Abstract
Pancreatic cancer is one of the most lethal diseases with no effective treatment. Previously, we have shown that FAK is overexpressed in pancreatic cancer and plays a key role in cancer cell survival and proliferation. FAK has been shown to interact with growth factor receptors including cMET and IGF-1R. As a novel therapeutic approach, we targeted the protein interaction of FAK with growth factor receptors to block tumor growth, alter signaling pathways and sensitize cells to chemotherapy. We have selected a small molecule compound (INT2-31) that decreases phosphorylation of AKT via disrupting interaction of FAK with cMET and IGF-1R. Our results demonstrate that interaction of a small molecule compound with FAK decreases phosphorylation of FAK Y397 while increasing FAK Y407 phosphorylation, without inhibiting the kinase activity of FAK and dramatically reduces downstream signaling to AKT. Our lead compound, INT2-31, demonstrates significant inhibition of tumor cell growth in two orthotopic models of pancreatic cancer. In addition, INT2-31increases sensitivity to gemcitabine chemotherapy in a direct fresh biopsy xenograft model of pancreatic cancer growth.
Keywords: FAK, IGF-1R, pancreatic cancer, protein interactions
INTRODUCTION
Pancreatic cancer has the worst prognosis of all carcinomas, with an extremely low survival rate (<5%). For decades, no significant advancements have been made in the treatment of this cancer. According to published cancer statistics for the United States, pancreatic cancer is the fourth leading cause of cancer-related deaths [1]. Due to an often late presentation of symptoms, detection is frequently delayed until the disease is quite advanced, which severely undermines the effectiveness of existing treatments. Most cases are not candidates for surgical excision of tumors, due to pervasive local invasion as well as metastases [2]. Pancreatic carcinoma is also highly resistant to current chemotherapy and radiation therapy techniques [3]. There is an urgent need for the development of novel therapeutic remedies that specifically and preferentially target pancreatic cancer cells. Elucidation of the molecular mechanism of cancer cell survival and proliferation in these tumors is of utmost importance in the development of efficacious treatments.
Multiple signaling pathways have been implicated in pancreatic cancer [4]. One of these pathways includes the cMET tyrosine kinase, a cell surface receptor for hepatocyte growth factor (HGF). HGF/MET are essential mediators for morphogenetic processes in fetal development, and are also critical signaling pathways for tissue repair [5]. Inflammation-induced activation of the cMET pathway may initiate formation of cancer cells. It has been shown that cMET is overexpressed and aberrantly activated in pancreatic cancer and promotes survival, motility, proliferation, and invasive activity of cells [6,7]. Upon HGF-binding to cMET, autophosphorylation of Y1349 and Y1356 makes accessible multiple docking sites for subsequent binding partners, including FAK [8].
Similar to cMET, insulin-like growth factor receptor-1 (IGF-1R) is involved in many biological and pathological pathways [9]. Studies have shown that IGF-1R is overexpressed in multiple cancers, including pancreatic cancer [10]. Activation of IGF-1R results in receptor dimerization and cross-phosphorylation of the kinase domain, which initiates a downstream signaling cascade. We have found that intracellular interaction between IGF-1R and FAK promotes anchorage-independent survival and growth of cancer cells [11,12]. Targeted disruption of IGF-1R signaling has been shown to effectively inhibit tumor growth and invasive behavior [13–14].
Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase protein involved in many biological processes [15]. FAK is composed of three distinct moieties; a N-terminus FERM domain (protein 4.1, ezrin, radixin and moesin homology), a mid-kinase region, and a C-terminal focal adhesion targeting (FAT) domain. FAK is a significant convergence point for extracellular stimulations through multiple cell receptor tyrosine kinases, including IGF-1R and cMET [16]. Once activated, FAK can bind to multiple signaling molecules, such as p120RasGAP, GRB7, GRB2, p130Cas, and Src. It has been established that interaction of the FERM domain with receptor kinases phosphorylates the Y397 site, inducing a conformational change that activates the kinase domain [17,18]. As with IGF-1R, FAK is also found to be overexpressed in pancreatic cancer cells and is involved in cancer growth via multiple mechanisms, including preventing cell apoptosis and increasing cancer cell motility [19,20].
Studies have found that inhibition of FAK interactions shows promising results for decreased tumor growth and proliferation [21]. In this work, we demonstrate a potentially more effective approach for cancer treatment by targeting protein-protein interactions (PPIs), specifically FAK interactions with IGF-1R and cMET.
Materials and Methods
Cell Lines and Cell Culture
Human carcinoma cells were obtained from American Type Culture Collection (Rockville, MD). Panc-1 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS) and 1 µg/ml penicillin–streptomycin. MiaPaca-2 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS, 2.5% horse serum and 1 µg/ml penicillin–streptomycin. Other cell lines were obtained and cultured as we described previously [22].
Stable Transduction of Firefly Luciferase in Cell Lines
Infection of pancreatic cancer cell lines, Panc-1 and Mia paca-2 was done in the laboratory of Dr. Lung-Ji Chang at the University of Florida. Pancreatic cancer cell lines were trypsinized and counted. The cells were then plated in 24-well trays and incubated at 37°C until 60–80% confluent. In each well, firefly luciferase and red fluorescent protein (RFP) containing lentivirus particles were added to the medium. After gently swirling the plate, cells were incubated at 37°C in a humidified incubator in an atmosphere of 5% CO2, to allow for optimal transduction efficiency. Four hours later, viral containing medium was replaced with fresh medium. Based on expression of RFP protein and flow cytometric sorting of the cells, pure populations of transduced cells were obtained.
