Abstract
Nanoparticles (NPs) have promising applications in medicine. Immune system is an important protective system to defend organisms from non-self matters. NPs interact with the immune system and modulate its function, leading to immunosuppression or immunostimulation. These modulating effects may bring benefits or danger. Compositions, sizes, and surface chemistry, and so forth, affect these immunomodulations. Here we give an overview of the relationship between the physicochemical properties of NPs, which are candidates to be applied in medicine, and their immunomodulation properties.
1. Introduction
Large surface area, high aspect ratio, small size, and unique physical and chemical properties in NPs enable their potential applications in many biomedicine fields, such as drug and gene delivery, imaging, photodynamic therapy, and tissue engineering [1–3]. The small size of nanoparticls offers them the ability to overcome various biological barriers to transport and deliver therapeutic agents to the target tissue. NPs may overcome drug resistance when functionalized with targeting moiety [4–6]. The “nanophotosensitizers” used in photodynamic therapy (PDT) show higher solubility than normal photosensitizer playing an important role in the treatment of cancer [2]. Additionally, the increased resolution and sensitivity give nanostructure-based diagnostics an advantage over classical methods [7, 8]. Compared to traditional molecular medicine, NPs show advantages, such as intermixing, diffusion, sensoric response, and ultrafast kinetics make nanomedicine a local process at the nanoscale [9]. At the same time, NPs will enter and interact with human body during these processes.
As an important protective system to defend organisms from foreign matters and danger signals inside the body, the immune system plays a critical role in keeping homeostasis in human body. The immune system exerts its function through innate immunity and adaptive immunity. Innate immunity is the first line of defense against microbial invasion, which interacts with the foreign materials and cleans the pathogen or pathogen-infected cells, which is nonspecific to pathogen. The function of innate immunity was realized by the phagocytic cells (macrophages, dendritic cells (DCs), neutrophils, and mast cells (MCs), etc.), which phagocytose pathogen and release cytokine to clear pathogen. If the pathogen cannot be effectively cleared by innate immunity, the adaptive immunity, as the second line of defense in human body, will be activated. During these processes, some phagocytic cells act as antigen-presenting cells (APCs) and present specific antigens to specialized cells which are responsible for adaptive immunity, such as T cells and B cells. By this antigen-presenting process, pathogen (antigen) could be recognized by T cells and B cells and stimulate the adaptive immune response, which is specific to pathogen [10, 11]. The strong ability to eliminate pathogens makes the immune system important in most disease treatment. However, abnormal intensity of immune response, including immunosuppression and immunostimulation, will lead to disease [10]. Immunosuppression can be caused by impairment of any component of the immune system, which results in a decreased immune function and thereby leads to pathogen which cannot be effectively cleared and infection or tumor will occur [12]. Immunostimulation could enhance the ability to resist pathogen, but it may result in a strong adverse response such as autoimmune disease if it was hypersensitive.
When nanomedicines are applied in vivo, they act as foreign materials and induce the immune response, immunosuppression, or immunostimulation [13]. However, these modulations of immune system caused by NPs are undesirable in most cases when nanomedicine is applied, such as imaging. Furthermore, these immune modulations by NPs could be adverse in other conditions. Some nanobased anticancer therapeutic agents show antitumor properties in vitro but tumor-promoting effect in vivo [14]. This opposite effect may be due to the disturbed anticancer immune system [14]. However, some immunomodulation properties are good for disease prevention and treatment such as vaccine adjuvant and antiallergy therapeutic agents [15, 16]. Therefore, NPs play as a Janus' double-face in nanomedicine applications (Figure 1). Immunomodulating potential of NPs should be considered seriously because it could bring unexpected side effects in the clinical treatment. Understanding of nano-immuno-interactions is critical for the safe application of engineered NPs in medicine and safe design of nanomedicine.
Figure 1.
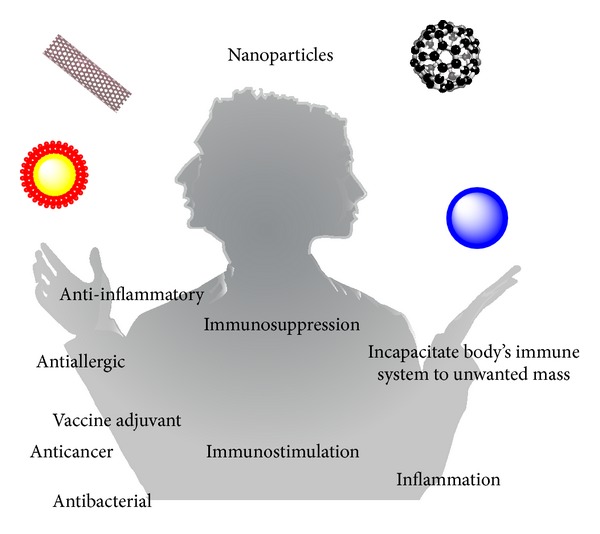
The immunomodulation of NPs presents a Janus' double-face in nanomedicine applications. On one hand, the effects to the immune system may benefit treatment of disease through enhancing immune response. On the other hand, the immunomodulation of NPs may bring harm.
In this review, we focus on the immunomodulating effects of NPs used in nanomedicine on immune system (Table 1). Effects of physicochemical properties of NPs on immune interactions and the underlying mechanisms are also reviewed.
Table 1.
Immunomodulation of various nanoparticles in nanomedicine applications.
| Nanomaterial | Size | Exposure routes/doses | Outcomes | Cytokines/chemokines | Animal | Reference |
|---|---|---|---|---|---|---|
| Carbon nanotube | ||||||
| MWCNT | L: 5–15 μm | Inhalation 5 mg/m3
6 h/day 14 days |
Immunosuppression | TGFβ↑, IL-10↑ | Male C57BL/6 | [17, 18] |
| D: 10–20 nm | ||||||
| SWCNT | L: 1–3 μm | Pharyngeal aspiration 40, 80, 120 μg/mouse | Inflammation immunosuppression | TNF-α↑, IL-6↑, MCP1↑ | Female BALB/c and C57BL/6 | [19] |
| D: 1–4 nm | ||||||
| MWCNT | L: several μm | Oropharyngeal aspiration 1, 2, and 4 mg/kg | Inflammation | IL-33↑, CCL3↑, CCL11↑ | C57BL/6 | [20, 21] |
| D: 12.5–25 nm | ||||||
| MWCNT | L: several μm | Oropharyngeal aspiration 4 mg/kg | Inflammation | IL-33↑, IL-5↑, IL-8↑, IL-13↑ | C57BL/6 | [22] |
| D: 12.5–25 nm | ||||||
| MWCNT | L: 15 ± 5 μm | Intravenously 1 mg/kg | Inflammation | IL-4↑, IL-33↑ | C57BL/6 | [23] |
| D: 25 ± 5 nm | ||||||
| MWCNT | L: 50 μm | Subcutaneous 0.05, 0.3, and 0.5 mg × 2/mouse | Acute inflammation | IL-17↑, IL-1β↑, IL-1α↑, IFN-γ↑ |
BALB/c | [24] |
| D: 20–30 nm | ||||||
| MWCNT | L: 0.3–50 μm | Inhalation 100 mg/m3 × 6 h |
Hypersensitivity | PDGF-AA↑, TGF-β↑, | Allergic asthma mice (C57BL/6) | [25] |
| D: 30–50 nm | ||||||
| SWCNT | L: 3–30 μm | Intratracheal 25, 50 μg × 6/mouse | Hypersensitivity | IL-4↑, IL-5↑, IL-13↑, IFN-γ↑, IL-17A↑, IL-23↑, IL-33↑ | Allergic inflammation mice (male ICR) | [26] |
| D: 67 nm | ||||||
|
| ||||||
| Graphene | ||||||
| Graphene | 4 ± 1 μm2 area | Intravenously 1 mg/kg | Activate Th2 immune response | IL-33↑, IL-5↑, IL-13↑ | C57BL/6 | [23] |
| 2 ± 1 nm thick | ||||||
|
| ||||||
| Fullerene | ||||||
| C60 | N/A | Intravenously 50 ng/mouse | Immunosuppression | Serum histamine↓, Lyn↓, Syk↓, ROS↓ | MC-dependent model of anaphylaxis (C57BL/6) | [16] |
| C60 | N/A | Intra-articular treatment 10.0 μM/week × 8 week | Immunosuppression | TNF-α↓, IL-1β↓ | Rat model of arthritis (female Sprague-Dawley rats) | [27] |
| C60 | N/A | Instillation 2 mg/kg | Inflammation | IL-1↑, TNF-α↑, IL-6↑, IL-12↑, IFN-γ↑ | Male ICR | [28, 29] |
| Carboxyfullerene | N/A | Peritoneum and air pouch 40 mg/kg | Activate immune system | N/A | C57BL/6 | [30] |
| Hydroxylated C60 | N/A | Intraperioneally injection 2 μg/g | Immunosuppression | IL-11↑, elastase2 gene↓ | Fathead minnow | [31] |
| C60 | N/A | Intraperitoneal injection 0.5 mL × 10 μg/mL × 14 days | Immunosuppression | IFN-γ↑ | Tumor-bearing mice (C57BL/6) | [14] |
|
| ||||||
| Gold nanoparticles | ||||||
| PfMSP-119/PvMSP-119 coated GNPs formulated with alum | 17 nm | Subcutaneously 25 μg/mouse | Immunogenic | Antibody titer↑ | BALB/c | [15] |
| PfMSP-119/PvMSP-119 coated GNPs | 17 nm | Subcutaneously 25 μg/mouse | Poor immunogenic | N/A | BALB/c | [15] |
| Short-chain PEG mixed-monolayer protected gold clusters | <5 nm | Subcutaneously injection 40 μM × 200 μL | Immunogenic | Antibody titer↑ | BALB/cAnNHsd | [32, 33] |
| PEG coated GNP | 13 nm | Intravenously 0, 0.17, 0.85 or 4.26 mg/kg | Acute inflammation | MCP-1/CCL-2↑, MIP-1α/CCL-3↑, MIP-1β↑, RANTES/CCL-5↑, IL-1β↑, IL-6↑, IL-10↑, IL-12β↑, TNF-α↑ | BALB/c | [34] |
| GNP functionalized with 2-mercaptoethanesulfonic acid (MES) or N,N,N-trimethylammoniumethanethiol (TMAT) | 1.5 nm | Media exposure 0.016–250 ppm | Activate immune response Inflammatory response | Il-5↓, IL-12↓, IL-15↓, IL-18↓ | Zebrafish embryos | [35] |
| Citrate-stabilized GNPs | 40 nm | Oropharyngeal aspiration 0.8 mg/kg | Hypersensitivity | MMP-9↑, MIP-2↑, TNF-α↓, IL-6↓ | TDI-sensitised mice (BALB/c) | [36] |
| GNP | 21 nm | Intraperitoneally injection 7.85 μg/g | Antiflammatory | TNF-αmRNA↓, IL-6 mRNA↓ | Male C57BL/6 | [37] |
| Citrate-stabilized GNPs | 5 nm | 100 nmol Au/kg | Antiflammatory | IL-1β↓ | IL-1β model mice (male C57BL/6) | [38] |
|
| ||||||
| Silver Nanoparticles | ||||||
| AgNP | 22.18 ± 1.72 nm | Inhalation 1.91 × 107 particles/cm3 × 6 h/day × 5 days/week × 2 weeks | Immunosuppression | Malt1 gene↓, Sema7a gene↓ | C57BL/6 | [39] |
| AgNP | 52.25 ± 23.64 nm | Intratracheal instillation 3.5 or 17.5 mg/kg once every 2 days for 5 weeks | Enhance immune function | IL-1↑, IL-6↑, TNF-α↑, GSH↓, T-SOD↓, MDA↑, NO↑ | Wistar rats | [40] |
| Ag conjugated to core nanobeads | 40–50 nm | Intradermally | Immunogenic | IFN-γ↑, antibody↑ | H-2Kb C57BL/6 | [41] |
|
| ||||||
| Magnetic Nanoparticles | ||||||
| Iron Oxide NP | 43 nm | Intratracheal instillation (4 or 20 μg × 3) | Activate immune response | IFN-γ↑, IL-4↑ | OVA-sensitized mice (BALB/c) | [42, 43] |
| Iron Oxide NP | 58.7 nm | Intravenously ≤10 mg iron/kg | Immunosuppression | IFN-γ↓, IL-6↓, TNF-α↓ | DTH mice (male BALB/c) | [44] |
| Iron Oxide NP | 35 ± 14 nm | Intratracheally 4 × 500 μg/mouse Intratracheally 4 × 250 μg/mouse |
Immunosuppression | IgE↓, IL-4↓ | OVA-sensitized mice (BALB/c) | [45] |
| Intratracheally 4 × 100 μg/mouse | Hypersensitivity | IgE↑, IL-4↑ | ||||
| 147 ± 48 nm | Intratracheally 4 × 500 μg/mouse Intratracheally 4 × 250 μg/mouse |
Immunosuppression | IgE↓, IL-4↓ | |||
| Intratracheally 4 × 100 μg/mouse | No significant effect | N/A | ||||
|
| ||||||
| Nanoceria | ||||||
