive association between obesity and selected CVD-related outcomes.4, 8, 9, 10, 12, 25, 26, 27 The findings from the Million Women Study,12 based on 1.2 million participants and published while this paper was under review, are the most comparable and demonstrate an identical 23% (95% CI: 22–25) increase in the risk of hospitalisation for IHD with each 5 kg m−2 increase in BMI. A meta-analysis conducted by the Asia Pacific Cohort Studies Collaboration, which included data on 60 718 people from Australia and New Zealand, reported a 10% (95% CI: 2–17) reduction in fatal and non-fatal IHD associated with 2 kg m−2 lower BMI in Australian and New Zealand cohorts.10 Another meta-analysis of 21 cohort studies including more than 300 000 people (mainly Caucasians) found that the increased risk of fatal and non-fatal IHD associated with a 5 kg m−2 increase in BMI was 29% (22–35%), after adjusting for age, sex, physical activity and smoking.9 Analyses undertaken by the Prospective Studies Collaboration,14 with nearly 900 000 participants in 57 prospective studies, showed that in BMI ranges 25–50 kg m−2, a 5 kg m−2 increase in BMI was associated with around 40% higher risk of fatal IHD. Hence, the magnitude of our findings regarding the relationship between IHD and BMI are in keeping with the evidence to date, although previous evidence relates predominantly to fatal events, which appear to show a stronger relationship to BMI than non-fatal events.12, 14 It is uncertain as to why, unlike previous studies including the Asia Pacific Cohort Studies Collaboration,10 we found a nonsignificant association between BMI and hospitalisation for stroke. This may, at least in part, reflect differences in study design, including the exclusion criteria. In the study reported here, we excluded people with previous CVD, but a previous 45 and Up Study examining the risk of hospitalisation without excluding people with previous CVD showed a 31% (95% CI: 4–66) increase in risk of hospitalisation for stroke in obese versus normal weight participants aged 45–64 years,28 a finding consistent with the Asia Pacific Cohort Studies Collaboration.10 Further, BMI levels may have different associations with stroke subtypes,10 but we had limited power to investigate subtypes in our study.
Although hospitalisation for certain subtypes of CVD has been shown to be significantly greater among obese adults than among normal weight adults,13, 26, 29, 30, 31, 32 we could not locate any studies demonstrating an increased risk of non-rheumatic aortic valve disorders or aortic aneurysm. Moreover, we are not aware of any studies presenting quantitative data on trends in risk for these conditions according to gradations of BMI.
Although representativeness is not necessary for valid and reliable estimates of relative risk from within-cohort comparisons,33 the prevalence of overweight and obesity within the cohort is similar to that in the general population. Using data from the cohort before exclusions, 47% of men and 30% of women aged 45–64 years were overweight (BMI 25.0–29.9 kg m−2); 23% of both men and women were obese (BMI 30 kg m−2 or more). This compares with an overweight prevalence of 44% in men and 30% in women and an obesity prevalence of 28% among men and 24% among women in the same age group from the self-reported height and weight in the representative population-based National Health Survey of Australia.34
With its large sample size, the current study provides the strongest evidence to date of increasing risk in overall as well as a spectrum of cause-specific CVD hospitalisation with small incremental changes in BMI. Another advantage of the study was the ability to link to administrative records, allowing virtually complete, and objective, ascertainment of outcomes over time and enhanced identification of people with previous CVD, so that they could be excluded from the study. The validity of administrative coding for the specific CVD outcomes varies, but is generally good, with kappa scores for agreement between chart review and recorded diagnoses of 0.6–0.835 and positive predictive values of 66–99%36, 37, 38 for acute myocardial infarction, cerebral infarction and heart failure. Where misclassification is present, it is unlikely to be biased by prior knowledge of BMI and would therefore tend to result in more conservative estimates of relative risk.
Alcohol consumption, tobacco smoking and socioeconomic status (annual household income, education, region of residence, private health insurance) are all related to BMI, and are also known risk factors for CVD hospitalisation, resulting in potential confounding of the BMI–CVD relationship. The ability to link administrative records to questionnaire data meant that we were able to adjust for these potential confounding factors in the main models. Consistent with this, the BMI–CVD relationship did not differ materially when examined separately in subgroups of these factors (Figure 4).
