Abstract
Background
Since the introduction of the cut-and-sew Cox-Maze procedure for atrial fibrillation (AF) there has been substantial innovation in techniques for ablation. Use of alternate energy sources for ablation simplified the procedure and has resulted in dramatic increase in the number of AF patients treated by surgical ablation. Despite its increasingly widespread adoption, there is lack of rigorous clinical evidence to establish this as an effective clinical therapy.
Methods and Results
This paper describes a comparative effectiveness randomized trial, supported by the Cardiothoracic Surgical Trials Network, of surgical ablation with left atrial appendage (LAA) closure versus LAA closure alone in patients with persistent and longstanding persistent AF undergoing mitral valve surgery. Nested within this trial, is a further randomized comparison of 2 different lesions sets: pulmonary vein isolation and full Maze lesion set. This paper addresses trial design challenges, including how to best characterize the target population, operationalize freedom from AF as a primary endpoint, account for the impact of anti-arrhythmic drugs, and measure and analyze secondary endpoints, such as post-operative AF load.
Conclusions
This paper concludes by discussing how insights that emerge from this trial may affect surgical practice and guide future research in this area.
INTRODUCTION
Atrial fibrillation (AF) coexists in 50% of patients presenting for mitral valve surgery, and, in at least half of patients, this dysrhythmia has been long-standing and refractory to medical treatment (persistent or long-standing persistent in current consensus terminology) (1). The rhythm is presumed to relate to left atrial enlargement from the valvular heart disease. Preoperative AF is associated with reduced postoperative survival and increased risk of stroke. The cut-and-sew Cox-Maze procedure --introduced in the late 1980s-- provided surgeons with a therapeutic approach to help restore sinus rhythm, and avert the mortality and morbidity associated with this disease (2, 3). Despite its reported success, its complexity initially prevented widespread adoption. The more recent development of tissue ablation technologies and energy sources, however, has simplified the technique and significantly reduced operative time. These improvements have led to substantial growth in the number of procedures performed.
Despite this increased adoption, there is lack of rigorous clinical evidence to establish surgical ablation as an effective clinical therapy. The majority of studies are retrospective, conducted in single centers, and not rigorously controlled. Whereas 9 randomized trials (4–12) compared the benefits of ablation surgery to mitral valve surgery (MVS) alone, most of them had sample sizes insufficient to draw definitive conclusions (none enrolled more than 100 patients). Moreover, both randomized and non-randomized studies were characterized by significant variations in the ablation procedure, heterogeneous patient populations, and variations in definition and measurement of the primary endpoint. Furthermore, few studies included the full gamut of relevant clinical endpoints. A recent meta-analysis of 5 trials using freedom from AF within 12 months as a primary endpoint in non-paroxysmal AF patients found that surgical ablation greatly increased the odds of being free from AF at 12 months, but with wide confidence intervals and from trials that had considerable heterogeneity in their design (13). As such, large-scale trials are needed to provide better evidence for guiding physician and patient decision-making.
The need for such evidence is highlighted by two recent developments. First, the RACE trial found that treatment with anti-arrhythmic drugs (AADs), with the intent to restore normal sinus rhythm, did not improve survival or reduce stroke risk when compared to the simpler strategy of pharmacologic rate control in persistent AF patients; thus, casting doubts about the benefits of restoring sinus rhythm (14). Second, the PROTECT trial, which evaluated percutaneous closure of the left atrial appendage (LAA) in non-valvular AF patients with elevated risk of stroke, found that the efficacy of LAA closure was non-inferior to standard therapy with warfarin (15). These trials raise the question whether LAA closure with rate control alone would be sufficient in a population with AF and mitral valve disease. Only one of the randomized trials on ablation therapy in MVS patients used LAA closure in both treatment arms. An important outstanding question is whether MVS with LAA closure alone is a viable treatment option as compared to surgical ablation.
This paper describes a comparative effectiveness randomized trial of surgical ablation with LAA closure versus LAA closure alone in patients with persistent and longstanding persistent AF undergoing MVS. This trial has been designed and is conducted within the Cardiothoracic Surgical Clinical Trials Network, which is supported by the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Neurological Disorders and Stroke (NINDS), and the Canadian Institute for Health Research (see Appendix A). Nested within this trial, is a further randomized comparison of 2 different lesions sets. This paper addresses trial design challenges, including how to best characterize the target population, operationalize freedom from AF as a primary endpoint, account for the impact of anti-arrhythmic drugs, and measure and analyze secondary endpoints, such as post-operative AF load. This paper concludes by discussing how insights that emerge from this trial may affect surgical practice and guide future research in this area.
