INTRODUCTION
Tick-borne relapsing fever (TBRF), is caused by several species of Borrelia spirochetes, which are transmitted to humans through the bites of Ornithodoros spp. soft ticks. Wild rodents and insectivores are common reservoir hosts. TBRF is responsible for recurring fever associated with spirochetemia. The epidemiology of TBRF has not been well documented in South America where three endemic ticks are suspected to act as vectors (Guglielmone et al., 2006). Ticks referred as Ornithodoros talaje are prevalent in Ecuador, Colombia, Venezuela, Argentina and Brazil, as well as in Guatemala, Panama, Mexico (Guglielmone et al., 2006). This tick was shown to transmit a relapsing fever Borrelia in Panama by human experimentation (Bates et al., 1921). It has been associated with ‘Borrelia mazzottii’ in Mexico (Davis, 1956). This bacterium has, however, been incompletely described, as neither an isolate nor DNA of this bacterium is available (Davis, 1956). Ornithodoros rudis is found in Ecuador, Colombia, Venezuela, Peru, Paraguay and Brazil, and has been associated with ‘B. venezuelensis’ (Davis, 1955). Also, a borrelia called ‘B. brasiliensis’ has been associated with O. brasiliensis in Brazil (Davis, 1952). All three of these borreliae were incompletely described in the 1950s and neither an isolate nor DNA is currently available. In this work, using specific semi-quantitative real-time polymerase chain reaction (PCR) with original primers pairs and probes, we aimed to detect relapsing fever Borrelia spp. in ticks collected in Bolivia.
METHODS
The area of tick collection was rocky outcrops composed of large blocks and located in the Cochabamba valley (alt. 2500 m) in the central zone of the Eastern Cordillera in the Cochabamba Department (Bolivia), close to the town of Cotapachi (−17°25′47.31″, −66°17′33.80″). In May 2009, a prototype baited trap combining heat, carbon dioxide and attractive odours was evaluated in Bolivia as a new means to capture triatomine bugs. A total of 35 ticks were also captured, stored in 90% ethanol and sent to Marseille, France. Morphological features indicated that the ticks were argasid ticks (soft ticks). Four specimens were sent to Rafaela, Argentina for expert morphological identification (A.A. Guglielmone). DNA was extracted from each tick using a QIAamp kit (QIAGEN, Hilden, Germany). DNA extracted from a nymph was used in a PCR to amplify and sequence a 460-bp fragment of the tick 16S rRNA-encoding gene as previously described (Mangold et al., 1998; Norris et al., 1996). To confirm the species-level identification of the ticks, a 446-bp sequence of the 16S rRNA-encoding gene was obtained and compared to sequences available in GenBank. The following mitochondrial 16S rDNA sequences of argasid ticks available in the GenBank were used for phylogenetic analyses: Argas persicus (GenBank accession number AF001402), A. monachus (EU283344), A. neghmei (DQ295781), Ornithodoros puertoricensis (AF113932), O. rioplatensis (EU283343), O. moubata (L34328), O. porcinus porcinus (L34329) and O. porcinus domesticus (L34330). Sequences for Ornithodoros spp. collected as larvae from Rodentia: Cricetidae were also included in the analysis. There is however no 16S rDNA sequence available for O. talaje. Phylogenetic relationships were analysed using distance measure, and a neighbour-joining tree was generated from Kimura’s two-parameter method. Support for the neighbour-joining topology was tested by boot-strapping over 1000 replicates. The Argas sequences were used as outgroups. All positions containing alignment gaps and missing data were eliminated only in pairwise sequence comparisons (pairwise deletion option).
Borrelia DNA was initially detected using specific semi-quantitative real-time PCR with primers (Bor16S3F, AGC CTT TAA AGC TTC GCT TGT AG; Bor16S3R, GCC TCC CGT AGG AGT CTG G) and a probe (Bor16S3P, 6FAM-CCG GCC TGA GAG GGT GAA CGG) that were designed for the amplification of a 148-bp fragment of a 16S rRNA-encoding gene. All PCR reactions were performed using LightCycler 2.0 equipment and software (Roche Diagnostics GmbH, Mannheim, Germany). Positive samples were used to PCR-amplify a 344-bp fragment of Borrelia spp. flagellin (fla). The amplification and sequencing was performed using primers designed for B. duttonii: Bfpbu (5′-GCTGAAGAGCTTGGAATGCAACC-3′) and Bfpcr (5′-TGATCAGTTATCATTCTAATAGCA-3′) (Fukunaga et al., 2001). The obtained sequences were compared to GenBank.
RESULTS
Morphological identification of ticks determined that they belonged to the Ornithodoros talaje species group (Acari: Ixodida: Argasidae) (Guglielmone et al., 2006). For the phylogenetic analysis, there were a total of 431 positions in the final dataset. There is, however, no 16S rDNA sequence available for O. talaje itself. A phylogenetic tree using a neighbour-joining analysis showed a high bootstrap value (99%) supporting the group of Ornithodoros sp. from Bolivia and two Ornithodoros sp. from Argentina (Mangold, personal unpublished data), both branching with members of the O. talaje group including O. puertoricensis and O. rioplatensis (Fig. 1). The sequence of the 16S rRNA gene fragment from Bolivian ticks has been deposited under the GenBank accession number is JF895756.
