Abstract
Targeting the molecular pathways associated with carcinogenesis remains the greatest opportunity to reduce treatment-related morbidity and mortality. Extracellular matrix metalloproteinase inducer (EMMPRIN), also known as CD147, is a cell surface molecule known to promote tumor growth and angiogenesis in preclinical studies of head and neck carcinoma making it an excellent therapeutic target. To evaluate the feasibility of anti-EMMPRIN therapy, an ex-vivo human head and neck cancer model was established using specimens obtained at the time of surgery (n=22). Tumor slices were exposed to varying concentrations of anti-EMMPRIN monoclonal antibody and cetuximab for comparison purposes. Cetuximab is the only monoclonal antibody currently approved for the treatment of head and neck carcinoma. After treatment, tumor slices were assessed by immunohistochemistry and western blot analysis for apoptosis (TUNEL) and EMMPRIN expression. Of the tumor specimens 33% showed a significant reduction in mean ATP levels after treatment with cetuximab compared with untreated controls, whereas 58% of the patients responded to anti-EMMPRIN therapy (P<0.05). Samples, which showed reactivity to anti-EMMPRIN, also had greater EMMPRIN expression based on immunohistochemistry staining (49%) when compared with nonresponders (25%, P=0.06). In addition, TUNEL analysis showed a larger number of cells undergoing apoptosis in antibody-treated tumor slices (77%) compared with controls (30%, P<0.001) with activation of apoptotic proteins, caspase 3 and caspase 8. This study shows the potential of anti-EMMPRIN to inhibit proliferation and promote apoptosis and suggests its future role in the targeted treatment of head and neck carcinoma.
Keywords: anti-EMMPRIN antibody, CNTO3899, ex vivo, head and neck squamous cell carcinoma, tissue slices
Introduction
Head and neck squamous cell carcinoma (HNSCC) accounts for 34000 new cases and nearly 7500 cancer-related deaths in the United States every year [1]. In spite of both surgical and medical advances, the 5-year survival rate has remained relatively unchanged (50–55%) over the last three decades [1], with locoregional recurrence being the most common cause for treatment failure [2]. Morbidity and mortality related to current chemotherapeutic and radiation regimens have limited further advances. Targeted therapy provides a new treatment option for patients with advanced disease offering additional survival benefits without overlapping toxicity [3–5].
Extracellular matrix metalloproteinase inducer (EMMPRIN), or CD147, represents a novel target for head and neck cancer treatment. EMMPRIN is expressed in high levels in HNSCC [6], is located on the cell surface, and is known to promote tumor growth and lymphatic metastasis [6–9]. An extensive list of proteins, including integrins, syndecans and most recently, cyclophylin-mediated isomerization has been linked to EMMPRIN expression and downstream signal transduction pathways [10]. Although the exact mechanism by which EMMPRIN exerts its effects is unclear [11,12], it has been implicated in MMP production and collagen degradation through tumor stromal interactions [13,14] and is shown to promote tumor growth and angiogenesis [12,15,16].
We have earlier shown antitumor activity in head and neck cancer cell lines in vivo and a reduction in proinflammatory and proangiogenic factors in vitro after treatment with anti-EMMPRIN monoclonal antibody (mAb, CNTO3899) [17]. As EMMPRIN plays an important role in tumor-stromal-mediated events and immunocompromised murine models often do not reflect the complex stromal elements found in human tumors, we assessed EMMPRIN as a therapeutic target in ex-vivo human head and neck cancer specimens. On the basis of earlier findings, we hypothesized that anti-EMMPRIN therapy would show significant antitumor effects. Anti-epidermal growth factor receptor (EGFR) therapy currently represents the gold standard in targeted treatment of head and neck carcinoma. Therefore, we provide a comparison of results seen with anti-EMMPRIN and anti-EGFR (cetuximab) mAb treatment using an ex-vivo human head and neck cancer model.
Materials and methods
Patient specimen collection
A prospective nonrandomized study of patients presenting with upper aerodigestive tract neoplasms from October 2008 to May 2009 was carried out at the University of Alabama at Birmingham. Institutional Review Board approval was obtained for ex-vivo treatment of tissue specimens. Only patients with histologically proven squamous cell carcinoma were included in the study. Demographic and clinical data consisted of patient age, sex, tumor site, stage and earlier treatment.
Specimen processing
Tumor specimens were obtained and immediately placed in complete culture media (DMEM supplemented with 10% FBS, 1% penicillin-streptomycin, and 1% gentamycin). Heat-treated or complement-inactivated FBS was used to avoid complications with antibody-dependent cytotoxicity because of complement deposition. Within 30 min of specimen retrieval, multiple tissue slices measuring approximately 5 mm in diameter and 800–1000 µm in thickness were cut by sharp dissection and weighed to control for variation. The slices were randomly selected and placed into individual wells of 24-well plates, in 1.5 ml of complete media supplemented with or without antibody and incubated at 37°C in 5% CO2 for 48 h [18].
