Abstract
With the use of planar substrates and collagen gels, the field of mechanotransduction has focused on the role of extracellular matrix stiffness, mechanical tension, and TGF-β1 in generating a more contractile fibroblast. However, little is known about the role of cell-cell interactions in inducing cellular contraction. We used 3-dimensional self-assembled microtissues, in which cell-cell interactions dominate, and a recently developed cell power assay (an assay for mechanotransduction) to quantify the effects of TGF-β1 vs. the heterotypic cell interface on the power exerted by pure normal human fibroblast (NHF) and pure rat hepatocyte 35 (H35) microtissues and their mixes. As a control, we found that TGF-β1 only doubled the power output of pure NHF and pure H35 microtissues, whereas the heterotypic environment resulted in a 5-fold increase in cell power (0.24±0.05 to 1.17±0.13 fJ/h). Seeding TGF-β1-treated NHFs with untreated H35 cells demonstrated that the heterotypic environment and TGF-β1 synergistically increase cell power by 22× by maximizing heterotypic cell interactions. Using a mathematical simulation of stress generation, we showed that tensile forces can be enhanced by heterotypic cell interactions. These data render a new understanding of how heterotypic cell interactions may increase cellular force generation during wound healing.—Youssef, J., Chen, P., Shenoy, V. B., Morgan, J. R. Mechanotransduction is enhanced by the synergistic action of heterotypic cell interactions and TGF-β1.
Keywords: 3-dimensional cell culture, cell-cell adhesion, cellular mechanics, multicellular aggregate, wound healing
Cell-cell interactions are of wide fundamental importance to a myriad of processes that occur during development, wound healing, and metastasis. In addition to generating biochemical signals that trigger intracellular cascades, cell-cell interactions generate and sense mechanical forces, and these processes are important for controlling the behavior of cells and the surrounding tissue (1). This field of mechanotransduction is examining the effects of various mechanical forces including adhesive forces (e.g., cadherins) and tensile forces, (e.g., myosin contraction) as well as the effects of the stiffness of cell types and their surrounding extracellular matrix (ECM). Compelling evidence has accumulated that mechanical forces not only mediate cell signaling but also direct morphogenesis and cell migration and are altered in certain disease states, such as metastasis and fibrosis (2–8).
In a recent study, we reported on an assay to quantify the collective forces that drive cell aggregation and the self-assembly of 3-dimensional (3D) microtissues (9). This complex process is now thought to be driven by numerous factors, including the number of surface adhesion proteins, cytoskeletal motors, and metabolic rate. Conversely, it is conceivable that self-assembly is opposed by other factors, such as cell stiffness, intransient receptor binding, and other sources of friction. Our cell power assay measures all of these events by quantifying the work that cells do against the force of gravity as they self-assemble a toroid that ascends a cone-shaped peg. We used the assay to quantify the power associated with the self-assembly of two cell types, normal human fibroblasts (NHFs) and the rat hepatocyte 35 (H35) cell line and to quantify the contribution of Rho kinase (ROCK)-mediated cell contraction to the assembly of these cells. Here, we have used this assay to measure power behind the assembly of mixed (NHF/H35) microtissues and examined the role of heterotypic adhesion in generating cell tension and creating a more active cell. We identified the heterotypic environment as a very potent inducer of cell-mediated tension and found its contribution to cell power to be significantly greater than that of a very well-known inducer of contractility, TGF-β1. Further demonstrating the importance of heterotypic cell-cell interactions, we found that when heterotypic interactions were increased, by changing the ratio of NHFs to H35 cells and/or by treating the NHFs with TGF-β1, cell power was substantially increased. Mathematical simulation of stress distribution shows that tensile forces can be enhanced and further propagated over longer distances because of this heterotypic interface. With relevance to wound healing and fibrosis, these data imply that the initial heterotypic interactions between fibroblasts and parenchymal cells may be more important than TGF-β1 in the activation of fibroblast and the generation of tension in tissue.
MATERIALS AND METHODS
Micromold design and gel casting
Toroid molds suitable for side-view microcopy were designed as described previously (9). Toroid molds were designed using the computer-aided design (CAD) software Solid Works (Solid Works Corp., Concord, MA, USA). The mold was designed with 12 features to create wells. Each feature is a rounded edged (350 μm in diameter) cylinder (1.1 mm) with a cone indent in the center. The slope of the central cone was 65°, and it was 650 μm in diameter. CAD files were used to produce thermowax molds with a rapid prototyping machine (3D Systems Corp., Valencia, CA, USA).
Wax molds were used to cast 13% polyacrylamide gels. Gels were removed from wax molds and transferred to 6-well culture plates. Each of the resulting wells was a circular trough confined by the hydrogel wall at the outer edge of the trough and by a conical peg on the inner edge of the trough. The gels were rinsed with fresh culture medium and then were equilibrated overnight at 37°C in 4 ml of DMEM supplemented with 1% penicillin/streptomycin. After equilibration, the medium was removed, and the gels were rinsed with fresh medium.
Cell culture and gel seeding
NHFs (passages 4–10), derived from neonatal foreskins, and H35 cells (passages 5–11) were grown in T-175 flasks in DMEM with 10% FBS and 1% penicillin/streptomycin at 37°C and 10% CO2. Cells were removed from flasks using a standard trypsin process. In brief, cells were exposed to 0.05% trypsin for 10 min, quenched with serum-containing medium, spun down at 800 rpm for 6 min, resuspended in a known volume of medium, and counted using a hemocytometer. Then 70 μl of cell solution was added to each hydrogel. After 30 min, 4 ml of fresh medium was added to each of the wells. After seeding, images of the samples were taken every 2 h for 8 h. Pure NHF microtissues were seeded with 10,500, 21,000, 25,000, or 35,000 cells/well. Heterotypic microtissues were seeded at ratios of 1:1, 2:3, 1:4, 1:6, 1:10, 1:16, and 1:20 (NHF/H35). The seeding per well for heterotypic samples was kept constant at 21,000 cells/well. For TGF-β1 experiments, NHFs and H35 cells were incubated for 48 h in DMEM with 10% FBS, 1% penicillin/streptomycin, and 5 ng/ml human recombinant TGF-β1 (Invitrogen, Carlsbad, CA, USA), passed according to standard protocol, and seeded at 21,000 cells/well. NHFs treated with TGF-β1 (NHFTGF-β1 cells) coseeded with H35 cells were seeded in DMEM with 10% FBS and 1% penicillin/streptomycin without additional TGF-β1.