CHARACTERIZATION OF TARGET POPULATION
Surgical ablation does not yet have a clear-cut role as a stand-alone procedure, and the obvious population for evaluating this procedure is patients undergoing concomitant cardiac surgery. The CTSN trial, therefore, focuses on adult patients undergoing MVS for several reasons. It is the most common indication among concomitant procedures; around 50% of patients undergoing MVS with AF also have an ablation procedure (1). Patients who remain in AF after MVS have lower survival rates than those in sinus rhythm. Moreover, because the left atrium is routinely opened for MVS, ablation adds little time or risk to the surgical procedure; the surgeon is “already there” for the mitral valve procedure. The protocol targets adult patients with a clinical indication for surgery for the following: organic mitral valve disease, functional non-ischemic mitral regurgitation (MR), or ischemic MR with evidence of concomitant structural mitral valve disease. The rationale for defining the target population in this manner is to avoid competition for patients that would qualify for two other Network trials in patients with severe and moderate ischemic mitral regurgitation (both these protocols exclude patients with structural valvular disease).
Defining Atrial Fibrillation
Interpretation of the current literature on surgical ablation is challenging, in part, due to varying and inconsistent terminology used to describe the pattern and chronicity of AF. Traditionally studies used continuous and intermittent labels, proposed by Cox (10), where continuous AF is defined as AF that does not self terminate, and intermittent AF as any AF that self-terminates, but may be recurrent. To facilitate comparison of outcomes across trials, we adopted the classification from the 2001 Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation (16): Persistent AF is defined as non self-terminating AF lasting greater than 7 days, or lasting less than 7 days but necessitating pharmacologic or electrical cardioversion. Longstanding persistent AF is defined as continuous AF of greater than one year duration. This definition applies only to AF episodes that are of at least 30 seconds’ duration and do not have a reversible cause such as acute pulmonary disease or hyperthyroidism. When AF is present for less than 3 months and is paroxysmal, MVS results in conversion to sinus rhythm approximately 80% of the time. By choosing patients with persistent or longstanding persistent AF, we exclude those patients who move between AF and sinus rhythm sporadically and without intervention, enabling us to attribute elimination of AF to the assigned treatment.
Another critical consideration in trial design is ensuring that the eligibility criteria do not unnecessarily restrict recruitment of patients. To assess the available patient population and highlight those eligibility criteria that may have a major impact on enrollment, CTSN investigators used detailed prospective screening logs. Based of these data, eligibility criteria were streamlined, and the protocol no longer excludes patients needing concomitant surgical management of functional tricuspid regurgitation, patent foramen ovale, CABG, aortic arch or aortic valve procedures (see Box 1).
Box 1.
Selected Inclusion Criteria
-
1
Clinical indications for mitral valve surgery for the following:
Organic mitral valve disease; or
Functional non-ischemic mitral regurgitation; or
Ischemic mitral regurgitation with evidence of concomitant structural mitral valve disease
-
2a
Persistent AF within 6 months prior to randomization, defined as non self-terminating AF lasting greater than 7 days or lasting less than 7 days but necessitating pharmacologic or electrical cardioversion.
Duration of AF documented by medical history and
Presence of AF documented by a direct electro-cardiographic assessment <6 months prior to randomization
-
2b
Longstanding persistent AF is defined as continuous AF of greater than one year duration.
Duration of AF documented by medical history and
Presence of AF documented by direct electrocardiographic assessment upon arrival in the OR.
Exclusion Criteria
AF without indication for mitral valve surgery
AF is paroxysmal
Evidence of left atrial thrombus by intra-operative TEE
Evidence of active infection
Mental impairment or other conditions that may not allow subject to understand the nature, significance, and scope of study
Surgical management of hypertrophic obstructive cardiomyopathy
Previous catheter ablation for AF
Life expectancy of less than one year
Absolute contraindications for anticoagulation therapy
Enrollment in concomitant drug or device trials
Uncontrolled hypo- or hyperthyroidism
FEV1 < 30% of predicted value and/or need for home oxygen therapy
TREATMENT ARMS
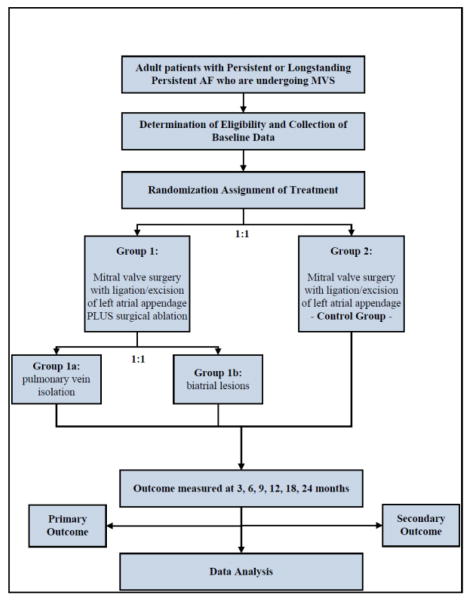
Patients are randomized in a 1:1 fashion to surgical ablation and LAA closure versus LAA closure alone at the induction of anesthesia. Nested within this trial, is a further randomized comparison within the ablation arm of 2 different lesions sets: pulmonary vein isolation (PVI) vs. biatrial lesion set patterned after the Cox-Maze III procedure; (figure 1). Randomization will be performed intraoperatively following verification of the absence of left atrial thrombus by transesophageal echocardiography.