| Nanoceria | D: 8 nm | Oropharyngeal instillation of 10, 30, or 100 μg/mouse | Inflammation | TNF-α↑, IL-6↑, osteopontin↑ | C57BL/6 | [46] |
| A: 44 m2/g | ||||||
| Nanoceria | 20 nm | Single intratracheal instillation at 0.15–7 mg/kg | Inflammation | NO↓, IL-12↑ | Specific pathogen-free male Sprague-Dawley (Hla: SD-CVF) rats | [47] |
| Nanoceria | D: 20–30 nm | Intratracheal instillation at 50 and 150 m2/mouse | Inflammation | IL-1β↑ | Female Wistar rats | [48] |
| A: 24.1 m2/g | ||||||
| Nanoceria | D: 55 nm | Inhalation of 641 mg/m3 for 24 h, 48 h, and 14 days | Inflammation | IL-1β↑, TNF-α↑, IL-6↑, MDA↑, GSH↓ | Wistar rats | [49] |
| A: 30–50 m2/g | ||||||
|
| ||||||
| Quantum Dots | ||||||
| CdTe NP | N/A | 1.6, 4, and 8 mg/L for 24 h | Immunosuppression | N/A | Elliption complanata | [50] |
| CdS/CdTe NP | N/A | 5, 10 and 20 nm for 96 h | Immunosuppression | N/A | Juvenile rainbow trout | [51] |
|
| ||||||
| Silica Nanoparticles | ||||||
| Amorphous silica NP | 30 and 70 nm | Intraperitoneal injection of 1 mg/mice | Inflammation | IL-5↑, IL-6↑, MCP-1↑, keratinocyte chemoattractant↑ | Female BALB/c | [52] |
| Amorphous silica NP modificated with carboxyl groups | 70 nm | Intraperitoneal injection of 1 mg/mice | Suppression of inflammation | N/A | ||
| Nonporous nanosilica NP | 15 nm | Intravenous injections at single dose at 50 mg/kg | Inflammation oxidative stress | ROS↑, TNF-α↑, NO↑ | Male SD rats | [53] |
|
| ||||||
| Polymer | ||||||
| Polystyrene NP | 50 nm | Intratracheal administration of 200 μg/mouse | Anti-inflammation immunosuppression | IL-4↓, IL-5↓, IL-13↓ | Allergen challenge mice | [54] |
| Polystyrene beads (PSB) coupled with the immunodominant myelin proteolipid protein PLP139–151 epitope (PLP139–151-PSB) | 500 nm | intravenous injection of approximately 9 × 109 microparticles | T-cell tolerance | IL-17↓, INF-γ↓ | Peptide-induced experimental autoimmune encephalomyelitis SJL/J mice | [55] |
|
| ||||||
| Dendrimer | ||||||
| Pan-DR-binding epitope (PADRE)-derivatized-dendrimer (PDD) | N/A | Intravenous injection with 6.25 mg/kg/day of LAmB at a ratio of 10 : 1 (PDD : LAmB) for 10 days | enhanced adaptive immunity | IFN-γ↑ | Female BALB/c mice inoculated intraperitoneally with metacyclic promastigotes of L. major | [56] |
|
| ||||||
| Lipid Nanoparticles | ||||||
| cSLN-pDNA (a DNA vaccine harbouring the L. donovani A2 antigen along with L.infantum cysteine proteinases) complexes | 241 ± 12 nm | immunized in the right-hind footpad with 50 μg of Qiagen purified pDNA | Enhanced immunity | Ratio of IFN-γ : IL-10↑ | L. infantum promastigotes challenged female BALB/c mice | [57] |
| MPLA : NLP | 6–25 nm | intraperitoneal injection ion of 1, 5, 10, 20 μg/mouse | Enhanced immunostimulatory | IL-6↑, TNF-α↑, MIP-1α↑ | Female BALB/c mice | [58] |
| CpG : NLP constructs | 6–25 nm | intraperitoneal injection ion of 10, 20, 40 or 80 μg/mouse | Enhanced immunostimulatory | IL-6↑, TNF-α↑, MIP-1α↑ | Female BALB/c mice | [58] |
| Pegylated liposomal doxorubicin (Doxil) | 85–100 nm | Infuse in accordance with the administration guideline of Doxil | Hypersensitivity reactions occurred in 45% of patients | N/A | Patients with solid tumors (n = 29) treated with Doxil for the first time | [59] |
2. NPs Candidates Used in Nanomedicine
Nanotechnology has a great potential in medicine applications such as medical diagnostics [60] and therapy [61]. As an inorganic fluorophore, quantum dots (QDs) have photostability which makes them ideal candidates for imaging tools in vivo [62]. Recent study showed a technique to track lymph flow in real time using quantum dots optical imaging in mice [22]. In addition, superparamagnetic iron oxide NPs (SPION) were also applied to trace neurodegenerative diseases by magnetic resonance imaging (MRI) [63]. Some carbon-based NPs are also applied in clinical use. Carbon nanotubes (CNTs) have unique physical properties such as electrical, thermal, and spectroscopic properties, which make them an advantage in detection and therapy of diseases [64]. It was reported that CNTs could prolong survival of tumor-bearing animals [65]. Graphene has good biocompatibility, biofunctionalization, and its unique mechanical, electronic, and optical properties for imaging and cancer phototherapy [66]. And it was demonstrated that graphene oxide (GO) have antibacterial properties [67], making them candidates as antibacterial agent. Besides, graphene derivatives are also good candidates for drug delivery as they can bind with aromatic drugs through π-π stack and/or van der Waals interactions [66]. Gold NPs (GNPs) are also potential materials in cancer therapies and imaging due to their biocompatibility, plasmon resonances, and diverse functionalizations [68]. It is promising to apply GNPs to targeted therapy of cancer [69] and overcome drug resistance [6]. Silver NPs (AgNPs) are important metal nanomaterial. They have antibacterial, antifungal, and antiviral effects [70]. Lipid NPs and liposome have been widely applied for drug delivery because of their improved drug potency and low off-target effects [71]. Other NPs such as polymer, CeO2, silica NPs, dendrimer, and protein NPs are also used in nanomedicine [72–78].
As foreign materials, NPs could be recognized by the immune system and induce immunosuppression or immunostimulation when used as nanomedicine. How to utilize or control these immunomodulation effects is largely based on NPs' different applications. NPs with immunosuppression effects might be used as anti-inflammatory or antiautoimmune disease therapeutic agents. On the contrast, NPs which activate immune system might be used as vaccines, or vaccine adjuvants. An advanced nanomedicine in drug delivery or imaging should not induce undesired immune-activation or immunosuppression effect. The detailed immunomodulation effects of these NPs in nanomedicine applications are discussed below.
3. Immunomodulation by Different NPs
3.1. Immunosuppression
3.1.1. Carbon Nanotubes
After inhalation exposure, CNTs induced systemic immunosuppression in mice, including production of prostaglandin and IL-10 [17, 18] and T cell dysfunction [18, 19, 23]. For example, inhalation of CNTs (0.3, 1, or 5 mg/m³, 6 h/day, 14 days) hardly induced injury in lungs but resulted in nonmonotonic systemic immunosuppression (reduced T-cell-dependent antibody against sheep erythrocytes and T-cell proliferative ability and decreased natural killer cell activity). This suppression was accompanied by increased spleen gene expression of interleukin-10 (IL-10), which is an anti-inflammatory cytokine, and NAD(P)H oxidoreductase [17]. Other studies showed that pharyngeal aspiration of SWCNTs (40 μg/mouse) in BALB/c mice induced pulmonary inflammation and suppressed the responsiveness of T cell 7 days postexposure. This immunosuppression was associated with the direct effects of SWCNTs on DCs [19].
3.1.2. Fullerene
As a strong free-radical scavenger [79], fullerene has anti-inflammatory effects. The antioxidative/anti-inflammatory activities of novel fullerenes have been reported [80]. Fullerene could suppress Ag-driven type I hypersensitivity when human MCs and peripheral blood basophils were preincubated with C60 fullerenes. This suppression involved decreasing the level of reactive oxygen species (ROS) [16]. In a MC-dependent model of anaphylaxis, fullerenes prevented the release of histamine [16]. In addition, polyhydroxylated fullerene derivatives might protect against oxidative stress in ischemia-reperfused lungs [81]. C60 also suppressed the tumor necrosis factor alpha (TNF-α) induced production of proinflammatory cytokines in vitro and inhibited the arthritis in vivo [27]. Other studies in different animal models also showed the immunosuppression effects of fullerenes. For example, hydroxylated fullerenes could interfere with the innate immune system in fathead minnow [31]. Nanocrystalline fullerene showed cytotoxicity and promotive effects on tumor cell growth in vitro and in vivo, respectively, which might be due to the suppression of anticancer immune response of mice by fullerene [14].
3.1.3. Gold NPs
The anti-inflammatory properties citrate-coated GNPs were also reported. Citrate coated GNPs (21 nm) did not cause detectable organ or cell toxicity in mice [37]. Studies also indicated that citrate-stabilized 5 nm and 15 nm GNPs inhibited cellular responses induced by interleukin 1 beta (IL-1β) and showed anti-inflammatory activity [38].
3.1.4. Silver NPs
The studies on the immunotoxicity of AgNPs are very limited. AgNPs induced ROS and inflammation [82, 83], indicating its potential interference of immune system. AgNPs (22 nm) exposure caused the downregulation of expression of Malt1 and Sema7a genes, which were associated with immune cell function, followed by aberrant T cell differentiation [39].
3.1.5. Magnetic NPs
Some in vitro studies showed that iron oxide NPs did not induce inflammatory response on human monocyte-macrophages [84] and aortic endothelia cells [85]. However, high doses of iron oxide NPs may induce oxidative stress [86]. When treated with PVA-coated SPION, human monocyte-derived DCs showed a decreased antigen processing and CD4 (+) T cell stimulation capacity [87]. These studies suggested the potential immune impact of magnetic NPs. The immunomodulation of the iron oxide NPs was much more complex in vivo. Intratracheally administration of high dose (4 × 500 μg/mouse) and intermediate dose (4 × 250 μg/mouse) of iron oxide NPs with a diameter of 35 ± 14 nm or 147 ± 48 nm inhibited the allergic Th2-dominated response induced by ovalbumin (OVA). The low dose (4 × 100 μg/mouse) of iron oxide particles (147 ± 48 nm) had no significant effect, while the low dose (4 × 100 μg/mouse) of particles (35 ± 14 nm) had an adjuvant effect on the Th2 response to OVA [45]. A single intratracheal instillation (250, 375 or 500 μg/mouse) or four-time repeated instillation (500 μg/mouse × 4) showed that both NPs induced lung inflammation and decreased pulmonary immune responses against sheep erythrocytes. In another study, intravenously administration of iron oxide NPs (58.7 nm) in doses ≤ 10 mg iron/kg shifted the Th1/Th2 immunobalance towards the Th2-dominant direction and suppressed the delayed-type hypersensitivity in OVA-sensitized mice [44]. Furthermore, repeated instillations resulted in a reduction of inflammation than single instillation [88].