For sorting experiments, NHFs and H35 cells were incubated with 2.5 μM CellTracker Red CMPTX and CellTracker Green CMFDA (Invitrogen), respectively, in serum-free medium for 30 min. After incubation, the dye was aspirated, and the cells were equilibrated in serum medium for 1 h before passing. For NHFTGF-β1:H35 sorting experiments, NHFs were incubated for 48 h in DMEM with 10% FBS, 1% penicillin/streptomycin, and 5 ng/ml TGF-β1. After incubation, the NHFTGF-β1 and H35 cells were fluorescence-labeled and passed as described previously.
To investigate the effect of paracrine factors, we collected the media from homotypic toroids containing 10,500 H35 cells and from homotypic toroids containing 10,500 NHFs. We then took these conditioned media and seeded 10,500 NHFs with the medium collected from the H35 cells and also seeded 10,500 H35 cells with the medium collected from the NHFs. We then analyzed power at 4 h because this is the time when we found enhanced power for heterotypic toroids, which contained 10,500 H35 cells and 10,500 NHFs (1:1 samples).
To determine whether extracellular calcium disintegrated the microtissues, we cultured 1:1 (NHF:H35) microtissues for 4 h. Culture medium was removed, and microtissues were washed with PBS and then incubated overnight at 37°C in 5mM EDTA in PBS. Control samples were kept in regular culture medium or PBS.
Microscopy and image analysis
Conventional view fluorescent and phase images were captured using Carl Zeiss Axio Observer.Z1 with an AxioCam MRm camera (Carl Zeiss MicroImaging, Thornwood, NY, USA). To capture side view images, a Mitutoyo FS-110 microscope (Mitutoyo, Kawasaki, Japan) was modified to lie on its back, and a translational stage was added to hold samples. Samples were imaged in bright field through the eyepiece of the microscope. ImageJ software (U.S. National Institutes of Health, Bethesda, MA, USA) was used to measure the height of the toroid, the major radius, and the minor radius of the toroid.
Immunohistochemistry and confocal microscopy
Before passing, NHFs were incubated with CellTracker Red CMPTX in serum-free DMEM for 30 min. Fluorescence-labeled NHFs were seeded with unlabeled H35 cells. At 8 h postseeding, microtissues were fixed overnight in 4% paraformaldehyde. Samples were then rinsed 3 times with 0.002% Triton X-100 and permeabilized for 6 h in 0.5% Triton X-100. Microtissues were then incubated with 1 ml of 300 nM DAPI dihydrochloride and Oregon Green 488 Phalloidin (Invitrogen) for 1 h. Confocal images were captured with a Zeiss LSM 510 confocal microscope (Carl Zeiss MicroImaging).
Principle stress modeling
To model the tensile stresses generated by the contractility of the NHFs in homotypic and heterotypic environments, we performed finite element simulations by assuming linear elastic constitutive relations (Young's modulus E=2 kPa and Poisson's ratio=0.5; ref. 5) for both NHFs and H35 cells. The stresses in the toroids were computed in the finite element framework using the package Abaqus 6.10 (Simulia, Providence, RI, USA). Because there are no constraints along the z direction and because the thicknesses of cell aggregates are smaller than their lateral dimensions in the x–y plane, plane stress elements CPS3 were used in all the finite element simulations. The peg was assumed to be rigid, and the contact between the peg and the cells was modeled using normal hard-contact elements. From a mechanistic perspective, the deformation and stresses created in an actin network by myosin motors can be modeled by treating the motors as force dipoles (10–13). This is because the motors exert equal but opposite forces along the actin filaments. When a large number of these motors are involved as in the case of cell aggregates, a coarse-grained description based on contractile strain, which gives the measure of the dipole strength per unit volume can be adopted. Mathematically, the elastic fields arising from the contractile strain due to myosin motors is similar to the fields created by sources of internal stress in solid materials, e.g., temperature fields, where thermal strain leads to the body forces (14). In our simulations, thermal strain induced by the spatially varying temperature fields, implemented in Abaqus version 6.10, is used to model the contractility in the cell aggregates. In all the simulations, the contractile strain in NHFs is assumed to be uniform and isotropic (magnitude=0.01), whereas the contractile strain in the H35 cells is assumed to be negligible. To investigate the effect of shape and stiffness of the cells on the enhancement of stresses in heterotypic mixes, we considered the changes in stress with NHF aspect ratios of 2 and 5 (major/minor radius) and H35 stiffness increased 5-fold.
RESULTS
We measured the range of power exerted by increasing numbers of NHFs (>3-fold) in a homotypic environment by seeding the cells into nonadhesive hydrogels with toroid recesses, each with a central cone (65° slope). Cells settled and formed cell-cell adhesions that drove the toroid-shaped microtissue up the cone. We calculated the power necessary to move the NHF toroid up the cone as P = ΔW/Δt, where ΔW is work performed against gravity to move a toroid of a known mass to a given height, and Δt is the time over which the work is performed (9). NHF toroid height (Fig. 1) did not change with increasing NHF cell numbers (186±19 μm, 2 h). Toroid power increased linearly as cell numbers increased, but power per NHF (cell power) was independent of cell numbers, with a peak cell power of 0.22 ± 0.02 fJ/h (2 h) and an average cell power (over all time points) of 0.16 ± 0.03 fJ/h.
Figure 1.
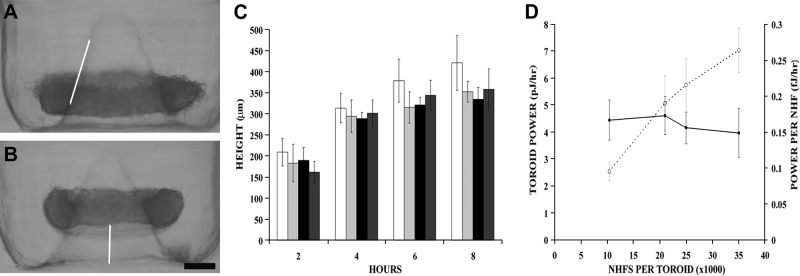
Toroid height and NHF cell power were constant regardless of cell numbers. A, B) NHFs were seeded into toroid micromolds with increasing cells per toroid. Side view images of the toroids were taken at 2 h (A) and 4 h (B). As early as 2 h, the toroid had begun to move up the cone (line added to show slope) and continued up the cone. C) Toroid height was measured from the bottom of the well to the bottom of the toroid at 2-h intervals for 8 h for 10,500 (open bars), 21,000 (light shaded bars), 25,000 (solid bars), and 35,000 (dark shaded bars) cells/toroid. D)Toroid power (○) was directly proportional to the number of cells within the toroid (R2=0.97), whereas cell power (●) was constant over the range of cell numbers tested (P>0.05). n = 5, 8, 7, and 4 for the 10,500, 21,000, 25,000, and 35,000 cells/toroid samples, respectively. Scale bar = 200 μm.