Reagents
Anti-EMMPRIN mAb, CNTO3899, was obtained from Centocor, Inc. (Radnor, Pennsylvania, USA). The recombinant human/mouse chimeric mAb binds specifically to the extracellular domain of human EMMPRIN and is composed of the V-region of murine anti-EMMPRIN antibody and human IgG1 [17]. Cetuximab (C225) was purchased from ImClone Systems Inc. (Branchburg, New Jersey, USA) and purified human IgG was purchased from Fisher Scientific (Pittsburgh, Pennsylvania, USA).
ATP viability assay
To determine cell viability, ATP assays (ATPlite-Perkin Elmer, Waltham, Massachusetts, USA) were performed on all tissue slices. The tissue slices were exposed to varying concentrations of anti-EMMPRIN (0, 50, 100, 200 µg/ml), cetuximab (0, 5, 10, 20 µg/ml) and IgG (0, 50, 100, 200 µg/ml). Six replicate slices were prepared per treatment group. After 48 h, the tissue slices were sonicated for 20 s in a 50:50 mixture of complete media and ATP mammalian cell lysis buffer. Intracellular ATP levels were measured in four aliquots per tissue slice by ATP-dependent light emission (counts per second) and mean ATP levels were determined for each slice. To determine the viability of tumor slices over time, untreated control slices (six replicates per time point) were processed at 0, 24, 48 and 72 h as described above.
Immunohistochemistry
Anti-EMMPRIN mAb-treated and mAb-untreated tumor slices were evaluated by immunohistochemistry (IHC and compared with tumor specimens fixed immediately after slicing. Formalin-fixed, paraffin-embedded tissues were cut at 5 µm, placed on treated slides and heated for 2 h at 60°C. Tissue sections were deparaffinized with xylene and rehydrated with absolute, 95 and 70% ethanol. Antigen retrieval was performed using 1 mmol/l EDTA (pH 9) and tumor sections were incubated with 3% goat serum to reduce nonspecific immunostaining. To evaluate EMMPRIN expression, slides were incubated with prediluted rabbit anti-EMMPRIN antibody (Zymed, Carlsbad, California, USA), exposed to biotinylated goat anti-rabbit antibody (Jackson Immuno Research, West Grove, Pennsylvania, USA) followed by streptavidin HRP (Signet Pathology Systems, Dedham, Massachusetts, USA), and the antibody-antigen complex was visualized with a 3,3′-diaminobenzidine tetrachloride substrate kit (ScyTek Laboratories, Logan, Utah, USA).
Slide preparation and staining for TUNEL were performed as described earlier using the Apop Tag Peroxidase In Situ Apoptosis Detection Kit 37100 (Chemicon International, Temecula, California, USA) [15]. All tissue sections were counterstained using weak Myers hematoxylin, dehydrated with graded alcohols, and soaked in xylene. EMMPRIN and TUNEL staining was evaluated by two of the authors (N. R. D and J. C. A) as described earlier [15]. For each tissue section, the fraction of immunostained cells to the total number of cells per × 400 field was recorded.
Western blot analysis
Treated and untreated tumor specimens were analyzed for caspase-3 and caspase-8 expression. Tissue samples were homogenized in radioimmunoprecipitation assay buffer and total protein was determined for each sample [6]. Protein lysates were electrophoresed using the Novex Mini-Gel system (Invitrogen, Carlsbad, California, USA) and immunoblotting was carried out using mAbs: anticaspase 3 (Santa Cruz Biotechnologies, Santa Cruz, California, USA) and anti-caspase 8 (BD Pharmingen, San Jose, California, USA), followed by secondary antimouse antibody and chemiluminescent detection. Mouse mAb to β-actin (anci-β-actin antibody, Santa Cruz Biotechnologies) was used to assess for variable protein concentrations.
Statistical analysis
Fractional tumor slice survival was calculated as the ratio of mean ATP level in control slices at time 0 versus all other time points and cytotoxicity data were transformed to reflect a reduction in cell proliferation as a percentage change from untreated controls. A pair-wise comparison of mean ATP and staining levels for control versus antibody-treated tissue was conducted using the t-test. ImageJ (http://rsb.info.nih.gov/ij/) was used for caspase-3 and caspase-8 quantification on western blot analysis. P < 0.05 was considered statistically significant. Variability in ATP levels for each treatment group and bias between IHC measurements were expressed as standard error of the mean.
Results
Patient characteristics
Twenty-two tissue specimens were collected from patients undergoing surgery for HNSCC at the University of Alabama at Birmingham from October 2008 to May 2009. Demographic data and tumor characteristics are summarized in Table 1. The majority of patients was diagnosed with stage IV (86.4%) squamous cell carcinoma of the oropharynx (54.5%). For two patients organ preservation therapy had been given earlier; one was treated with chemoradiation, and one with surgical resection and postoperative radiation.