Other factors (physical activity, physical functional impairment, diabetes, treatment for hypertension, treatment for high cholesterol and aspirin intake) were not treated as potential confounders and adjusted for in the main models because they are likely to lie, at least in part, on the causal pathway in the BMI–CVD relationship (mediators) or may reflect reverse causality; it is not appropriate to adjust for factors that lie on the causal pathway in the main analysis.39 Subgroup analyses enabled us to investigate whether the relationship of BMI to IHD hospitalisation varied significantly between the different subgroups, defined according to different levels of these factors (Figure 4). Elevated HRs with BMI above 20 kg m−2 were observed in all of these subgroups, and were similar in magnitude to that observed in the whole cohort for subgroups of physical activity, diabetes and treatment for hypercholesterolaemia (no significant interaction with BMI was observed either). Among people with diabetes and/or lower physical functioning (indicators of greater baseline levels of ill health), the raised HRs of IHD among individuals with lower BMI observed illustrate the potential for reverse causality to influence the findings (Figure 3).
Study limitations that may influence the interpretation of results include: (i) there was relatively short follow-up, thereby making it difficult to exclude completely the potential effect of reverse causality and subclinical CVD at baseline, although this was minimised by excluding those with a history of CVD and by examining results in those without diabetes and in those with minimal physical functional impairment at baseline; (ii) BMI was calculated using self-reported weight and height; (iii) data on potential confounding factors were mostly based on self-report; and (iv) measured data on CVD risk factors such as blood pressure and blood lipids were not available. Regarding self-reported BMI, although people tend to underestimate their weight and overestimate their height,40 and consequently BMI is underestimated, a validation study involving participants in the 45 and Up Study revealed that the mean difference between self-reported and measured BMI was not large (on average −0.74 kg m−2) and correlations between self-reported and measured height and weight were 0.95 and 0.99, respectively,41 results that are consistent with other studies. Other measures of central obesity were not measured in our study. Primary diagnoses of CVD outcomes, including severe disease such as myocardial infarction and stroke, were a focus of the analyses. These are unlikely to be overly influenced by biases resulting from differential use of health services according to BMI. However, it is not possible to exclude entirely the theoretical possibility that the increased risk of hospitalisation for CVD among people with high BMI could be influenced to some extent by the increased probability of hospitalisation for any cause with increasing BMI.28
The dose–response relationship in IHD hospitalisation that was found after adjustment for a range of important confounders gives strength to the argument that above-normal BMI is causally linked to IHD, with the gradient seen even in the absence of diabetes or physical functioning limitations. Although the evidence to date supports a direct causal relationship, it is not known precisely how excess body weight increases the risk of CVD. Excess body weight contributes directly to a range of known risk factors for CVD, such as diabetes mellitus, hypertension and hypercholesterolaemia, and these remain the most likely pathway by which increases in BMI cause increased risk of CVD.14 Although some commentators have considered that the higher incidence of coronary events in obese subjects is only partly explained by conventional associated risk factors, and could be related to factors such as inflammation and fibrinogen,42 the contribution of BMI to risk above and beyond conventional risk factors remains difficult to quantify.14 The biological mechanism by which obesity increases the risk of venous thromboembolism is not fully understood, but raised levels of fibrinogen and some clotting factors, low-grade systemic inflammation and raised intra-abdominal pressure and reduced venous return from the lower limbs are thought to be involved.43
The findings suggest that the risk of hospitalisation for a wide range of CVD subtypes increases with relatively small increases in BMI. Prevention strategies are likely to benefit from focusing on bringing down the mean BMI at the population level.44 However, continuation of strategies targeting the population above specific thresholds of BMI is also likely to be necessary.