To understand the effects of TGF-β1 on power, NHFs were treated with TGF-β1 and seeded into the toroid recesses. As early as 2 h, height of the NHFTGF-β1 toroid increased 2-fold, resulting in a significant increase in toroid power (9.82±1.32 vs. 5.08±1.04 pJ/h for controls; Fig. 2). Peak cell power (2 h) for NHFTGF-β1 cells was 0.47 fJ/h (2 h), and average cell power was 0.16 ± 0.21 fJ/h. TGF-β1 also increased the power of homotypic toroids of H35 cells. Untreated H35 toroids required 48 h to move the same distance that NHF toroids moved in 2 h (9). The effects of TGF-β1 on H35 cells were evident at 24 h. H35TGF-β1 toroid and cell power both doubled to 0.45 ± 0.08 pJ/h and 0.022 ± 0.004 fJ/h, respectively, compared with untreated controls.
Figure 2.
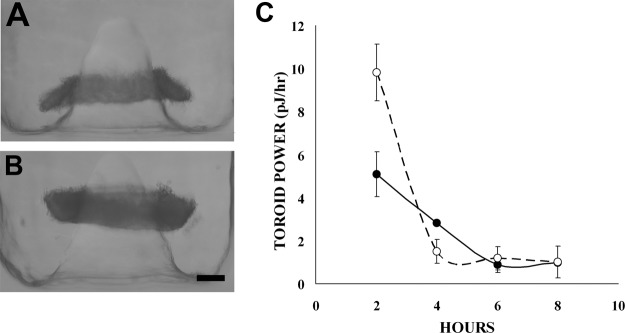
TGF-β1 treatment increased NHF power. A, B) NHFs were treated for 48 h in 5 ng/ml TGF-β1 and then were seeded into the toroid recess. After 2 h, toroids of NHFs treated with TGF-β1 (B) moved twice as far up the peg as untreated NHFs (A). C) TGF-β1 treatment (○) resulted in doubling in cell power (P<0.05) relative to that of the untreated controls (●). n = 8/group. Scale bar = 200 μm.
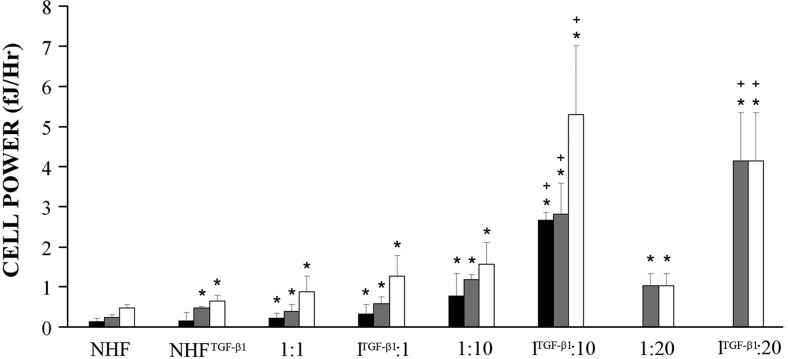
To examine cell power in a heterotypic environment, mixtures of NHFs and H35 cells were seeded with the total number of cells per toroid held constant (∼21,000). Heterotypic toroids moved at different rates and reached different heights (Supplemental Fig. S1). Power of the heterotypic toroids decreased as the percentage of NHFs decreased, and there was a delay to reach peak toroid power (Supplemental Fig. S1). To determine the effect of the heterotypic environment in conjunction with TGF-β1 treatment, NHFTGF-β1 cells were mixed with untreated H35 cells at varying ratios. All treated heterotypic toroids moved further up the peg with the 1:1, 1:10, and 1:20 samples moving 1.4, 4.4, and 6.3 times higher than their respective controls.
Interestingly, when NHFs or NHFTGF-β1 cells were seeded with H35 cells, there was enhanced power. To make quantitative comparisons of the enhancement in cell power due to the heterotypic environment, we calculated the projected power of a toroid and compared it with its actual measured power to derive a value for enhanced toroid power (Table 1). For each mixture, because the power of a homotypic H35 toroid is undetectable in 8 h, projected toroid power was calculated from the number of NHFs present in the mix multiplied by cell power value as measured in the corresponding homotypic environment (NHF or NHFTGF-β1). The enhanced toroid power can be attributed to the increased activity of one of the two cell types in the mix (NHF or H35), or it can be attributed to the heterotypic interface where both cell types interact. Because the homotypic H35 toroid power is undetectable at 8 h, we first looked at the resulting NHF cell power as if NHFs were the sole power generator in the system. This is an upper bound to just how powerful the NHF could become. Surprisingly, NHF cell power increased as the percentage of NHFs decreased, with the 1:10 ratio exerting the greatest peak power per NHF (1.17±0.13 fJ/h). This NHF cell power value in the heterotypic environment was 5 times greater than NHF cell power in the homotypic environment (100% NHFs; 0.24 fJ/h), and 2.5 times greater than the effect of TGF-β1 treatment.
Table 1.
Heterotypic toroids have enhanced power
| Heterotypic toroids | Measured toroid power (pJ/h) | Projected toroid power (pJ/h) | Enhanced toroid power (pJ/h) | NHF has enhanced power (fJ/h/NHF) | Relative increase in NHF cell power | H35 has enhanced power (fJ/h/H35) |
|---|---|---|---|---|---|---|
| 1:1 (NHF:H35) | 4.02 | 2.50 | 1.52 | 0.39 | 1.6× | 0.15 |
| 1:10 (NHF:H35) | 2.21 | 0.45 | 1.75 | 1.17 | 4.9× | 0.09 |
| 1:20 (NHF:H35) | 1.01 | 0.24 | 0.77 | 1.02 | 4.2× | 0.04 |
| 1:1 (NHFTGF-β1:H35) | 6.05 | 4.90 | 1.16 | 0.58 | 2.4× | 0.11 |
| 1:10 (NHFTGF-β1:H35) | 5.30 | 0.89 | 4.41 | 5.30 | 22.1× | 0.23 |
| 1:20 (NHFTGF-β1:H35) | 4.10 | 0.47 | 3.63 | 4.13 | 17.2× | 0.18 |
Measured toroid power was the toroid power exhibited at the time of peak power for each of the samples (1:10 is the combination of 6 and 8 h because there were 2 peaks in power). Projected toroid power equals the power per NHF (homotypic toroid) or TGF-β1-treated NHFs multiplied by the number of NHFs in the mixed toroid. Enhanced toroid power equals the difference between the measured toroid power and the projected toroid power. When treated with TGF-β1 and in the heterotypic environment, NHFs are 22 times more powerful if enhanced power is distributed to NHFs. If enhanced power is distributed to H35s, the H35s have enhanced power in the range of untreated NHFs.