Table 1.
Patient characteristics
| N | % | |
|---|---|---|
| Age (years) | ||
| Median | 59 | |
| Range | 40–75 | |
| Tumor site | ||
| Oral cavity | 7 | 31.8 |
| Oropharynx | 12 | 54.5 |
| Neck | 2 | 9.2 |
| Sinus | 1 | 4.5 |
| Stage | ||
| I | 0 | 0 |
| II | 3 | 13.6 |
| III | 0 | 0 |
| IV | 19 | 86.4 |
Anti-EMMPRIN therapy mediates ATP reduction in an ex-vivo HNSCC model
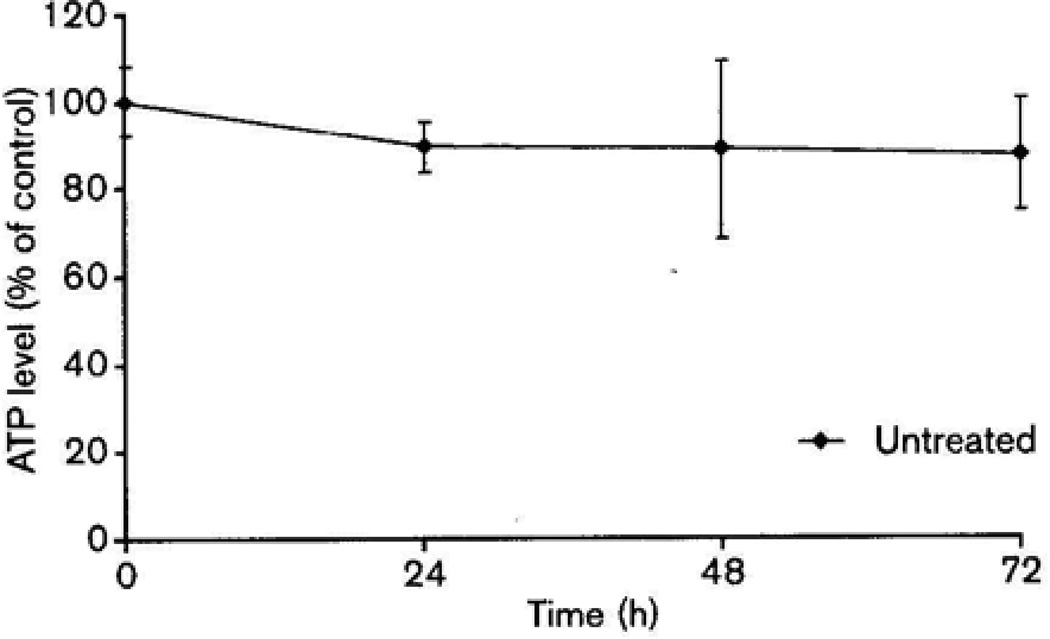
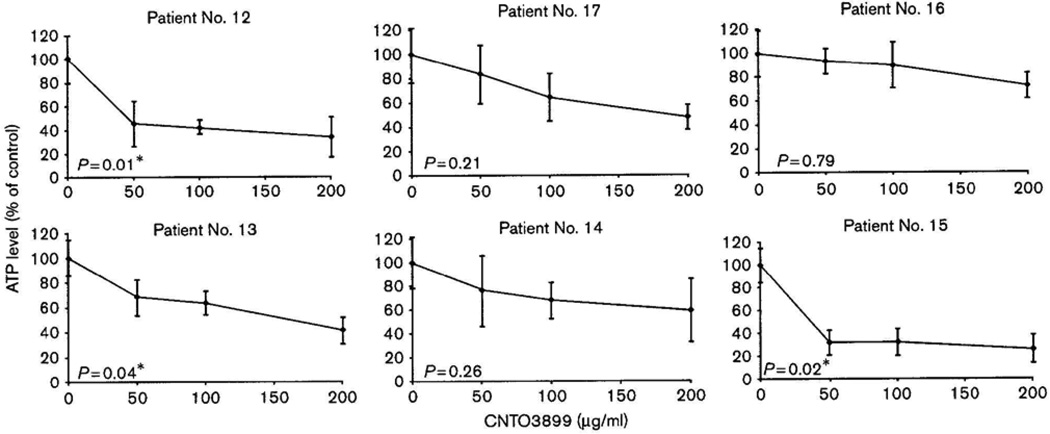
Untreated head and neck cancer tissue slices showed excellent viability over 72h of ex-vivo culture (Fig. 1). Tissue specimens were treated with 0, 50, 100 and 200 µg/ml of anti-EMMPRIN antibody. Examples of antibody-induced cytotoxicity curves are shown in Fig. 2 and P values are reported for mean ATP levels at a concentration of 100 µg/ml compared with untreated controls. As insufficient tissue was available for three patients, a total of 19 specimens were assayed for anti-EMMPRIN mAb-induced cytotoxicity (Table 2). Of all patients 47% showed a significant reduction in mean ATP levels after treatment with 100 µg/ml of CNTO3899 (P < 0.05). These patients were classified as responders while two additional patients (patients 8 and 11) were classified as moderate responders showing a significant reduction in ATP levels after treatment with 200 µg/ml of anti-EMMPRIN antibody. Sensitivity to anti-EMMPRIN therapy did not correlate with stage, tumor site or earlier treatment. A significant reduction in ATP levels for eight patients (42%) did not occur even after treatment with the highest dose of CNTO3899. These patient specimens were classified as nonresponders. To confirm that anti-EMMPRIN-mediated cytotoxicity was antibody specific, tumor slices were treated with purified human IgG (supplemental data). Tissue specimens treated with IgG did not show a significant reduction in ATP levels. ATP levels for tissue treated with 100 µg/ml IgG were 90% of control (P = 0.45), similar to reductions seen in tissue viability assays of untreated controls at 48 and 72 h.