3.1.6. CeO2 NPs
Due to their reducibility, cerium oxide NPs (nanoceria) were found to have the ability to reduce ROS and may be used as a novel therapeutic tool for inflammation treatment [73]. Some in vitro studies indicated that nanoceria with a small diameter caused a significant anti-inflammatory effect [73, 89, 90]. For example, nanoceria with a diameter of 3–5 nm scavenged free radicals inhibited inflammatory mediator production in J774A.1, the murine macrophages [73]. A recent study reported that the same size of nanoceria induced APCs to secrete IL-10, and induced a Th2-dominated T cell proliferation. The nanoceria (5–8 nm) showed an effective antioxidant property in cardiac progenitor cells and protected the cardiac progenitor cells from H2O2-induced cytotoxicity. In addition, in vivo investigation on immune cells of the sea urchin indicated that nanoceria suppressed the innate immunity when force-fed 1 mL 10−2 g/L 50–60 nm of nanoceria [91].
3.1.7. Quantum Dots
As efficient energy donors [92], QDs can induce the generation of ROS by transferring energy to nearby oxygen molecules. In vitro studies have shown that QDs induced production of ROS and led to multiple organelle damage and cell death [93, 94]. Preexposure to a dose at 10−7 to 10−3 μg/mL CdTe QDs suppressed the immune responses of J774A.1 macrophage to bacteria by reducing NO, TNF-α, KC/CXCL-1, and IL-8 production [95]. These in vitro studies suggested that QDs might have high immunotoxicity. In vivo studies also showed similar results. CdTe QDs (1.6, 4, and 8 mg/L for 24 h at 15°C) significantly decreased the viability of hemocytes, as well as number of hemocytes capable of ingesting fluorescent beads in Elliption complanata mussels [50]. The immunosuppression was also observed in Juvenile rainbow trout. When 5, 10 and 20 nM CdS/CdTe QDs were exposed to Juvenile rainbow trout for 96 h at 15°C, the leukocyte counts, viability, and both resting and active phagocytic activity were significantly reduced [51]. QDs affected the proliferation of immune cells, but did not induce immune response including cytokine production [96]. Size of QDs aggregates may affect the immune response of QDs. Large CdS/CdTe QDs aggregates (25–100 nm) reduced phagocytosis more than smaller NPs (<25 nm) on bivalves (Mytilus edulis and Elliptio complanata) and fish (Oncorhynchus mykiss) [97]. Therefore, caution is needed to overcome this barrier when QDs are applied in clinical treatment.
3.1.8. Polymeric NPs
In vivo studies indicated that some polymeric NPs inhibited inflammation but had no effect on host immunity [54, 98]. NPs produced by particle replication in nonwetting template technology remained in the lungs for up to 7 days without triggering host immunity after intratracheal administration of 50 μg/mouse [98]. Polystyrene NPs (50 nm) inhibited lung inflammation by intratracheal administration of 200 μg/mouse after allergen challenge. This inhibition was due to the modulation of DCs functions. NPs inhibited expansion of CD11c+MHCIIhi DCs in the lungs and draining lymph node and allergen-laden CD11bhiMHCIIhi DCs in the lungs [54]. In addition, polystyrene particles have the potential to halt the disease process in autoimmunity. For example, antigen-decorated polystyrene particles with a diameter of 500 nm induced T-cell tolerance and ameliorated experimental autoimmune encephalomyelitis by inactivating pathogenic T cells [55].
3.2. Immunostimulation
3.2.1. Carbon Nanotubes
CNTs induced immunostimulation in vitro and in vivo. The oropharyngeal aspiration of MWCNTs (1, 2, and 4 mg/kg) in C57BL/6 mice induced inflammation (30 days postinstillation) in lungs [20, 21]. MWCNTs were translocated progressively into the spleen reached a maximum of 48 h after intraperitoneally (i.p.) administration, which caused the lymphocytic hyperplasia and increased oxidative stress in the spleen [99]. Subcutaneous administration of MWCNTs with total dose of 1.0 mg for two s.c injections in BALB/c mice induced acute immunological reactions for 1 week (activation of complement and increased proinflammatory cytokines). However, the accumulation of MWCNTs and injury was not observed in spleen [24]. MWCNTs injected intravenously activated Th2 immune response by elevating Th2 cytokines and increasing number of CD4+ and CD8+ T cell in the spleen [23]. In other studies, CNTs showed allergy adjuvant effect in inflammatory mass. For example, SWCNTs and MWCNTs exhibited adjuvant activity to the OVA-sensitized mice [25, 100]. MWCNTs aggravated asthma and induced fibrosis in OVA-sensitized lungs but showed no response with healthy pulmonary. The results indicated that these NPs could bring harm to asthma patients but not health ones [25]. In recent in vitro studies, MWCNTs increased the release of a series of cytokines in peripheral blood mononuclear cells (PBMCs) from healthy donors after stimulation with toll-like receptor (TLR) agonists or T cell mitogen. However, MWCNTs suppressed immune responses in PBMCs from mite-allergic subjects. These studies suggest that MWCNTs may either stimulate or suppress immune system depending on their immune cell target [101].
3.2.2. Graphene
GO could induce healthy DCs to differention and maturation at varying degrees [102] but suppress the antigen-delivering ability of OVA-loaded DCs to T lymphocytes [103]. This inhibition was associated with downregulation of subunit LMP7 of immunoproteasome in cells, which is responsible for antigens processing in DCs [103]. When macrophage cells RAW264.7 were incubated with GO, toll-like receptor (TLR4/TLR9-) 6 modulated autophagy and inflammatory responses occurred [104]. In addition, PVP-coated GO exhibited lower immunogenicity than GO on the aspect of inducing maturation and differentiation of DCs [102]. PVP-coated GO enhanced the physiological activity of macrophages, which showed anti-phagocytosis ability against macrophages and delayed the apoptotic process of T lymphocytes [102]. This advantage makes PVP-coated GO a promising candidate of immunoadjuvant. The effect of two sizes (2 μm and 350 nm) of the GO in response to microphages was investigated [105]. These two NPs had equal uptake amount in macrophages, but microsized GO induced stronger inflammation responses and showed divergent intracellular locations compared to nanosized GO [105]. This result demonstrated that lateral dimension of GO plays an important role in the regulation of cellular responses. Recent studies demonstrated that intravenously delivered graphene nanosheets induced site-specific Th2 inflammatory responses in the lungs via the IL-33/ST2 axis [23]. This effect may cause host defense and exacerbation of allergic diseases. However, more studies in vivo are needed to assess and eliminate the potential immunomodulation of graphene-based materials to ensure their safety for applications in biomedicine.
3.2.3. Fullerene
Some studies showed that C60 have immunostimulatory properties [28–30, 103, 106]. After instillation, C60 upregulated gene expression of various proinflammatory cytokines (IL-1, TNF-α, IL-6) and Th1 cytokines (IL-12,IFN-γ) in mice. Besides inflammation, C60 could activate the immune system. The carboxyfullerene could prolong the infiltrating neutrophils to enhance the bactericidal activity of neutrophils [30]. Other studies indicated that fullerene may enhance the ability of DCs to stimulate T cells and furthermore activated cells of innate immune system by enhancing production of IL-6 and an activation of natural killer (NK) cells [103, 106]. In addition, immunization of mice with a C60 fullerene derivative conjugated to bovine thyroglobulin could produce a population of fullerene-specific antibodies, which included a subpopulation that cross-reacted with a C70 fullerene [29].
3.2.4. Gold NPs
GNPs with different surface modification showed different immunogenicity in organisms. The immunogenicity of GNPs coated with C-terminal 19 kDa fragment of merozoite surface protein 1 (MSP-119) was an important vaccine candidate. In this study, GNPs showed poor immunogenicity in mice but enhanced antibody response when formulated with alum [15]. However, GNPs coated with monosaccharide or disaccharides could initiate the immune response by activating the macrophages [107]. Some studies indicated that high concentrations of PEG coated on GNPs could induce antibody production and trigger immune responses. High doses of injected PEG-coated GNPs were cleared through these mechanisms [32, 33].
GNPs can also induce inflammatory responses in vivo. Well-dispersed PEG-coated GNPs (13 nm) can be recognized by host defense mechanism and induce acute inflammation and apoptosis in the liver [34]. If inflamed tissues are exposed, stronger immune responses may be induced [108]. When exposed to sensitized mice, 40 nm GNPs could lead to a threefold increase in airway hyperreactivity and increase the number of neutrophils and macrophages [36].
3.2.5. Silver NPs
Intratracheal instillation of AgNPs with a diameter of 52.25 ± 23.64 nm could enhance the respiratory immune function through oxidative stress and induced inflammation in the respiratory. When alveolar macrophages were activated by AgNPs to cause phagocytosis, the alveolar macrophages generated ROS and free radicals which resulted in oxidative stress. The normal function of alveolar macrophages and epithelial cells was subsequently affected. This led to oversecretion of cytokines and oxides, which then caused the stimulation of the respiratory immune function [40]. These opposite results may be due to the different diameters of NPs. Covalent conjugation of Ag to solid core nanobeads with different diameters ranging from 0.02 to 2 μm was found localized into DCs (DEC205+, CD40+, CD86+) in draining lymph nodes and induced high levels of IFN-γ production and high antibody titers in tumor-bearing mice [41].
3.2.6. CeO2 NPs
Instillation of 100 μg nanoceria with a diameter of 8 nm in C57BL/6 mice revealed that the NPs induced inflammation in pulmonary system by activating MCs [46]. Other studies using bigger sizes showed the same effect. Single intratracheal instillation of 20 nm nanoceria at 0.15–7 mg/kg caused a dose-dependent inflammation and lung injury [47]. The intratracheal instillation of 20–30 nm nanoceria (24.1 m2/g) with doses of 50 and 150 cm2/mouse induced both acute and chronic neutrophilic/mildly cytotoxic inflammation [48]. Inhalation of 55 nm nanoceria with an average aerosol concentration of 641 mg/m3 for 4 h induced cytotoxicity via oxidative stress and led to a chronic inflammatory response including up regulation of IL-1β, TNF-α and IL-6 [49].
3.2.7. Silica NPs
30 nm amorphous silica NPs were investigated in PBMCs and purified monocytes. These NPs could induce inflammatory response as production of IL-1β, IL-8, and ROS. This result indicated the potential of silica NPs to evoke innate immune reactions [109]. Other reports showed that 30 and 70 nm silica NPs induced higher production of TNF-α in RAW264.7 cells and stronger inflammatory responses (IL-5↑, IL-6↑, MCP-1↑, keratinocyte chemoattractant↑) than 300 and 1000 nm particles in vivo through intraperitoneally injection. The 70 nm particle-induced TNF-α production was dependent on the production of ROS and activation of mitogen activated protein kinases (MAPKs). However, modification of carboxyl groups on 70 nm particles dramatically suppressed the inflammatory responses [52]. As well, Kupffer cells stimulated by 15 nm silica NPs released large amounts of ROS, TNF-α and NO. The viability of Buffalo rat liver (BRL) cells was reduced after cultured with silica NP-stimulated Kupffer cells. The authors also studied the intravenous injections of silica NPs with a single dose of 50 mg/kg. It caused hepatic inflammation and oxidative stress [53].