Likewise, NHFTGF-β1 cell power increased in the heterotypic environment as the percentage of NHFTGF-β1 cells decreased, with the 1:20 mix exerting the greatest peak cell power (4.13±1.22 fJ/h) and the 1:10 mix exerting the greatest total cell power of 5.3 ± 1.32 fJ/h between 4 and 8 h (Fig. 3), meaning that the cell power of the NHFTGF-β1 cells is increased by >11 times in the heterotypic environment. When compared with untreated NHFs in a homotypic toroid, TGF-β1 treatment in conjunction with the heterotypic environment causes cell power to increase by >22 times. Because TGF-β1 alone only doubles cell power, and the heterotypic environment alone only increases power 5-fold, the combination of these two factors is synergistic.
Figure 3.
NHF cell power increased with TGF-β1 and the heterotypic environment. Average cell power (solid bars), peak cell power (shaded bars), and total cell power (open bars) were calculated for each sample with the assumption that all power is generated by the NHFs. Average cell power was calculated as the mean power per cell value for each sample (over the time during which the samples exerted power), peak cell power is the maximum cell power output of each sample at a single time point, and total cell power is the sum of the cell power over the duration of the experiment. *P < 0.05 vs. homotypic NHFs; +P < 0.05 for TGF-β1 treatment vs. untreated controls; n ≥ 6/group.
Alternatively, if the enhanced toroid power is attributed solely to the H35 and is distributed among all H35 cells in the toroid, the power per H35 is increased from near 0 (homotypic environment) up to 0.23 fJ/h (1:10 mix). This enhanced cell power value for an H35 is very large, considering that baseline H35 cell power in a homotypic toroid is very small (0.022 fJ/h, detectable 24 h after seeding; ref. 9) and that such an enhanced value would be in the range of untreated NHFs in a pure-NHF toroid.
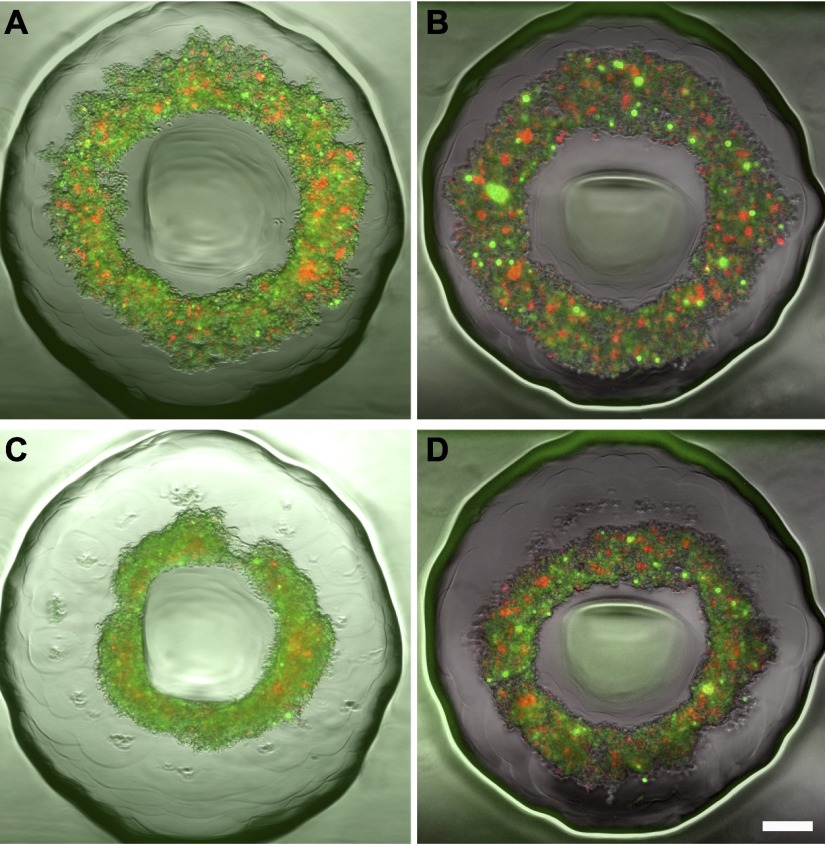
For each of the mixes, there was a delay in toroid motion before peak power was reached. For homotypic NHF toroids, peak power was reached at the first time point (2 h). As the percentage of NHFs in the mixes decreased, the time to reach peak power increased. To determine whether this delay was due to the time for cells to self-sort, NHFs and H35 cells were fluorescently labeled, and sorting was assessed (Supplemental Fig. S2). When the proportion of NHFs was high, the cells self-sorted within 8 h, with NHFs forming a contiguous inner toroid with a circumferential coating of H35 cells. When the percentage of NHFs was reduced (<1:4), the NHFs no longer sorted within the 8 h nor formed a contiguous inner toroid but were distributed throughout the toroid. In the 1:10 mix, sorting was evident after 24 h, with the NHFs clustering into pockets centrally located in the toroid. Interestingly, this sorting was eliminated by treating the NHFs with TGF-β1 (Fig. 4). Thus, neither sorting nor the formation of a contiguous NHF toroid is necessary (or the mechanism) for the movement of the toroid at low percentages of NHFs, and TGF-β1 treatment inhibits self-sorting.
Figure 4.
Delay in peak power was not related to the time to self-sort, and TGF-β1 treatment decreased sorting. NHF/NHFTGF-β1 (red) and H35 cells (green) were seeded at a 1:10 ratio. For untreated samples (A, C), sorting is visible at 24 h (C) with NHFs (red) taking the interior position and H35 cells (green) the exterior, but sorting was absent in the peak power time (A). TGF-β1 treatment of NHFs prevented sorting (B, D), because sorting was absent at both the peak power time (B) and the 24-h time point (D). Scale bar = 200 μm.