Figure 1.
AF Trial Design Schematic
An important issue in trial design, especially of complex surgical procedures, is standardization of treatment interventions. Such standardization removes a potentially large source of random variation, and, thereby, improves the efficiency of design and precision of trial results. The investigators developed treatment guidelines (eAppendix B), which includes a pacing protocol (before and after PVI) to confirm acute conduction block at the pulmonary vein level. To date, none of the studies in the literature assess acute procedural success (conduction block). Bipolar energy sources are preferred for PVI alone and for the PVI component of the biatrial maze lesion set. For PVI two separate encircling lesions will be made around left and right pulmonary veins (Figure 2). For PVI with unipolar energy sources, a “box lesion” will be made, consisting of a continuous ablation line around all 4 pulmonary veins. After PVI, the heart will be arrested and the LAA excised or excluded. The remainder of the procedure for patients randomized to the biatrial maze lesion set will be performed at a point in the operation dictated by the surgeon’s standard practice.
Figure 2.
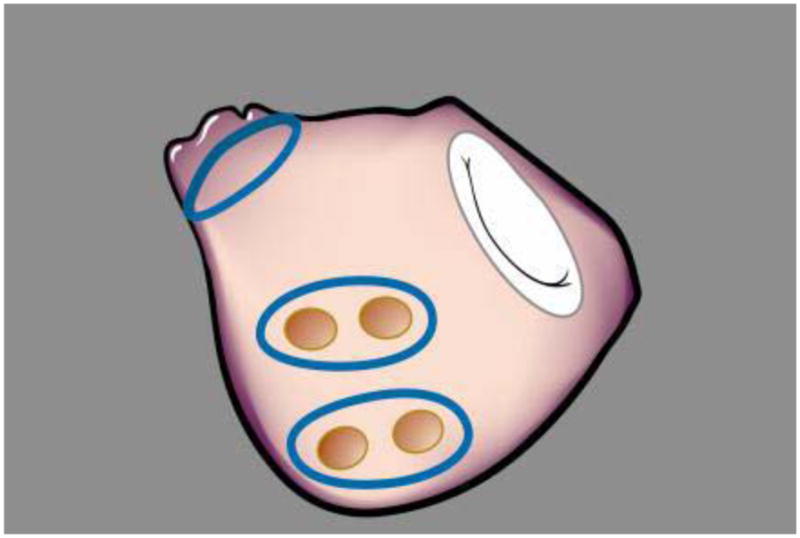
PVI lesion set with bipolar device
In recent years, several energy sources for ablation have been introduced into clinical practice. There do not appear to be significant differences in efficacy or safety of different energy sources. Radiofrequency (RF) is the oldest energy source with the largest clinical experience; thus, results from a trial that does not incorporate RF ablation may lack generalizability to the broader surgical community. In this trial, we limited the number of devices to those based on a RF energy source. In addition to the linear lesions created by unipolar or bipolar radiofrequency, additional spot lesions will be created at the mitral annulus and isthmus using either unipolar radiofrequency or cryotherapy.
Closure (amputation) of the LAA may be achieved by cut-and-sew technique or by application of a surgical stapler. Unless contraindicated, all patients will receive Class I/III anti-arrhythmic drugs (AADs) beginning within 24 hours of surgery, which will be terminated at 3 months. Warfarin is prescribed to patients in both arms throughout the follow-up period.
DEFINING SUCCESS IN ABLATION TRIALS – ESTABLISHING A PRIMARY ENDPOINT
The primary efficacy endpoint is freedom from AF assessed by 3-day continuous Holter monitoring at 6 and 12 months post-ablation. Freedom from postoperative AF will be defined as absence of any episode of AF lasting >30 seconds at both 6- and 12-month monitoring time points. The null hypothesis is that there is no difference in the proportion of patients meeting the primary outcome between patients randomized to surgical ablation plus LAA closure or to LAA closure alone. The primary null hypothesis will be tested in an intent-to-treat analysis using a 0.05 level Mantel-Haenszel chi-squared test with stratification by clinical center, the factor upon which randomization is stratified. For simplicity, the benefit of ablation between treatment arms will be quantified as a simple difference in the proportion of patients free of AF in the two groups (expressed as a relative risk with associated 95% confidence intervals).