Fig. 1.
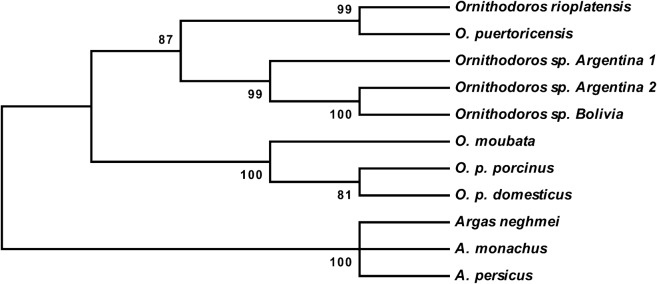
Phylogenetic tree (neighbour-joining analysis) for 13 taxa within Ornithodoros ticks, derived from mitochondrial 16S rRNA gene sequences. The sequence of the 16S rRNA gene fragment from Bolivian ticks branches with members of the O. talaje group including O. puertoricensis and O. rioplatensis.
Two ticks tested positive for Borrelia DNA with a Ct (cycle number at the threshold level of log-based fluorescence) value lower than 36 (approximately 10–20 copies of spacer). These samples were used to PCR-amplify a 344-bp fragment of Borrelia spp. flagellin (fla).
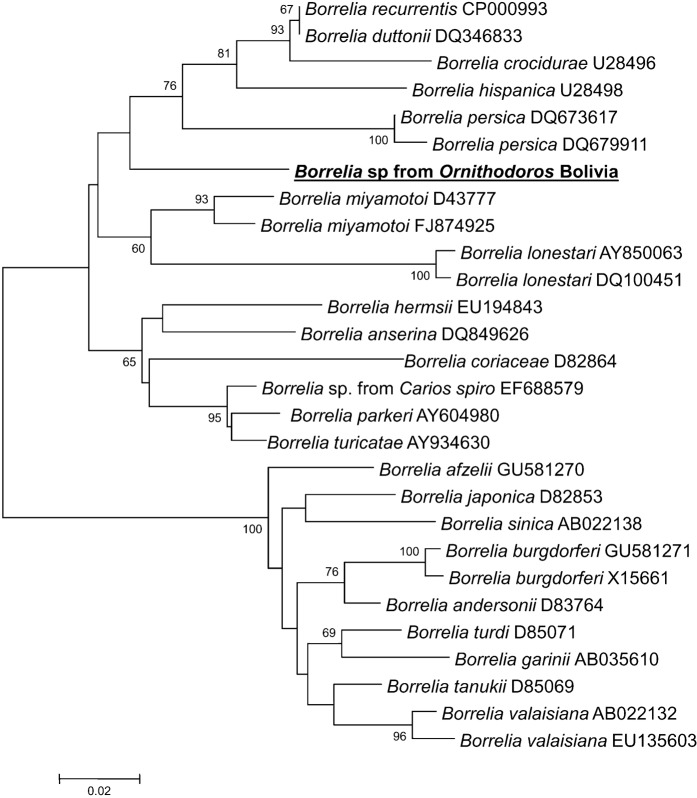
The sequences obtained were identical (GenBank HM583797) and appeared to differ from all corresponding sequences available in GenBank. Those of Borrelia duttonii and B. crocidurae were the closest with 92.4% identity. In a neighbour-joining phylogenetic tree based on the alignments of 302 bp of fla, the sequence clustered with those of relapsing fever Borrelia spp (Fig. 2).
Fig. 2.
Phylogenetic tree based on aligned 302-bp sequences of fla and constructed by the neighbour-joining method. The numbers in the nodes represent bootstrap values when above 60. The tree shows the position of Borrelia identified in Ornithodoros talaje group ticks from Bolivia. The GenBank accession number is indicated at the end of the line for each sequence.
DISCUSSION
Our baited traps captured ticks as well as triatomine bugs, revealing their potential as ‘generalist’ lures for different haematophagous arthropods (Ryelandt et al., 2011). O. talaje ticks have been reported to bite humans (Guglielmone et al., 2006). However, most ticks previously named as ‘talaje’ in the literature have been ascribed to other species, and no new collections of ‘talaje’ are known. Thus, the previously wide distributional range of O. talaje recorded in the literature may actually be restricted to Central America. Given the results of our phylogenetic analysis, our Bolivian ticks may represent a new species belonging to the ‘talaje’ group with other closely related species (Venzal et al., 2008). The systematics of Argasidae remains, however, controversial (Estrada-Peña et al., 2010).