Acknowledgments
We thank the men and women participating in the 45 and Up Study. The 45 and Up Study is managed by The Sax Institute in collaboration with major partner Cancer Council New South Wales; and partners the National Heart Foundation of Australia (New South Wales Division); New South Wales Ministry of Health; beyondblue; Ageing, Disability and Home Care, Family and Community Services NSW; the Red Cross Blood Service; and Uniting Care Ageing. We also acknowledge the support of the Centre for Health Record Linkage. This specific project was supported by Australian National Health and Medical Research Council (NHMRC) Project Grant number 585402, which arose as an initiative of the MBF Policy in Action Roundtable, funded solely by the Bupa Health Foundation. The NHMRC had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; nor in the decision to submit the article for publication. Emily Banks and Bette Liu are supported by the NHMRC.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary Material
References
- Organisation for Economic Co-operation and Development . Obesity and the Economics of Prevention: Fit Not Fat. OECD: Paris, France; 2010. [Google Scholar]
- Thompson D, Edelsberg J, Colditz GA, Bird AP, Oster G. Lifetime health and economic consequences of obesity. Arch Intern Med. 1999;159:2177–2183. doi: 10.1001/archinte.159.18.2177. [DOI] [PubMed] [Google Scholar]
- Kortt MA, Langley PC, Cox ER. A review of cost-of-illness studies on obesity. Clin Ther. 1998;20:772–779. doi: 10.1016/s0149-2918(98)80140-9. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew–Paisley study) Eur Heart J. 2006;27:96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- Guh D, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis A. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogers RP, Bemelmans WJE, Hoogenveen RT, Boshuizen HC, Woodward M, Knekt P, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167:1720–1728. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- Asia Pacific Cohort Studies Collaboration Body mass index and cardiovascular disease in the Asia-Pacific Region: an overview of 33 cohorts involving 310 000 participants. Int J Epidemiol. 2004;33:751–758. doi: 10.1093/ije/dyh163. [DOI] [PubMed] [Google Scholar]
- Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- Canoy D, Cairns BJ, Balkwill A, Wright FL, Green J, Reeves G, et al. Body mass index and incident coronary heart disease in women: a population-based prospective study. BMC Med. 2013;11:87. doi: 10.1186/1741-7015-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin L, Sweetland S, Balkwill A, Green J, Reeves G, Beral V. Body mass index, surgery, and risk of venous thromboembolism in middle-aged women: a cohort study. Circulation. 2012;125:1897–1904. doi: 10.1161/CIRCULATIONAHA.111.063354. [DOI] [PubMed] [Google Scholar]
- Prospective Studies Collaboration Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks E, Redman S, Jorm L, Armstrong B, Bauman A, Beard J, et al. Cohort profile: the 45 and up study. Int J Epidemiol. 2008;37:941–947. doi: 10.1093/ije/dym184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Centre for Classification in Health International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD-10-AM)5th edn.National Centre for Classification in Health: Sydney, NSW, Australia; 2006 [PubMed] [Google Scholar]
- National Centre for Classification in Health The Australian Classification of Health Interventions (ACHI) Tabular List of Interventions and Alphabetic Index of Interventions6th edn.National Centre for Classification in Health: Sydney, NSW, Australia; 2007 [Google Scholar]
- Population Health Division . The Health of the People of New South Wales—Report of the Chief Health Officer. NSW Department of Health: Sydney, NSW, Australia; 2010. [Google Scholar]
- World Health Organization WHO: Global Database on Body Mass Index , 2006. Available at: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html .