Patients who die prior to the 12-month assessment, or who are determined by an independent adjudicator to be too ill to undergo AF assessment, will be considered as treatment failures. In the primary analysis, patients in both treatment arms who undergo ablation therapy for AF (including surgical ablation or percutaneous catheter ablation) subsequent to the index procedure will be considered treatment failures. The trial incorporates a three-month blanking period from the time of randomization, which allows for recovery from the inflammatory effects of the ablation. Events that occur during this period of time are not taken into account into the primary efficacy analysis. In the primary analysis, the use of AADs after the first 3 months is not considered a treatment failure as the trial is not able to standardize the use of AADs by referring cardiologists during the follow-up period.
SAMPLE SIZE ESTIMATION
Sample size estimates must be based on data from the clinical literature and the ability to detect, with high probability, a clinically meaningful presumed benefit of treatment. The reported absence of AF one year post MVS among control patients in previously executed randomized clinical trials ranges from approximately 15% to 35%. These trials all reported a relatively large but imprecise, benefit of ablation. For example, Doukas and colleagues found an absolute benefit of 35% for ablation in the proportion of patients free of AF at one year (95% confidence interval from 17% to 53%), corresponding to a slightly more than 3-fold increase in the proportion of patients free of AF at one year (10).
For a primary endpoint assessing freedom from AF at both six months and one year, we assume that 25% of patients treated with MVS and LAA closure will be free of AF. A total of 260 patients (i.e., 130 in each group) will provide 90% power to detect an absolute increase of 20% (25% versus 45%) in the proportion of patients free of AF, based on a two-tailed 0.05 level continuity corrected chi-squared test. The sample size takes into account a single interim analysis, in addition to the final analysis. The interim analysis will take place when 50% of patients have been followed up for one year and will be conducted at the 0.003 significance level. The results will be reported only to an independent data and safety monitoring board (DSMB), which will assess if the trial should stop early if the results favor one treatment. The final analysis will be conducted at the 0.049 significance level. The DSMB will also monitor for futility, that is, they will recommend stopping the trial if the probability of detecting an absolute 20% benefit for those randomized to MVS plus ablation is less than 0.20. Thus, it is possible to carry out a well-designed, prospective clinical trial with a relatively small sample size given the anticipated difference in freedom from AF between the two study arms.
AF SECONDARY ENDPOINTS
Designing endpoints to capture response to treatment in this trial requires an understanding of the episodic nature of AF and the need for rigorous evaluation of post-operative rhythm status. In addition to Holter monitoring at 6 and 12 months, this trial uses trans-telephonic monitoring (TTM) technology to obtain electrocardiographic data from weekly rhythm strips (90 second) to better assess rhythm activity during the interval periods. The downside to weekly TTM is that it (1) requires a higher level of compliance from patients; (2) requires patient education on the use of such devices; and (3) requires consistency in timing of patient transmission to avoid bias from circadian rhythm. As such, this modality of rhythm assessment is probably not reliable enough for use as the primary endpoint. Subsequent to designing the current trial, the FDA approved an implantable continuous rhythm monitoring device, which the network is now considering for use in a sub-study.
AF Burden
One endpoint that arises from the use of more frequent rhythm monitoring is AF load, which is defined as the proportion of recordings documenting AF in a given patient during spot recordings. Here patients are required to submit rhythm strips on a regular basis from 3 months after surgery until the time of the primary endpoint assessment. While this method places greater burden on patients and can result in noncompliance, this form of analysis prevents any single arrhythmia from significantly affecting the endpoint as the overall proportion of arrhythmias encountered is examined at the conclusion of the study.
Freedom from Any Electrocardiographically Documented Atrial Tachyarrhythmia Recurrences
In addition to AF load, another secondary endpoint of interest is freedom from any electrocardiographically documented atrial tachyarrhythmia recurrences. Although the primary interest is the success of AF ablation, there exists the possibility for induction of other arrhythmias during the process. Freedom from AF, atrial flutter or atrial tachycardia will be defined by absence of any of these electrocardiographically documented events lasting > 30 seconds.
