Abstract
The application of nanogold in biopharmaceutical field is reviewed in this work. The properties of nanogold including nanogold surface Plasmon absorption and nanogold surface Plasmon light scattering are illustrated. The physical, chemical, biosynthesis methods of nanogold preparation are presented. Catalytic properties as well as biomedical applications are highlighted as one of the most important applications of nanogold. Biosensing, and diagnostic and therapeutic applications of gold nanoparticles are evaluated. Moreover, gold nanoparticles in drugs, biomolecules and proteins’ delivery are analyzed. Gold nanoparticles for the site-directed photothermal applications are reviewed as the most fruitful research area in the future.
Keywords: Gold, Nanotechnology, Nanogold, Drug delivery, Gold catalysis
1. What is nanogold?
The term nano originates from the greek word “nanos” which means dwarf or small. When nano is used as a prefix it means one billionth part of (10−9). In nanotechnology, nano refers to things in the range of 1–100 nanometers (abbreviated as nm) in size. Atoms are less than one nm in size; molecules and cells vary in size from one to several nanometers. One definition of nanogold is that it concerns itself.
2. Properties of nanogold
2.1. The nanogold surface plasmon absorption
A strong absorption band in the visible region is shown with gold nanoparticles when the frequency of the electromagnetic field is resonant with the coherent electron motion, which is called surface plasmon resonance absorption (Ivan et al., 2005). Interaction with the electric field results in a polarization of the electrons with respect to and relative to the ionic core of a nanoparticle (Fig. 1). The dipole oscillations of the free electrons with respect to the ionic core of a spherical nanoparticle result in the so-called surface plasmon absorption (Stephan and Mostafa, 2003). When the frequency of the electromagnetic field becomes resonant with the coherent electron motion, a strong absorption band around 520 nm in the spectrum is observed, which is the origin of the observed brilliant color of the nanoparticles in solution (Gustav and Mie, 1908).
Figure 1.
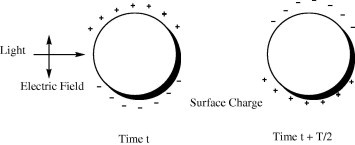
A scheme of surface plasmon absorption of spherical nanoparticles illustrating the excitation of the dipole surface plasmon oscillation.
The peak intensity and position of the surface plasmon absorption band are dependent on the size, the shape of nanoparticles and the dielectric constant of the metals as well as the medium surrounding the particles (Stephen and Mostafa, 2000). As the size increases, the absorption maximum is red shifted slightly. Link and El-Sayed (Stephen and Mostafa, 1999) have shown that the bandwidth decreases with the increase of the nanoparticles’ size particularly when the nanoparticles are lesser than 20 nm in diameter. On the other hand, the bandwidth increases with the increase of the nanoparticles’ size when the nanoparticles are larger than 420 nm. They also found that the absorption coefficient is linearly dependent on the volume of the nanoparticles which is in agreement with the Mie theory (Gustav and Mie, 1908).
2.2. The nanogold surface plasmon light scattering
Upon illumination by a white light beam, the gold particle suspensions scatter colored light (Stephen and Mostafa, 2004). The suspensions of light-scattering nanogold particles show appearance similar to fluorescent solutions (Yguerabide, 1998). The light scattering is sensitive to the size, the shape and the composite of the nanoparticles. Nanoparticles of 58 nm diameter scatter green light while those of 78 nm diameter scatter yellow light and gold nanorods scatter red light under illumination of a beam of white light (Sönnichsen et al., 2002).
3. Nanogold preparation methods
3.1. Physical methods
Laser ablation method is used to produce gold nanoparticles by using the pulsed laser irradiation of gold target in water in the absence of any additives, at (532 nm, 10 ns, 10 Hz), or (266 nm) wavelengths (Ko et al., 2006). Inert gas condensation can be used for the preparation of gold nanoparticles (Lee et al., 2005). In this method, the gold nanoparticles as soon as they are formed rapidly collide with inert gas in a low-pressure environment and thus smaller and controlled nanoparticles are formed. The advantage of these methods is the narrow particle size distribution of the produced gold nanoparticles, while its limitation is the need for expensive equipment. Other physical methods such as thermolysis of gold(I) complex at 180 °C for 5 h under nitrogen atmosphere (Yamamoto and Nakamoto, 2003), radiolysis of gold salts in aqueous solution using γ-irradiation-induced reduction in the field of a 60Co γ-ray source (Henglein and Meisel, 1998, Dawson and Kamat, 2000), photochemistry, e.g. in the HAuCl4 solution containing certain amounts of protective agent and acetone, the colloidal gold particles with an average diameter of 5 nm (σ = 0.86) were prepared by UV 300 nm irradiation (Mallick et al., 2001, Sau et al., 2001), and sonochemistry using ultrasound-induced reduction of gold salts in aqueous solution (Chen et al., 2001, Pol et al., 2003, Reed et al., 2003) have been used to prepare a variety of gold nanoparticles.
3.2. Chemical methods
Emulsification procedure produces gold nanoparticles but with a wide distribution of particle diameters (Pal et al., 2007). Nanogold particles are prepared by reduction of the gold ions in the presence of a dispersant in order to avoid excessive gold agglomeration. Frens, (1973) and Turkevitch et al. (1951) initially introduced a sodium citrate reduction of HAuCl4 for the synthesis of stable gold nanoparticles. In addition there are other reductants such as sodium borohydride (Wagner et al., 2008), stannous chloride (Vaškelis et al., 2007) and ascorbic acid (Sun et al., 2009). Amine-containing molecules for example, branched poly(ethyleneimine) (PEI) Note et al., 2006, third-generation poly(propyleneimine) dendrimer (PPI-G3) in the presence of sunlight (Luo, 2008), azacryptand at room temperature (Lee et al., 2007), amino acid (Selvakannan et al., 2004), polysaccharide (Huang and Yang, 2004), gallic acid (Wang et al., 2007), alcohols, chitosan (Shih et al., 2009) or other organic compounds are also used as a reductant for the synthesis of nanogold.
There are several reported modified chemical methods including seed-mediated growth where small particles produced by other techniques like irradiation were exploited as seeds and fresh Au(III) ions were reduced onto the surface of the seed particles by reducing agents like ascorbic acid (Brown and Natan, 1998, Jana et al., 2001), use of reverse micelles which involves reduction of HAuCl4 in sodium bis (2-ethylhexyl)sulfosuccinate/isooctane reverse micelles system using reducing agents like ascorbic acid (Chiang, 2000), phase transfer reactions as a representative reaction in a novel water–cyclohexane two-phase system, the aqueous formaldehyde is transferred to cyclohexane phase via reaction with dodecylamine to form reductive intermediates in cyclohexane; the intermediates are capable of reducing gold ions in aqueous solution to form gold nanoparticles in cyclohexane solution at room temperature. (Esumi et al., 2000, Haifeng et al., 2005). In view of all these methods, it was reported that the chemical methods are still the preferred method for the preparation of gold nanoparticles than other methods.
3.3. Biosynthesis methods
Biosynthesis of nanoparticles as an emerging highlight of the intersection of nanotechnology and biotechnology has received considered attention due to a growing need to develop environmentally benign technologies in material syntheses. Sastry and coworkers have reported the extracellular synthesis of gold nanoparticles by fungus Fusarium oxysporum and actinomycete Thermomonospora sp., respectively (Ahmad et al., 2003a, Ahmad et al., 2003b). The same group has reported the intracellular synthesis of gold nanoparticles by fungus Verticillium sp. as well (Mukherjee et al., 2001). The reaction of AuCl4− ions with the extract of geranium leaves and an endophytic fungus, Colletotrichum sp., present in the leaves, leads to the formation of AuNPs (Shankar et al., 2003). Nair and Pradeep reported the growth of nanocrystals and nanoalloys using Lactobacillus (Nair and Pradeep, 2002). Daizy has reported a successful attempt on the extract of Volvariella volvacea, an edible mushroom, as reducing and protecting agent for the synthesis of gold, silver and Au–Ag alloy nanoparticles. Gold nanoparticles of different sizes (20–150 nm) and shapes, from triangular to nearly spherical and hexagonal nanoprisms, are obtained by this novel method. The size and shape of gold nanoparticles are also found to depend on the temperature of the extract (Philip, 2009).