To examine whether enhanced power was transduced through cell-cell interactions, 1:1 (NHF:H35) microtissues were cultured for 4 h and then incubated in EDTA to destabilize the calcium-dependent cell-cell adhesions. Control samples were kept in regular culture medium or PBS. Microtissues incubated in EDTA disintegrated into monodispersed cells, whereas control samples in both medium and PBS maintained their integrity, indicating that cell-cell junctions are needed for mechanotransduction.
To determine whether enhanced power was due to paracrine factors secreted by NHFs and/or H35 cells, the media from homotypic toroids containing 10,500 H35 cells and from homotypic toroids containing 10,500 NHFs were collected. We then took these conditioned media and seeded 10,500 NHFs with the medium collected from the H35 cells and also seeded 10,500 H35 cells with the medium collected from the NHFs. Power was analyzed at 4 h because this is the time when the heterotypic samples that contained 10,500 H35 cells and 10,500 NHFs (1:1 samples) had enhanced power. We found that, similar to control H35 microtissues, H35 cells seeded in NHF conditioned medium had no power. For NHF microtissues seeded in H35 conditioned medium, there was no enhancement in power compared with controls (P=0.6). In particular, the power for control NHF microtissues was 0.19 ± 0.06 fJ/h/cell, and power for NHF microtissues in conditioned medium was 0.18 ± 0.03 fJ/h/cell.
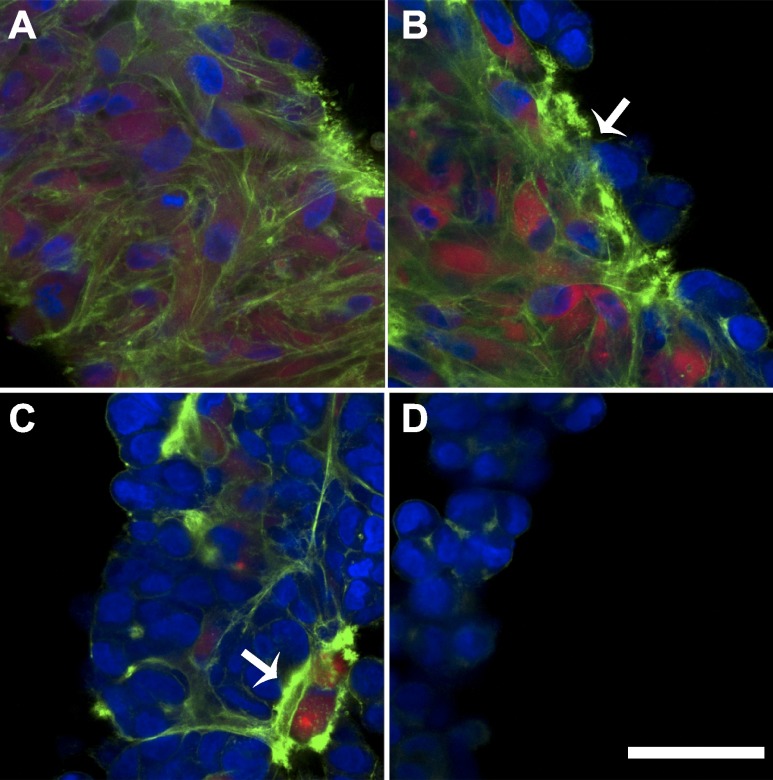
To investigate cytoskeletal changes in the heterotypic environment, toroids were stained for F-actin. Confocal images revealed that the gross cytoskeletal architecture was very different for homotypic vs. heterotypic toroids (Fig. 5). Homotypic NHF toroids had a continuous and dense F-actin network spanning the thickness of the toroid, whereas homotypic H35 toroids had weak punctate F-actin staining only at cell junctions. Heterotypic toroids (1:1 and 1:4) exhibited a dense F-actin network throughout the central NHF portion of the toroid up to the H35 junction. The 1:10 mix had dense F-actin networks focused around the NHFs that were randomly distributed through the thickness of the toroid, because NHFs had not yet sorted to the center.
Figure 5.
Cytoskeletal architecture was altered in the heterotypic environment. Pure NHF (A), 1:1 (B), 1:10 (C), and pure H35 (D) microtissues were fixed and stained after 8 h. The nuclei and F-actin of all cells were labeled with DAPI (blue) and phalloidin (green), respectively, and NHFs were labeled with CellTracker Red before self-assembly. H35 cells are identified as cells with a blue nuclei without red staining. Pure NHF microtissues had a dense, continuous F-actin network, whereas pure H35 microtissues had a weak punctate F-actin signal. In heterotypic mixes, there was an enhanced F-actin signal at the junction between NHFs and H35 cells, which met the nuclei of the H35 cells. Scale bar = 50 μm.
H35 cells in direct contact with NHFs had a stronger F-actin signal than either H35 cells that were not in contact with NHFs or H35 cells in a homotypic toroid. In the 1:20 mix, there were three different local cellular environments with distinct F-actin staining (Supplemental Fig. S3). One region had a high density of NHFs (Supplemental Fig. S3A, I) with an F-actin network similar to that of a homotypic NHF toroid. Another region had a high density of H35 cells distant from NHFs (Supplemental Fig. S3A, II) and an F-actin network similar to that of a homotypic H35 toroid. A third region had a single NHF surrounding a cluster of H35 cells (Supplemental Fig. S3A, III) with a distinct cytoskeletal structure at the NHF/H35 interface, a strong F-actin signal extending from the NHF to the nuclei of the surrounding H35 cells.