ADDITIONAL CLINICAL SECONDARY ENDPOINTS
Whereas the impact on the occurrence of AF episodes is important to patients, mortality, important adverse events, such as stroke, and quality of life (QoL) are critical for defining the value of a therapy. Although these events themselves have profound effects on patients and their families, their frequencies are low and evaluating the impact of therapy on survival or stroke as a primary endpoint with precision would require extremely large sample sizes.
We, therefore, included a composite primary safety endpoint, which is defined as a composite of death, stroke, serious cardiac adverse events, cardiac re-hospitalizations, transient ischemic attack, pulmonary embolism, peripheral embolism, excessive bleeding, deep sternal wound infection/mediastinitus, damage to specialized conduction system requiring permanent pacemaker, damage to peripheral structures, such as the esophagus, within 30 days post-procedure or hospital discharge (whichever is later). In addition, secondary endpoints include assessing MACCE, which is relevant to cardiac procedures in general, defined as a non-weighted composite score of death, stroke, worsening heart failure (+1 NYHA Class), CHF hospitalization, and mitral valve re-intervention within 12 months of randomization. Survival and differences in the incidence of serious adverse events within 12 months of randomization will also be compared between groups using Poisson regression (with exact 95% confidence intervals). In addition to looking at events that are life-threatening or life-altering, we will be measuring overall impact on QoL and hospitalization burden. The long-term impact on QoL will be assessed at 12 months and will include a general health status measure (SF-12) and a disease-specific one (the Atrial Fibrillation Severity Scale). All hospitalization readmissions and total hospital days will be measured over the duration of follow-up, which is two years in order to assess long-term clinical endpoints.
DISCUSSION
The prevalence of AF among patients with mitral valve disease presenting for surgery, combined with the development of new ablation techniques that have simplified the cut-and-sew Maze technique, have led to renewed interest in surgical ablation. Subsequently, questions have emerged whether the results from the PROTECT trial, which showed equivalence of LAA closure to warfarin in non-valvular AF patients, would be applicable in (long-standing) persistent AF patients undergoing mitral valve surgery. What then would be the value of surgical ablation and LAA closure versus excising the LAA alone in this patient group?
The current literature provides insufficient evidence to address this important clinical issue. Among the numerous device technologies to enter the clinical arena for treatment of AF, only devices using cryoablation are specifically approved by the FDA for the treatment of AF. The remaining energy sources (radiofrequency, laser, microwave, and ultrasound) have been approved for the ablation of cardiac tissue but not specifically for AF therapy. Clinical trials evaluating the efficacy of these devices for this indication are now underway, but none of these trials provide a randomized comparison to mitral valve surgery with LAA closure alone. Clearly, surgical ablation procedures have been extensively used in practice and have been the subject of evaluative research. However, the literature evaluating the effectiveness of surgical ablation as a therapeutic approach is difficult to interpret due to paucity of rigorously controlled studies, and the lack of standardization in procedures, AF classification schemes, and primary endpoints. The paucity of rigorous clinical evidence regarding the effectiveness of surgical ablation, the fact that nearly half of MV surgery patients do not receive a concomitant surgical ablation procedure, and recent evidence that LAA closure is non-inferior to anticoagulation in a non-valvular patient group supports the argument that there is equipoise to design a trial in (long-standing) persistent AF patients that compares ablation and LAA closure to LAA closure alone.
The CT Surgery Trials Network has designed and is conducting such a comparative effectiveness trial, which required different manufacturers to support an FDA investigational device exemption (IDE) application. The trial design, however, is geared towards evaluation of ablation as a therapeutic approach (not a specific device), and will not support FDA approval for any individual device.
The results from this trial will provide several important insights. First, it will offer evidence regarding the comparative benefits of surgical ablation. An open question remains how to best assess benefit in ablation trials, given the episodic nature of this disease. There is currently no accepted standard for defining a “successful” surgical ablation procedure and the variations in primary endpoints have led to difficulty in interpreting the current literature. Some studies have used time to first recurrence of AF as a primary endpoint, employing standard Kaplan-Meier methods for analysis, which may be more appropriate for sustained clinical events such as stroke or death (17). Rhythm status or freedom from AF, however, represent clinical states with intermittent occurrences, thereby rendering the first occurrence less relevant. When AF is detected on an ECG, the time of detection does not necessarily represent the time the arrhythmia began, and time-to-event methods are less appropriate. Freedom from AF during the first year has become increasingly used as a primary endpoint. In some trials, success is defined only as “freedom from AF” at a single postoperative time point (e.g. 3 months), while in others, a successful outcome requires documentation of normal rhythm that is sustained over a period of time, or success is defined more stringently as absence of AF after discontinuation of all AADs. In this trial, AF will be measured by 3-day continuous monitoring at 6 and 12 months post-ablation; and freedom of AF will be defined by absence of AF (lasting > 30 seconds) at both time points. This trial will also provide important information on survival, safety, QoL, functional status and hospitalization time to help guide treatment decisions.