Gold nanoparticles of 20–100 nm diameter were synthesized within HEK-293 (human embryonic kidney), HeLa (human cervical cancer), SiHa (human cervical cancer), and SKNSH (human neuroblastoma) cells. Incubation of 1 mM tetrachloroaurate solution, prepared in phosphate buffered saline (PBS), pH 7.4, with human cells grown to ∼80% confluency yielded systematic growth of nanoparticles over a period of 96 h. The cells, stained due to nanoparticle growth, were adherent to the bottom of the wells of the tissue culture plates, with their morphology preserved, indicating that the cell membrane was intact. Transmission electron microscopy of ultrathin sections showed the presence of nanoparticles within the cytoplasm and in the nucleus, the latter being much smaller in dimension. Scanning near field microscopic images confirmed the growth of large particles within the cytoplasm. Normal cells gave UV–visible signatures of higher intensity than the cancer cells. Differences in the cellular metabolism of cancer and noncancer cells were manifested, presumably in their ability to carry out the reduction process. The differential ability with which nanoparticles are synthesized by cancer and normal cells can have implications to cancer diagnostics (Anshup et al., 2005).
4. Applications of nanogold
4.1. Catalytic properties
Few years ago, nanogold received wide interests due to the availability of small sized nanoparticles which provide large surface-to-volume ratio compared to the bulk materials and thus became active for catalysis. Fig. 2 shows the publication of gold catalysis since 1901 from Science finder Scholar and Fig. 3 shows the patents on the gold catalysis in the past decades (Hutchings, 2004). Recent years have witnessed a tremendous growth in the number of gold-catalyzed highly selective chemical transformations (Arcadi and Di Giuseppe, 2004). The catalysis of organic reactions by gold compounds has been shown to be a powerful tool in organic synthesis (Thompson, 1999). Although gold was considered to be an inert metal for a long time, its ability to behave as a soft Lewis acid has only been recognized recently. Such a property allows gold to activate unsaturated functionalities such as alkynes, alkenes, and allenes to create carbon–carbon and carbon–heteroatom bonds under extremely mild conditions (Georgy et al., 2005, Balme et al., 2003). Moreover, by pre-coordination gold may activate sp, sp2, and sp3 carbon–hydrogen bonds efficiently. This may provide new opportunities in organic chemistry using gold as a catalyst. In 1986, the Ito–Hayashi asymmetric aldol reaction catalyzed by homogeneous gold was successfully reported for the first time (Sawamura et al., 1995). Thereafter, Fukuda and Utimoto, (1991) and Teles et al., (1998) as well as the Hashmi group (Hashmi et al., 2000) and others (Hayashi et al., 1992) initiated an impressive growth of activities on homogeneous gold catalysis. Reactions types are as follows.
Figure 2.
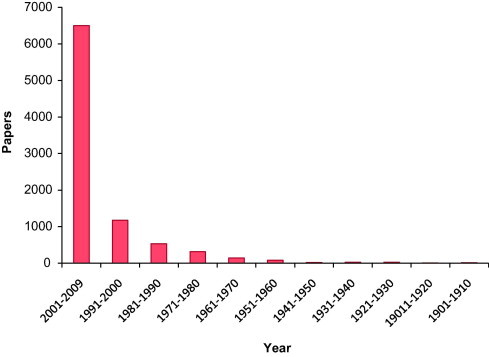
Gold catalysis publications from 1901 to 2009.
Figure 3.
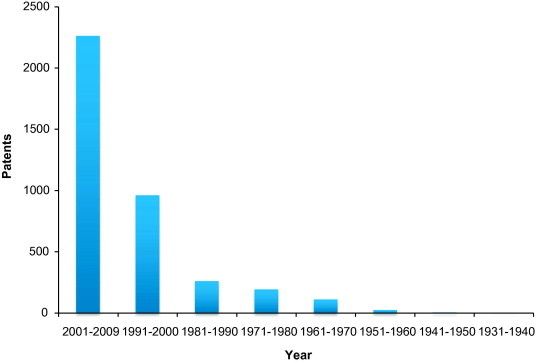
Gold catalysis patents from 1991 to 2009.
4.1.1. Gold-catalyzed C–C bond formations
4.1.1.1. Addition of terminal alkyne to C X group
The strong coordination of terminal alkyne to gold catalyst results in gold-acetylides (Fig. 4) which is used for the alkynylation of orthoalkynylaryl aldehydes (Yao and Li, 2006). In addition, gold further catalyzes an intramolecular cyclization of the hydroxyl–alkyne intermediate, leading to 1-alkynyl-1H-isochromenes directly (Scheme 1). Such isochromenes are common structural units in natural products and exhibit interesting biological activities such as antibiotic properties (Wang et al., 1998). Au(III) and Au(I) catalyzes efficiently the direct coupling of aldehyde, alkyne, and a secondary amine (A3-coupling) (Fig. 5) Wei and Li, 2003a.
Figure 4.
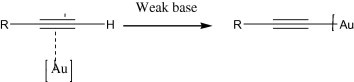
Gold-acetylide complex.
Scheme 1.
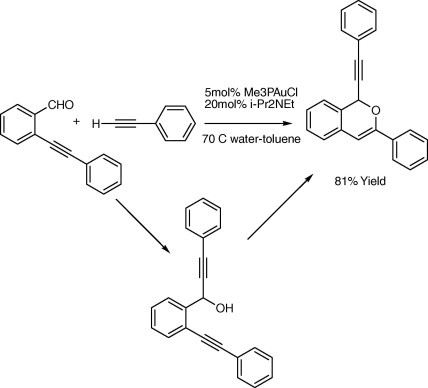
Figure 5.
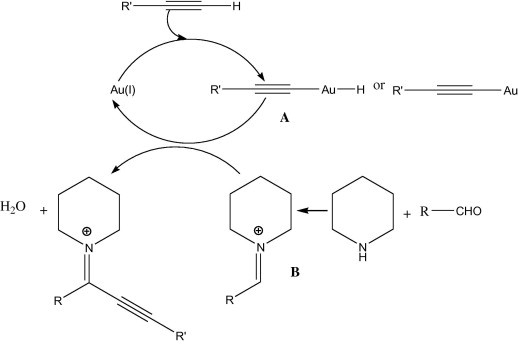
Catalyzes efficiently the direct coupling of aldehyde, alkyne, and a secondary amine (A3-coupling).
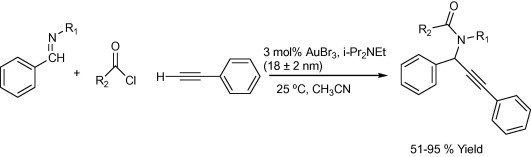
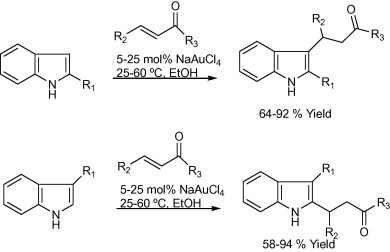
The one-step synthesis of 1-substituted 3-aminoindolizines (Scheme 2) using 1 mol% of NaAuCl4-catalyzed three-component reactions of aldehydes, amines, and alkynes followed by cycloisomerization reaction under solvent-free conditions or in H2O at 60 °C was successfully reported by Yan and Liu (2007). Kidwai et al. Mazaahir et al. (2007) reported an efficient and recyclable Au-nanoparticle, particle size 18 ± 2 nm, catalyst for the A3-coupling reaction of aldehyde, amine, and alkyne via C–H activation (Scheme 3). In 2005, Wei and Li, (2005b) reported a gold-catalyzed direct addition of alkynes to an active acyliminium ion, which was generated in situ from an imine and an acidchloride, to afford propargylamides (Scheme 4). The reaction was efficiently catalyzed with 3 mol% of AuBr3 in the presence of i-Pr2NEt base in acetonitrile.
Scheme 2.