Reagents and Antibodies
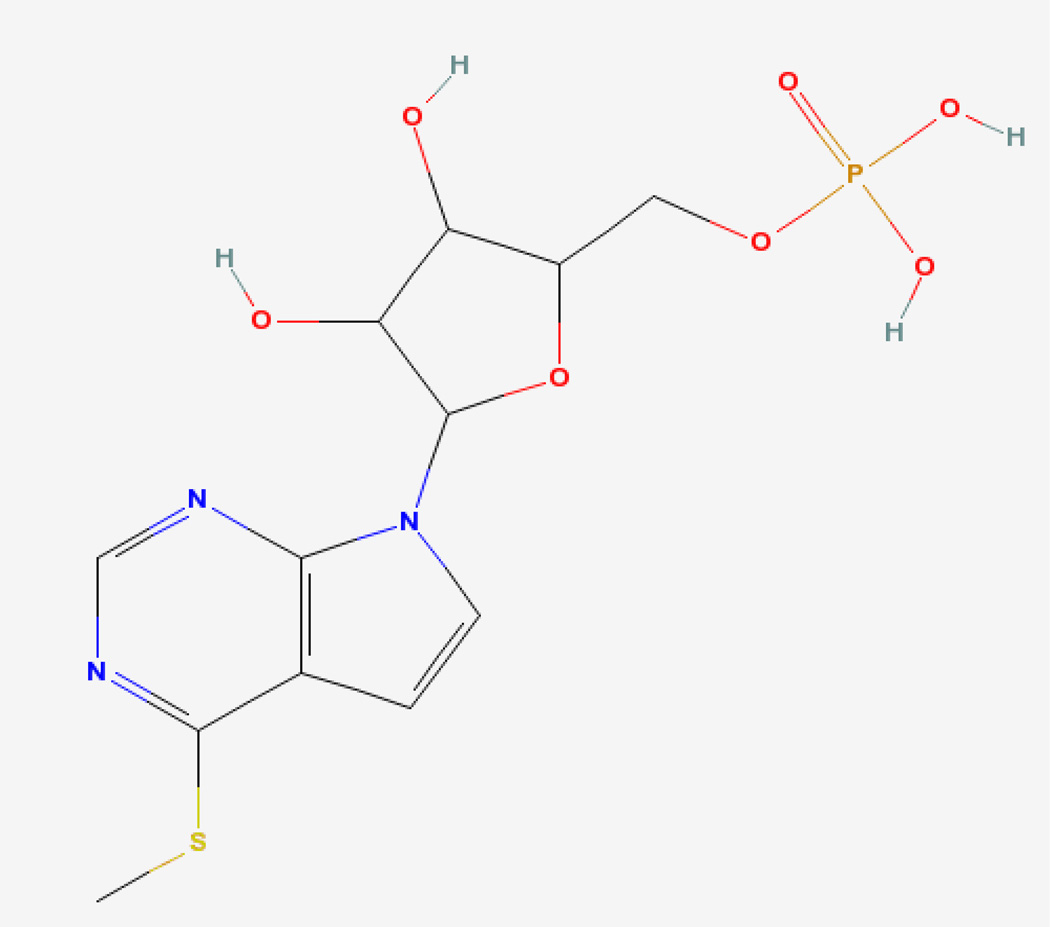
INT2-31 (C12H16N3O7PS) (7H-Pyrrolo[2,3-d]pyrimidine, 4-(methylthio)-7-(5-O-phosphono-.beta.-D-ribofuranosyl) (Figure 1) was originally obtained from the National Cancer Institute Developmental Therapeutics Program and subsequently synthesized by Alchem (Gainesville, FL) (Figure 1). Gemcitabine was obtained from Eli Lilly and Company (Indianapolis, IN). Anti-FAK, anti-IGF-1Rβ, anti-IRS-1, anti-phospho-IGF-1R, anti-caspase 8, anti-caspase 9, anti-phospho-AKT (Ser473), anti-AKT, anti-phospho-ERK1/2, anti-ERK1/2 and anti-PARP antibodies was from Cell Signaling Technology (Beverly, MA). Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody from Advanced ImmunoChemical (Long Beach, CA). Polyclonal anti-phospho-Met (Tyr-1234/Tyr-1235) was purchased from Upstate Biotechnology (Lake Placid, NY). Polyclonal anti-phospho-Met (Tyr-1349) was from Cell Signaling Technology (Beverly, MA).
Figure 1.
Structure of INT2-31.
Kinase Profile Screening
Kinase specificity screening was performed with Millipore Kinase Profiling Services. The screening was performed with 10 µM compound, INT2-31, 10µM ATP, and kinase substrates against 288 recombinant kinases.
Orthotopic model of pancreatic cancer
For orthotopic model of pancreatic cancer, the University of Florida IACUC approved the following protocol (IACUC Study# 2000801506). The luciferase-RFP expressing pancreatic cancer cell lines, Miapaca-2 and Panc-1, were implanted into the pancreas of nude mice. For intra-pancreatic implantation of cells, mice were anesthetized with Isoflurane. Under sterile surgical conditions, via 1.0 cm incision of the skin, abdominal wall and peritonium, the spleen was retracted and cells were injected in 30µL volume into the tail of the pancreas using a 27-gauge needle. The abdominal wall and peritoneum were sutured using 5.0 absorbable surgical sutures and the skin was closed with clips. A cryogenically cooled IVIS Imaging System (Xenogen) with Living Image acquisition and analysis software (Version 2.11, Xenogen) was used for detecting the bioluminescence signals in mice. When tumors reach ~ 100 mm3, mice were randomized into groups, with 7 mice in each group.
Direct fresh biopsy patient xenografts
Tumor samples from human patients with pancreatic cancer were obtained at the time of surgery and implanted into nude mice. Initially small pieces (0.3 × 0.3 × 0.3 cm) from fresh pancreatic tumor samples obtained from surgical specimens of patients operated at the University of Florida Shands Hospital, were implanted subcutaneously in group of 4 mice for each patient. When they reached 1 cm3, tumors were excised, cut into small pieces, and transplanted subcutaneously into another 20 mice. When tumors reached ~ 100 mm3, mice were randomized into treatment groups.
FERM domain targeting peptide
A 16 aa peptide (SVKAKTLRKLIQQTFR, N terminal acetyl and C-terminla amide; HPLC-MS purity >95%) containing a conserved sequence corresponding to the FAK FERM domain, shown to be important for interaction with cMET[23] was prepared by Genscript USA Inc. (Piscataway, NJ).
Results
INT2-31 Reduces the Viability of Cancer Cells
To evaluate the effect of INT2-31 on cell viability of melanoma, esophageal, pancreatic and breast cancer cell lines the IC50 value was determined for each cell line (Table 1). To obtain the average IC50 value, each cell line was treated in triplicate with increasing concentrations of the compound for 72 hours, and the median IC50 value was calculated. A majority of the cancer cell lines were highly sensitive to 72-hour treatment with INT2-31. In contrast, normal breast epithelial cells MCF10A and melanocyte cells were found to be highly resistant to INT2-31, (average IC50 of 100 µM and 99.3 µM, respectively, highlighting the selective targeting of tumor cells.
Table 1.