3.2.8. Polymeric NPs
Polymer-based NPs were shown to be effective adjuvants in vaccination [72, 110, 111]. These polymers have the ability to activate cellular immune responses in the host [112]. For example, N-trimethyl chitosan-mono-N-carboxymethyl chitosan (TMC/MCC) NPs appeared to be very promising as an adjuvant and delivery system for antigens. Intranasal vaccination with tetanus toxoid loaded TMC/MCC NPs (283.5 ± 2.5 nm) was shown to induce both the mucosal and systemic immune response (enhanced antibody response). The enhanced immunoglobulin G (IgG) immune response could be explained by the sustained release of the toxoid [113].
Some polymer NPs were reported to activate immune system through modulating the activation and capability of immune cells, such as dendritic cells and T cells [114–116]. Amphiphilic NPs possessed pathogen-mimicking properties by activating DCs similar to lipopolysaccharide (LPS); thus, it has the ability to activate innate immune response [114]. Poly(methyl vinyl ether-co-maleic anhydride) NPs (149 ± 2 nm) activated DCs through TLR stimulation in innate immune system [115]. As well, the sulfonate (245 nm) and phosphonate-functionalized (227 nm) polystyrene NPs induced the maturation of immature DCs and significantly enhanced T cells stimulatory capacity, indicating a shift to Th1 response [116].
3.2.9. Other NPs
Dendrimers have the ability to stimulate immune system and can be used as potential candidates for vaccines [76]. Maltose-functionalized dendrimer-peptide complex is a potential DC-based vaccine candidate by stimulating DC and activating the immune system [117]. Research also showed that Pan-DR-binding epitope-derivatized-dendrimer could reduce the effective dose of liposomal amphotericin B in murine cutaneous leishmaniasis and enhance adaptive immunity by activating strong parasite specific T-cell responses [56].
Protein NPs have shown immunostimulating properties in recent studies [118–120]. Self-assembled protein NPs that displayed epitopes of the repeat sequence in circumsporozoite protein of plasmodium falciparum (PfCSP) elicited a strong immune response against PfCSP [119, 121]. In addition, protein NPs mimic viruses have the ability to facilitate DCs activation and cross-presentation. These protein NPs codelivered with peptide epitopes to DCs showed an increased and prolonged CD8+ T cell activation [120].
A lipid NP was investigated in a DNA vaccine application. This NP which decorated with stearyl-conjugated KALA, an α-helical peptide (sequence WEAKLAKALAKALAKHLAKALAKALKACEA) showed enhancement of transgene expression; this enhancement was closely related to immune-activation [122]. A cationic solid-lipid NP was used as a vaccine to deliver a DNA vaccine against visceral leishmaniasis. High levels of IFN-γ and low levels of IL-10 production were detected in BALB/c mice after administration of the DNA vaccine delivered by this cationic solid-lipid NP. This NP induced a strong Th1 immune response, indicating its potential as therapeutic agent against visceral leishmaniasis [57]. Nanolipoprotein conjugated with TLR agonists monophosphoryl lipid A or CpG oligodeoxynucleotides significantly enhanced the immunostimulatory profiles compared to the agonists alone. Moreover, the BALB/c mice pretreated with CpG/nanolipoprotein coloaded nanoconstructs, but not CpG alone, survived a lethal influenza challenge [58]. Research also indicated that intravenous injection of liposome-DNA complexes elicited production of IFN-α and IFN-β in vivo, which suggested that the liposome-DNA complexes can induce inflammation and cause systemic toxicity [123]. In another study, it was reported that Doxil, an PEGylated liposomal formulation of doxorubicin, may cause hypersensitivity reactions while the standard doxorubicin did not, indicating that liposomes might be responsible for this hypersensitivity [59]. It was speculated that the complement system activation by Doxil may play a key role in this effect [59].
4. The Factors Affecting Immunomodulation of NPs
Many factors contribute to immunomodulation of NPs. The nature of NPs such as composition, surface chemistry, size, shape, and protein-binding ability dominates these interactions. Besides, individual difference and exposure route also contribute to immunomodulation of NPs.
4.1. Composition
Composition of NPs lays a vital role in the interactions between NPs and immune system. For example, QDs showed high immunotoxicity [50, 51] because they release heavy metal ions. Some other NPs exhibit less immunotoxicity [17, 23, 39, 44, 54], immunogenic [15, 32], or no effect [45]. The different core of NPs gave different reaction in allergy mass. For example, CNTs and GNPs showed adjuvant effect and led to hypersensitivity in these allergy masses [25, 26, 36] while fullerene often showed immunosuppression [14, 31].
4.2. Surface Chemistry
For NPs with the same composition, surface properties may also affect the immune system. Engineered NPs such as CNTs, fullerenes, GNPs, and silica NPs can be modified with diverse surface chemistry. This may alter their immune response both in vitro and in vivo.
Eighty diversely functionalized multiwall nanotube (MWNT) induced different levels of protein binding, cytotoxicity, and immune responses (Figure 2) [124]. The modification of MWCNTs significantly alleviated nuclear factor kappa B (NF-κB) activation and reduced immunotoxicity of MWCNTs in BALB/c mice [125]. Carboxyfullerene activated immune system of C57BL/6 mice by prolonging the infiltrating neutrophils to enhance the bactericidal activity of neutrophils [30], while hydroxylated fullerenes interfered with the innate immune system in fathead minnow [31]. MWCNTs-PEG induced less generation of ROS and cytotoxicity in macrophages than MWCNTs-COOH, which was in correspondence with the lower cellular uptake of MWCNTs-PEG [126]. The monolayer-protected GNPs in vivo were studied. Simple place-exchange reactions within the monolayer by short chain, mercaptotetraethylene glycol, have been used. The short chain at lower concentrations did not trigger the immune system to produce anti-PEG antibody [33]. However, high concentration mixed monolayer coated NPs initiated an immune response [32]. Silica NPs (70 nm) induced strong inflammation by intraperitoneal injection, but these inflammatory responses could be dramatically suppressed by surface modification by carboxyl groups [52]. The toxicity of porous silica NPs to immune cells was surface chemistry and surface charge-dependent. Compare to surface hydrophobicity, surface charge had stronger impact on NPs' immunotoxicity in vitro and in vivo. Positively charged hydrophobic NPs showed more DNA damage than negatively charged hydrophilic NPs [127].
Figure 2.
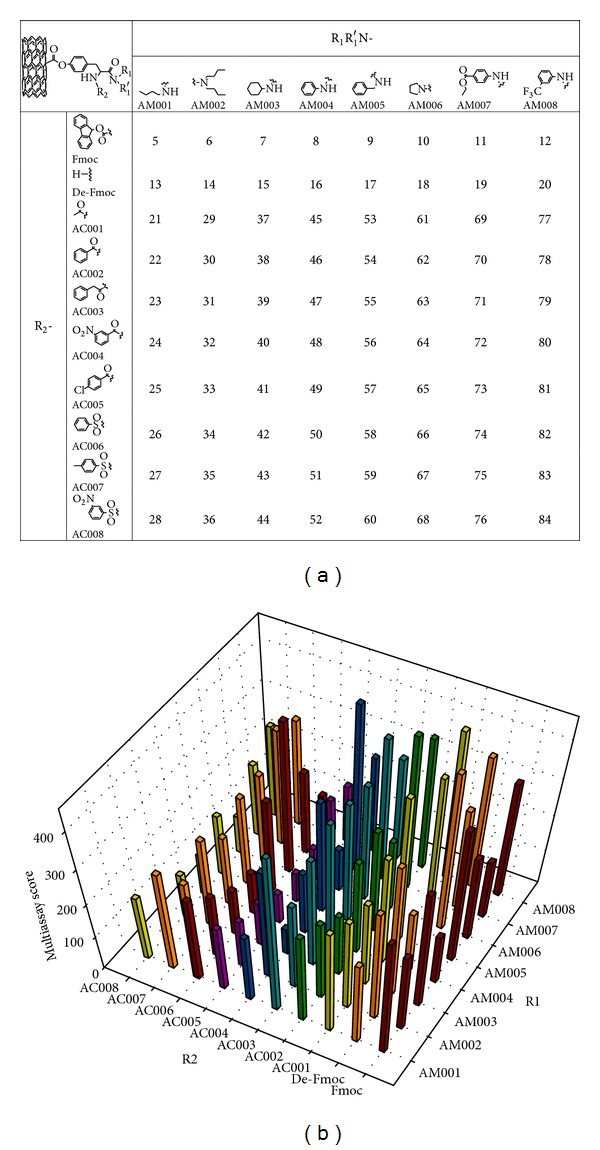
Multiassay score of the functional MWNT library. (a) Surface molecular compositions of combinatorial MWNT library members. (b) Findings from four protein (BSA, carbonic anhydrase, chymotrypsin, and hemoglobin) binding assays, cytotoxicity, and immune response assays (MWNT-induced NO release) at 50 μg/mL in THP-1 macrophages were ranked for all library members. The sum of their ranks was designated the multiassay score and is shown as vertical bars in the graph. Reprinted with permission from [124].
4.3. Size
Size is another important parameter that determines the interaction with organisms. When examined immunity is induced by a series of differentially sized (20, 40, 49, 67, 93, 101, and 123 nm) polystyrene nanobeads, IFN-γ induction from CD8+ T cells was limited to 40 and 49 nm beads, while 93–123 nm beads induced CD4+ T cell activation and increased IL-4 level. These results showed that the size of nanobead for vaccination could influence the type 1/type 2 cytokine balance. This would be useful in the development of vaccines against common human pathogens [128]. 200 nm NPs increased more antigen-specific polyfunctional CD4+ T cells as compared to 30 nm NPs. The immunoactivity of disaccharides coated GNPs is strongly dependent on size. They used 2 and 5 nm GNPs coated with disaccharides. These NPs activated the macrophages and induced the proliferation of T cells and the increase of IL-2 levels. The 5 nm NPs performed far better than 2 nm ones (increased APC proliferation, MHC II expression, T cell proliferation, and IL-2 expression) [107]. Other researches indicated that single instillation (250, 375, or 500 μg/mouse) of 35 ± 14 nm iron oxide NPs induced higher levels of inflammation and immunodepression than 147 ± 48 nm ones [88]. Repeated intratracheal administration of high dose (4 × 500 μg/mouse) and intermediate dose (4 × 250 μg/mouse) of the same NPs inhibited the allergic Th2-dominated response induced by OVA. The low dose (4 × 100 μg/mouse) of 147 ± 48 nm iron oxide particles had no significant effect, but the low dose (4 × 100 μg/mouse) of 35 ± 14 nm particles had an adjuvant effect on the Th2 response to OVA [45]. Aggregate size may also affect the immunotoxicity of QDs. The toxicity of CdS/CdTe QDs was size dependent. Large CdS/CdTe QD aggregates (25 nm–100 nm) reduced phagocytosis of fish macrophages more than did smaller ones (<25 nm) [97].
4.4. Protein Binding
When NPs enter the body through injection, they firstly interact with blood [129]. The plasma protein binding on the NPs surface, such as apolipoprotein E and transferrin, may contribute to the activation/deactivation of receptor-dependent signaling [130].
The amount and types of proteins adsorbed on the NPs affect the interactions of cells and NPs and the biological responses [131, 132]. The composition, surface characteristics, and shape of NPs affect the manners that the proteins bind to them [129, 133–136]. The blood proteins adsorbed onto the NPs include immunoglobulins, apolipoproteins and proteins of the complement system among many others. These proteins may act as signals for immune responses [137, 138]. Furthermore, NPs may induce conformational changes in the structure of adsorbed proteins. Negatively charged poly(acrylic acid-) conjugated GNPs bound with fibrinogen and induced the unfolding of this protein, which promoted interaction with Mac-1, an integrin receptor. This activation increased the NF-κB signalling pathway and released inflammatory cytokines [139].