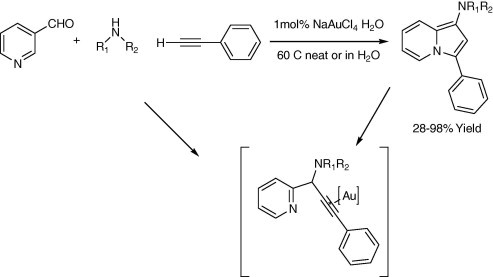
Scheme 3.
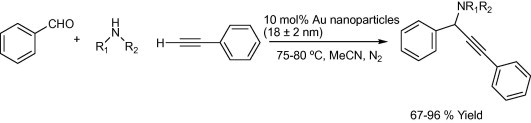
Scheme 4.
4.1.1.2. Addition of aryl group to terminal alkyne (hydroarylation)
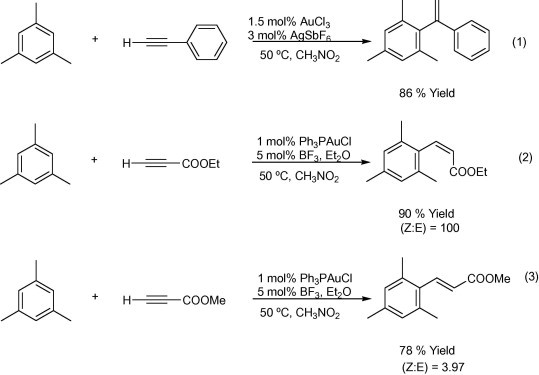
(Reetz et al., (2003) reported the direct addition of arenes to triple bonds with different gold catalysts; for example, 1,3,5-trimethylbenzene added to the internal alkyne carbon in the presence of AuCl3/AgSbF6 (Eq. 1, Scheme 5). Both the regio- and stereo-selectivities of hydroarylation have been completely reversed with different electron-deficient terminal alkynes; the use of ethyl propiolate in the presence of Ph3PAuCl/BF3.OEt gave exclusive (Z)-selectivity (Eq. 2, Scheme 5) whereas in the case of 3-butyne-2-one (E)-selectivity was observed (Eq. 3, Scheme 5).
Scheme 5.
4.1.1.3. Addition of aryl group to alkenes (hydroarylation)
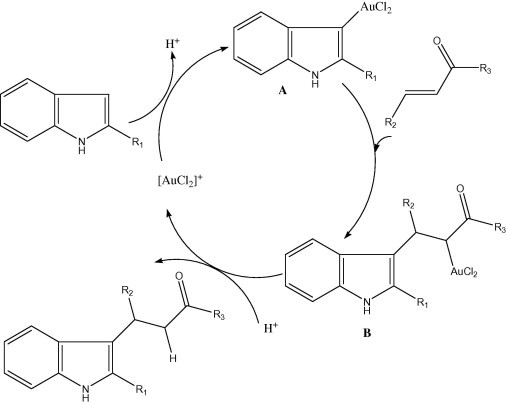
NaAuCl4 has been used as a catalyst for the addition of indole/pyrrole derivatives to α,β-unsaturated ketones or 1,3-dicarbonyls (scheme 6) (Arcadi et al., 2004, Maria et al., 2006). The gold catalyst makes electrophilic attach at the indole forming indolyl-gold species A, which subsequently adds to the α,β-unsaturated ketones resulting in the s-alkyl-gold species B. Protonation of B produced the final addition product and regenerates the catalyst (Itahara et al., 1984). A tentative mechanism for this addition is illustrated in Scheme 7. Regioselective direct electrophilic attack of the gold catalyst at
Scheme 6.
Scheme 7.
4.1.1.4. Addition of aryl group to imine
In Gold-catalyzed imino Friedel–Crafts-type reaction the electron-rich arenes add readily to imines in the presence of 5 mol% of AuOTf as a catalyst (Luo and Li, 2004) (Eq. 1, Scheme 8). Various amino acid derivatives were prepared under the same conditions in high yield (Eq. 2, Scheme 8).
Scheme 8.
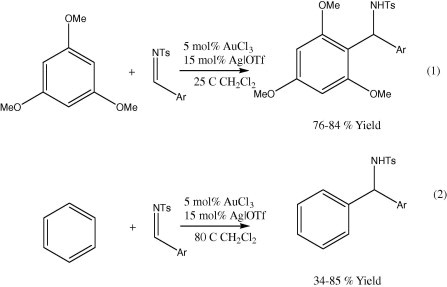
4.1.1.5. Addition of aryl group to epoxide
Several publications reported a cyclization of electron-rich arenes with tethered epoxides catalyzed by gold(III) (Eq. 1, Scheme 9). Trimethoxybenzene explored SN2-type addition at the less hindered primary carbon of propylene oxide (Eq. 2, Scheme 9) (Shi and He, 2004a).
Scheme 9.
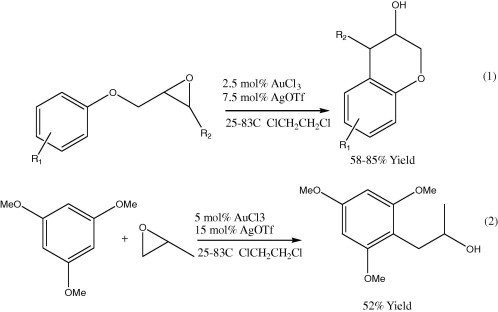
4.1.1.6. Intramolecular nucleophilic substitution of primary alcohol mesylate (SN2 reaction)
Scheme 10 shows the synthesis of Chroman and benzopyranone derivatives by this method (Scheme 10). The reaction mechanism involves arylgold(III) as a reaction intermediate rather than a delocalized carbocation in a typical Friedel–Crafts-type reaction. The arylgold intermediate then reacts with the sulfonate ester to give the final product (Shi and He, 2004b).
Scheme 10.
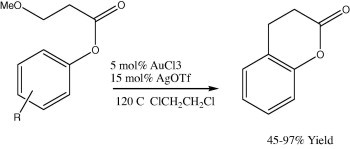
4.1.2. Gold-catalyzed aliphatic carbon–oxygen bond formations
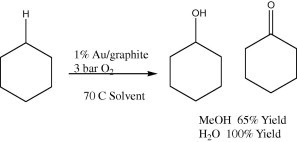
Methane was oxidized into methanol using the combination of metallic gold as a catalyst and selenic acid (H2SeO4) as an oxidant in 96% of sulfuric acid (H2SO4) as a solvent at 180 °C (Jones et al., 2004). Cyclohexane was oxidized at 150 °C using gold supported on ZSM-5 (Au/ZSM-5) as a catalyst with 1 MPa of oxygen as a source of oxidant (Scheme 11) Zhao et al., 2004. Cyclohexene was oxidized, catalyzed by NaAuCl4 using O2 as an oxidant, into cyclohexen-3-ol as the main product together with smaller amounts of cyclohexene-3-one and the epoxide (Shul’pin et al., 2001).
Scheme 11.
4.1.3. Alcohols selective oxidation
The gold-based catalysts have demonstrated very interesting and promising activity, and different types of gold-based homogeneous and heterogeneous catalysts in the form of metal complex or nanoparticles have been developed for the oxidation of alcohols into corresponding aldehydes or ketones (Hu et al., 2007).
4.1.4. Gold-catalyzed C–N formation
Scheme 12 shows the use of arylgold(III) to C–N bond formations via the activation of aromatic or benzylic C–H bonds using 2 mol% of AuCl3 in CH2Cl2 at room temperature (Li et al., 2007, Kharasch and Isbell, 1931, de Graaf et al., 1976).
Scheme 12.