Fig. 1.
Viability of ex-vivo control tissue slices (n = 6) at 24, 48, and 72h compared to mean ATP level at time zero.
Fig. 2.
Tissue slice cytotoxicity assays in head and neck squamous cell carcinoma patient specimens cultured with varying concentrations of CNTO3899 (0, 50, 100, and 200 µg/ml). Six replicate slices were prepared per treatment group. A significant reduction in ATP levels was obtained with the treatment of 100 µg/ml anti-extracellular matrix metalloproteinase inducer monoclonal antibody in patients 12, 13, and 15.
Table 2.
Head and neck cancer patient ATP levels after treatment with CNTO3899 (100 µg/ml)
| Patient ID | TNM classification | Stage | ATP level (% of control) | P |
|---|---|---|---|---|
| 1 | T4N2c | IV | 40 | < 0.001* |
| 2 | T3N2b | IV | 47 | 0.002* |
| 3 | TxN2c | IV | 48 | <0.001* |
| 4 | TxN3 | IV | 33 | <0.001* |
| 5 | T4N2b | IV | 78 | 0.31 |
| 6 | T4N2C | IV | 27 | <0.001* |
| 7 | T4N2c | IV | 44 | 0.004* |
| 8 | T4N0 | IV | 60 | 0.09 |
| 9 | T2N2b | IV | 60 | 0.15 |
| 10 | T4N2b | IV | 75 | 0.42 |
| 11 | T2N0 | II | 58 | 0.12 |
| 12 | T4N2b | IV | 42 | 0.01* |
| 13 | T4N0 | IV | 63 | 0.04* |
| 14 | T4N0 | IV | 68 | 0.26 |
| 15 | T4N0 | IV | 32 | 0.02* |
| 16 | T4N2c | IV | 90 | 0.79 |
| 17 | T2N2c | IV | 65 | 0.21 |
| 18 | T4N2b | IV | 90 | 0.11 |
| 19 | T2N0 | II | 70 | 0.37 |
TNM, tumor node metastasis.
P value < 0.05, statistically significant.
Comparison of anti-EMMPRIN and cetuximab treatment response
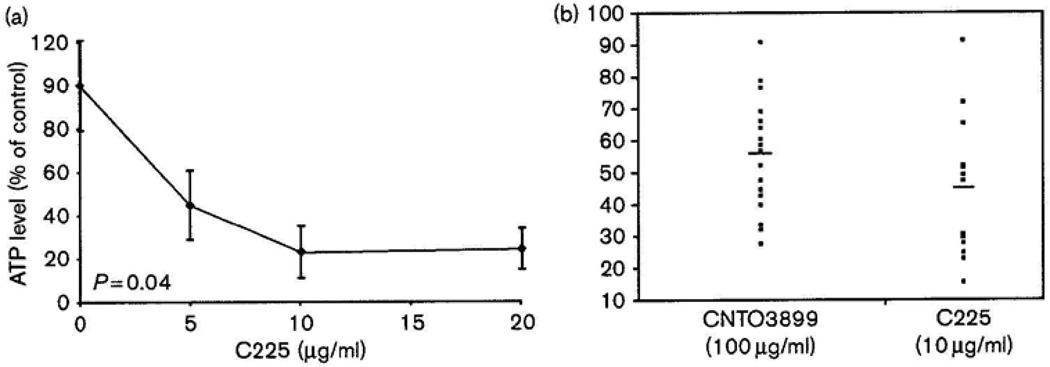
To determine the relative benefit of anti-EMMPRIN treatment we compared results to those seen with cetuximab. Cetuximab is an anti-EGFR mAb and currently the only targeted agent with known efficacy in head and neck carcinoma [4,5,19]. To determine the therapeutic dose of cetuximab, patient specimens were treated with 0, 5, 10 and 20 µg/ml (Fig. 3a). Of all patients 33% treated with 10 µg/ml cetuximab showed a significant reduction in ATP levels and were classified as responders. No patient specimens were responsive to doses of cetuximab greater than 10 µg/ml. To determine the relative use of anti-EMMPRIN mAb as a potential therapeutic agent, a side-by-side comparison of all CNTO3899 and cetuximab-treated tumor specimens was conducted (Fig. 3b). A larger percentage of patients responded to anti-EMMPRIN therapy (58%) when compared with cetuximab treatment (33%). Cetuximab-responsive specimens showed a slightly greater reduction in mean ATP levels when both groups were compared with untreated controls (µCNTO3899 = 57% of control, µCetuximab = 45% of control); however, no statistical difference was observed (P = 0.13).