4.5. Exposure Route
Exposure route is another factor affecting the immunomodulation of NPs. The outcomes of immune response are dependent on entrance of NPs. In the lungs, DCs, pulmonary epithelium, and macrophages play an important role in handling foreign materials. In the blood, leukocyte such as neutrophil, eosinophil, basophil, lymphocyte, and monocyte play a vital role. Single intratracheal instillation of 500 μg/mouse iron oxide NPs (35 ± 14 nm and 147 ± 48 nm) induced lung inflammation and decreased pulmonary immune responses against sheep erythrocytes [88]. Pharyngeal aspiration of SWCNTs modified systemic immunity by modulating DCs function [19]. Intravenously administration of iron oxide NPs (58.7 nm) could suppress the infiltration and functional activity of Th1 cells and macrophages [44]. Intravenous injection of graphene nanosheets activated a Th2 immune response, which consisted of neutrophilic influx and a significant increase in IL-5, IL-13, IL-33 in the bronchoalvelar lavage fluid [23]. The dosage of administration is also important. Intratracheal injection of iron oxide NPs (35 ± 14 nm) inhibited the allergic response in OVA-sensitized mice at a dosage of 4 × 250 μg/mouse or 4 × 500 μg/mouse, but showed adjuvant effect at a dosage of 4 × 100 μg/mouse [45].
5. The Mechanisms of Immunomodulation Induced by NPs
NPs interact with both innate and adaptive immune cells, affect their functions, and disturb immune system (Figure 3).
Figure 3.
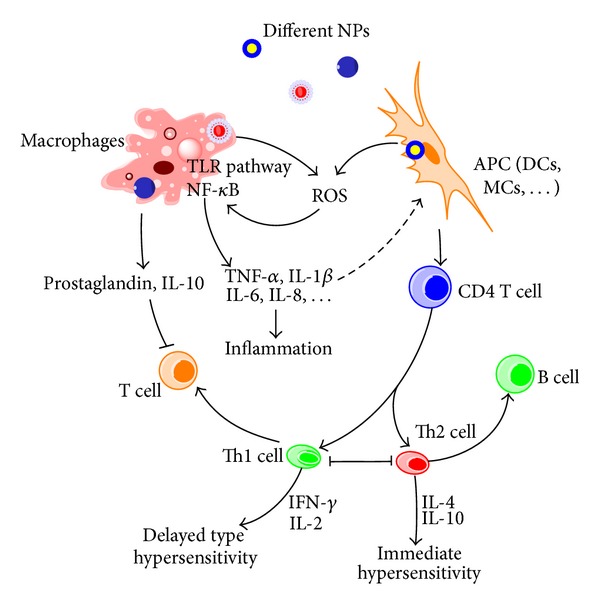
Mechanisms involved in NPs-induced immunomodulation. The stimulation/suppression to immune system depends on the nature of NPs and results in different outcomes. NPs, nanoparticles; NF-κB, nuclear factor kappa B; TLR pathway: toll-like receptor pathway; APC, antigen-presenting cell; DCs, dendritic cells; MCs, mast cells; GM-CSF, granulocyte-macrophage colony-stimulating factor; Th0, type 0 T-helper lymphocyte; Th1, type 1 T-helper lymphocyte; Th2, type 2 T-helper lymphocyte; solid line with arrow, activate/release/induce; solid line with vertical dashes at ends, inhibit; dotted line, possible influence; broken line, polarization/differentiation.
Inflammation is an important response of immune system, which can be induced by NPs, evidenced by the production of cytokines or chemokines. Oxidative stress caused by NPs is reported to be the main downstream events of the inflammation. NPs have large surface areas and strong oxidative abilities than normal particles [140]. Oxidative damage induced by NPs is an important factor of immune imbalance [11]. Many types of NPs have been shown to produce ROS in vitro and in vivo and enhance immune function or inflammatory response [40, 49, 53, 86, 141]. Free radical-induced tissue damage plays an important role in inflammatory diseases [142, 143].
The signal pathway to induce ROS and mediate inflammation was reported. Among them, TLR4 signaling pathway was documented. GO could induce intracellular ROS which decreased the viability of macrophages and induced necrosis by a TLR4 signaling pathway (Figure 4) [144]. TLR is a receptor of the innate immune system and innate immunity could be triggered by stimulating TLRs and lead to strong adaptive immunity [115]. Activation of the TLR pathways could induce chronic inflammation and ROS [145]. Resent research indicated that quantum dots could activate myeloid differentiation primary response gene 88 (MyD88, which is an adapter protein to activate NF-κB) dependent-TLRs in mcrophages and activated NF-κB [146].
Figure 4.
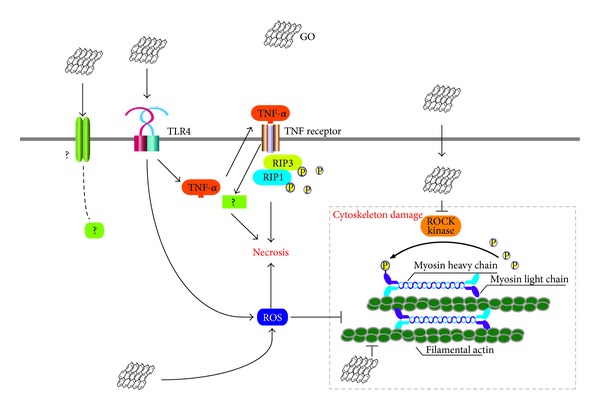
A schematic diagram elucidating the mechanisms responsible for GO-induced cytotoxicity to macrophages. Reprinted with permission from [144].
NF-κB pathway is another key regulator of immune response [125, 147–149]. As an important regulator of proinflammatory gene expression, synthesis of cytokines such as IL-1β, IL-6, IL-8, and TNF-α is mediated by NF-κB. Activation of NF-κB may induce human inflammatory diseases [150, 151]. It was reported that negatively charged poly(acrylic acid-) conjugated GNP activated the NF-κB signalling pathway in THP-1 cells. The cells released inflammatory cytokines including TNF-α and IL-8 [139]. And citrate-stabilized 10 nm GNPs could induce activation of an NF-κB regulated luciferase reporter in murine B-lymphocyte cell line (CH12.LX) and altered the cell function [152].
NPs may also disturb the immune balance by inappropriate maturation/activation of APCs such as DCs [26, 153]. Intratracheal instillation of 43 nm MIONs, the alveolar region of BALB/c mice could generate a significant number of exosomes. These exosomes were quickly eliminated from the alveolar region into systemic circulation and transferred their signals to the immune system, which resulted in maturation of DCs and activation of splenic T cells, and the exosome-induced T-cell activation is more efficient in OVA-sensitized mice [42, 43]. Cytotoxicity to immune cells may contribute to immunosuppression of NPs. QDs may decrease the viability of hemocytes in Elliption complanata mussels [50] and reduced phagocytosis on bivalves and fish [97].
Recent reports indicated that NPs could modulate the homeostasis of immune cells, including the shift of Th1/Th2 balance [43, 44, 89] and monocyte homeostasis [154]. For example, magnetic iron oxide NPs activated the T cells, induced a Th1 polarization, and aggravated inflammation [43]. Other studies showed that SWCNTs accentuated Th cells immunity including Th2 (IL-4↑, IL-5↑, and IL-13↑) and Th17 (IL-17A↑, IL-23↑). The inappropriate maturation/activation of APCs such as DCs might be responsible for these accentuated Th cells immunity [26, 153]. The normal immune system keeps a Th1/Th2 balance in order to achieve an appropriate immune response. Selectively activating Th1 or Th2 cells results in immune deviation and breaks the balance of immune system. In addition, MWCNTs could selectively decrease phagocytosis-competent monocytes and promote adhesion of the phagocytosis-incompetent monocytes in blood flow [154].
6. Conclusion
The immune response of NPs is like a double-edged sword in nanomedicine applications by bringing both benefits and harms. We should take advantage of the benefits from the immunomodulating properties of NPs and, on the other hand, avoid the undesirable immune responses in order to minimize the systemic side effects. The factors affecting the immune response are complex, including particle composition, size, surface chemistry, plasma protein binding, and exposure route. Investigation of the relationship between properties of NPs and systemic immune response is crucial for their application in medicine and other areas. Although treatments of acute and long-term immune toxicities have been developed, current approaches of prediction, prevention, and treatment of nanoimmunomodulation are still lacking, encouraging further in-depth studies.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21137002, 21077068, and 21307077), the Doctoral Fund of the Ministry of Education of China (20130131120007), China Postdoctoral Science Foundation funded Project (2013M541920), the Natural Science Foundation of Shandong Province (ZR2013BQ026), and the National Key Basic Research and Development Program (973) (2010CB933504).