IC50 values following treatment with INT2-31 in multiple cell lines
| Breast Cancer | Conc (µM) |
|---|---|
| MCF7 | 0.03 |
| BT474 | 10.5 |
| MCF10A | 100 |
| Melanoma | |
| A375 | 2.7 |
| C8161 | 1.5 |
| SK-ML-28 | 22.1 |
| Melanocytes | 99.3 |
| Pancreatic Cancer | |
| Miapaca2 | 0.5 |
| Panc1 | 6.8 |
| AsPC-1 | 15.8 |
| Esophageal Cancer | |
| KYSE70 | 4.6 |
| KYSE140 | 2.5 |
INT2-31 Disrupts cMET and IGF-1R Interactions with FAK
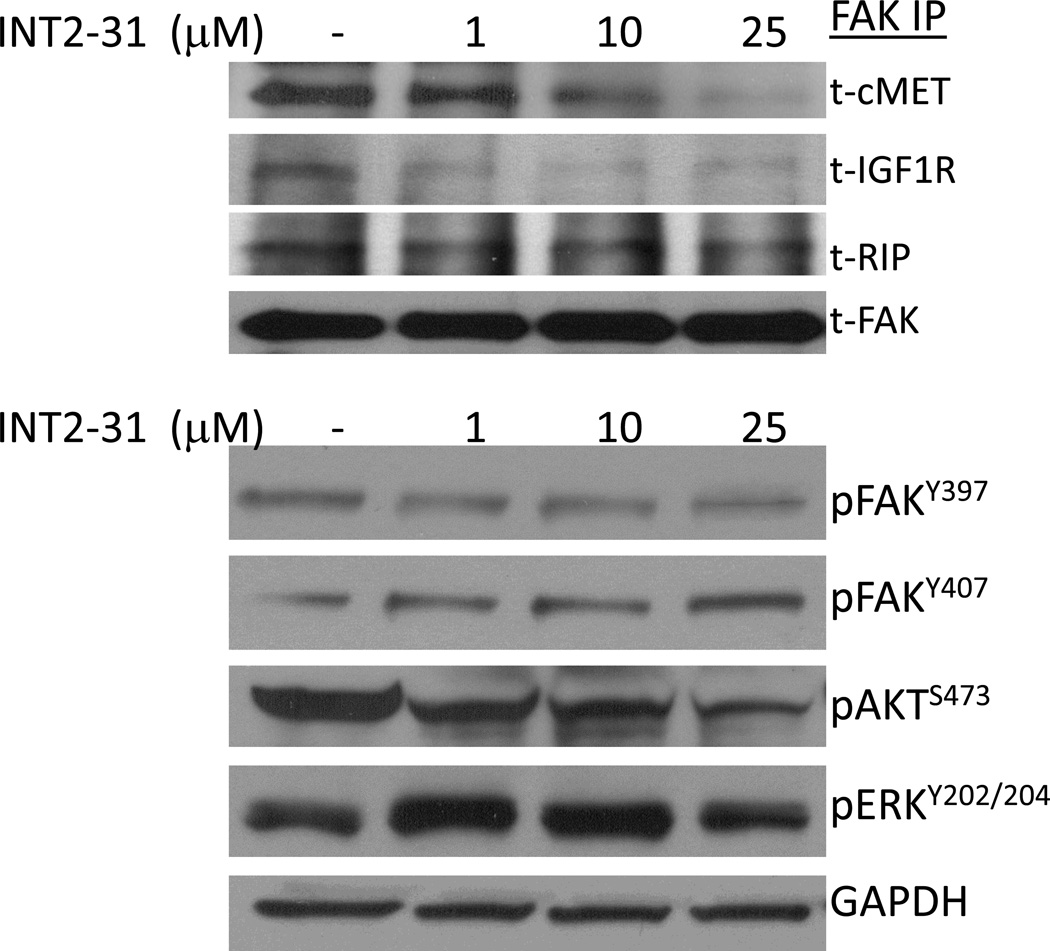
To test the ability of our lead small molecule compound to disrupt the protein interactions of FAK, Panc-1 cells were treated with 1, 10 and 25 µM of INT2-31 for an hour and harvested cell lysates were used for immunoprecipitation with FAK-4.47 antibody. INT2-31 disrupted binding of cMET and IGF-1R proteins at low micromolar concentration as shown by immunoblot analysis (Figure 2, upper panel). Another FERM domain binding partner, receptor interacting protein (RIP), level remained comparable to those observed in the control cells. In addition, serum starved cells displayed a reduced basal level interaction of cMET and IGF-1R with FAK protein (Supplemental Figure 1). Upon induction of cells with 10 nM IGF-1 for 10 minutes, the interaction of FAK and IGF-1R was restored to normal medium control levels. Similar to serum free conditions, increasing INT2-31 concentrations in full serum, and in the absence of IGF-1 stimulation, prevented the interaction of FAK with cMET and IGF-1R.
Figure 2. INT2-31 disrupts binding of cMET and IGF-1R with FAK.
Panc-1 cells were treated with increasing doses of INT2-31 for one hour duration. An antibody to FAK was utilized to pulldown FAK and coimmunoprecipitation of IGF-1R, cMET and RIP was evaluated (top panel). Western blot analysis following treatment with INT2-31 demonstrates a slight decrease in FAKY397 with an increase in FAK407. This is associated with a decrease in p-Akt and an increase in p-ERK.
Disruption of Protein-Protein Interactions Alters Phosphorylation of Proteins in a Time and Dose-Dependent Manner
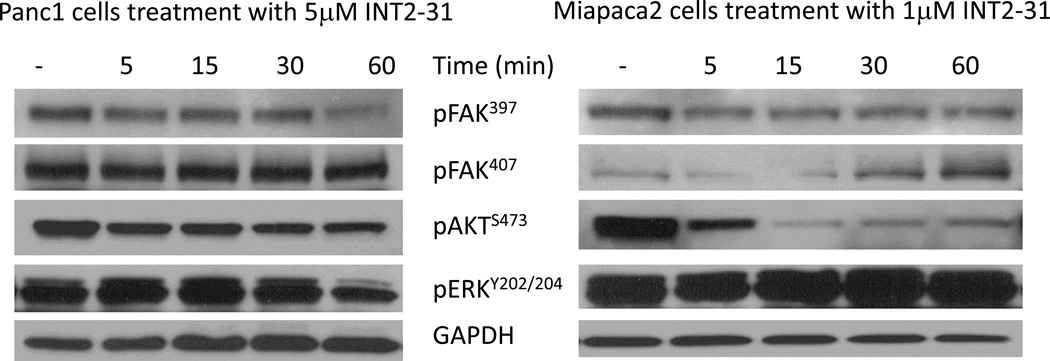
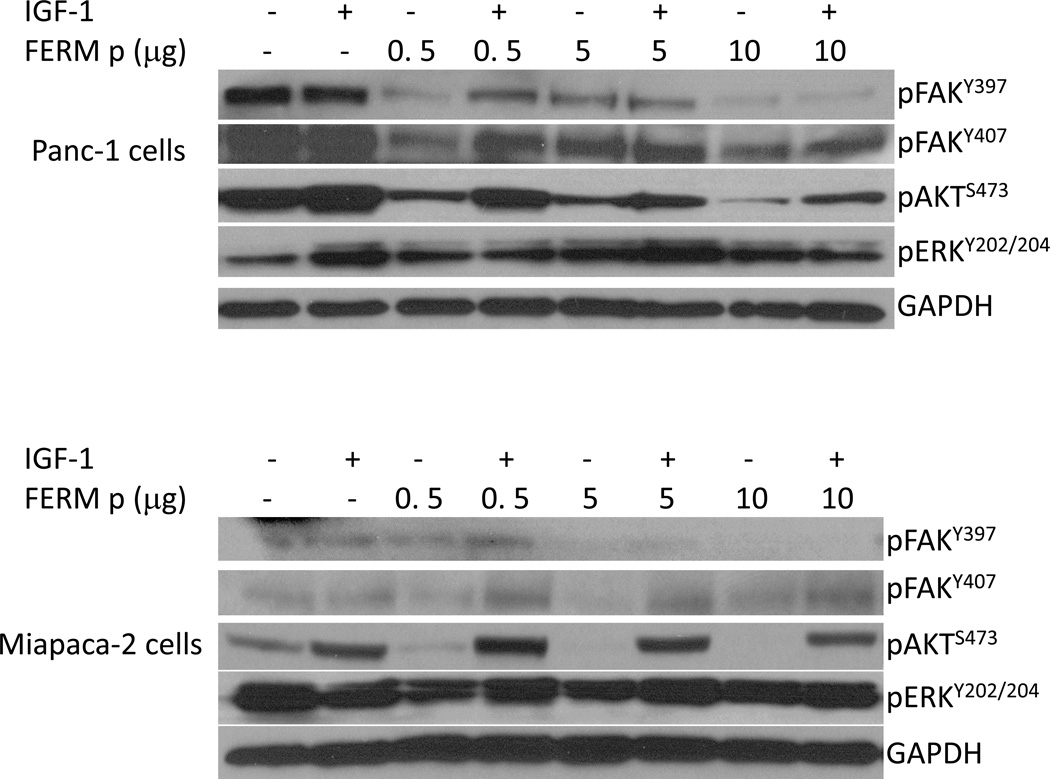
Due to interfering with FAK protein interactions, possible alterations in signal transduction pathways were expected with INT2-31 treatment. Therefore, we monitored the effects of INT2-31 on the phosphorylation status of targeted proteins. In dose dependent assays, Panc-1 cells were treated with increasing concentrations of INT2-31. Although there was only a slight decrease in FAK397, there was a more significant increase in FAK407, and decrease in AKT phosphorylation (Figure 2, lower panel). In time course assays, Miapaca2 and Panc-1 cells were treated with 1 and 5 µM INT2-31, respectively, for one hour period of time. We observed a decreased phosphorylation of FAK Y397 in both cell lines, with an increase of phosphorylation of the inhibitory site of Y407 on the FERM domain