Fig. 3.
Comparison of anti-extracellular matrix metalloproteinase inducer antibody and cetuximab-induced cytotoxicity in head and neck squamous cell carcinoma patient specimens. (a) Cytotoxicity curve for head and neck cancer tissue slices (n = 6 per treatment group) treated with varying concentrations of cetuximab (C225) (0, 5, 10, and 20 µg/ml). A significant reduction in ATP levels was obtained with 10 µg/ml. (b) Side-by-side comparison of CNTO3899 (100 µg/ml) and C225 (10 µg/ml)-treated tissue specimens for all patients as a percentage of control. No significant difference was observed between mean ATP levels for anti-extracellular matrix metalloproteinase inducer (57%) and cetuximab (45%)-treated tumor specimens (P = 0.13).
Anti-EMMPRIN treatment promotes apoptosis
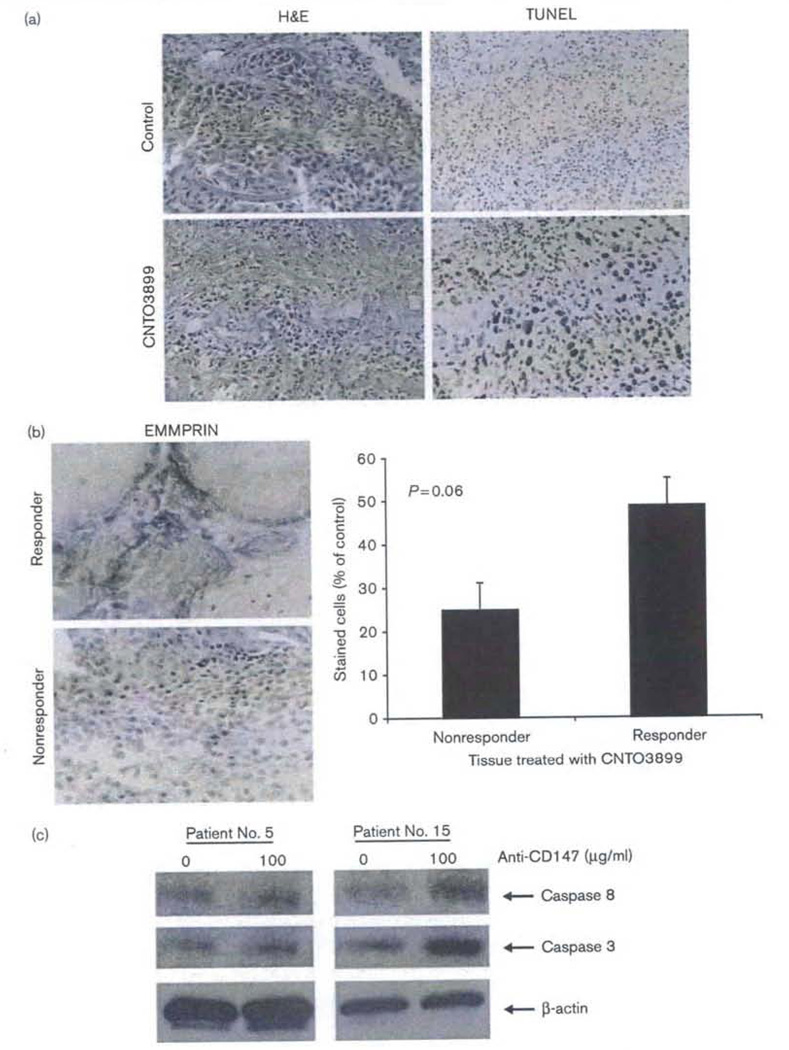
A total of nine patient specimens underwent IHC analysis by TUNEL assay and EMMPRIN staining. Before evaluating tumor specimens for IHC, slices were stained with hematoxylin and eosin to assess slice architecture and tumor burden. Both stromal and inflammatory cells are present in tissue specimens, and intact vasculature, similar to that of the tumor environment in vivo (Fig. 4a). To assess for necrosis during ex-vivo tissue culture, pathological review of all available specimens (n = 9/19) was performed and necrosis was measured as a percentage of the entire tissue specimen, Less than 5% (4.8%, range 0–8%) of any given specimen showed necrosis. This finding further clarifies the viability of the ex-vivo ATP assay and maintains that minimal necrosis is observed in patient specimens even after 48 h in culture. Increased apoptosis was observed in anti-EMMPRIN-treated tumor slices (77%) when compared with controls (30%, P < 0.001) and tumor sections from patients who responded to CNTO3899 therapy showed greater EMMPRIN staining (49%) when compared with nonresponders (25%, P = 0.06) (Fig. 4b). To further clarify that tissue slice cytotoxicity was because of antibody-mediated apoptosis rather than tissue necrosis, patient specimens were evaluated for caspase-3 and caspase-8 expression. Examples of anti-EMMPRIN-mediated caspase expression are shown for two patients (Fig. 4c). A significant difference in caspase-3 and caspase-8 levels was observed between the controls and anti-EMMPRIN-treated tissue slices for patient 15 (responder, P < 0.001). Tissue specimens from patient 5 (nonresponder) showed greater caspase-3 expression after anti-EMMPRIN treatment (P = 0.007) whereas no difference in caspase-8 expression was observed (P = 0.07). Insufficient tissue was available for detailed analysis of all patients.