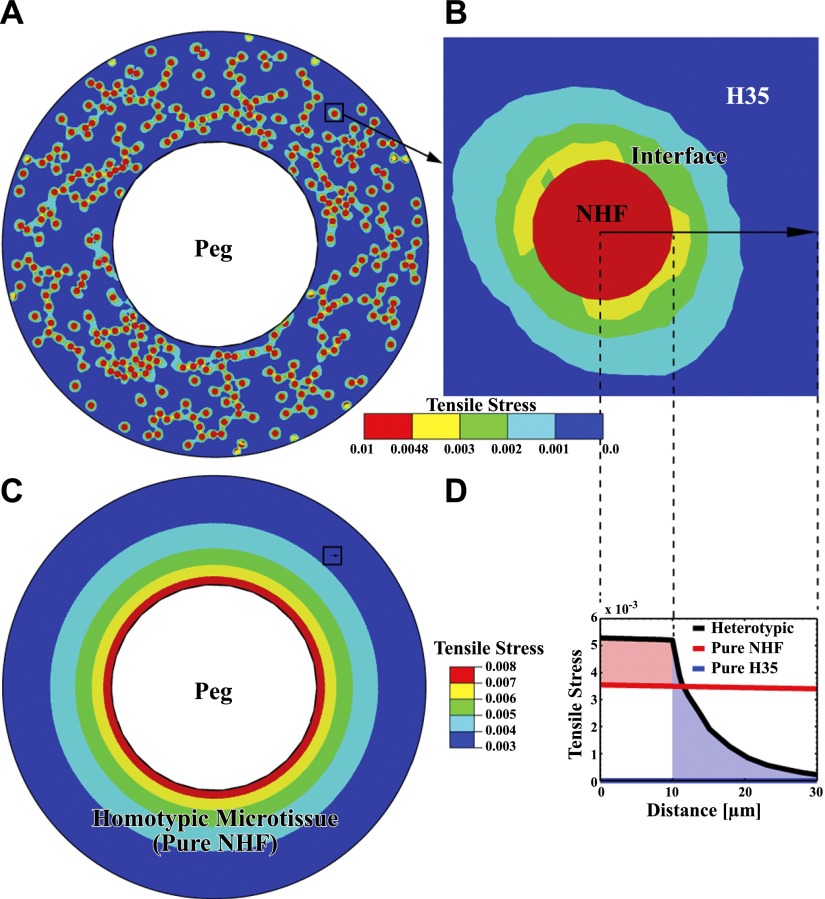
Tensile stress modeling demonstrated that the heterotypic environment increases stresses. In homotypic NHFs, the peg constrains the tissue from contracting, leading to tensile stresses that decrease in magnitude radially outward from the surface of the peg (Fig. 6). For the heterotypic tissue, in addition to the constraints from the peg, there is a new and more significant mechanism generating tensile stresses. Because the H35 cells that surround the NHFs are less contractile, they act as resistance to the NHFs that are trying to contract. This generates tensile stresses both in the NHFs and the H35 cells (refer to Fig. 6B). The tensile stresses decrease from the NHF boundary to the surrounding H35 cells. The tension in both the NHF and the adjacent H35 cells is significantly higher than the corresponding tension in the respective homotypic environments.
Figure 6.
Simulation of stress distribution in heterotypic microtissues demonstrates that the heterotypic environment increased stress in the NHF and the surrounding H35 cells. A) For the 1:10 ratio, NHFs (red circles) are randomly distributed with the H35 cells in a toroid with peg and toroid radii of 325 and 675 μm, respectively. The contractility of NHFs results in tension in the NHF, the NHF/H35 heterotypic interfacial region, and the H35. B) Variation in tensile stresses near one of the NHFs surrounded by H35 cells (black square in A). Tension induced by the contractility of NHFs decreases from the interface of the NHF with the surrounding H35 cells. Note that the tension in the heterotypic interface region is much larger than that in the surrounding H35 region (blue). C) Tensile stresses in homotypic microtissues decrease radially from the peg and are uniform circumferentially. D) Comparison of tensile stresses of heterotypic and homotypic microtissues in the same location (black squares in A, C). Black curve corresponds to stresses in the heterotypic tissue; red and blue curves represent stresses in homotypic NHF and H35 tissues of the same dimensions. Red and blue shaded regions indicate enhancement in tension for the NHFs and H35 cells in the heterotypic tissue, compared with tension in the corresponding homotypic environments. Tensile stress plots correspond to the maximum in-plane principal stress and are normalized by the elastic modulus of cells. Arrow in B denotes the path along which the distance is measured. Decay of tension in the heterotypic interface region is consistent with the F-actin distribution shown in Fig. 5.
Enhancement of mechanical tension would also induce actomyosin activity in both cell types, which would increase overall power in the heterotypic environment. This finding is consistent with F-actin distribution, which shows that the cortical actin of the H35 is rearranged and more aligned with the radial actin of the adjacent NHFs. Changes in tensile stresses in a heterotypic environment can be further enhanced for both NHFs and H35 cells by considering factors such as the shape of the NHFs and differences in elastic stiffness of the cell types (Supplemental Table S1). Modeling the NHF as an elongated ellipsoid (aspect ratio=5) doubles the tensile stress, and if the stiffness of the H35 cells surrounding the NHFs is larger by a factor of 5, than the tensile stress is increased 4-fold. For a given level of contractile strain in the NHF, the tensile stress generated in the heterotypic environment will depend on the shape of the NHFs. For an elliptic shape, the largest (tensile) principal component of stress in the NHF increases with increasing aspect ratio compared with the principal stresses in a circular shape. Because an increase in tensile stresses leads to an increase in contractility, our calculations show that an enhancement in power can be expected in more elongated NHFs.
DISCUSSION
Cell-mediated mechanical forces, implicated in tissue remodeling and wound healing, are often the focus of pathological conditions such as fibrosis (15–17). Much work has focused on the contractile forces of cells embedded in an ECM, and quantitative studies have helped to define the complex interplay between matrix composition and stiffness and the role of growth factors in regulating contractile forces in 3D analogs (15, 18–20). However, in many circumstances, cells exert contractile forces on other cells, and yet there is little quantitative understanding of the factors influencing direct cell-cell mechanotransduction. In fact, much of the work with cells in ECM analogs is assumed to be applicable to cell-cell interaction. Here, we quantify the forces of cell-cell interactions and show that the effect of heterotypic cell interactions is significantly greater than the effect of TGF-β1, a well-known inducer of cell contractility.
Previously, we have shown that monodispersed cells seeded onto nonadhesive hydrogels with toroidal-shaped recesses aggregate and form a multicellular toroid that moves up the central cone (21). The complex cell-cell interactions driving this simple event were quantified in terms of power and shown to vary significantly among cell types. As a tool for quantifying cell-cell aggregation, the toroid/cone assay does not interfere with cell function via cell contact and requires little, if any, calibration because it relies only on gravity, a well-characterized external load. Cell power is a quantitative measure of the multicomponent system (mechanical, chemical, and surface energy) that drives toroid motion up the cone. In addition to gravity, the cell power measurement also takes into account all forces (e.g., friction) that oppose the motion of the toroid up the cone. The assay measures self-assembly in terms of work performed against gravity in a consistent and well-defined environment (i.e., nonadhesive synthetic hydrogel cone of defined geometry). These consistencies in the load and environmental test-bed conditions enable precise quantitative comparisons to be made between cell types as well as the quantification of the contributions of proteins or protein systems to the complex process of cell aggregation. We showed that more than 50% of the power of a toroid could be reduced by blocking ROCK-mediated contraction (9). Here, we have used the assay and cell power measurement to quantify the cell-cell mechanics that occur in mixes of two cell types in a 3D cellular environment.