of the FAK protein in Miapaca-2 cells (Figure 3 and Supplemental Figure 2). More importantly, disruption of cell surface receptor protein interactions with FAK efficiently altered the downstream signaling to AKT, and reduced AKT phosphorylation in both cell lines. Furthermore, 24h serum starvation, and 10 min HGF treatment of Panc-1 cells in the presence of increasing doses of INT2-31 significantly reduced the phosphorylation of Y1345 on cMET in a dose dependent manner, whereas Y1230-34-35 remained unchanged. These results indicate that cMET was induced by HGF, yet disruption of its binding to FAK suppressed its full activation (Figure 4). Furthermore, to validate that the changes in the phosphorylation of FAK and AKT are due to disruption of protein-protein interactions, we incubated the cells with increasing concentrations of a 16 aa peptide (SVKAKTLRKLIQQTFR) that contains the targeted FAK FERM domain site. As demonstrated in Figure 5, incubation with a peptide corresponding to 16aa of the FERM domain yields similar results to INT2-31 treatment including reduction in p-Y397 FAK and p-S473 AKT in both cell lines, as well as increased p-Y407 FAK in Miapaca-2 cells.
Figure 3. INT2-31 increases p-FAK407 and decreases p-Akt.
Panc-1 and Miapaca-2 cells were treated with INT2-31 for 5, 15, 30 and 60 minutes in full serum. Western blot analysis demonstrates an increase in p-FAK407 in Miapaca-2 cells associated with a decrease in p-Akt.
Figure 4. INT2-31 decreases phosphorylation of Y13454 on cMET.
Following 24 hours of serum starvation, and 10 min HGF treatment of Panc-1 cells in the presence of increasing doses of INT2-31, phosphorylation of Y1345 on cMETis reduced in a dose dependent manner, whereas Y1230-34-35 remained unchanged. These results indicate that cMET was induced by HGF, yet disruption of its binding to FAK suppressed its full activation.
Figure 5. A peptide corresponding to 16aa of the FAK FERM domain yields similar results to INT2-31.
Panc-1 (upper panel) and Miapaca-2 (lower panel) cells were incubated with a 16 aa peptide (SVKAKTLRKLIQQTFR) corresponding to the FAK FERM domain for 12 hours. This resulted in a reduction in p-Y397 FAK and p-S473 AKT, as well as increased p-Y407 FAK, similar to INT2-31 treatment.
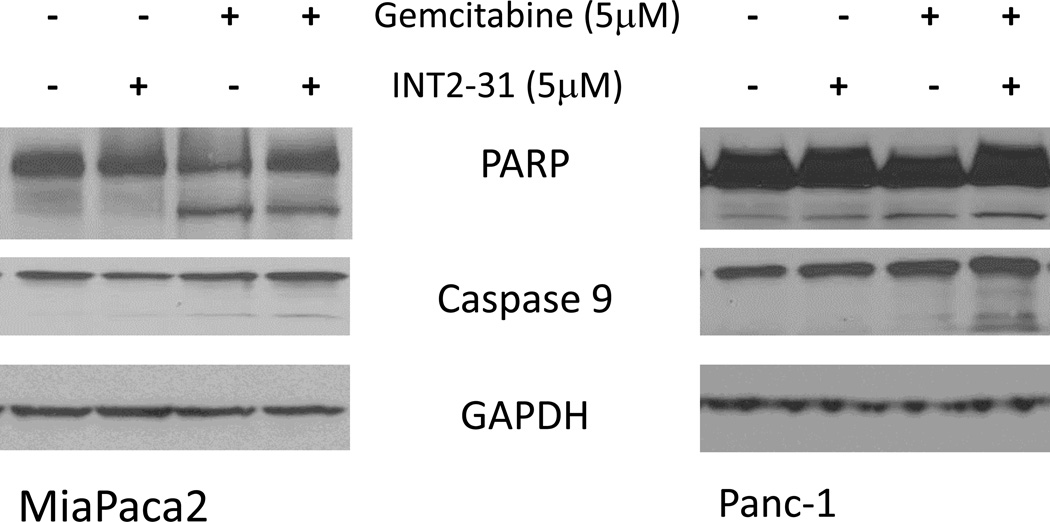
INT2-31 and Gemcitabine Synergistically Induces Apoptosis
In addition to the significant effect of INT2-31 on pancreatic cancer cell viability, immunoblot analysis demonstrated that the combination of INT2-31 and gemcitabine increased apoptosis at 48h in both Panc-1 and Miapaca2 cells. PARP or caspase-9 cleavage was demonstrated starting at 48h of treatment with INT2-31. Interestingly, gemcitabine significantly activated caspase 9 cleavage in both Miapaca2 and Panc-1 cells. However, the combination of INT2-31 and gemcitabine resulted in an additive effect on caspase-9 cleavage in both cell lines (Figure 6).