In addition to providing evidence for making treatment decisions, this trial will generate insights to help shape future clinical research. For example, if ablation is found to offer better AF control over LAA closure alone, the nested sub-comparison of different lesion sets will provide preliminary data to inform the design of subsequent trials comparing specific lesion sets and ablation devices. Moreover, the trial compares 2 different techniques for post-ablation heart rhythm monitoring (long-term holter monitoring vs. weekly rhythm strips), which should provide the evidence needed to determine optimal methods for evaluating post-operative rhythm control in the context of a clinical trial. Since more frequent rhythm assessment may reveal otherwise unappreciated episodes of AF, this comparison may also redefine the general definition of freedom from AF, thereby impacting clinical decision making algorithms (such as those used for anticoagulation). The investigators are also designing a sub-study that includes an implantable monitoring device. Finally, current methods to analyze longitudinal data for complex temporal patterns seen in AF are limited, and, as mentioned, are the subject of methodological exploration by the investigators. Building a rigorous evidence basis for surgical ablation therapies for persistent or longstanding persistent AF ablation is much needed. This comparative effectiveness trial should provide an important first step in achieving this.
Figure 3.
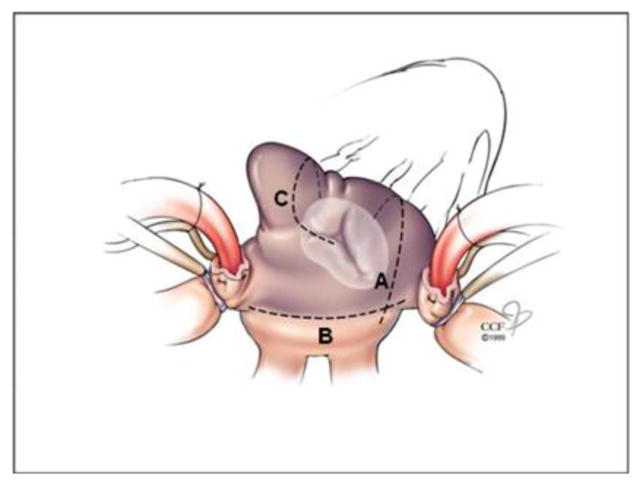
Right atrial lesions
APPENDIX A. Cardiothoracic Surgical Trials Network (CTSN)
National Heart, Lung and Blood Institute: Marissa A. Miller, Wendy C. Taddei-Peters, Dennis Buxton, Ron Caulder, Nancy L. Geller, David Gordon, Neal O. Jeffries, Albert Lee
National Institute of Neurological Disorders and Stroke: Claudia S. Moy
Canadian Institutes of Health Research: Ilana Kogan Gombos, Jennifer Ralph
Network Chairs: Christiana Care Health System, Timothy J. Gardner, (Chair); Brigham and Women’s Hospital, Patrick T. O’Gara, (Co-Chair)
Data Coordinating Center: International Center for Health Outcomes and Innovation Research (InCHOIR) in the Department of Health Evidence and Policy, Mount Sinai School of Medicine, New York, NY, Annetine C. Gelijns, Michael K. Parides, Deborah D. Ascheim, Alan J. Moskowitz, Ellen Moquete, Eric A. Rose, Melissa Chase, Yingchun Chen, Rosemarie Gagliardi, Alejandra Guerchicoff, Lopa Gupta, Alexander Iribarne, Edlira Kumbarce, Ron Levitan, Karen O’Sullivan, Mark J. Russo, Milerva Santos, William Slavik, Alan Weinberg, Martin Wells, Paula Williams, Carrie Wood, Xia Ye
Core Clinical Site Investigators
Cleveland Clinic Foundation, Eugene H. Blackstone (PI), A. Marc Gillinov, Tomislav Mihaljevic, Richard A. Grimm, Ben Barzilai, Bruce D. Lindsay, Christine Whitman, Denise Kosty Sweeney, Roberta Palumbo; NHLBI Clinical Research Scholars: Gregory Pattakos, Pamela A. Clarke;
Columbia University Medical Center, Michael Argenziano (PI), Mathew Williams, Lyn Goldsmith, Rebecca T. Hahn, Linda Gillam, Craig R. Smith, Yoshifumi Naka, Allan Stewart, Allan Schwartz;
Duke University, Peter K. Smith (PI), Stacey Welsh, John H. Alexander, Carmelo A. Milano, Donald D. Glower, Joseph P. Mathew, J. Kevin Harrison; NHLBI Clinical Research Scholars: Mark F. Berry, Cyrus J. Parsa, Betty C. Tong, Judson B. Williams;
East Carolina Heart Institute, T. Bruce Ferguson (PI), Alan P. Kypson, Evelio Rodriguez, Malissa Harris, Brenda Akers, Allison O’Neal;
Emory University, John D. Puskas (PI), Vinod H. Thourani, Robert Guyton, Jefferson Baer, Kim Baio, Alexis Neill;