In this study, when small numbers of NHFs were mixed with H35 cells to form heterotypic toroids, there was a significant increase in cell power due solely to the heterotypic environment. NHF cell power in the heterotypic environment was 5 times greater than that in the homotypic environment. In comparison, the cell power of NHFTGF-β1 increased only 2-fold compared with cell power for the NHF in the homotypic environment. Interestingly, the maximal increase in NHF cell power was cell ratio-dependent. NHF cell power in the 1:10 ratio was 3 times higher than that in the 1:1 ratio. The 1:10 ratio approximates the ideal 1:12 ratio in close spheres packing, where one sphere contacts 12 nearest neighbors, maximizing heterotypic cell interactions. Within the toroid, we identified foci of heterotypic interactions by staining for F-actin. NHFs were located at these foci of F-actin staining, and the signal was significantly stronger than that for areas of the toroid where H35 homotypic interactions predominated. The F-actin staining at these heterotypic foci was not confined to just the NHF but extended into neighboring H35 cells, suggesting that they were experiencing increased tension and had a reorganized cytoskeleton (17).
Another interesting finding was that as the number of NHFs decreased, the time to reach peak power also decreased. For the 1:10 mix, power was undetectable for the first 4 h and then rapidly rose to its peak power. One possibility is that this lag was necessary for NHFs to self-sort and form an inner toroid of NHFs within the heterotypic toroid. This was ruled out because these small numbers of NHFs are not able to form a contiguous toroid (9), and cell labeling showed that self-sorting did not coincide with peak power. In addition, self-sorting of NHFTGF-β1 cells was reduced compared with that for untreated NHFs in the heterotypic environment. It is possible that the lag time is required for the changes to occur at the heterotypic interface that will result in increased power. NHFs may require time to sense and adapt to the increased load and/or make cytoskeletal changes at the heterotypic interface. Consistent with this possibility is the observation that after 1 h of contact between a fibroblast and an epitheliocyte, the cortical actin of the epitheliocyte is disassembled and aligned with the radial actin of the adjacent fibroblast (22).
Although the cause of the increased power in the heterotypic environment is unclear, our data suggest that the changes are due to the heterotypic interface between NHFs and H35 cells. The modeling data suggest that when highly contractile fibroblasts are surrounded by noncontractile H35 cells, there is a significant enhancement in stress for both the NHFs and the adjacent H35 cells compared with that in the homotypic environment. The increase in tensile stresses can lead to actomyosin recruitment (23), and the strong foci of F-actin staining suggests that the heterotypic interface causes a reorganization of the actin cytoskeletons of the H35 cells surrounding NHFs. The H35 cortical actin that is now realigned would be part of a new contiguous hepatofibro contractile unit with significantly more contractile force that could transmit stresses over greater distances. In this heterotypic contractile unit, both hepatocytes and fibroblasts could make contributions to the enhanced power, perhaps via an increase in efficiency or recruitment of a power source that is only tapped through heterotypic interactions. Whatever the mechanism, the time for these changes is fairly rapid because the peak power of the 1:10 toroid is manifest at 6 h. Although NHFs and H35 cells could secrete growth factors known to activate contractility in the opposite cell type, leading to increased power (24–26), we show that this is a minor contribution, if at all, because power did not significantly increase when the cells were seeded in conditioned medium. Furthermore, F-actin staining is specifically increased only at NHF/H35 interfaces and not at H35/H35 interfaces in the same heterotypic toroid, indicating that it is not soluble factors diffusing through the tissue that cause enhanced power.
Although the effect of the heterotypic environment was greater than that of TGF-β1 treatment, their combined effects were synergistic and resulted in a 22-fold increase in cell power compared with that of NHFs. TGF-β1 is pleiotropic, and its actions on fibroblasts grown on 2D substrates and embedded in 3D gels have been well defined (27–31). The ability of TGF-β1 to induce contractility of the fibroblasts is certainly one means by which power is increased in the homotypic and heterotypic environments. However, increased contractility alone does not explain the synergistic action of TGF-β1 treatment and the heterotypic environment. One possible explanation is that NHFTGF-β1 cells engage in more heterotypic interactions. This is supported by the observation that self-sorting of NHFTGF-β1 cells is reduced compared with that of untreated NHFs in the heterotypic environment. As self-sorting proceeds, NHFs segregate away from H35 cells, and heterotypic interactions of higher power are exchanged with homotypic interactions of lower power. By inhibiting self-sorting, TGF-β1 treatment sustains the heterotypic interactions that lead to the most significant increase in power. This was evident in the 1:10 sample for which there was not a single peak but rather two peaks in power.
The nature of the heterotypic interactions and how they give rise to increased power is unclear, whereas homotypic interactions of fibroblasts are well characterized and include the formation of large, stable cell-cell adherens junctions that transmit contractile stress (6) and cadherin expression that changes from N-cadherin to stronger OB-cadherins (32). In addition to being mechanically coupled, fibroblasts engaged in homotypic interactions that are also electrochemically coupled via gap junctions (6). It remains to be determined how the heterotypic interface is coupled and how this coupling differs from the homotypic interface.
Change in the mechanical environment after tissue injury is an immediate and significant ongoing stimulus for scarring and fibrosis of numerous organs and tissues, including, but not limited to, the liver. Soon after tissue injury, fibroblasts migrate out of the stable, stress-shielding ECM and into a heterotypic environment where they interface for the first time with parenchymal cells, such as the hepatocyte, and the ratio of cell-cell interactions compared with cell-ECM interactions increase (6). Our data suggest that regardless of whether the fibroblasts are 22 times more powerful or the hepatocytes are as powerful as normal fibroblasts, the heterotypic interface is a stimulus significantly greater than TGF-β1, and that it may serve to increase contractility and/or be an initial event activating the fibroblast, which in turn increases stress in the parenchyma, factors that could contribute to tissue fibrosis. The role of TGF-β1 in this early stage is synergistic and serves to sustain and increase these heterotypic interactions. Inhibition of these very early events at the heterotypic interface may be a useful target for an antifibrotic strategy.