Figure 6. Effect of INT2-31 treatment in combination with gemcitabine.
Incubation of Panc-1 and Miapaca-2 cells for 48 hours with the combination of gemcitabine (5µM) and INT2-31 (5µM) resulted in a PARP and caspase-9 cleavage.
Inhibition of Tumor Growth with INT2-31 Treatment in an Orthotopic Pancreatic Cancer Xenograft Model
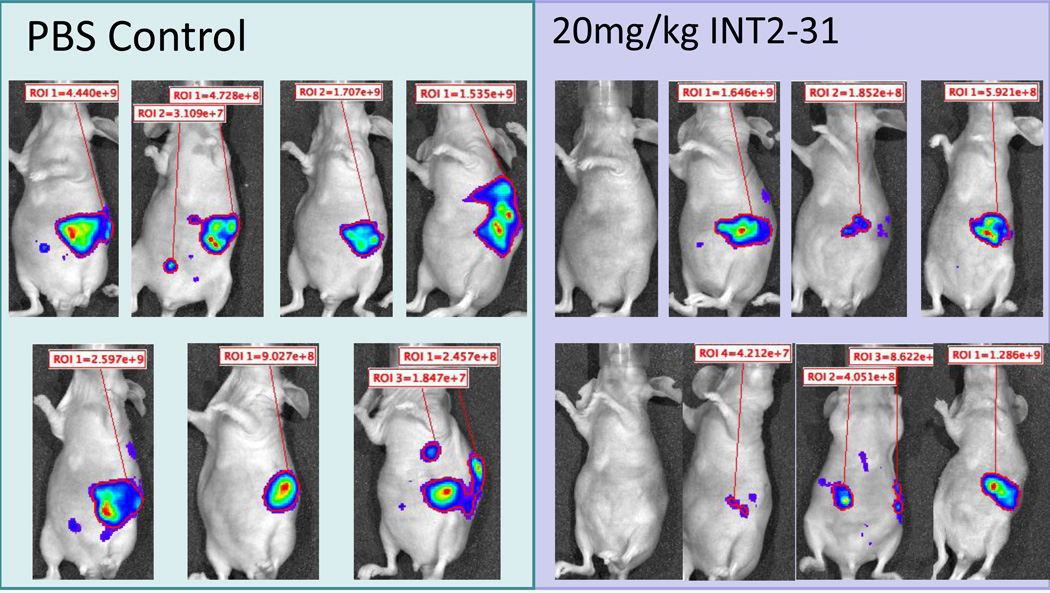
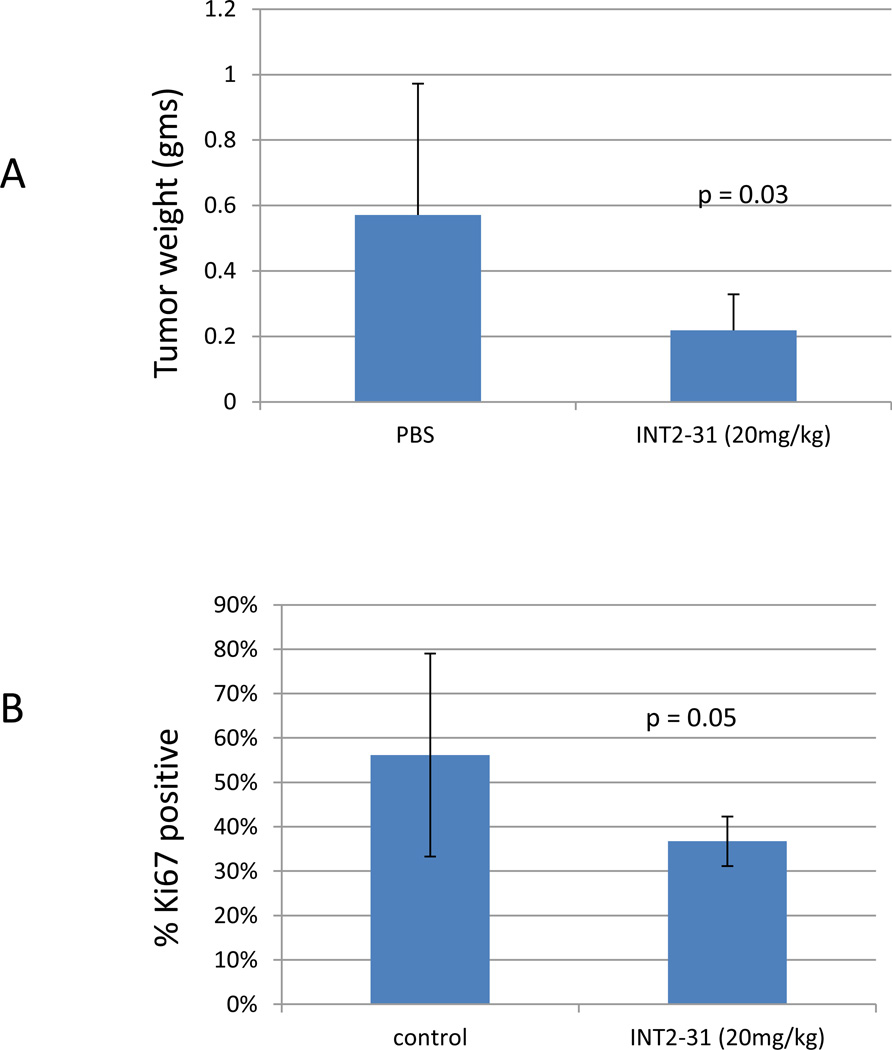
To further validate the activity and specificity of INT2-31, we used an orthotopic pancreatic cancer mouse model. The pancreatic cancer cell lines, Miapaca-2 and Panc-1 cells were stably infected using luciferase-RFP (red fluorescent protein) reporter gene for in vivo imaging of the xenografts. Following expansion and sorting of RFP positive cells, cells were expanded in culture and 5 × 106 tumor cells were implanted into the pancreas of nude mice. Ten days following implantation, mice were equally randomized to treatment with INT2-31 vs PBS control based on the presence of photon emission from the tumor. All mice had luciferase imaging demonstrating presence of tumor at the initiation of treatment. As shown in Figures 7 and 8, daily intraperitoneal treatment with 50 mg/kg or 20 mg/kg of INT2-31 for twenty days significantly decreased tumor growth of both Miapaca2 and Panc-1 tumors, respectively, as determined by the tumor weight (Figure 9A), without any significant side effects on body weights and the appearances of the animals. Although the luciferase photon emission appears less in both Miapaca-2 and Panc-1 tumors treated with INT2-31, quantification of the photon emission between the two groups failed to reach statistical significance, possibly due to the large variation in the photon signal between each animal. In addition, the Ki67 index was reduced in Panc-1 tumors treated with INT2-31 (p=0.05, Figure 9B).