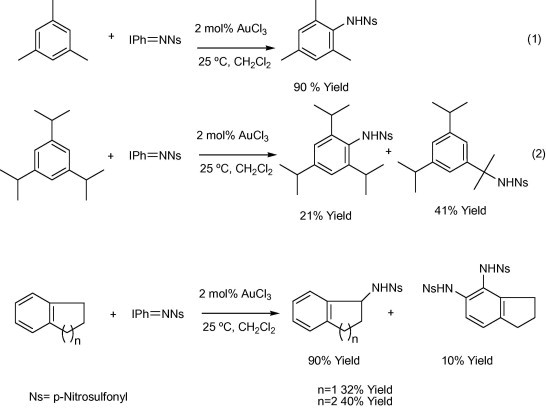
4.2. Biomedical applications
4.2.1. Biosensing and diagnostic
4.2.1.1. Immunohistochemistry
Gold nanoparticles are useful in the construction of electrochemical immunosensors where it plays a crucial role both in the enhancement of the electrochemical signal transducing the binding reaction of antigens at antibody immobilized surfaces and in the ability of increasing the amount of immobilized immunoreagents in a stable mode. Hepatitis B virus surface antigen was detected using electrochemical impedance spectroscopy (EIS) through immobilization of the antibody onto gold nanoparticles-modified 4-aminothiophenol self-assembled monolayers (Wang et al., 2004). Potentiometric and amperometric immunosensors for Hepatitis B virus surface antigen detection were also constructed by electrostatic adsorption of the antibody onto gold nanoparticles/tris(2,2-bipyridyl)cobalt (III) multilayer films (Tang et al., 2005) or by immobilization of the antibody onto gold nanoparticles-modified thiol-containing sol–gel network (Tang et al., 2006). The antigen–antibody reaction is detected through measuring the changes in the electric potential before and after. Furthermore, a different strategy was used to fabricate an amperometric immunosensor based on immobilization of hepatitis B antibody on a gold electrode modified with gold nanoparticles and horseradish peroxidase (Zhuo et al., 2005) in order to avoid non-specific adsorption and also amplify the response of the antigen–antibody reaction. Also, a gold nanoparticles-based potentiometric immunosensor was developed for detecting diphtheria antigen and diphtherotoxin (Dianping et al., 2005). An electrode fabricated from gold nanoparticles was used for the determination of aflatoxin B1 based on immunoreactions (Liu et al., 2006). Amperometric immunosensor made of modified nanogold monolayer was used for Schistosoma japonicum (Sj) antigen Lei et al., 2003, Xia et al., 2005.
4.2.1.2. Detection of disease biomarker
Gold nanoparticles have been used in the assembly of electrochemical and amperometric biosensors for the diagnosis of patients with germ cell tumors and hepatocellular carcinoma. This is done by the detection of a tumor marker, alpha-fetoprotein (AFP), an oncofetal glycoprotein (Ying et al., 2005). Carbohydrate antigen 19-9 (CA19-9) is one of the most important carbohydrate tumor markers expressed in many malignancies as pancreatic, colorectal, gastric and hepatic carcinomas (Reetz et al., 2003). Another carbohydrate antigen CA125 is also an important marker determined by nanogold immunosensors (Xiao-Hong, 2007). Carcinoembryonic antigen (CEA) is a well-known marker associated with the progression of colorectal tumors. PSA is prostate-specific antigen in prostate cancer (Liu, 2008). Electrochemical detection of these disease markers was accomplished by the amperometric changes occurring before and after the antigen–antibody interaction Xiao-Hong, 2007).
4.2.1.3. DNA-modified nanogold electrochemical biosensors
Gold nanoparticles-modified electrodes are used in the assembly of electrochemical DNA biosensors. They constitute useful analytical tools for sequence-specific DNA diagnosis and detection due to their inherent advantages of low cost, sensitivity and rapidity of response (Odenthal and Gooding, 2007). Pathogens, bacteria and viruses, can be detected via their unique, respective nucleic acid sequences. An exemplary target mixture containing Escherichia coli and Stachybotrys chartarum, an airborne pathogen, was then introduced. These DNA biosensors use the unique binding event between E. coli single-stranded DNA-binding protein (SSB) and single-stranded oligonucleotides conjugated to gold (Au) nanoparticles. The amplified oxidation signal of Au nanoparticles provided a detection limit of 2.17 pM target DNA (Kerman et al., 2004).
An important associated aspect is the accurate, sensitive, and rapid detection of the transgenic plants. DNA electrochemical sensors are most likely to become an analytical tool for the transgenic plant products. Nanogold-modified electrodes represent DNA electrochemical biosensor and are described as accurate, rapid and sensitive for the electrochemical impedance spectroscopy detection of the sequence-specific DNA related to transgene in the transgenic plants. These DNA-modified gold electrodes are useful electrochemical genosensors for gene analysis, detection of genetic disorders, tissue matching, and forensic applications due to their high sensitivity, small dimensions, low cost and compatibility.
4.2.2. Therapeutic application of gold nanoparticles
Gold nanoparticles exploit their unique chemical and physical properties for transporting and unloading the pharmaceuticals. First, the gold core is essentially inert and is their ease of synthesis; monodisperse nanoparticles can be formed with core sizes ranging from 1 to 150 nm (Connor et al., 2005). Second advantage is imparted by their ready functionalization, generally through thiol linkages. In addition, their photophysical properties could trigger drug release at remote place (Skirtach et al., 2006). Fig. 6 shows various drug, gene, and protein delivery using GNPs.
Figure 6.
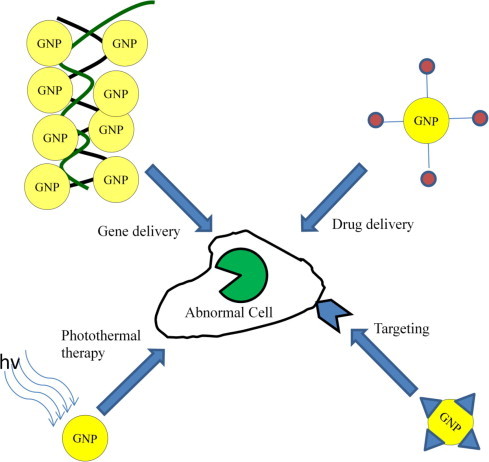
Various applications of gold nanoparticles in therapy.
4.2.2.1. Gold nanoparticles in drug delivery
Drug delivery systems (DDSs) provide positive attributes to a ‘free’ drug by improving solubility, in vivo stability, and biodistribution. They can also alter unfavorable pharmacokinetics of some ‘free’ drugs. Moreover, huge loading of pharmaceuticals on DDSs can render ‘drug reservoirs’ for controlled and sustained release to maintain the drug.
Hong et al. (2006) have demonstrated cellular delivery and glutathione-mediated, GSH-mediated release of a hydrophobic dye (BODIPY), as a model of hydrophobic drugs, using functionalized gold nanoparticles (fGNPs). The particles (core d = ∼2 nm) featured a mixed monolayer composed of tetra(ethyleneglycol)ylated cationic ligands (TTMA) and fluorogenic ligands (HSBDP). The cationic nature facilitates the crossing of cell-membrane barrier, and the fluorophore probes possible drug release mechanism. Controlled release of the dye was verified by treating mouse embryonic fibroblast cells with varying concentration of glutathione monoester. A recent study has reported on the therapeutic ability of a novel cyclodextrin-covered gold nanoparticle (AuNP) carrier for noncovalent encapsulation of an anti-cancer drug (Xiaohua et al., 2006). The surface of the AuNPs was functionalized with cyclodextrin as a drug pocket, anti-epidermal growth factor receptor (anti-EGFR) antibody as a targeting moiety, and poly(ethyleneglycol) (PEG) as an anti-fouling shell. β-Lapachone, an anti-cancer drug, was efficiently encapsulated into the hydrophobic cavity of cyclodextrin on the surface of the AuNP carriers (AuNP-1). The glutathione-mediated release of β-lapachone from the surface of AuNP-1 was demonstrated by an experiment with MCF-7 (low glutathione concentration) and A549 cells (high glutathione concentration) (Xiaohua et al., 2006).
GNPs could possibly be employed in the delivery of diatomic therapeutic agents, like singlet oxygen, or nitric oxide. Singlet oxygen (1O2), a cytotoxic species, is involved in the photodynamic therapy of cancer (Xiaohua et al., 2008). Russell and coworkers decorated the surface of GNPs with phthalocyanines (PCs), a photosensitizer to generate singlet oxygen with good quantum yield (Hone et al., 2002). Nitric oxide (NO) regulates multiple cellular processes including angiogenesis, vasodilatation, and the immune response (Mocellin et al., 2007). Controlled release of NO could be an effective therapy for hypoxic respiratory failure associated with pulmonary hypertension. Schoenfisch et al. demonstrated that NO can be efficiently stored by covalent linking with polyamine-stabilized GNP via formation of acid-labile N-diazeniumdiolate (Polizzi et al., 2007). They were able to show effective release of NO from the water-soluble nanocontainers in acidic condition (pH = 3). pH responsive materials are applicable for drug delivery due to the presence of mild acidic environment inside inflammatory and tumor tissues (pH ∼ 6.8), or cellular vesicles such as endosomes (pH ∼ 5.5–6) and lysosomes (pH ∼ 4.5–5.0) Engin et al., 1995, Yang et al., 2005.