Montefiore-Einstein Heart Center, New York, NY, Robert E. Michler (PI), Ricardo Bello, David A. D’Alessandro, Joseph J. DeRose, Jr., Daniel J. Goldstein, Cynthia Taub, Daniel Spevak, Roger Swayze;
Montreal Heart Institute, Louis P. Perrault (PI), Arsène-Joseph Basmadjian, Denis Bouchard, Michel Carrier, Philippe Demers, Michel Pellerin, Sophie Robichaud;
NIH Heart Center at Suburban Hospital, Keith A. Horvath (PI), Philip C. Corcoran, Michael P. Siegenthaler, Mandy Murphy, Margaret Iraola, Ann Greenberg;
University of Pennsylvania, Michael A. Acker (PI), Y. Joseph Woo, Wilson Y. Szeto, Mary Lou Mayer;
University of Virginia, Irving L. Kron (PI), Gorav Ailawadi, Karen Johnston, John M. Dent, Sandra Burks, Kim Gahring
Affíliate/Ancillary Sites
Hôpital du Sacré-Cœur de Montréal, Pierre Pagé (PI), Hugues Jeanmart, Philippe Demers, Claude Sauvé, Carole Sirois;
Institut Universitaire de Cardiologie et de Pneumologie de Québec, Pierre Voisine (PI), François Dagenais, Eric Dumont, Gladys Dussault;
Inova Fairfax Hospital, Alan M. Speir (PI), Niv Ad, Minh Dang;
The Ohio State University Medical Center, Chittoor B. Sai-Sudhakar (PI), Danielle Jones;
WellStar Health System, Kennestone Hospital, William A. Cooper (PI), Rajnish Prasad, Richard J. Myung, Jennifer LaCorte, Melinda Mock;
Satellite Sites
Baylor Research Institute, Michael J. Mack (PI), Robert Smith, William Ryan, Jennifer Withers;
Brigham and Women’s Hospital, Frederick Y. Chen (PI), R. Morton Bolman III, Anne M. Burgess, Debra Conboy;
Jewish and St. Mary’s Hospital, Mark S. Slaughter (PI), Matthew Williams, Marcus Stoddard, Heather Moody;
Mission Hospital, Mark A. Groh (PI), Ben Trichon, Todd Hansen, Claudine Cuento;
University of Southern California, Vaughn A. Starnes (PI), Michael Bowdish, Becky Lopez;
University of Maryland, James S. Gammie (PI), Mandeep Mehra, Bartley Griffith, Dana Beach;
Washington University, Ralph J. Damiano, Jr. (PI), Scott Silvestry, Marc Moon, Jennifer Lawton
Cardiopulmonary Exercise Core Laboratory: Henry Ford Hospital, Steven J. Keteyian, Clinton A. Brawner
Echo Core Laboratory: Massachusetts General Hospital, Judy Hung, Xin Zeng
Electrophysiology Core Laboratory: University of Rochester Medical Center, Jean-Philippe Couderc
Neurocognitive Core Laboratories: Duke University, Joseph P. Mathew
Protocol Review Committee: David A. Bull (Chair); Patrice Desvigne-Nickens, Executive Secretary; Dennis O. Dixon, Mark Haigney, Richard Holubkov, Alice Jacobs, Frank Miller, John M. Murkin, John Spertus, Andrew S. Wechsler
Data and Safety Monitoring Board: Frank Selke (Chair); Cheryl L. McDonald, Executive Secretary; Robert Byington, Neal Dickert, Dennis O. Dixon, John S. Ikonomidis, David O. Williams, Clyde W. Yancy
Medical Monitors: James C. Fang, Wayne Richenbacher
Overall Event Adjudication Committee: Vivek Rao (Chair); Karen L. Furie, Rachel Miller, Sean Pinney, William C. Roberts
Infection Event Adjudication Committee: Rachel Miller (Chair); Shirish Huprikar, Marilyn Levi
Footnotes
Disclosures
Funding
National Heart Lung and Blood Institute; Canadian Institute of Health Research; National Institute of Neurological Diseases and Stroke
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gammie JS, Haddad M, Milford-Beland S, Welke KF, Ferguson TB, O’Brien SM, Griffith BP, Peterson ED. Atrial Fibrillation Correction Surgery: Lessons from the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg. 2008;85:909–15. doi: 10.1016/j.athoracsur.2007.10.097. [DOI] [PubMed] [Google Scholar]
- 2.Cox JL. Cardiac surgery for arrhythmias. J Cardiovasc Electrophysiol. 2004 Feb;15(2):250–62. doi: 10.1046/j.1540-8167.2004.03656.x. [DOI] [PubMed] [Google Scholar]