Figure 7. Effect of in vivo administration of INT2-31 in an orthotopic models of Miapaca-2 cells.
Bioluminescent imaging of luciferase expressing Miapaca-2 cells reveals a visual decrease in tumor signal following daily intraperitoneal treatment with 50 mg/kg of INT2-31 as compared to PBS control. Quantification of the photon emission failed to reach statistical significance, possibly due to the large variation in the photon emission from each tumor.
Figure 8. Effect of in vivo administration of INT2-31 in orthotopic models of Panc-1 cells.
Bioluminescent imaging of luciferase expressing Panc-1 cells reveals a visual decrease in tumor signal following daily intraperitoneal treatment with 20 mg/kg of INT2-31 as compared to PBS control. Quantification of the photon emission failed to reach statistical significance, possibly due to the large variation in the photon emission from each tumor.
Figure 9. Tumor weights and Ki67 proliferative index in animals implanted with orthotopic Panc-1 cells.
Treatment with INT2-31 was associated with a significant decrease in tumor weights (A) and Ki67 proliferative index (B) in those animals treated with INT2-31 compared to PBS control.
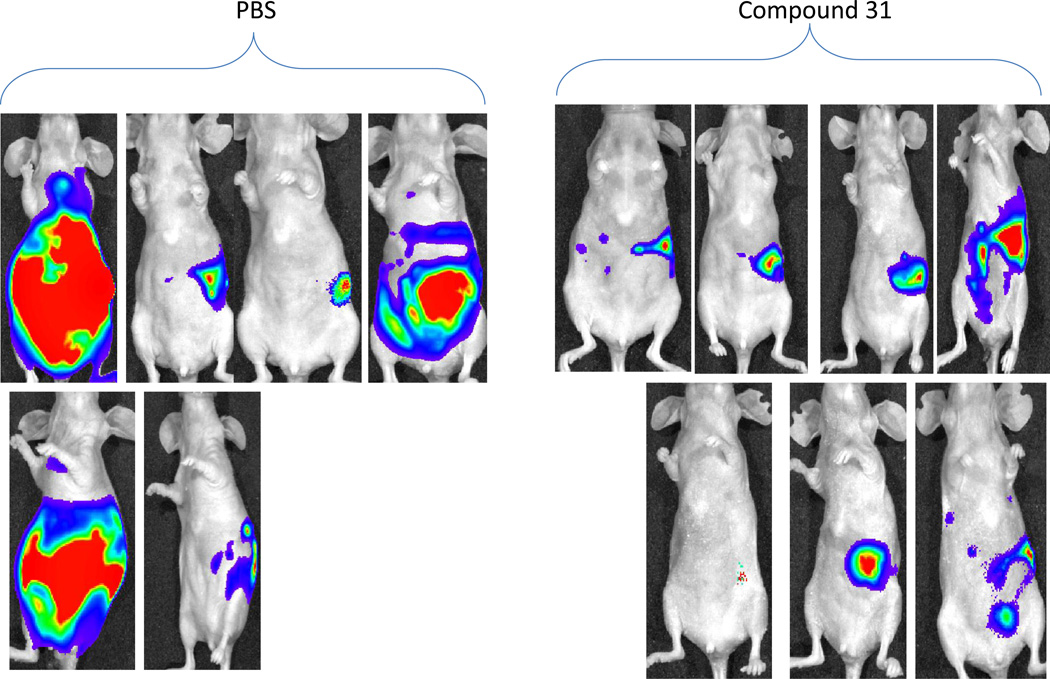
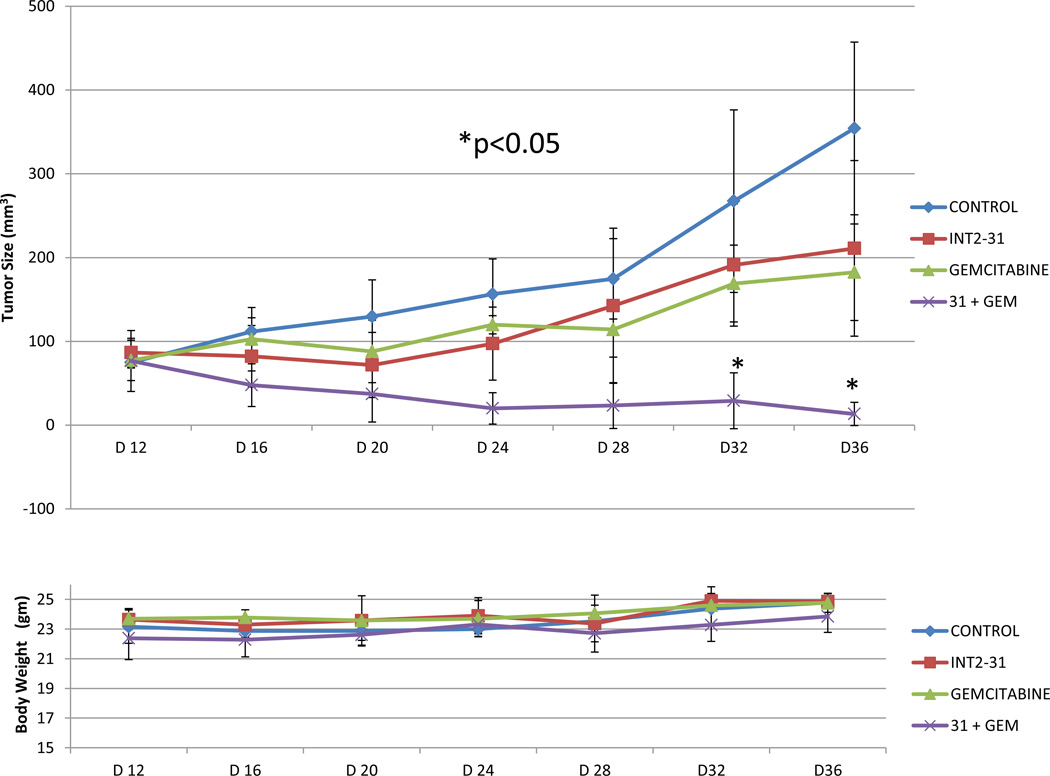
Subsequently, the effect of INT2-31 was evaluated in a direct fresh biopsy pancreatic cancer xenograft growing in the subcutaneous position of nude mice. Daily IP administration of INT-31 (20 mg/kg) in combination with gemcitabine chemotherapy (25 mg/kg)-significantly decreased growth of a direct pancreatic cancer xenograft compared to any therapy alone (Figure 10).