Fig. 4.
Anti-extracellular matrix metalloproteinase inducer (EMMPRIN) monoclonal antibody induces caspase-mediated apoptosis; treatment response correlates with EMMPRIN expression. (a) Tissue specimens maintain intact tumor cells with surrounding vasculature and stroma on hematoxylin and eosin (H&E) staining. An increase in apoptosis in treated (100 µg/ml CNTO3899) (77%) versus untreated slices (30%, P < 0.001) was confirmed through TUNEL analysis. (b) EMMPRIN staining correlates with treatment response. Patient specimens classified as responders showed greater EMMPRIN expression (49%) than nonresponders (25%, P = 0.06) following treatment with anti-EMMPRIN monoclonal antibody. (c) CNTO3899 stimulates caspase-mediated apoptosis. Control and CNTO3899 treated tissue specimens were analyzed for caspase-3 and caspase-8 expression. An increase in caspase-3 expression was observed in both patients following treatment (P<0.01). Caspase-8 expression was significantly greater for anti-EMMPRIN treated tumor tissue from patient No. 15 (responder) when compared with control (P < 0.001).
Discussion and conclusion
As a cell surface molecule, which is upregulated in multiple cancer types including head and neck carcinoma, EMMPRIN is an attractive target for antibody-based therapy. We show that anti-EMMPRIN treatment inhibits tumor cell proliferation and induces caspase-mediated apoptosis in an ex-vivo human head and neck cancer model. This model has been used earlier [18,20] and provides a unique opportunity to evaluate antineo-plastic agents that disrupt tumor-stromal interactions. Unlike in-vitro or in-vivo xenograft studies, fresh tissue slices maintain multiple tumor components that can influence therapeutic outcomes.
In this study, antibody-induced cytotoxicity was determined for each patient specimen after 48 h of incubation. The length of incubation was based on results from earlier studies [21] and untreated tissue slice viability was maintained over 72 h of culture with a minimal reduction in ATP levels. A significant reduction in ATP levels was shown for 11 patient specimens after anti-EMMPRIN antibody treatment. Changes in ATP levels in these experiments were considered to be a result of antibody treatment rather than cell decay in culture. Furthermore, reduction in ATP levels seem to be antibody specific, given no significant decrease, was observed after treatment with purified human IgG (P = 0.45). In a similar study by Baba et al. [22], colon carcinoma cells showed a significant reduction in intracellular ATP levels after anti-human CD147 mouse mAb treatment. Decreased lactate transport in CD147 knockdown and anti-CD147-treated cells, was associated with glycolytic inhibition and cell death as a result of ATP depletion. Earlier studies have shown that cancer cells typically exhibit increased glycolysis from which main ATP stores are generated. Inhibition of this pathway through anti-EMMPRIN therapy may be a novel strategy for cancer treatment.
To further evaluate the efficacy of anti-EMMPRIN therapy and compare results with those of an antibody currently approved for the treatment of head and neck carcinoma, tissue specimens were treated with cetuximab. Of 19 patient specimens, 58% responded to treatment with anti-EMMPRIN whereas only 33% responded to treatment with cetuximab. Cytotoxicity dose curves were established to show the optimal therapeutic dose for each antibody. A maximal effect was noted to occur at different concentrations for anti-EMMPRIN (100 µg/ml) and cetuximab (10 µg/ml). No additional patient samples responded to a higher dose of cetuximab therapy. When mean ATP levels (as a percentage of control) were compared between anti-EMMPRIN and cetuximab treatment groups, no significant difference was observed (57 vs. 45%, P = 0.13).