4.2.2.2. Gold nanoparticles in biomolecules delivery
In addition to the delivery of small molecules, tunable size and functionality of gold nanoparticles make them a useful scaffold for efficient recognition and delivery of biomolecules such as peptides, proteins, or nucleic acids like DNA or RNA.
Efficient and safe nonviral gene delivery systems are a prerequisite for the clinical application of therapeutic genes. In some studies, it was reported that an enhancement of the transfection efficiency of plasmid DNA happened via the use of positively charged colloidal gold nanoparticles (GNPs). The results of this study suggested that the GNP/DNA complexes may harbor the potential for development into efficient and safe gene delivery vehicles (Noh et al., 2007). Gold nanoparticles chemically modified with primary amine groups were developed as intracellular delivery vehicles for therapeutic small interfering RNA (siRNA).
The electrostatic binding of siRNA and gold nanorods has been recently reported by the Prasad group (Bonoiu et al., 2009). They conjugated cetyltrimethylammonium bromide (CTAB) gold nanorods to siRNA (against DARPP-32 gene in dopaminergic neuronal (DAN) cells) and studied the uptake of conjugates inside the DAN cells. They found that the siRNA was efficiently delivered into DAN cells after treatment with the gold nanorod–siRNA conjugates and cell viability was 98%. Moreover, there was still about 67% knockdown of DARPP-32 gene expression after 120 h for the cells that were incubated with the gold nanorod–siRNA conjugates, compared to only about 30% knockdown for cells treated with a commercial transfection agent as a control. This study also confirms that gold nanoparticles can be used as an innovative vehicle to deliver genes into neuron cells. According to the authors of that study, this might one day provide the basis for a nanotherapy to treat drug addiction in patients (Bonoiu et al., 2009).
Recent work has shown that the combination of phototherapy with conventional gene therapy offers a high possibility to improve the efficiency of gene delivery into cells (Mariko et al., 2005). For example, Niidome et al., (2006) have investigated the release of plasmid DNA from spherical gold nanoparticles after exposure to pulsed laser irradiation.
Interference of gene expression can also be performed using gold nanorods. Lee et al. have demonstrated the idea of a remote optical switch for localized and selective control of gene interference. Thiol-modified sense oligonucleotides were conjugated with gold nanorods. Thereafter, the antisense oligonucleotides were hybridized to the sense oligonucleotides. After laser irradiation, the double strands of the oligonucleotides were denatured and the antisense oligonucleotides were released from the complex structure. These antisense oligonucleotides can bind to the corresponding mRNA while the sense oligonucleotides were still attached to gold nanorods through thiol bonds. Once the mRNA/oligodeoxynucleotide heteroduplex is formed, its structure will be recognized and degraded by RNaseH enzymes inside the cell, thereby inhibiting the normal genetic function of the mRNA (Lee et al., 2009).
Electroporation is another external stimulus that can be used to release genes from a gold nanoparticle. Kawano et al. (2006) have investigated gene delivery in vivo using gold nanoparticles excited with electrical pulses. In this study, gold nanoparticles were modified with mPEG-SH5000 and conjugated with plasmid DNA. These were then injected into anesthetized mice. After a suitable delay to allow the conjugates to spread in the mouse, electrical pulses were then applied to the left lobe of its liver. The result was that gene expression was detected in the major mouse organs. In contrast, the injection of naked DNA resulted in a 10-fold lower level of detection. The degradation of DNA in blood, which occurs in times as short as 5 min, is evidently the reason for the inefficient transfection in the latter case. This study illustrates yet another interesting approach to improve gene delivery using gold nanoparticles.
4.2.2.3. Gold nanoparticles for protein delivery
Gold nanoparticles can be novel nanocarriers of peptides and proteins of interest. Cationic tetraalkylammonium-functionalized GNPs recognize the surface of an anionic protein through complementary electrostatic interaction and inhibit its activity (Verma et al., 2004). The activity was recovered due to the release of free protein by treating the protein–particle complex with glutathione, showing GNPs as potential protein transporters. Pokharkar and coworkers have demonstrated functionalized gold nanoparticles as the carriers of insulin (Bhumkar and Joshi, 2007). Chitosan-coated particles strongly adsorb insulin on their surface, and are effective for transmucosal delivery of insulin.
4.2.2.4. Gold nanoparticles for site-directed photothermal applications
Gold nanoparticles cause local heating when they are irradiated with light (800–1200 nm). El-Sayed et al. have recently reported about the potential use of GNPs in photothermal destruction of tumors (Huang et al., 2007). Citrate-stabilized GNPs (core d = 30 nm) were coated with anti-EGFR (epidermal growth factor receptor) to target HSC3 cancer cells (human oral squamous cell carcinoma). The use of GNPs enhanced the efficacy of photothermal therapy by 20 times. In another approach, optically responsive delivery systems have been designed by incorporation of gold nanospheres into the shells of capsules. Caruso et al. prepared microcapsules via layer-by-layer (LBL) technique to encapsulate macromolecules like fluorescein-labeled dextrans (Angelatos et al., 2005). The capsule-shells were doped with GNPs, which respond against near infrared (NIR) light. FITC-dextran was released upon laser (1064 nm) treatment due to the rupture of the shell. Skirtach and coworkers have extended this strategy to deliver encapsulated materials from polyelectrolyte-multilayer capsules inside living cancer cells (Skirtach et al., 2006).
4.2.2.5. Gold nanocarriers applied in vivo
Patra et al. (2008) have developed a nanoparticle-based targeted drug delivery system (DDS), which contains cetuximab (C225) anti-epidermal growth factor receptor (EGFR) antibody as the targeting agent, gemcitabine as the anti-cancer drug, and gold nanoparticles as the delivery vehicle. The administration of this targeted delivery system resulted in the significant inhibition of pancreatic tumor cell proliferation in vitro and orthotropic pancreatic tumor growth in vivo. In the near future, this strategy could be used as a generalized approach for the treatment of a variety of cancers including pancreatic cancer.
Wu and coworkers have demonstrated that MTX–GNP conjugate inhibits tumor growth in a mouse ascites model of Lewis lung carcinoma (LL2) Chen et al., 2007. Paciotti et al. carried out an in vivo study to investigate the therapeutic effect of PEGylated gold colloids (cAu-PEG-TNF) with adsorbed protein (tumor necrosis factor, TNF) (Paciotti et al., 2004). When cAu-PEG-TNF was injected intravenously into mice, they largely accumulated in MC-38 colon carcinoma tumors compared to liver, spleen, or other healthy organs. TNF provided both targeting and therapeutic action via killing the targeted cells. Importantly, the particles were more effective in diminishing tumor mass than ‘free’ TNF. Recent studies by this group have demonstrated enhanced tumor therapy by grafting paclitaxel, an anti-cancer drug, onto cAu-PEG-TNF (Paciotti et al., 2006).