Figure 9. Effect of in vivo administration of INT2-31 plus gemcitabine in a direct fresh biopsy xenograft model of pancreatic cancer.
Daily IP administration of low dose INT2-31 (20 mg/kg) in combination with gemcitabine (25mg/kg every 3 days) chemotherapy decreased growth of a direct pancreatic cancer xenograft compared to any therapy alone. (* p<0.05 for combination treatment)
DISCUSSION
Pancreatic cancer is a unique disease that warrants special attention in the area of research and development for novel therapeutic approaches. Appropriate selection and targeting of specific molecular sites in pancreatic cancer cells should increase efficiency of treatment and minimize side effects. The dual function of FAK as both a kinase and scaffolding protein, a recipient of external signals and a transmitter of intracellular signals, renders it an excellent candidate for inhibition with an organic small molecule compound [15,24]. However, preferentially targeting desired protein kinase activity can be challenging due to similarities in the amino acid sequence and structure of the active site of kinases [25]. Non-selective kinase inhibition can result in side effects, as observed with the FAK inhibitor TAE226 (Novartis Pharm) [26]. Therefore, in this study, we focus on specific targeting of protein-protein interactions of FAK with growth factor receptors as an alternative and potentially more selective way of inhibiting FAK function.
We have defined IGF-1R as the binding partner of FAK at the FERM domain[27], while others have shown that cMET, PDGF (platelet-derived growth factor), and EGFR (epidermal growth factor receptor) also bind to the FERM domain of FAK [8,28]. Many structural and sequence similarities have been found between the cytoplasmic regions of IGF-1R and cMET. To define the similarities between IGF-1R and cMET proteins, using the NCBI blast program (http://blast.ncbi.nlm.nih.gov/Blast.cgi,) two proteins were aligned and their amino acids were overlapped (Supplemental Figure 3). Their structural similarities are pronounced (Supplemental Figure 4). Chen et al have previously identified the interaction site of the FERM domain (216KAKTLRK222) with cMET [23]. Therefore we initially evaluated the disruption of both the FAK-IGF-1R and FAKcMET interactions using a highly selective small molecule compound (INT2-31). However, it is also possible that INT2-31 disrupts FAK interactions with other growth factor receptors or other non specific protein interactions. Specifically, our cell viability assay results demonstrate the sensitivity of breast cancer cell lines to INT2-31 (Table 1) indicating that the critical interactions between FAK and growth factor receptors that is affected by INT2-31 is not important for pancreatic cancer cells only but worth evaluating in multiple cell types. Of note, normal cell lines such MCF10A and melanocytes are much less sensitive to INT2-31, demonstrating the increased sensitivity of cancer cells to this small molecule compound. Indeed, our previous results support this as well [22].
Our results also show that INT2-31, which was selected by virtual screening to bind to the FERM domain of FAK, effectively disrupts the interaction of both cMET and IGF-1R with FAK. Treatment with INT2-31 decreases slightly the phosphorylation of Y397 and increases phosphorylation of Y407 on FAK in Miapaca-2 cells, which is associated with a decreased phosphorylation of AKT. These effects are seen without alteration of the kinase activity of FAK (Supplemental Table 1). Since FAK has been shown to autoinhibit its own activation with the FERM and kinase domains forming a direct interaction blocking access of ATP and substrate to the active site, it is possible that INT2-31 binds to FAK and increases this autoinhibition configuration [29]. Similar to the effects of INT2-31, we have also found that serum free medium conditions decreases the interaction of FAK with cMET and IGF-1R as shown in Supplemental Figure 1.
In addition to the effects in vitro, INT2-31 had potent effects in vivo with inhibition of tumor growth in both orthotopic and direct fresh biopsy xenograft models of pancreatic cancer. Previously, we have shown that INT2-31 penetrates tumor cells in vitro and can be identified in the tumor xenograft in vivo [30]. The additive effect of INT2-31 with gemcitabine, for inducing apoptosis and inhibiting tumor growth, demonstrates the possible use of INT2-31 in combination with standard chemotherapeutic regimens for pancreatic cancer.
To ascertain that the mechanism of decreased phosphorylation was via disruption of the protein-protein interactions, and not due to inhibition of kinase activity, INT2-31 was analyzed for inhibition of 288 kinases using a Millipore kinase screening service. The data revealed no significant inhibition of kinase activity with 10 uM INT2-31 (Supplemental Table 1). This provided the necessary confirmation that the decreased phosphorylation is due to blocking the protein-protein interaction of critical growth factor receptors and FAK.
It has been shown that the receptor interacting protein (RIP) also binds with the FERM domain of FAK [31]. For this reason, we examined whether INT2-31 interferes with RIP binding to FAK. Co-IP results showed that in the presence of INT2-31, the level of RIP pull down by FAK was comparable to that in the control. Furthermore, when we evaluated cancer cells treated with INT2-31 for activation of apoptotic pathways, intrinsic caspase 9 pathways were activated within 48 hours of treatment. If the RIP interaction with FAK is disrupted, RIP gains access to the FADD complex, which induces extrinsic cell death via activation of caspase 8. This data provided additional verification for the results from the pull down experiment.
It is also important to note that 128 other proteins in the human protein sequence database have been found to contain the conserved amino acid sequence “KAKTLRK” found in the FAK FERM domain. Therefore, a small molecule compound, such as INT2-31, if it targets this sequence, could have off target side effects. Additional binding studies are necessary to confirm specific binding of INT2-31 to FAK.
Supplementary Material
Acknowledgments
Supported by: This work was supported by the following grants: NIH CA113766 (S.N.H.) and the Bankhead Coley Research Program (S.N.H.)