Supplementary Material
Acknowledgments
This work was funded in part by the Materials Research Science and Engineering Centers Program of the National Science Foundation (NSF) under award DMR-0520651, NSF CMMI-1129172, and U.S. National Institutes of Health grant NIH R01-EB008664-01A1. J.R.M. has an equity interest in MicroTissues, Inc. This relationship has been reviewed and managed by Brown University in accordance with its conflict of interest policies.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 3D
- 3-dimensional
- CAD
- computer-aided design
- ECM
- extracellular matrix
- H35
- rat hepatocyte 35
- H35TGF-β1
- rat hepatocyte 35 treated with TGF-β1
- NHF
- normal human fibroblast
- NHFTGF-β1
- normal human fibroblast treated with TGF-β1
- ROCK
- Rho kinase
REFERENCES
- 1. Chen C. S., Tan J., Tien J. (2004) Mechanotransduction at cell-matrix and cell-cell contacts. Annu. Rev. Biomed. Eng. 6, 275–302 [DOI] [PubMed] [Google Scholar]
- 2. Makrilia N., Kollias A., Manolopoulos L., Syrigos K. (2009) Cell adhesion molecules: role and clinical significance in cancer. Cancer Invest. 27, 1023–1037 [DOI] [PubMed] [Google Scholar]
- 3. Ingber D. E. (2006) Mechanical control of tissue morphogenesis during embryological development. Int. J. Dev. Biol. 50, 255–266 [DOI] [PubMed] [Google Scholar]
- 4. Krieg M., Arboleda-Estudillo Y., Puech P. H., Kafer J., Graner F., Muller D. J., Heisenberg C. P. (2008) Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 10, 429–436 [DOI] [PubMed] [Google Scholar]
- 5. Discher D. E., Janmey P., Wang Y. L. (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 [DOI] [PubMed] [Google Scholar]
- 6. Hinz B., Gabbiani G. (2003) Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb. Haemost. 90, 993–1002 [DOI] [PubMed] [Google Scholar]
- 7. Albelda S. M. (1993) Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab. Invest. 68, 4–17 [PubMed] [Google Scholar]
- 8. Mammoto T., Ingber D. E. (2010) Mechanical control of tissue and organ development. Development 137, 1407–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Youssef J., Nurse A. K., Freund L. B., Morgan J. R. (2011) Quantification of the forces driving self-assembly of three-dimensional microtissue. Proc. Natl. Acad. Sci. U. S. A. 108, 6993–6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zemel A., Safran S. A. (2007) Active self-polarization of contractile cells in asymmetrically shaped domains. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 76, 021905 [DOI] [PubMed] [Google Scholar]
- 11. Carlsson A. E. (2006) Contractile stress generation by actomyosin gels. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 74, 051912 [DOI] [PubMed] [Google Scholar]
- 12. MacKintosh F. C., Levine A. J. (2008) Nonequilibrium mechanics and dynamics of motor-activated gels. Phys. Rev. Lett. 100, 018104 [DOI] [PubMed] [Google Scholar]
- 13. Chen P., Shenoy V. B. (2010) Strain stiffening induced by molecular motors in active crosslinked biopolymer networks. Soft Matter 2, 355–358 [Google Scholar]
- 14. Timoshenko S. P., Goodier J. N. (1970) Theory of Elasticity, 3rd Ed., McGraw-Hill, New York [Google Scholar]
- 15. Tomasek J. J., Gabbiani G., Hinz B., Chaponnier C., Brown R. A. (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349–363 [DOI] [PubMed] [Google Scholar]
- 16. Desmouliere A., Chaponnier C., Gabbiani G. (2005) Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 13, 7–12 [DOI] [PubMed] [Google Scholar]
- 17. Gabbiani G. (2003) The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 200, 500–503 [DOI] [PubMed] [Google Scholar]
- 18. Hinz B. (2009) Tissue stiffness, latent TGF-β1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr. Rheumatol. Rep. 11, 120–126 [DOI] [PubMed] [Google Scholar]
- 19. Hinz B., Celetta G., Tomasek J. J., Gabbiani G., Chaponnier C. (2001) α-Smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell 12, 2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Freyman T. M., Yannas I. V., Yokoo R., Gibson L. J. (2001) Fibroblast contraction of a collagen-GAG matrix. Biomaterials 22, 2883–2891 [DOI] [PubMed] [Google Scholar]
- 21. Dean D. M., Napolitano A. P., Youssef J., Morgan J. R. (2007) Rods, tori, and honeycombs: the directed self-assembly of microtissues with prescribed microscale geometries. FASEB J. 21, 4005–4012 [DOI] [PubMed] [Google Scholar]
- 22. Omelchenko T., Fetisova E., Ivanova O., Bonder E. M., Feder H., Vasiliev J. M., Gelfand I. M. (2001) Contact interactions between epitheliocytes and fibroblasts: formation of heterotypic cadherin-containing adhesion sites is accompanied by local cytoskeletal reorganization. Proc. Natl. Acad. Sci. U. S. A. 98, 8632–8637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fernandez-Gonzalez R., Zallen J. A. (2009) Cell mechanics and feedback regulation of actomyosin networks. Sci. Signal. 2, pe78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Desmouliere A., Geinoz A., Gabbiani F., Gabbiani G. (1993) Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 122, 103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lamouille S., Derynck R. (2007) Cell size and invasion in TGF-β-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J. Cell Biol. 178, 437–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Birchmeier C., Gherardi E. (1998) Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 8, 404–410 [DOI] [PubMed] [Google Scholar]
- 27. Park J. S., Kim J. Y., Cho J. Y., Kang J. S., Yu Y. H. (2000) Epidermal growth factor (EGF) antagonizes transforming growth factor (TGF)-β1-induced collagen lattice contraction by human skin fibroblasts, Biol. Pharm. Bull. 23, 1517–1520 [DOI] [PubMed] [Google Scholar]
- 28. Montesano R., Orci L. (1988) Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc. Natl. Acad. Sci. U. S. A. 85, 4894–4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen J., Li H., SundarRaj N., Wang J. H. (2007) α-Smooth muscle actin expression enhances cell traction force. Cell Motil. Cytoskeleton 64, 248–257 [DOI] [PubMed] [Google Scholar]
- 30. Roberts A. B., Heine U. I., Flanders K. C., Sporn M. B. (1990) Transforming growth factor-β. Major role in regulation of extracellular matrix. Ann. N. Y. Acad. Sci. 580, 225–232 [DOI] [PubMed] [Google Scholar]
- 31. Vaughan M. B., Howard E. W., Tomasek J. J. (2000) Transforming growth factor-β1 promotes the morphological and functional differentiation of the myofibroblast, Exp. Cell Res. 257, 180–189 [DOI] [PubMed] [Google Scholar]
- 32. Hinz B., Pittet P., Smith-Clerc J., Chaponnier C., Meister J. J. (2004) Myofibroblast development is characterized by specific cell-cell adherens junctions, Mol. Biol. Cell 15, 4310–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.