References
- Ahmad A., Senapati S., Khan M.I., Kumar R., Ramani R., Srinivas V., Sastry M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology. 2003;14:824–828. [Google Scholar]
- Ahmad A., Mukherjee P., Senapati S., Mandal D., Khan M.I., Kumar R., Sastry M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. 2003;B28:313–318. [Google Scholar]
- Angelatos A.S., Radt B., Caruso F. Light-responsive polyelectrolyte/gold nanoparticle microcapsules. J. Phys. Chem. B. 2005;109:3071–3076. doi: 10.1021/jp045070x. [DOI] [PubMed] [Google Scholar]
- Anshup A., Venkataraman J.S., Chandramouli S., Rajeev K.R., Suma P., Santhosh K.T.R., Omkumar R.V., Annie J., Pradeep T. Growth of gold nanoparticles in human cells. Langmuir. 2005;21:11562–11567. doi: 10.1021/la0519249. [DOI] [PubMed] [Google Scholar]
- Arcadi A., Di Giuseppe S. Recent applications of gold catalysis in organic synthesis. Curr. Org. Chem. 2004;8:795–812. [Google Scholar]
- Arcadi A., Bianchi G., Chiarini M., D’Anniballe G., Marinelli F. Gold-catalyzed conjugate addition type reaction of indoles with α,β-enones. Synlett. 2004:944–950. [Google Scholar]
- Balme G., Bossharth E., Monteiro N. Pd-assisted multicomponent synthesis of heterocycles. Eur. J. Org. Chem. 2003:4101–4102. [Google Scholar]
- Bhumkar D.R., Joshi H.M., Sastry M., Pokharkar V.B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm. Res. 2007;24:1415–1426. doi: 10.1007/s11095-007-9257-9. [DOI] [PubMed] [Google Scholar]
- Bonoiu A.C., Mahajan S.D., Ding H., Roy I., Yong K.T., Kumar R., Hu R., Bergey E.J., Schwartz S.A., Prasad P.N. Nanotechnology approach for drug addiction therapy: gene silencing using delivery of gold nanorod–siRNA nanoplex in dopaminergic neurons. Proc. Natl. Acad. Sci. USA. 2009;106:5546–5550. doi: 10.1073/pnas.0901715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K.R., Natan M.J. Hydroxylamine seeding of colloidal Au nanoparticles in solution and on surfaces. Langmuir. 1998;14:726–728. [Google Scholar]
- Chen W., Cai W.P., Liang C.H., Zhang L.D. Synthesis of gold nanoparticles dispersed within pores of mesoporous silica induced by ultrasonic irradiation and its characterization. Mater. Res. Bull. 2001;36:335–342. [Google Scholar]
- Chen Y.H., Tsai C.Y., Huang P.Y., Chang M.Y., Cheng P.C., Chou C.H., Chen D.H., Wang C.R., Shiau A.L., Wu C.L. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol. Pharm. 2007;4:713–722. doi: 10.1021/mp060132k. [DOI] [PubMed] [Google Scholar]
- Chiang C.L. Controlled growth of gold nanoparticles in aerosol-OT/sorbitan monooleate/isooctane mixed reverse micelles. Colloid Interface Sci. 2000;230:60–66. doi: 10.1006/jcis.2000.7040. [DOI] [PubMed] [Google Scholar]
- Connor E.E., Mwamuka J., Gole A., Murphy C.J., Wyatt M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- Dawson A., Kamat P.V. Complexation of gold nanoparticles with radiolytically generated thiocyanate radicals J. Phys. Chem. B. 2000;104:11842–11846. [Google Scholar]
- de Graaf P.W.J., Boersma J., van der Kerk G.J.M. Preparation and properties of arylgold compounds. Scope and limitations of the auration reaction. J. Organomet. Chem. 1976;105:399–406. [Google Scholar]
- Dianping T., Ruo Y., Yaqin C., Linyan Z., Xia Z., Yan L., Jianyuan D. Preparation and application on a kind of immobilization method of anti-diphtheria for potentiometric immunosensor modified colloidal Au and polyvinyl butyral as matrixes. Sens. Actuators B. 2005;104:199–206. [Google Scholar]
- Engin K., Leeper D.B., Cater J.R., Thistlethwaite A.J., Tupchong L., Mcfarlane J.D. Extracellular pH distribution in human tumors. Int. J. Hypertherm. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- Esumi K., Hosoya T., Suzuki A. Preparation of hydrophobically modified poly(amidoamine) dendrimer-encapsulated gold nanoparticles. J. Colloid Interface Sci. 2000;229:303–306. doi: 10.1006/jcis.2000.6970. [DOI] [PubMed] [Google Scholar]
- Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973;241:20–22. [Google Scholar]
- Fukuda Y., Utimoto K. Effective transformation of unactivated alkynes into ketones or acetals with a gold(III) catalyst. J. Org. Chem. 1991;56:3729–3731. [Google Scholar]
- Georgy M., Boucard V., Campagne J.-M.J. Gold(III)-catalyzed nucleophilic substitution of propargylic alcohols. J. Am. Chem. Soc. 2005;127:14180–14181. doi: 10.1021/ja0534147. [DOI] [PubMed] [Google Scholar]
- Gustav M., Mie G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. Vierte Folge. 1908;25:377. [Google Scholar]
- Haifeng Z., Cheng T., Suping Z., Junbai L. One step synthesis and phase transition of phospholipid-modified Au particles into toluene. Colloids Surf. A: Physicochem. Eng. Aspects. 2005;257–258:411–414. [Google Scholar]
- Hashmi A.S.K., Schwarz L., Choi J.H., Frost T.M. A new gold-catalyzed C–C bond formation. Angew. Chem. Int. Ed. 2000;39:2285–2288. doi: 10.1002/1521-3773(20000703)39:13<2285::aid-anie2285>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Sawamura M., Ito Y. Asymmetric-synthesis catalyzed by chiral ferrocenylphosphine-transition metal-complexes; gold(I)-catalyzed asymmetric aldol reaction of isocyanoacetate. Tetrahedron. 1992;48:1999–2012. [Google Scholar]
- Henglein A., Meisel D. Radiolytic control of the size of colloidal gold nanoparticles. Langmuir. 1998;14:7392–7396. [Google Scholar]
- Hone D.C., Walker P.I., Evans-Gowing R., FitzGerald S., Beeby A., Chambrier I., Cook M.J., Russell D.A. Generation of cytotoxic singlet oxygen via phthalocyanine stabilized gold nanoparticles: a potential delivery vehicle for photodynamic therapy. Langmuir. 2002;18:2985–2987. [Google Scholar]
- Hong R., Han G., Fernandez J.M., Kim B.J., Forbes N.S., Rotello V.M. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc. 2006;128:1078–1079. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- Hu J., Chen L., Zhu K., Suchopar A., Richards R. Aerobic oxidation of alcohols catalyzed by gold nano-particles confined in the walls of mesoporous silica. Catal. Today. 2007;122:277–283. [Google Scholar]
- Huang H., Yang X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohydr. Res. 2004;339:2627–2631. doi: 10.1016/j.carres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Huang X., Qian W., El-Sayed I.H., El-Sayed M.A. The potential use of the enhanced nonlinear properties of gold nanospheres in photothermal cancer therapy. Laser Surg. Med. 2007;39:747–753. doi: 10.1002/lsm.20577. [DOI] [PubMed] [Google Scholar]
- Hutchings G.J. New direction in gold catalysis. Gold Bull. 2004;37:3–11. [Google Scholar]
- Itahara T., Kawasaki K., Ouseto F. Alkenylation of 1-benzenesulfonylindole with olefins bearing electron-withdrawing substituents. Synthesis. 1984:236–237. [Google Scholar]
- Ivan H.E., Xiaohua H., Mostafa A.E. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5:829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- Jana N.R., Gearheart L., Murphy C.J. Evidence for seed-mediated nucleation in the formation of gold nanoparticles from gold salts. Chem. Mater. 2001;13:2313–2322. [Google Scholar]
- Jones C.J., Taube D., Ziatdinov V.R., Periana R.A., Nielsen R.J., Oxgaard J., Goddard W.A. Selective oxidation of methane to methanol catalyzed, with C–H activation, by homogeneous, cationic gold. Angew. Chem. Int. Ed. 2004;43:4626–4629. doi: 10.1002/anie.200461055. [DOI] [PubMed] [Google Scholar]