Abbreviations
- APC:
Antigen-presenting cell
- CNT:
Carbon nanotube
- SWCNT:
Single walled carbon nanotube
- MWCNT:
Multiwalled carbon nanotube
- DC:
Dendritic cell
- OVA:
Ovalbumin
- BSA:
Bull serum albumin
- TGF-β:
Transforming growth factor beta
- PBMCs:
Peripheral blood mononuclear cells
- Th cells:
Helper T cells
- IL:
Interleukin
- GO:
Grapheme oxide
- TRL:
Toll-like receptor
- PVP:
Polyvinyl pyrrolidone
- MC:
Mast cell
- ROS:
Reactive oxygen species
- TNF-α:
Tumor necrosis factor alpha
- IFN-γ:
Interferon gamma
- PEG:
Polyethylene glycol
- GNP:
Gold nanoparticle
- AgNP:
Silver nanoparticle
- QDs:
Quantum dots
- PfCSP:
Circumsporozoite protein of plasmodium falciparum
- LPS:
Lipopolysaccharide
- MCP-1:
Monocyte chemoattractant protein-1
- MAPKs:
Mitogen activated protein kinases
- NF-κB:
Nuclear factor kappa B
- MIONs:
Magnetic iron oxide nanoparticles
- DNA:
Deoxyribonucleic acid
- MHC II:
Major histocompatibility complex class II.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Vanić Ž, Škalko-Basnet N. Nanopharmaceuticals for improved topical vaginal therapy: can they deliver? The European Journal of Pharmaceutical Sciences. 2013;50(1):29–41. doi: 10.1016/j.ejps.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 2.Lim C-K, Heo J, Shin S, et al. Nanophotosensitizers toward advanced photodynamic therapy of cancer. Cancer Letters. 2013;334(2):176–187. doi: 10.1016/j.canlet.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Corot C, Petry KG, Trivedi R, et al. Macrophage imaging in central nervous system and in carotid atherosclerotic plaque using ultrasmall superparamagnetic iron oxide in magnetic resonance imaging. Investigative Radiology. 2004;39(10):619–625. doi: 10.1097/01.rli.0000135980.08491.33. [DOI] [PubMed] [Google Scholar]
- 4.Kim CK, Ghosh P, Pagliuca C, Zhu Z, Menichetti S, Rotello VM. Entrapment of hydrophobic drugs in nanoparticle monolayers with efficient release into cancer cells. Journal of the American Chemical Society. 2009;131(4):1360–1361. doi: 10.1021/ja808137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Li J, Wang Y, et al. A folate receptor-targeting nanoparticle minimizes drug resistance in a human cancer model. ACS Nano. 2011;5(8):6184–6194. doi: 10.1021/nn200739q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li R, Wu R, Zhao L, Wu M, Yang L, Zou H. P-glycoprotein antibody functionalized carbon nanotube overcomes the multidrug resistance of human leukemia cells. ACS Nano. 2010;4(3):1399–1408. doi: 10.1021/nn9011225. [DOI] [PubMed] [Google Scholar]
- 7.Nam JM, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003;301(5641):1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 8.Chang H-H, Moura JMF, Wu YL, Ho C. Automatic detection of regional heart rejection in USPIO-enhanced MRI. IEEE Transactions on Medical Imaging. 2008;27(8):1095–1106. doi: 10.1109/TMI.2008.918329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Nanomedicine: challenge and perspectives. Angewandte Chemie: International Edition. 2009;48(5):872–897. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janeway C, Travers P, Hunt S. Immunobiology: The Immune System in Health and Disease. Vol. 7. Current Biology; 1996. [Google Scholar]
- 11.Hussain S, Vanoirbeek JAJ, Hoet PHM. Interactions of nanomaterials with the immune system. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2012;4(2):169–183. doi: 10.1002/wnan.166. [DOI] [PubMed] [Google Scholar]
- 12.Descotes J, Choquet-Kastylevsky G, van Ganse E, Vial T. Responses of the immune system to injury. Toxicologic Pathology. 2000;28(3):479–481. doi: 10.1177/019262330002800319. [DOI] [PubMed] [Google Scholar]
- 13.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nature Nanotechnology. 2007;2(8):469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 14.Zogovic NS, Nikolic NS, Vranjes-Djuric SD, et al. Opposite effects of nanocrystalline fullerene (C60) on tumour cell growth in vitro and in vivo and a possible role of immunosupression in the cancer-promoting activity of C60. Biomaterials. 2009;30(36):6940–6946. doi: 10.1016/j.biomaterials.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Parween S, Gupta PK, Chauhan VS. Induction of humoral immune response against PfMSP-119 and PvMSP-119 using gold nanoparticles along with alum. Vaccine. 2011;29(13):2451–2460. doi: 10.1016/j.vaccine.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Ryan JJ, Bateman HR, Stover A, et al. Fullerene nanomaterials inhibit the allergic response. Journal of Immunology. 2007;179(1):665–672. doi: 10.4049/jimmunol.179.1.665. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell LA, Gao J, Wal RV, Gigliotti A, Burchiel SW, McDonald JD. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicological Sciences. 2007;100(1):203–214. doi: 10.1093/toxsci/kfm196. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell LA, Lauer FT, Burchiel SW, McDonald JD. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nature Nanotechnology. 2009;4(7):451–456. doi: 10.1038/nnano.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tkach AV, Shurin GV, Shurin MR, et al. Direct effects of carbon nanotubes on dendritic cells induce immune suppression upon pulmonary exposure. ACS Nano. 2011;5(7):5755–5762. doi: 10.1021/nn2014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Katwa P, Podila R, et al. Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Particle and Fibre Toxicology. 2011;8(1, article 24) doi: 10.1186/1743-8977-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katwa P, Wang X, Urankar RN, et al. A carbon nanotube toxicity paradigm driven by mast cells and the IL-33/ST2 axis. Small. 2012;8(18):2904–2912. doi: 10.1002/smll.201200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosaka N, Mitsunaga M, Choyke PL, Kobayashi H. In vivo real-time lymphatic draining using quantum-dot optical imaging in mice. Contrast Media & Molecular Imaging. 2013;8(1):96–100. doi: 10.1002/cmmi.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Podila R, Shannahan J, Rao A, Brown J. Intravenously delivered graphene nanosheets and multiwalled carbon nanotubes induce site-specific Th2 inflammatory responses via the IL-33/ST2 axis. International Journal of Nanomedicine. 2013;8:1733–1748. doi: 10.2147/IJN.S44211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng J, Yang M, Jia F, Xu Z, Kong H, Xu H. Immune responses of BALB/c mice to subcutaneously injected multi-walled carbon nanotubes. Nanotoxicology. 2011;5(4):583–591. doi: 10.3109/17435390.2010.523483. [DOI] [PubMed] [Google Scholar]
- 25.Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong BA, Bonner JC. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. The American Journal of Respiratory Cell and Molecular Biology. 2009;40(3):349–358. doi: 10.1165/rcmb.2008-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue K-I, Koike E, Yanagisawa R, Hirano S, Nishikawa M, Takano H. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicology and Applied Pharmacology. 2009;237(3):306–316. doi: 10.1016/j.taap.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Yudoh K, Karasawa R, Masuko K, Kato T. Water-soluble fullerene (C60) inhibits the development of arthritis in the rat model of arthritis. International Journal of Nanomedicine. 2009;4:217–225. doi: 10.2147/ijn.s7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park E-J, Kim H, Kim Y, Yi J, Choi K, Park K. Carbon fullerenes (C60s) can induce inflammatory responses in the lung of mice. Toxicology and Applied Pharmacology. 2010;244(2):226–233. doi: 10.1016/j.taap.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 29.Chen BX, Wilson SR, Das M, Coughlin DJ, Erlanger BF. Antigenicity of fullerenes: antibodies specific for fullerenes and their characteristics. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(18):10809–10813. doi: 10.1073/pnas.95.18.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsao N, Luh T, Chou C, Wu J, Lin Y, Lei H. Inhibition of group A streptococcus infection by carboxyfullerene. Antimicrobial Agents and Chemotherapy. 2001;45(6):1788–1793. doi: 10.1128/AAC.45.6.1788-1793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jovanović B, Anastasova L, Rowe EW, Palić D. Hydroxylated fullerenes inhibit neutrophil function in fathead minnow (Pimephales promelas Rafinesque, 1820) Aquatic Toxicology. 2011;101(2):474–482. doi: 10.1016/j.aquatox.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Simpson CA, Huffman BJ, Gerdon AE, Cliffel DE. Unexpected toxicity of monolayer protected gold clusters eliminated by PEG-thiol place exchange reactions. Chemical Research in Toxicology. 2010;23(10):1608–1616. doi: 10.1021/tx100209t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson CA, Agrawal AC, Balinski A, Harkness KM, Cliffel DE. Short-chain PEG mixed monolayer protected gold clusters increase clearance and red blood cell counts. ACS Nano. 2011;5(5):3577–3584. doi: 10.1021/nn103148x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho W-S, Cho M, Jeong J, et al. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicology and Applied Pharmacology. 2009;236(1):16–24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Truong L, Tilton SC, Zaikove T, et al. Surface functionalities of gold nanoparticles impact embryonic gene expression responses. Nanotoxicology. 2013;7(2):192–201. doi: 10.3109/17435390.2011.648225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hussain S, Vanoirbeek JAJ, Luyts K, et al. Lung exposure to nanoparticles modulates an asthmatic response in a mouse model. European Respiratory Journal. 2011;37(2):299–309. doi: 10.1183/09031936.00168509. [DOI] [PubMed] [Google Scholar]
- 37.Chen H, Dorrigan A, Saad S, Hare DJ, Cortie MB, Valenzuela SM. In vivo study of spherical gold nanoparticles: inflammatory effects and distribution in mice. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0058208.e58208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sumbayev VV, Yasinska IM, Garcia CP, et al. Gold nanoparticles downregulate interleukin-1-induced pro-inflammatory responses. Small. 2013;9(3):472–477. doi: 10.1002/smll.201201528. [DOI] [PubMed] [Google Scholar]
- 39.Lee H-Y, Choi Y, Jung E, et al. Genomics-based screening of differentially expressed genes in the brains of mice exposed to silver nanoparticles via inhalation. Journal of Nanoparticle Research. 2010;12(5):1567–1578. [Google Scholar]
- 40.Liu H, Yang D, Yang H, et al. Comparative study of respiratory tract immune toxicity induced by three sterilisation nanoparticles: silver, zinc oxide and titanium dioxide. Journal of Hazardous Materials. 2013;248-249:478–486. doi: 10.1016/j.jhazmat.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 41.Fifis T, Gamvrellis A, Crimeen-Irwin B, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. Journal of Immunology. 2004;173(5):3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 42.Zhu M, Tian X, Song X, et al. Nanoparticle-induced exosomes target antigen-presenting cells to initiate Th1-type immune activation. Small. 2012;8(18):2841–2848. doi: 10.1002/smll.201200381. [DOI] [PubMed] [Google Scholar]
- 43.Zhu M, Li Y, Shi J, Feng W, Nie G, Zhao Y. Exosomes as extrapulmonary signaling conveyors for nanoparticle-induced systemic immune activation. Small. 2012;8(3):404–412. doi: 10.1002/smll.201101708. [DOI] [PubMed] [Google Scholar]
- 44.Shen CC, Liang HH, Wang CC, Liao MH, Jan TR. Iron oxide nanoparticles suppressed T helper 1 cell-mediated immunity in a murine model of delayed-type hypersensitivity. International Journal of Nanomedicine. 2012;7:2729–2737. doi: 10.2147/IJN.S31054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ban M, Langonné I, Huguet N, Guichard Y, Goutet M. Iron oxide particles modulate the ovalbumin-induced Th2 immune response in mice. Toxicology Letters. 2013;216(1):31–39. doi: 10.1016/j.toxlet.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Wingard CJ, Walters DM, Cathey BL, et al. Mast cells contribute to altered vascular reactivity and ischemia-reperfusion injury following cerium oxide nanoparticle instillation. Nanotoxicology. 2011;5(4):531–545. doi: 10.3109/17435390.2010.530004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma JY, Zhao H, Mercer RR, et al. Cerium oxide nanoparticle-induced pulmonary inflammation and alveolar macrophage functional change in rats. Nanotoxicology. 2011;5(3):312–325. doi: 10.3109/17435390.2010.519835. [DOI] [PubMed] [Google Scholar]
- 48.Cho W-S, Duffn R, Poland CA, et al. Metal oxide nanoparticles induce unique infammatory footprints in the lung: important implications for nanoparticle testing. Environmental Health Perspectives. 2010;118(12):1699–1706. doi: 10.1289/ehp.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srinivas A, Rao PJ, Selvam G, Murthy PB, Reddy PN. Acute inhalation toxicity of cerium oxide nanoparticles in rats. Toxicology Letters. 2011;205(2):105–115. doi: 10.1016/j.toxlet.2011.05.1027. [DOI] [PubMed] [Google Scholar]