- Fay MP, Feuer EJ. Confidence intervals for directly adjusted rates: a method based on the gamma distribution. Stat Med. 1997;16:791–801. doi: 10.1002/(sici)1097-0258(19970415)16:7<791::aid-sim500>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Hays RD, Liu H, Spritzer K, Cella D. Item response theory analyses of physical functioning items in the medical outcomes study. Med Care. 2007;45 (Suppl 1:S32–S38. doi: 10.1097/01.mlr.0000246649.43232.82. [DOI] [PubMed] [Google Scholar]
- Australian Institute of Health and Welfare . The Active Australia Survey: A Guide and Manual for Implementation, Analysis and Reporting. AIHW: Canberra, ACT, Australia; 2003. [Google Scholar]
- SAS Institute Inc . SAS Version 9.3 [computer program] SAS Institute: Cary, NC, USA; 2011. [Google Scholar]
- Kaplan GA, Haan MN, Wallace RB. Understanding changing risk factor associations with increasing age in adults. Annu Rev Public Health. 1999;20:89–108. doi: 10.1146/annurev.publhealth.20.1.89. [DOI] [PubMed] [Google Scholar]
- Jensen MK, Chiuve SE, Rimm EB, Dethlefsen C, Tjønneland A, Joensen AM, et al. Obesity, behavioral lifestyle factors, and risk of acute coronary events. Circulation. 2008;117:3062–3069. doi: 10.1161/CIRCULATIONAHA.107.759951. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ôunpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case–control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, et al. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322:882–889. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- Korda RJ, Liu B, Clements MS, Bauman AE, Jorm LR, Bambrick HJ, et al. Prospective cohort study of body mass index and the risk of hospitalisation: findings from 246,361 participants in the 45 and Up Study. Int J Obes. 2013;37:790–799. doi: 10.1038/ijo.2012.155. [DOI] [PubMed] [Google Scholar]
- Han E, Truesdale KP, Taber DR, Cai J, Juhaeri J, Stevens J. Impact of overweight and obesity on hospitalization: race and gender differences. Int J Obes. 2009;33:249–256. doi: 10.1038/ijo.2008.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabrhel C, Varraso R, Goldhaber SZ, Rimm EB, Camargo CA. Prospective study of BMI and the risk of pulmonary embolism in women. Obesity. 2009;17:2040–2046. doi: 10.1038/oby.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: The Danish Diet, Cancer, and Health Study. Am J Med. 2005;118:489–495. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118:978–980. doi: 10.1016/j.amjmed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Mealing NM, Banks E, Jorm LR, Steel DG, Clements MS, Rogers KD. Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs. BMC Med Res Methodol. 2010;10:26. doi: 10.1186/1471-2288-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Survey . Summary of Results, 2007–2008 (Reissue) Australian Bureau of Statistics: Canberra, ACT, Australia; 2010. [Google Scholar]
- Quan H, Parsons GA, Ghali WA. Assessing accuracy of diagnosis-type indicators for flagging complications in administrative data. J Clin Epidemiol. 2004;57:366–372. doi: 10.1016/j.jclinepi.2003.01.002. [DOI] [PubMed] [Google Scholar]
- Teng TH, Finn J, Hung J, Geelhoed E, Hobbs M. A validation study: how effective is the Hospital Morbidity Data as a surveillance tool for heart failure in Western Australia. Aust N Z J Public Health. 2008;32:405–407. doi: 10.1111/j.1753-6405.2008.00269.x. [DOI] [PubMed] [Google Scholar]
- Ruigomez A, Martin-Merino E, Rodriguez LA. Validation of ischemic cerebrovascular diagnoses in the health improvement network (THIN) Pharmacoepidemiol Drug Saf. 2010;19:579–585. doi: 10.1002/pds.1919. [DOI] [PubMed] [Google Scholar]
- Boyle CA, Dobson AJ. The accuracy of hospital records and death certificates for acute myocardial infarction. Aust N Z J Med. 1995;25:316–323. doi: 10.1111/j.1445-5994.1995.tb01896.x. [DOI] [PubMed] [Google Scholar]
- Szklo M, Nieto FJ.Epidemiology Beyond the Basics2 edn.Jones and Barlett Publishers: Sudbury, ON, Canada; 2007 [Google Scholar]
- Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Ng SP, Korda R, Clements M, Latz I, Bauman A, Bambrick H, et al. Validity of self-reported height and weight and derived body mass index in middle-aged and elderly individuals in Australia. Aust N Z J Public Health. 2011;35:557–563. doi: 10.1111/j.1753-6405.2011.00742.x. [DOI] [PubMed] [Google Scholar]
- Rossi R, Iaccarino D, Nuzzo A, Chiurlia E, Bacco L, Venturelli A, et al. Influence of body mass index on extent of coronary atherosclerosis and cardiac events in a cohort of patients at risk of coronary artery disease. Nutr Metab Cardiovasc Dis. 2011;21:86–93. doi: 10.1016/j.numecd.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Darvall KA, Sam RC, Silverman SH, Bradbury AW, Adam DJ. Obesity and thrombosis. Eur J Vasc Endovasc Surg. 2007;33:223–233. doi: 10.1016/j.ejvs.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Williams PT. Evidence that obesity risk factor potencies are weight dependent, a phenomenon that may explain accelerated weight gain in western societies. PLoS One. 2011;6:e27657. doi: 10.1371/journal.pone.0027657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.