References
- 1.Yang GY, Wagner TD, Fuss M, Thomas CR., Jr Multimodality approaches for pancreatic cancer. CA Cancer J Clin. 2005;55(6):352–367. doi: 10.3322/canjclin.55.6.352. [DOI] [PubMed] [Google Scholar]
- 2.Kircher SM, Krantz SB, Nimeiri HS, Mulcahy MF, Munshi HG, Benson AB., 3rd Therapy of locally advanced pancreatic adenocarcinoma: unresectable and borderline patients. Expert Rev Anticancer Ther. 2011;11(10):1555–1565. doi: 10.1586/era.11.125. [DOI] [PubMed] [Google Scholar]
- 3.Philip PA, Mooney M, Jaffe D, Eckhardt G, Moore M, Meropol N, Emens L, O'Reilly E, Korc M, Ellis L, Benedetti J, Rothenberg M, Willett C, Tempero M, Lowy A, Abbruzzese J, Simeone D, Hingorani S, Berlin J, Tepper J. Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol. 2009;27(33):5660–5669. doi: 10.1200/JCO.2009.21.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korc M. Signaling pathways in pancreatic cancer. Crit Rev Eukaryot Gene Expr. 2011;21(2):115–129. doi: 10.1615/critreveukargeneexpr.v21.i2.20. [DOI] [PubMed] [Google Scholar]
- 5.Peters S, Adjei AA. MET: a promising anticancer therapeutic target. Nat Rev Clin Oncol. 2012;9(6):314–326. doi: 10.1038/nrclinonc.2012.71. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterolog, 2011;141(6):2218–2227. doi: 10.1053/j.gastro.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14(12):3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 8.Chen SY, Chen HC. Direct interaction of focal adhesion kinase (FAK) with Met is required for FAK to promote hepatocyte growth factor-induced cell invasion. Mol Cell Biol, 2006;26:5155–5167. doi: 10.1128/MCB.02186-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 10.Ioannou N, Seddon AM, Dalgleish A, Mackintosh D, Modjtahedi H. Expression pattern and targeting of HER family members and IGF-IR in pancreatic cancer. Front Biosci. 2012;1:2698–2724. doi: 10.2741/4081. 17. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Bloom DA, Cance WG, Kurenova EV, Golubovskaya VM, Hochwald SN. FAK and IGF-IR interact to provide survival signals in human pancreatic adenocarcinoma cells. Carcinogenesis. 2008;29(6):1096–1107. doi: 10.1093/carcin/bgn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng D, Golubovskaya V, Kurenova E, Wood C, Massoll NA, Ostrov D, Cance WG, Hochwald SN. A novel strategy to inhibit FAK and IGF-1R decreases growth of pancreatic cancer xenografts. Mol Carcinog. 2010;49(2):200–209. doi: 10.1002/mc.20590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tognon CE, Sorensen PH. Targeting the insulin-like growth factor 1 receptor (IGF1R) signaling pathway for cancer therapy. Expert Opin Ther Targets. 2012;16(1):33–48. doi: 10.1517/14728222.2011.638626. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Ylipää A, Sun Y, Zheng H, Chen K, Nykter M, Trent J, Ratner N, Lev DC, Zhang W. Genomic and molecular characterization of malignant peripheral nerve sheath tumor identifies the IGF1R pathway as a primary target for treatment. Clin Cancer Res. 2011;17(24):7563–7573. doi: 10.1158/1078-0432.CCR-11-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer. 2005;5(7):505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 16.Hochwald SN, Golubovskaya V. FAK as a target for cancer therapy. Gene Therapy and Molecular Biology. 2009;13:26–35. [Google Scholar]
- 17.Ucar DA, Dang L, Hochwald SN. FAK signaling and function in pancreatic cancer. Frontiers in Bioscience. 2011;3(1):750–756. doi: 10.2741/e283. [DOI] [PubMed] [Google Scholar]
- 18.Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nature Reviews Molecular Cell Biology. 2010;11(11):802–814. doi: 10.1038/nrm2996. [DOI] [PubMed] [Google Scholar]
- 19.Ucar DA, Hochwald SN. FAK and interacting proteins as therapeutic targets in pancreatic cancer. Anti-Cancer Agents in Medicinal Chemistry. 2010;10(10):742–746. doi: 10.2174/187152010794728675. [DOI] [PubMed] [Google Scholar]
- 20.Ucar D, Hochwald SN. FAK as a therapeutic target in malignancy. Recent advances in carcinogenesis. 2010;1:1–7. [Google Scholar]
- 21.Kurenova EV, Hunt DL, He D, Magis AT, Ostrov DA, Cance WG. Small molecule chloropyramine hydrochloride (C4) targets the binding site of focal adhesion kinase and vascular endothelial growth factor receptor 3 and suppresses breast cancer growth in vivo. J Med Chem. 2009;52(15):4716–4724. doi: 10.1021/jm900159g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ucar DA, Cox A, He DH, Ostrov DA, Kurenova E, Hochwald SN. A novel small molecule inhibitor of FAK and IGF-1R protein interactions decreases growth of human esophageal carcinoma. Anticancer Agents Med Chem. 2011;11(7):629–637. doi: 10.2174/187152011796817718. [DOI] [PubMed] [Google Scholar]
- 23.Chen TH, Chan PC, Chen CL, Chen HC. Phosphorylation of focal adhesion kinase on tyrosine 194 by Met leads to its activation through relief of autoinhibition. Oncogene. 2011;30(2):153–166. doi: 10.1038/onc.2010.398. [DOI] [PubMed] [Google Scholar]
- 24.Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, Richter D, Emerson E, Lin J, Kath J, Coleman K, Yao L, Martinez-Alsina L, Lorenzen M, Berliner M, Luzzio M, Patel N, Schmitt E, LaGreca S, Jani J, Wessel M, Marr E, Griffor M, Vajdos F. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68(6):1935–1944. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nature Rev. Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe N, Takaoka M, Sakurama K, Tomono Y, Hatakeyama S, Ohmori O, Motoki T, Shirakawa Y, Yamatsuji T, Haisa M, Matsuoka J, Beer DG, Nagatsuka H, Tanaka N, Naomoto Y. Dual tyrosine kinase inhibitor for focal adhesion kinase and insulin-like growth factor-I receptor exhibits anticancer effect in esophageal adenocarcinoma in vitro and in vivo. Clin Cancer Res. 2008;14(14):4631–4639. doi: 10.1158/1078-0432.CCR-07-4755. [DOI] [PubMed] [Google Scholar]
- 27.Zheng D, Kurenova E, Ucar D, Golubovskaya V, Magis A, Ostrov D, Cance WG, Hochwald SN. Targeting of the protein interaction site between FAK and IGF-1R. Biochem Biophys Res Commun. 2009;388(2):301–305. doi: 10.1016/j.bbrc.2009.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2(5):249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 29.Hall JE, Fu W, Schaller MD. Focal adhesion kinase: exploring Fak structure to gain insight into function. Int Rev Cell Mol Biol. 2011;288:185–225. doi: 10.1016/B978-0-12-386041-5.00005-4. [DOI] [PubMed] [Google Scholar]
- 30.Ucar DA, Kurenova E, Garrett TJ, Cance WG, Nyberg C, Cox A, Massoll N, Ostrov D, Lawrence N, Sebti S, Zajac-Kaye M, Hochwald SN. Disruption of the protein interaction of FAK and IGF-1R inhibits melanoma tumor growth. Cell Cycle. 2012;11(17):3250–3259. doi: 10.4161/cc.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurenova E, Xu LH, Yang X, Baldwin AS, Jr, Craven RJ, Hanks SK, Liu ZG, Cance WG. Focal adhesion kinase suppresses apoptosis by binding to the death domain of receptor-interacting protein. Mol Cell Biol. 2004;24(10):4361–4371. doi: 10.1128/MCB.24.10.4361-4371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.