- 50.Gagné F, Auclair J, Turcotte P, et al. Ecotoxicity of CdTe quantum dots to freshwater mussels: impacts on immune system, oxidative stress and genotoxicity. Aquatic Toxicology. 2008;86(3):333–340. doi: 10.1016/j.aquatox.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Gagné F, Fortier M, Yu L, et al. Immunocompetence and alterations in hepatic gene expression in rainbow trout exposed to CdS/CdTe quantum dots. Journal of Environmental Monitoring. 2010;12(8):1556–1565. doi: 10.1039/c0em00031k. [DOI] [PubMed] [Google Scholar]
- 52.Morishige T, Yoshioka Y, Inakura H, et al. Suppression of nanosilica particle-induced inflammation by surface modification of the particles. Archives of Toxicology. 2012;86(8):1297–1307. doi: 10.1007/s00204-012-0823-5. [DOI] [PubMed] [Google Scholar]
- 53.Chen Q, Xue Y, Sun J. Kupffer cell-mediated hepatic injury induced by silica nanoparticles in vitro and in vivo. International Journal of Nanomedicine. 2013;8:1129–1140. doi: 10.2147/IJN.S42242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardy CL, LeMasurier JS, Belz GT, et al. Inert 50-nm polystyrene nanoparticles that modify pulmonary dendritic cell function and inhibit allergic airway inflammation. Journal of Immunology. 2012;188(3):1431–1441. doi: 10.4049/jimmunol.1100156. [DOI] [PubMed] [Google Scholar]
- 55.Getts DR, Martin AJ, McCarthy DP, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nature Biotechnology. 2012;30(12):1217–1224. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daftarian PM, Stone GW, Kovalski L, et al. A targeted and adjuvanted nanocarrier lowers the effective dose of liposomal amphotericin B and enhances adaptive immunity in murine cutaneous leishmaniasis. Journal of Infectious Diseases. 2013;208(11):1914–1922. doi: 10.1093/infdis/jit378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saljoughian N, Zahedifard F, Doroud D, et al. Cationic solid-lipid nanoparticles are as efficient as electroporation in DNA vaccination against visceral leishmaniasis in mice. Parasite Immunology. 2013;35(12):397–408. doi: 10.1111/pim.12042. [DOI] [PubMed] [Google Scholar]
- 58.Weilhammer DR, Blanchette CD, Fischer NO, et al. The use of nanolipoprotein particles to enhance the immunostimulatory properties of innate immune agonists against lethal influenza challenge. Biomaterials. 2013;34(38):10305–10318. doi: 10.1016/j.biomaterials.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chanan-Khan A, Szebeni J, Savay S, et al. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Annals of Oncology. 2003;14(9):1430–1437. doi: 10.1093/annonc/mdg374. [DOI] [PubMed] [Google Scholar]
- 60.Mahajan S, Koul V, Choudhary V, et al. Preparation and in vitro evaluation of folate-receptor-targeted SPION–polymer micelle hybrids for MRI contrast enhancement in cancer imaging. Nanotechnology. 2013;24(1) doi: 10.1088/0957-4484/24/1/015603.015603 [DOI] [PubMed] [Google Scholar]
- 61.Eldar-Boock A, Polyak D, Scomparin A, Satchi-Fainaro R. Nano-sized polymers and liposomes designed to deliver combination therapy for cancer. Current Opinion in Biotechnology. 2013;24(4):682–689. doi: 10.1016/j.copbio.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 62.Jaiswal JK, Simon SM. Belling the cat—tagging live cells with quantum dots. Clinical Chemistry. 2013;59(6):995–996. doi: 10.1373/clinchem.2012.199166. [DOI] [PubMed] [Google Scholar]
- 63.Liu CH, Huang S, Cui J, et al. MR contrast probes that trace gene transcripts for cerebral ischemia in live animals. The FASEB Journal. 2007;21(11):3004–3015. doi: 10.1096/fj.07-8203com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nature Nanotechnology. 2009;4(10):627–633. doi: 10.1038/nnano.2009.241. [DOI] [PubMed] [Google Scholar]
- 65.Podesta JE, Al-Jamal KT, Herrero MA, et al. Antitumor activity and prolonged survival by carbon-nanotube-mediated therapeutic siRNA silencing in a human lung xenograft model. Small. 2009;5(10):1176–1185. doi: 10.1002/smll.200801572. [DOI] [PubMed] [Google Scholar]
- 66.Zhang H, Grüner G, Zhao Y. Recent advancements of graphene in biomedicine. Journal of Materials Chemistry B. 2013;1(20):p. 2542. doi: 10.1039/c3tb20405g. [DOI] [PubMed] [Google Scholar]
- 67.Akhavan O, Ghaderi E. Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon. 2012;50(5):1853–1860. [Google Scholar]
- 68.Loo C, Lin A, Hirsch L, et al. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technology in Cancer Research & Treatment. 2004;3(1):33–40. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- 69.Cheng Y, Samia AC, Meyers JD, Panagopoulos I, Fei B, Burda C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. Journal of the American Chemical Society. 2008;130(32):10643–10647. doi: 10.1021/ja801631c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tran QH, Nguyen VQ, Le A-T. Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives. Advances in Natural Sciences: Nanoscience and Nanotechnology. 2013;4(3)033001 [Google Scholar]
- 71.Kraft JC, Freeling JP, Wang Z, Ho RJY. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. Journal of Pharmaceutical Sciences. 2013;103(1):29–52. doi: 10.1002/jps.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silva JM, Videira M, Gaspar R, Préat V, Florindo HF. Immune system targeting by biodegradable nanoparticles for cancer vaccines. Journal of Controlled Release. 2013;168(2):179–199. doi: 10.1016/j.jconrel.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 73.Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM. Anti-inflammatory properties of cerium oxide nanoparticles. Small. 2009;5(24):2848–2856. doi: 10.1002/smll.200901048. [DOI] [PubMed] [Google Scholar]
- 74.Park J-H, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nature Materials. 2009;8(4):331–336. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salonen J, Laitinen L, Kaukonen AM, et al. Mesoporous silicon microparticles for oral drug delivery: loading and release of five model drugs. Journal of Controlled Release. 2005;108(2-3):362–374. doi: 10.1016/j.jconrel.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 76.Heegaard PM, Boas U, Sorensen NS. Dendrimers for vaccine and immunostimulatory uses: a review. Bioconjugate Chemistry. 2010;21(3):405–418. doi: 10.1021/bc900290d. [DOI] [PubMed] [Google Scholar]
- 77.Yang Y, Neef T, Mittelholzer C, et al. The biodistribution of self-assembling protein nanoparticles shows they are promising vaccine platforms. Journal of Nanobiotechnology. 2013;11(1):p. 36. doi: 10.1186/1477-3155-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Advanced Drug Delivery Reviews. 2008;60(8):876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 79.Chueh SC, Lai MK, Lee MS, Chiang LY, Ho TI, Chen SC. Decrease of free radical level in organ perfusate by a novel water-soluble carbon-sixty, hexa(sulfobutyl)fullerenes. Transplantation Proceedings. 1999;31(5):1976–1977. doi: 10.1016/s0041-1345(99)00234-1. [DOI] [PubMed] [Google Scholar]
- 80.Magoulas GE, Garnelis T, Athanassopoulos CM, et al. Synthesis and antioxidative/anti-inflammatory activity of novel fullerene-polyamine conjugates. Tetrahedron. 2012;68(35):7041–7049. [Google Scholar]
- 81.Chen YW, Hwang KC, Yen C, Lai Y. Fullerene derivatives protect against oxidative stress in RAW 264.7 cells and ischemia-reperfused lungs. The American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2004;287(1):R21–R26. doi: 10.1152/ajpregu.00310.2003. [DOI] [PubMed] [Google Scholar]
- 82.Rahman MF, Wang J, Patterson TA, et al. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicology Letters. 2009;187(1):15–21. doi: 10.1016/j.toxlet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 83.Samberg ME, Oldenburg SJ, Monteiro-Riviere NA. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environmental Health Perspectives. 2010;118(3):407–413. doi: 10.1289/ehp.0901398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Müller K, Skepper JN, Posfai M, et al. Effect of ultrasmall superparamagnetic iron oxide nanoparticles (Ferumoxtran-10) on human monocyte-macrophages in vitro. Biomaterials. 2007;28(9):1629–1642. doi: 10.1016/j.biomaterials.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 85.Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, Barakat AI. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environmental Health Perspectives. 2007;115(3):403–409. doi: 10.1289/ehp.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naqvi S, Samim M, Abdin MZ, et al. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. International Journal of Nanomedicine. 2010;5(1):983–989. doi: 10.2147/IJN.S13244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Blank F, Gerber P, Rothen-Rutishauser B, et al. Biomedical nanoparticles modulate specific CD4(+) T cell stimulation by inhibition of antigen processing in dendritic cells. Nanotoxicology. 2011;5(4):606–621. doi: 10.3109/17435390.2010.541293. [DOI] [PubMed] [Google Scholar]
- 88.Ban M, Langonné I, Huguet N, Goutet M. Effect of submicron and nano-iron oxide particles on pulmonary immunity in mice. Toxicology Letters. 2012;210(3):267–275. doi: 10.1016/j.toxlet.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 89.Schanen BC, Das S, Reilly CM, et al. Immunomodulation and T helper TH1/TH2 response polarization by CeO2 and TiO2 nanoparticles. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0062816.e62816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pagliari F, Mandoli C, Forte G, et al. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano. 2012;6(5):3767–3775. doi: 10.1021/nn2048069. [DOI] [PubMed] [Google Scholar]
- 91.Falugi C, Aluigi MG, Chiantore MC, et al. Toxicity of metal oxide nanoparticles in immune cells of the sea urchin. Marine Environmental Research. 2012;76:114–121. doi: 10.1016/j.marenvres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 92.Clapp AR, Medintz IL, Mauro JM, Fisher BR, Bawendi MG, Mattoussi H. Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. Journal of the American Chemical Society. 2004;126(1):301–310. doi: 10.1021/ja037088b. [DOI] [PubMed] [Google Scholar]
- 93.Samia AC, Chen X, Burda C. Semiconductor quantum dots for photodynamic therapy. Journal of the American Chemical Society. 2003;125(51):15736–15737. doi: 10.1021/ja0386905. [DOI] [PubMed] [Google Scholar]
- 94.Lovrić J, Cho SJ, Winnik FM, Maysinger D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chemistry & Biology. 2005;12(11):1227–1234. doi: 10.1016/j.chembiol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 95.Nguyen KC, Seligy VL, Tayabali AF. Cadmium telluride quantum dot nanoparticle cytotoxicity and effects on model immune responses to Pseudomonas aeruginosa . Nanotoxicology. 2013;7(2):202–211. doi: 10.3109/17435390.2011.648667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoshino A, Hanada S, Manabe N, Nakayama T, Yamamoto K. Immune response induced by fluorescent nanocrystal quantum dots in vitro and in vivo. IEEE Transactions on Nanobioscience. 2009;8(1):51–57. doi: 10.1109/TNB.2009.2016550. [DOI] [PubMed] [Google Scholar]
- 97.Bruneau A, Fortier M, Gagne F, et al. Size distribution effects of cadmium tellurium quantum dots (CdS/CdTe) immunotoxicity on aquatic organisms. Environmental Science: Processes & Impacts. 2013;15(3):596–607. doi: 10.1039/c2em30896g. [DOI] [PubMed] [Google Scholar]
- 98.Roberts RA, Shen T, Allen IC, Hasan W, DeSimone JM, Ting JPY. Analysis of the murine immune response to pulmonary delivery of precisely fabricated nano- and microscale particles. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0062115.e62115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clichici S, Biris AR, Catoi C, Filip A, Tabaran F. Short-term splenic impact of single-strand DNA functionalized multi-walled carbon nanotubes intraperitoneally injected in rats. Journal of Applied Toxicology. 2013;34(4):332–344. doi: 10.1002/jat.2883. [DOI] [PubMed] [Google Scholar]
- 100.Nygaard UC, Hansen JS, Samuelsen M, Alberg T, Marioara CD, Løvik M. Single-walled and multi-walled carbon nanotubes promote allergic immune responses in mice. Toxicological Sciences. 2009;109(1):113–123. doi: 10.1093/toxsci/kfp057. [DOI] [PubMed] [Google Scholar]