- 3.Cox JL. The long-standing persistent confusion surrounding surgery for atrial fibrillation. The JTCVS. 2010;139:1374–86. doi: 10.1016/j.jtcvs.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Khargi K, Deneke T, Haardt H, et al. Saline-irrigated, cooled-tip radiofrequncy ablation is an effective technique to perform the mze procedure. Ann Thor Surg. 2001;72:S1090–S1095. doi: 10.1016/s0003-4975(01)02940-x. [DOI] [PubMed] [Google Scholar]
- 5.Jessurun ER, van Hemel NM, Defauw JJ, et al. A randomized study of combining maze surgery for atrial fibrillation with mitral valve surgery. J Cardiovasc Surg (Torino) 2003;44:9–18. [PubMed] [Google Scholar]
- 6.Akpinar B, Gulden M, Sagbas E, et al. Combined radiofrequency modified maze and mitral valve procedure through a port access approach: Early and mid term results. Eur J Cardiothorac Surg. 2003;24:223–230. doi: 10.1016/s1010-7940(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 7.Schuetz A, Schulze CJ, Sarvanakis KK, et al. Surgical treatment of permanent atrial fibrillation using microwave energy ablation: a prospective randomized clinical trial. Eur J Cardiothorac Surg. 2003;24:475–480. doi: 10.1016/s1010-7940(03)00377-4. [DOI] [PubMed] [Google Scholar]
- 8.Vasconcelos JT, Scanavacca MI, Sampaio RO, et al. Surgical treatment of atrial fibrillation through isolation of the left atrial posterior wall in patients with chronic rheumatic mitral valve disease. A randomized study with control group. Arq Bras Cardiol. 2004;83:211–218. doi: 10.1590/s0066-782x2004001500004. [DOI] [PubMed] [Google Scholar]
- 9.DeLima GG, Kalil RA, Leiria TL, et al. Randomized study of surgery for patients with permanent atrial fibrillation as a result of mitral valve disease. Ann Thor Surg. 2004;77:2089–2094. doi: 10.1016/j.athoracsur.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Doukas G, Samani NJ, Alexiou C, Oc M, Chin DT, Stafford PG, Ng LL, Spyt TJ. Left atrial radiofrequency ablation during mitral valve surgery for continuous atrial fibrillation: a randomized controlled trial. JAMA. 2005 Nov 9;294(18):2323–9. doi: 10.1001/jama.294.18.2323. [DOI] [PubMed] [Google Scholar]
- 11.Abreu Filho CA, Lisboa LA, Dallan LA, et al. Effectiveness of the maze procedure using cooled-tip radiofrequency ablation in patients with permanent atrial fibrillation and rheumatic mitral valve disease. Circulation. 2005;112:120–125. doi: 10.1161/CIRCULATIONAHA.104.526301. [DOI] [PubMed] [Google Scholar]
- 12.Blomstrom-Lundqvist C, Johansson B, Berglin E, et al. A randomized double-blind study of epicardial left atrial cryoablation for permanent atrial fibrillation in patients undergoing mitral valve surgery: The SWEDish Multicentre Atrial Fibrillation Study (SWEDMAF) Eur Heart J. 2007;28:2902–2908. doi: 10.1093/eurheartj/ehm378. [DOI] [PubMed] [Google Scholar]
- 13.Kong MH, Lopes RD, Piccini JP, et al. Surgical maze procedure as a treatment for atrial fribrillaion: A meta-analysis of randomized controlled trials. Cardiovascular Therapeutics. 2010;28:311–326. doi: 10.1111/j.1755-5922.2010.00139.x. [DOI] [PubMed] [Google Scholar]
- 14.van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation N. Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 15.Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomized non-inferiority trial. Lancet. 2004;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 16.Fuster V, Ryden LE, Asinger RW, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) Developed in Collaboration With the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol. 2001;38:1231. doi: 10.1016/s0735-1097(01)01587-x. [DOI] [PubMed] [Google Scholar]
- 17.Camm AJ, Reiffel JA. Defining endpoints in clinical trials on atrial fibrillation. European Heart Journal Supplements. 2008;10:H55–H78. [Google Scholar]