- Kawano T., Yamagata M., Takahashi H., Niidome Y., Yamada S., Katayama Y., Niidome T. Stabilizing of plasmid DNA in vivo by PEG-modified cationic gold nanoparticles and the gene expression assisted with electrical pulses. J. Controlled Release. 2006;111:382–389. doi: 10.1016/j.jconrel.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Kerman K., Morita Y., Takamura Y., Ozsoz M., Tamiya E. Modification of Escherichia coli single-stranded DNA binding protein with gold nanoparticles for electrochemical detection of DNA hybridization. Anal. Chim. Acta. 2004;510:169–174. [Google Scholar]
- Kharasch M.S., Isbell H.S.J. J. Am. Chem. Soc. 1931;53:3053–3059. [Google Scholar]
- Ko S.H., Choi Y., Hwang D.J., Grigoropoulos C.P., Chung J., Poulikakos D. Nanosecond laser ablation of gold nanoparticle films. Appl. Phys. Lett. 2006;89:141126-1–141126-3. [Google Scholar]
- Lee K.M., Park S.T., Lee D.J. Nanogold synthesis by inert gas condensation for immuno-chemistry probes. J. Alloys Compd. 2005;390:297–300. [Google Scholar]
- Lee K.Y., Hwang J., Lee Y.W., Kim J., Han S.W. One-step synthesis of gold nanoparticles using azacryptand and their applications in SERS and catalysis. J. Colloid Interface Sci. 2007;316:476–481. doi: 10.1016/j.jcis.2007.07.076. [DOI] [PubMed] [Google Scholar]
- Lee S.E., Liu G.L., Kim F., Lee L.P. Remote optical switch for localized and selective control of gene interference. Nano Lett. 2009;9:562–570. doi: 10.1021/nl802689k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C.-X., Gong F.-C., Shen G.-L., Yu R.-Q. Amperometric immunosensor for Schistosoma japonicum antigen using antibodies loaded on a nano-Au monolayer modified chitosan-entrapped carbon paste electrode. Sens. Actuators B Chem. 2003;96:582–588. [Google Scholar]
- Li Z., Capretto D.A., Rahaman R.O., He C. Gold(III)-catalyzed nitrene insertion into aromatic and benzylic C–H groups. J Am Chem Soc. 2007;129:12058–12059. doi: 10.1021/ja0724137. [DOI] [PubMed] [Google Scholar]
- Liu Y. Electrochemical detection of prostate-specific antigen based on gold colloids/alumina derived sol–gel film. Thin Solid Films. 2008;516:1803–1808. [Google Scholar]
- Liu Y., Qin Z., Wu X., Jiang H. Immune-biosensor for aflatoxin B1 based bio-electrocatalytic reaction on microcomb-type electrode. Biochem. Eng. J. 2006;32:211–217. [Google Scholar]
- Luo Y. Size-controlled preparation of dendrimer-protected gold nanoparticles: a sunlight irradiation-based strategy. Mater. Lett. 2008;62:3770–3772. [Google Scholar]
- Luo Y., Li C.-J. A highly efficient gold/silver-catalyzed addition of arenes to imines. Chem. Commun. 2004:1930–1931. doi: 10.1039/b404509b. [DOI] [PubMed] [Google Scholar]
- Mallick K., Wang Z.L., Pal T. Seed-mediated successive growth of gold particles accomplished by UV irradiation: a photochemical approach for size-controlled synthesis. J. Photochem. Photobiol. A: Chem. 2001;140:75–80. [Google Scholar]
- Maria A., Antonio A., Gabriele B., Fabio M., Antonella N. Gold-catalyzed C-3-alkylation of 7-azaindoles through michael-type addition to α,β-enones. Eur. J. Org. Chem. 2006:2393–2402. [Google Scholar]
- Mariko U., Mariko H.-S., Kingo U., Yasuhide N. Photo-control of the polyplexes formation between DNA and photo-cation generatable water-soluble polymers. Curr. Drug Deliv. 2005;2:207–214. doi: 10.2174/1567201054367986. [DOI] [PubMed] [Google Scholar]
- Mazaahir K., Vikas B., Ajeet K., Subho M. The first Au-nanoparticles catalyzed green synthesis of propargylamines via a three-component coupling reaction of aldehyde, alkyne and amine. Green Chem. 2007;9:742–745. [Google Scholar]
- Mocellin S., Bronte V., Nitti D. Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med. Res. Rev. 2007;27:317–352. doi: 10.1002/med.20092. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Ahmad A., Mandal D., Senapati S. Fungus mediated synthesis of silver nanoparticles and their immobilization in the mycellial matrix: a novel biological approach to nano particle synthesis. Nano Lett. 2001;1:515–519. [Google Scholar]
- Nair B., Pradeep T. Coalescence of nanoclusters and formation of submicron crystallites assisted by lactobacillus strains. Cryst. Growth Des. 2002;2:293–298. [Google Scholar]
- Niidome Y., Niidome T., Yamada S., Horiguchi Y., Takahashi H., Nakashima K. Pulsed-laser induced fragmentation and dissociation of DNA immobilized on gold nanoparticles. Mol. Cryst. Liq. Cryst. 2006;445:201/[491]–206/[496]. 491. [Google Scholar]
- Noh S.M., Kim W.-K., Kim S.J., Kim J.M., Baek K.-H., Oh Y.-K. Enhanced cellular delivery and transfection efficiency of plasmid DNA using positively charged biocompatible colloidal gold nanoparticles. Biochim. Biophys. Acta. 2007;1770:747–752. doi: 10.1016/j.bbagen.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Note C., Kosmella S., Koetz J. Poly(ethyleneimine) as reducing and stabilizing agent for the formation of gold nanoparticles in w/o microemulsions. Colloids Surf. A. 2006;290:150–156. [Google Scholar]
- Odenthal K.J., Gooding J.J. Electrochemical DNA biosensors. Analyst. 2007;132:603–610. doi: 10.1039/b701816a. [DOI] [PubMed] [Google Scholar]
- Paciotti G.F., Myer L., Weinreich D., Goia D., Pavel N., McLaughlin R.E., Tamarkin L. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11:169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- Paciotti G.F., Kingston D.G.I., Tamarkin L. Colloidal gold nanoparticles: a novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev. Res. 2006;67:47–54. [Google Scholar]
- Pal A., Shah S., Devi S. Preparation of silver, gold and silver–gold bimetallic nanoparticles in w/o microemulsion containing Triton X-100. Colloids Surf. A: Physicochem. Eng. Aspects. 2007;302:483–487. [Google Scholar]
- Patra C.R., Bhattacharya R., Wang E., Katarya A., Lau J.S., Dutta S., Muders M., Wang S., Buhrow S.A., Safgren S.L., Yaszemski M.J., Reid J.M., Ames M.M., Mukgerjee P., Mukhopadhyay D. Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Res. 2008;68:1970–1978. doi: 10.1158/0008-5472.CAN-07-6102. [DOI] [PubMed] [Google Scholar]
- Philip D. Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Chimica. Acta Part A. 2009;73:374–381. doi: 10.1016/j.saa.2009.02.037. [DOI] [PubMed] [Google Scholar]
- Pol V.G., Gedanken A., Calderro-Moreno J. Deposition of gold nanoparticles on silica spheres: a sonochemical approach. Chem. Mater. 2003;15:1111–1118. [Google Scholar]
- Polizzi M.A., Stasko N.A., Schoenfisch M.H. Water-soluble nitric oxide-releasing gold nanoparticles. Langmuir. 2007;23:4938–4943. doi: 10.1021/la0633841. [DOI] [PubMed] [Google Scholar]
- Reed J.A., Cook A., Halaas D.J., Parazolli P., Robinson A., Matula T.J., Griezer F. The effects of microgravity on nanoparticle size distributions generated by the ultrasonic reduction of an aqueous gold–chloride solution. Ultrason. Sonochem. 2003;10:285–289. doi: 10.1016/S1350-4177(03)00093-2. [DOI] [PubMed] [Google Scholar]
- Reetz M.T., Sommer K. Gold-catalyzed hydroarylation of alkynes. Eur. J. Org. Chem. 2003:3485–3496. [Google Scholar]
- Sau T.K., Pal A., Jana N.R., Wang Z.L., Pal T. Size controlled synthesis of gold nanoparticles using photochemically prepared seed particles. J. Nanopart. Res. 2001;3:257–261. [Google Scholar]