Sensitivity to anti-EMMPRIN therapy did not correlate with stage, tumor subsite or earlier treatment. As a significant amount of tissue is required for multiple treatment regimens the majority of specimens obtained w advanced stage lesions. Further studies are required to determine whether there is an association between treatment response and tumor characteristics. Response to a CNTO3899 does seem to correlate with EMMPRIN expression. Patient specimens that showed greater EMMPRIN staining trended toward superior response rates to anti-EMMPRIN therapy when compared with nonresponders (P = 0.06). EMMPRIN expression has been shown to be a prognostic indicator in head and neck carcinoma and is involved in tumor growth and metastasis [6,9]. We have shown earlier that EMMPRIN expression is associated with HNSCC xenograft response to bevacizumab therapy, and is likely related to EMMPRIN-stimulated vascular endothelial cell growth factor production [23].
Although earlier xenograft studies have shown a reduction in cell proliferation and an increase in apoptosis with the treatment of CNTO3899 [17], the exact mechanism by which the antibody mediates these effects is still unclear. Anti-EMMPRIN antibody-treated tumor slices show an increase in apoptosis (TUNEL staining) when compared with untreated controls (P < 0.001) and increased expression of extrinsic apoptosis pathway proteins caspase 3 and caspase 8. Many current cancer therapies, such as chemotherapy and γ-irradiation, exert their anti-tumor effects by triggering caspase-mediated apoptosis in cancer cells. Caspase activation is initiated either on ligation with the death receptor at the cell membrane (extrinsic pathways) or through proapoptotic mitochondrial signaling (intrinsic pathway). Stimulation of receptor-mediated apoptosis specifically results in pro-caspase-8 activation and downstream cleavage of effector protein caspase 3 [24]. In this study we show a moderate increase in expression of extrinsic pathway proteins caspase 3 and caspase 8 suggesting that the anti-EMMPRlN antibody may exert its effects through the stimulation of the death receptor. Links between the receptor and the mitochondrial pathway exist at different levels, however, and caspase-3 activation can occur with apoptotic mitochondrial signaling as well. The observation that caspase-8 expression was not elevated after anti-EMMPRIN treatment for tissue obtained from patient 15 (nonresponder) suggests caspase-3 activation may be more likely to occur through mitochondrial signaling, white lack of caspase-8 activation may be associated with resistance to anti-EMMPRIN therapy. Further studies will be required to define the precise molecular events that mediate anti-EMMPRIN-induced apoptosis.
Most targeted agents are used in combination with traditional cytotoxic therapies. Recent studies have shown that EMMPRIN expression promotes radiation resistance in cervical cancer [25] and is a strong prognostic factor for response to cisplatin-based chemotherapy in bladder cancer [26], We have shown earlier an enhanced radiation response in anti-EMMPRIN-treated head and neck cancer xenografts. Therefore, it is possible that greater sensitivity may occur in human head and neck cancer specimens with the combination of CNTO3899 and radiation or chemotherapy. In conclusion, anti-EMMPRIN therapy results in a reduction in cell proliferation and caspase-mediated apoptosis in ex-vivo human head and neck cancer specimens. EMMPRIN remains a novel target for future head and neck therapy.
Supplementary Material
Acknowledgements
This study was supported by grants from the National Cancer Institute (NCIK08CA102154) and the National Institute of Health (2T32 CA091078-06).
Footnotes
All supplementary data are available directly from the authors.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Silverman S., Jr Demographics and occurrence of oral and pharyngeal cancers. The outcomes, the trends, the challenge. J Am Dent Assoc. 2001;132(Suppl):7S–11S. doi: 10.14219/jada.archive.2001.0382. [DOI] [PubMed] [Google Scholar]
- 3.Gold KA, Lee HY, Kim ES. Targeted therapies in squamous cell carcinoma of the head and neck. Cancer. 2009;115:922–935. doi: 10.1002/cncr.24123. [DOI] [PubMed] [Google Scholar]
- 4.Bonner JA, De Los Santos J, Waksal HW, Needle MN, Trummel HO, Raisch KR. Epidermal growth factor receptor as a therapeutic target in head and neck cancer. Semin Radiat Oncol. 2002;12:11–20. doi: 10.1053/srao.2002.34864. [DOI] [PubMed] [Google Scholar]
- 5.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal EL, Shreenivas S, Peters GE, Grizzle WE, Desmond R, Gladson CL. Expression of extracellular matrix metalloprotease inducer in laryngeal squamous cell carcinoma. Laryngoscope. 2003;113:1406–1410. doi: 10.1097/00005537-200308000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, et al. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–439. [PubMed] [Google Scholar]