- 101.Laverny G, Casset A, Purohit A, et al. Immunomodulatory properties of multi-walled carbon nanotubes in peripheral blood mononuclear cells from healthy subjects and allergic patients. Toxicology Letters. 2013;217(2):91–101. doi: 10.1016/j.toxlet.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 102.Zhi X, Fang H, Bao C, et al. The immunotoxicity of graphene oxides and the effect of PVP-coating. Biomaterials. 2013;34(21):5254–5261. doi: 10.1016/j.biomaterials.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 103.Tkach AV, Yanamala N, Stanley S, et al. Graphene oxide, but not fullerenes, targets immunoproteasomes and suppresses antigen presentation by dendritic cells. Small. 2013;9(9-10):1686–1690. doi: 10.1002/smll.201201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen G-Y, Yang H-J, Lu C-H, et al. Simultaneous induction of autophagy and toll-like receptor signaling pathways by graphene oxide. Biomaterials. 2012;33(27):6559–6569. doi: 10.1016/j.biomaterials.2012.05.064. [DOI] [PubMed] [Google Scholar]
- 105.Yue H, Wei W, Yue Z, et al. The role of the lateral dimension of graphene oxide in the regulation of cellular responses. Biomaterials. 2012;33(16):4013–4021. doi: 10.1016/j.biomaterials.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 106.Bunz H, Plankenhorn S, Klein R. Effect of buckminsterfullerenes on cells of the innate and adaptive immune system: an in vitro study with human peripheral blood mononuclear cells. International Journal of Nanomedicine. 2012;7:4571–4580. doi: 10.2147/IJN.S33773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fallarini S, Paoletti T, Battaglini CO, et al. Factors affecting T cell responses induced by fully synthetic glyco-gold-nanoparticles. Nanoscale. 2013;5(1):390–400. doi: 10.1039/c2nr32338a. [DOI] [PubMed] [Google Scholar]
- 108.Pfaller T, Puntes V, Casals E, Duschl A, Oostingh GJ. In vitro investigation of immunomodulatory effects caused by engineered inorganic nanoparticles: the impact of experimental design and cell choice. Nanotoxicology. 2009;3(1):46–59. [Google Scholar]
- 109.Yang E-J, Choi I-H. Immunostimulatory effects of silica nanoparticles in human monocytes. Immune Network. 2013;13(3):94–101. doi: 10.4110/in.2013.13.3.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lou PJ, Cheng W, Chung Y, Cheng C, Chiu L, Young T. PMMA particle-mediated DNA vaccine for cervical cancer. Journal of Biomedical Materials Research A. 2009;88(4):849–857. doi: 10.1002/jbm.a.31919. [DOI] [PubMed] [Google Scholar]
- 111.Kreuter J, Liehl E. Protection induced by inactivated influenza virus vaccines with polymethylmethacrylate adjuvants. Medical Microbiology and Immunology. 1978;165(2):111–117. doi: 10.1007/BF02122746. [DOI] [PubMed] [Google Scholar]
- 112.Shakya AK, Nandakumar KS. Applications of polymeric adjuvants in studying autoimmune responses and vaccination against infectious diseases. Journal of the Royal Society Interface. 2012;10(79) doi: 10.1098/rsif.2012.0536.20120536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sayin B, Somavarapu S, Li XW, Sesardic D, Senel S, Alpar OH. TMC-MCC (N-trimethyl chitosan-mono-N-carboxymethyl chitosan) nanocomplexes for mucosal delivery of vaccines. The European Journal of Pharmaceutical Sciences. 2009;38(4):362–369. doi: 10.1016/j.ejps.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 114.Petersen LK, Ramer-Tait AE, Broderick SR, et al. Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials. 2011;32(28):6815–6822. doi: 10.1016/j.biomaterials.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 115.Camacho AI, da Costa Martins R, Tamayo I, et al. Poly(methyl vinyl ether-co-maleic anhydride) nanoparticles as innate immune system activators. Vaccine. 2011;29(41):7130–7135. doi: 10.1016/j.vaccine.2011.05.072. [DOI] [PubMed] [Google Scholar]
- 116.Frick SU, Bacher N, Baier G, et al. Functionalized polystyrene nanoparticles trigger human dendritic cell maturation resulting in enhanced CD4 + T cell activation. Macromolecular Bioscience. 2012;12(12):1637–1647. doi: 10.1002/mabi.201200223. [DOI] [PubMed] [Google Scholar]
- 117.Córdoba EV, Pion M, Rasines B, et al. Glycodendrimers as new tools in the search for effective anti-HIV DC-based immunotherapies. Nanomedicine: Nanotechnology, Biology and Medicine. 2013;9(7):972–984. doi: 10.1016/j.nano.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 118.McCoy ME, Golden HE, Doll TA, et al. Mechanisms of protective immune responses induced by the Plasmodium falciparum circumsporozoite protein-based, self-assembling protein nanoparticle vaccine. Malaria Journal. 2013;12(1):p. 136. doi: 10.1186/1475-2875-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Walker S. Biophysical characterization of optimized self-assembling protein nanoparticles as a malaria vaccine [Honors Scholar Thesis] Storrs, Conn, USA: University of Connecticut; 2013. [Google Scholar]
- 120.Molino NM, Anderson AKL, Nelson EL, Szu-Wen W. Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. ACS Nano. 2013;7(11):9743–9752. doi: 10.1021/nn403085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo Q, Dasgupta D, Doll TA, Burkhard P, Lanar DE. Expression, purification and refolding of a self-assembling protein nanoparticle (SAPN) malaria vaccine. Methods. 2013;160(3):242–247. doi: 10.1016/j.ymeth.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Akita H, Ishii S, Miura N, et al. A DNA microarray-based analysis of immune-stimulatory and transcriptional responses of dendritic cells to KALA-modified nanoparticles. Biomaterials. 2013;34(35):8979–8990. doi: 10.1016/j.biomaterials.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 123.Sellins K, Fradkin L, Liggitt D, Dow S. Type I interferons potently suppress gene expression following gene delivery using liposome-DNA complexes. Molecular Therapy. 2005;12(3):451–459. doi: 10.1016/j.ymthe.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 124.Zhou H, Mu Q, Gao N, et al. A nano-combinatorial library strategy for the discovery of nanotubes with reduced protein-binding, cytotoxicity, and immune response. Nano Letters. 2008;8(3):859–865. doi: 10.1021/nl0730155. [DOI] [PubMed] [Google Scholar]
- 125.Gao N, Zhang Q, Mu Q, et al. Steering carbon nanotubes to scavenger receptor recognition by nanotube surface chemistry modification partially alleviates NFκB activation and reduces its immunotoxicity. ACS Nano. 2011;5(6):4581–4591. doi: 10.1021/nn200283g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang Y, Zhang H, Wang Y, et al. Modulation of apoptotic pathways of macrophages by surface-functionalized multi-walled carbon nanotubes. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0065756.e65756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shahbazi MA, Hamidi M, Mäkilä EM, et al. The mechanisms of surface chemistry effects of mesoporous silicon nanoparticles on immunotoxicity and biocompatibility. Biomaterials. 2013;34(31):7776–7789. doi: 10.1016/j.biomaterials.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 128.Mottram PL, Leong D, Crimeen-Irwin B, et al. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Molecular Pharmaceutics. 2007;4(1):73–84. doi: 10.1021/mp060096p. [DOI] [PubMed] [Google Scholar]
- 129.Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA. What the cell “sees” in bionanoscience. Journal of the American Chemical Society. 2010;132(16):5761–5768. doi: 10.1021/ja910675v. [DOI] [PubMed] [Google Scholar]
- 130.Marano F, Hussain S, Rodrigues-Lima F, Baeza-Squiban A, Boland S. Nanoparticles: molecular targets and cell signalling. Archives of Toxicology. 2011;85(7):733–741. doi: 10.1007/s00204-010-0546-4. [DOI] [PubMed] [Google Scholar]
- 131.Cedervall T, Lynch I, Lindman S, et al. Understanding the nanoparticle-protein corona using methods to quntify exchange rates and affinities of proteins for nanoparticles. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(7):2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Deng ZJ, Liang M, Monteiro M, Minchin RF. Plasma protein binding of positively and negatively charged polymer-coated gold nanoparticles elicits different biological responses. Nanotoxicology. 2012;7(3):314–322. doi: 10.3109/17435390.2012.655342. [DOI] [PubMed] [Google Scholar]
- 133.Horie M, Nishio K, Fujita K, et al. Protein adsorption of ultrafine metal oxide and its influence on cytotoxicity toward cultured cells. Chemical Research in Toxicology. 2009;22(3):543–553. doi: 10.1021/tx800289z. [DOI] [PubMed] [Google Scholar]
- 134.Lynch I, Dawson KA. Protein-nanoparticle interactions. Nano Today. 2008;3(1-2):40–47. [Google Scholar]
- 135.Deng ZJ, Mortimer G, Schiller T, Musumeci A, Martin D, Minchin RF. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology. 2009;20(45) doi: 10.1088/0957-4484/20/45/455101.455101 [DOI] [PubMed] [Google Scholar]
- 136.Saptarshi SR, Duschl A, Lopata AL. Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. Journal of Nanobiotechnology. 2013;11(1):1–12. doi: 10.1186/1477-3155-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(38):14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sacchetti C, Motamedchaboki K, Magrini A, et al. Surface polyethylene glycol conformation influences the protein corona of polyethylene glycol-modified single-walled carbon nanotubes: potential implications on biological performance. ACS Nano. 2013;7(3):1974–1989. doi: 10.1021/nn400409h. [DOI] [PubMed] [Google Scholar]
- 139.Deng ZJ, Liang M, Monteiro M, Toth I, Minchin RF. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nature Nanotechnology. 2011;6(1):39–44. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- 140.Pantarotto D, Briand J, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chemical Communications. 2004;10(1):16–17. doi: 10.1039/b311254c. [DOI] [PubMed] [Google Scholar]
- 141.Guo D, Zhu L, Huang Z, et al. Anti-leukemia activity of PVP-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions. Biomaterials. 2013;34(32):7884–7894. doi: 10.1016/j.biomaterials.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 142.Chapple ILC. Reactive oxygen species and antioxidants in inflammatory diseases. Journal of Clinical Periodontology. 1997;24(5):287–296. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 143.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochemical Journal. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qu G, Liu S, Zhang S, et al. Graphene oxide induces toll-like receptor 4 (TLR4)-dependent necrosis in macrophages. ACS Nano. 2013;7(7):5732–5745. doi: 10.1021/nn402330b. [DOI] [PubMed] [Google Scholar]
- 145.Lucas K, Maes M. Role of the toll like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Molecular Neurobiology. 2013;48(1):190–204. doi: 10.1007/s12035-013-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ho C-C, Luo Y-H, Chuang T-H, Yang C-S, Ling Y-C, Lin P. Quantum dots induced monocyte chemotactic protein-1 expression via MyD88-dependent Toll-like receptor signaling pathways in macrophages. Toxicology. 2013;308(7):1–9. doi: 10.1016/j.tox.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 147.Pasparakis M. Regulation of tissue homeostasis by NF-B signalling: implications for inflammatory diseases. Nature Reviews Immunology. 2009;9(11):778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 148.Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. Journal of Clinical Investigation. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annual Review of Immunology. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 150.Wang SZ, Ma FM, Zhao JD. Expressions of nuclear factor-kappa B p50 and p65 and their significance in the up-regulation of intercellular cell adhesion molecule-1 mRNA in the nasal mucosa of allergic rhinitis patients. European Archives of Oto-Rhino-Laryngology. 2012;270(4):1329–1334. doi: 10.1007/s00405-012-2136-y. [DOI] [PubMed] [Google Scholar]
- 151.Patel JK, Clifford RL, Deacon K, Knox AJ. Ciclesonide inhibits TNFα- and IL-1β-induced monocyte chemotactic protein-1 (MCP-1/CCL2) secretion from human airway smooth muscle cells. The American Journal of Physiology: Lung Cellular and Molecular Physiology. 2012;302(8):L785–L792. doi: 10.1152/ajplung.00257.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sharma M, Salisbury RL, Maurer EI, Hussain SM, Sulentic CEW. Gold nanoparticles induce transcriptional activity of NF-κB in a B-lymphocyte cell line. Nanoscale. 2013;5(9):3747–3756. doi: 10.1039/c3nr30071d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Inoue K-I, Yanagisawa R, Koike E, Nishikawa M, Takano H. Repeated pulmonary exposure to single-walled carbon nanotubes exacerbates allergic inflammation of the airway: possible role of oxidative stress. Free Radical Biology and Medicine. 2010;48(7):924–934. doi: 10.1016/j.freeradbiomed.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 154.de Nicola M, Gattia DM, Traversa E, Ghibelli L. Maturation and demise of human primary monocytes by carbon nanotubes. Journal of Nanoparticle Research. 2013;15, article 1711 [Google Scholar]