The true burden of TBRF deserves more attention, particularly in tropical and subtropical areas, although it was recently shown to remain a significant problem in Africa (Cutler et al., 2009). Little information is available about the epidemiology of TBRF in Bolivia. In 1994, the results of a seroepidemiological survey of borrelioses conducted in the Cordillera Province, southeastern Bolivia, were published (Ciceroni et al., 1994). Borrelia turicatae and B. parkeri, two agents of TBRF in North America, were used as antigens in an indirect immunofluorescent assay, and sera were also tested after absorption with a Treponema phagedenis extract to eliminate cross-reactivity to common antigens of several bacteria. Anti-B. turicatae and anti-B. parkeri antibodies were detected in 16.1% and 8.2% of the serum samples tested, respectively, and confirmed after absorption in 1.3% and 1.0%, respectively.
The identification of DNA of a novel TBRF agent in ticks collected in the field does not definitely mean that this has significance for human health. We were unable to determine whether the Borrelia detected in the present study was the incompletely described ‘Borrelia mazzottii’ (Davis, 1956). Although the borrelia detected here deserves further investigation, our results confirm the possibility of the presence of TBRF in Bolivia. Specific surveys in patients with fever of unknown origin are warranted, particularly with the help of molecular tools, to estimate the public health importance of TBRF in Bolivia.
Acknowledgments
The authors acknowledge F. Noireau and his research team (IIBISMED, Cochabamba, Bolivia) for their valuable technical assistance during the field work. The field work in Bolivia was supported by the Agence Nationale de la Recherche, the Centre National de la Recherche Scientifique and the University of Tours (France). We thank Juline Collin for technical assistance, and Claudio R. Lazzari for his support.
REFERENCES
- Bates LB, Dunn LH, St John JH.(1921)Relapsing fever in Panama. The human tick, Ornithodoros Talaje, demonstrated to be the transmitting agent of relapsing fever in Panama by human experimentation. The American Journal of Tropical Medicine and Hygiene s1–1183–210. [Google Scholar]
- Ciceroni L, Bartoloni A, Guglielmetti P, Paradisi F, Barahona HG, Roselli M, Ciarrocchi S, Cacciapuoti B.(1994)Prevalence of antibodies to Borrelia burgdorferi, Borrelia parkeri and Borrelia turicatae in human settlements of the Cordillera Province, Bolivia. Journal of Tropical Medicine & Hygiene 9713–17. [PubMed] [Google Scholar]
- Cutler SJ, Abdissa A, Trape JF.(2009)New concepts for the old challenge of African relapsing fever borreliosis. Clinical Microbiology and Infection 15400–406. [DOI] [PubMed] [Google Scholar]
- Davis GE.(1952)Observations on the biology of the Argasid tick, Ornithodoros brasiliensis Aragão, 1923, with the recovery of a spirochaete Borreliabrasiliensis, N. Sp. The Journal of Parasitology 38473–476. [PubMed] [Google Scholar]
- Davis GE.(1955)Relapsing fever spirochetes: the present status of Borrelia venezuelensis Brumpt and Borrelia neotropicalis Bates and St. John. International Bull Bacteriol Nomenclature and Taxonomy 5107–109. [Google Scholar]
- Davis GE.(1956)A relapsing fever spirochete, Borrelia mazzottii (sp. nov.) from Ornithodoros talaje from Mexico. American Journal of Hygiene 6313–17. [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A, Mangold AJ, Nava S, Venzal JM, Labruna MB, Guidon-Attali C.(2011)A review of the systematics of the tick family Argasidae (Ixodida). Acarologia 50317–333. [Google Scholar]
- Fukunaga M, Ushijima L, Aoki LY, Talbert A.(2001)Detection of Borrelia duttoni, a tick-borne relapsing fever agent in Central Tanzania, within ticks by flagellin gene-based nested polymerase chain reaction. Vector-Borne and Zoonotic Diseases 1331–338. [DOI] [PubMed] [Google Scholar]
- Guglielmone AA, Beati L, Barros-Battesti DM, Labruna MB, Nava S, Venzal JM, Mangold AJ, Szabo MP, Martins JR, Gonzàlez-Acuna D, Estrada-Pena A.(2006)Ticks (Ixodidae) on humans in South America. Experimental and Applied Acarology 4083–100. [DOI] [PubMed] [Google Scholar]
- Mangold AJ, Bargues MD, Mas-Coma S.(1998)18S rRNA gene sequences and phylogenetic relationships of European hard-tick species (Acari: Ixodidae). Parasitology Research 8431–37. [DOI] [PubMed] [Google Scholar]
- Norris DE, Klompen JS, Keirans JE, Black WC.(1996)Population genetics of Ixodes scapularis (Acari: Ixodidae) based on mitochondrial 16S and 12S genes. Journal Medical Entomology 3378–89. [DOI] [PubMed] [Google Scholar]
- Ryelandt J, Noireau F, Lazzari CR.(2011)A multimodal bait for trapping blood-sucking arthropods. Acta Tropica 117131–136. [DOI] [PubMed] [Google Scholar]
- Venzal JM, Estrada-Pena A, Mangold AJ, Gonzalez-Acuna D, Guglielmone AA.(2008)The Ornithodoros (Alectorobius) talaje species group (Acari: Ixodida: Argasidae): description of Ornithodoros (Alectorobius) rioplatensis n. sp. from southern South America. Journal Medical Entomology 45832–840. [DOI] [PubMed] [Google Scholar]