- 8.Riethdorf S, Reimers N, Assmann V, Kornfeld JW, Terracciano L, Sauter G, et al. High incidence of EMMPRIN expression in human tumors. Int J Cancer. 2006;119:1800–1810. doi: 10.1002/ijc.22062. [DOI] [PubMed] [Google Scholar]
- 9.Bordador LC, Li X, Toole B, Chen B, Regezi J, Zardi L, et al. Expression of emmprin by oral squamous cell carcinoma. Int J Cancer. 2000;85:347–352. [PubMed] [Google Scholar]
- 10.Schlegel J, Redzic JS, Porter CC, Yurchenko V, Bukrinsky M, Labeikovsky W, et al. Solution characterization of the extracellular region of CD147 and its interaction with its enzyme ligand cyclophilin A. J Mol Biol. 2009;391:518–535. doi: 10.1016/j.jmb.2009.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenthal EL, Zhang W, Talbert M, Raisch KR, Peters GE. Extracellular matrix metalloprotease inducer-expressing head and neck squamous cell carcinoma cells promote tibroblast-mediated type I collagen degradation in vitro. Mol Cancer Res. 2005;3:195–202. doi: 10.1158/1541-7786.MCR-04-0203. [DOI] [PubMed] [Google Scholar]
- 12.Zucker S, Hymowitz M, Rollo EE, Mann R, Conner CE, Cao J, et al. Tumorigenic potential of extracellular matrix metalloproteinase inducer. Am J Pathol. 2001;158:1921–1928. doi: 10.1016/S0002-9440(10)64660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, Matrisian LM, Holmbeck K, Vick CC, Rosenthal EL. Fibroblast-derived MT1-MMP promotes tumor progression in vitro and in vivo. BMC Cancer. 2006;6:52. doi: 10.1186/1471-2407-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caudroy S, Polette M, Nawrocki-Raby B, Cao J, Toole BP, Zucker S, et al. EMMPRIN-mediated MMP regulation in tumor and endothelial cells. Clin Exp Metastasis. 2002;19:697–702. doi: 10.1023/a:1021350718226. [DOI] [PubMed] [Google Scholar]
- 15.Newman JR, Bohannon IA, Zhang W, Skipper JB, Grizzle WE, Rosenthal EL. Modulation of tumor cell growth in vivo by extracellular matrix metalloprotease inducer. Arch Otolaryngol Head Neck Surg. 2008;134:1218–1224. doi: 10.1001/archotol.134.11.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005;65:3193–3199. doi: 10.1158/0008-5472.CAN-04-3605. [DOI] [PubMed] [Google Scholar]
- 17.Dean NR, Newman JR, Helman EE, Zhang W, Safavy S, Weeks DM, et al. Anti-EMMPRIN monoclonal antibody as a novel agent for therapy of head and neck cancer. Clin Cancer Res. 2009;15:4058–4065. doi: 10.1158/1078-0432.CCR-09-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estes JM, Oliver PG, Straughn JM, Jr, Zhou T, Wang W, Grizzle WE, et al. Efficacy of anti-death receptor 5 (DR5) antibody (TRA-8) against primary human ovarian carcinoma using a novel ex-vivo tissue slice model. Gynecol Oncol. 2007;105:291–298. doi: 10.1016/j.ygyno.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Robert F, Ezekiel MP, Spencer SA, Meredith RF, Bonner JA, Khazaeli MB et al. Phase I study of anti-epidemial growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:3234–3243. doi: 10.1200/JCO.2001.19.13.3234. [DOI] [PubMed] [Google Scholar]
- 20.Kirby TO, Rivera A, Rein D, Wang M, Ulasov I, Breidenbach M, et al. A novel ex vivo model system for evaluation of conditionally replicative adenoviruses therapeutic efficacy and toxicity. Clin Cancer Res. 2004;10:8697–8703. doi: 10.1158/1078-0432.CCR-04-1166. [DOI] [PubMed] [Google Scholar]
- 21.Kendrick JE, Straughn JM, Jr, Oliver PG, Wang W, Nan L, Griule WE, et al. Anti-tumor activity of the TRA-8 anti-DR5 antibody in combination with cisplatin in an ex vivo human cervical cancer model. Gynecol Oncol. 2008;108:591–597. doi: 10.1016/j.ygyno.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 22.Baba M, Inoue M, Itoh K, Nishizawa Y. Blocking CD147 induces cell death in cancer cells through impairment of glycolytic energy metabolism. Biochem Biophys Res Commun. 2008;374:111–116. doi: 10.1016/j.bbrc.2008.06.122. [DOI] [PubMed] [Google Scholar]
- 23.Newman JR, Helman EE, Safavy S, Zhang W, Rosenthal EL. EMMPRIN expression is required for response to bevacizumab therapy in HNSCC xenografts. Cancer Lett. 2009;274:313–318. doi: 10.1016/j.canlet.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 25.Ju XZ, Yang JM, Zhou XY, Li ZT, Wu XH. EMMPRIN expression as a prognostic factor in radiotherapy of cervical cancer. Clin Cancer Res. 2008;14:494–501. doi: 10.1158/1078-0432.CCR-07-1072. [DOI] [PubMed] [Google Scholar]
- 26.Als AB, Dyrskjot L, von der Maase H, Koed K, Mansilla F, Toldbod HE, et al. Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13:4407–4414. doi: 10.1158/1078-0432.CCR-07-0109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.