- Sawamura M., Nakayama Y., Kato T., Ito Y. Gold(I)-catalyzed asymmetric aldol reaction of an α-isocyano weinreb amide. An efficient synthesis of optically active ß-hydroxyl α-amino aldehydes and ketones. J. Org. Chem. 1995;60:1727–1732. [Google Scholar]
- Selvakannan P.R., Mandal S., Phadtare S., Gole A., Pasricha R., Adyanthaya S.D., Sastry M. Water-dispersible tryptophan-protected gold nanoparticles prepared by the spontaneous reduction of aqueous chloroaurate ions by the amino acid. J. Colloid Interface Sci. 2004;269:97–102. doi: 10.1016/s0021-9797(03)00616-7. [DOI] [PubMed] [Google Scholar]
- Shankar S.S., Ahmad A., Pasricha R., Sastry M. Bioreduction of chloroautate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003;13:1822–1826. [Google Scholar]
- Shi Z., He C. An Au-catalyzed cyclialkylation of electron-rich arenes with epoxides to prepare 3-chromanols. J. Am. Chem. Soc. 2004;126:5964–5965. doi: 10.1021/ja031953a. [DOI] [PubMed] [Google Scholar]
- Shi Z., He C. Direct functionalization of arenes by primary alcohol sulfonate esters catalyzed by gold(III) J. Am. Chem. Soc. 2004;126:13596–13597. doi: 10.1021/ja046890q. [DOI] [PubMed] [Google Scholar]
- Shih C.-M., Shieh Y.-T., Twu Y.-K. Preparation of gold nanopowders and nanoparticles using chitosan suspensions. Carbohydr. Polym. 2009;78:309–315. [Google Scholar]
- Shul’pin G.B., Suess-Fink G., Shilov A.E. Alkane oxygenation catalysed by gold complexes. Tetrahedron Lett. 2001;42:7253–7256. [Google Scholar]
- Skirtach A.G., Javier A.M., Kreft O., Kohler K., Alberola A.P., Mohwald H., Parak W.J., Sukhorukov G.B. Laser-induced release of encapsulated materials inside living cells. Angew. Chem. Int. Ed. 2006;45:4612–4617. doi: 10.1002/anie.200504599. [DOI] [PubMed] [Google Scholar]
- Sönnichsen C., Franzl T., Wilk T., Plessen G.V., Feldmann J., Wilson O., Mulvaney P. Drastic reduction of plasmon damping in gold nanorods. Phys. Rev. Lett. 2002;88:077402/1. doi: 10.1103/PhysRevLett.88.077402. [DOI] [PubMed] [Google Scholar]
- Stephan L., Mostafa A.E. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu. Rev. Phys. Chem. 2003;54:331–366. doi: 10.1146/annurev.physchem.54.011002.103759. [DOI] [PubMed] [Google Scholar]
- Stephen L., Mostafa A.E. Oscillations in gold and silver nano-dots and nano-rods. J. Phys. Chem. B. 1999;103:8410–8426. [Google Scholar]
- Stephen L., Mostafa A.E. Shape and size dependence of radiative, nonradiative, and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. 2000;19:409–433. [Google Scholar]
- Stephen L., Mostafa A.E. In: Semiconductor and Metal Nanocrystals: Synthesis and Electronic and Optical Properties. Klimov V.I., editor. Marcel Dekker; New York: 2004. Optical spectroscopy of surface plasmons in metal nanoparticles; pp. 239–288. [Google Scholar]
- Sun K., Qiu J., Liu J., Miao Y. Preparation and characterization of gold nanoparticles using ascorbic acid as reducing agent in reverse micelles. J. Mater. Sci. 2009;44:754–758. [Google Scholar]
- Tang D., Yuan T.R., Chai Y., Fu Y., Dai J., Liu Y., Zhong X. New amperometric and potentiometric immunosensors based on gold nanoparticles/tris(2,2′-bipyridyl)cobalt(III) multilayer films for hepatitis B surface antigen determinations. Biosens. Bioelectron. 2005;21:539–548. doi: 10.1016/j.bios.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Tang D., Yuan R., Chai Y., Zhong X., Liu Y., Dai J. Electrochemical detection of hepatitis B surface antigen using colloidal gold nanoparticles modified by a sol–gel network interface. Clin. Biochem. 2006;39:309–314. doi: 10.1016/j.clinbiochem.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Teles J.H., Brode S., Chabanas M. Cataionic Gold(I) complexes: highly efficient catalysts for the addition of alcohols to alkynes. Angew. Chem. Int. Ed. 1998;37:1415–1418. doi: 10.1002/(SICI)1521-3773(19980605)37:10<1415::AID-ANIE1415>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Thompson D. New advances in gold catalysis part II. Gold Bull. 1999;32:12–19. [Google Scholar]
- Turkevitch J., Stevenson P.C., Hillier J. Nucleation and growth process in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951;11:55–75. [Google Scholar]
- Vaškelis A., Tarozaitė R., Jagminienė A., Tamašiūnaitė L.T., Juškėnas R., Kurtinaitienė M. Gold nanoparticles obtained by Au(III) reduction with Sn(II): preparation and electrocatalytic properties in oxidation of reducing agents. Electrochim. Acta. 2007;53:407–416. [Google Scholar]
- Verma A., Simard J.M., Worrall J.W.E., Rotello V.M. Tunable reactivation of nanoparticle-inhibited beta-galactosidase by glutathione at intracellular concentrations. J. Am. Chem. Soc. 2004;126:13987–13991. doi: 10.1021/ja046572r. [DOI] [PubMed] [Google Scholar]
- Wagner J., Tshikhudo T.R., Köhler J.M. Microfluidic generation of metal nanoparticles by borohydride reduction. Chem. Eng. J. 2008;135:S104–S109. [Google Scholar]
- Wang W., Breining T., Li T., Milbum R., Attardo G. Dehydrogenation by air: preparation of 1, 3-disubstituted-5,1-dioxo-5,10-dihydro-1H-benzo[g] isochromene scaffold. Tetrahedron Lett. 1998;39:2459–2462. [Google Scholar]
- Wang M., Wang L., Wang G., Ji X., Bai Y., Li T., Gong S., Li J. Application of impedance spectroscopy for monitoring colloid Au-enhanced antibody immobilization and antibody–antigen reactions. Biosens. Bioelectron. 2004;19:575. doi: 10.1016/s0956-5663(03)00252-5. [DOI] [PubMed] [Google Scholar]
- Wang W., Chena O., Jiang C., Yang D., Liu X., Xua S. One-step synthesis of biocompatible gold nanoparticles using gallic acid in the presence of poly-(N-vinyl-2-pyrrolidone) Colloids Surf. A: Physicochem. Eng. Aspects. 2007;301:73–79. [Google Scholar]
- Wei C., Li C.-J. A highly efficient three-component coupling of aldehyde, alkyne, and amines via C–H activation catalyzed by gold in water. J. Am. Chem. Soc. 2003;125:9584–9585. doi: 10.1021/ja0359299. [DOI] [PubMed] [Google Scholar]
- Wei C., Li C.-J. Gold-catalyzed coupling of alkynes and acyl iminiums. Lett. Org. Chem. 2005;2:410–414. [Google Scholar]
- Xia C., Zhi-Feng X., Xin F., Shi-Ping W., Guo-Li S., Ru-Qin Y. Silver-enhanced colloidal gold metalloimmunoassay for Schistosoma japonicum antibody detection. J. Immunol. Methods. 2005;301:77–88. doi: 10.1016/j.jim.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Xiao-Hong F. Electrochemical immunoassay for carbohydrate antigen-125 based on polythionine and gold hollow microspheres modified glassy carbon electrodes. Electroanalysis. 2007:1831–1839. [Google Scholar]
- Xiaohua H., Prashant K.J., Ivan H.El.-S., Mostafa A.El.-S. Determination of the minimum temperature required for selective photothermal destruction of cancer cells with the use of immunotargeted gold nanoparticles. Photochem. Photobiol. 2006;82:412–417. doi: 10.1562/2005-12-14-RA-754. [DOI] [PubMed] [Google Scholar]
- Xiaohua H., Prashant K.J., Ivan H.El.-S., Mostafa A.El.-S. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008;23:217–228. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Nakamoto M. New type of monodispersed gold nanoparticles capped by myristate and PPh3 ligands prepared by controlled thermolysis of [Au(C13H27COO)(PPh3)] Chem. Lett. 2003;32:452–453. [Google Scholar]
- Yan B., Liu Y. Gold-catalyzed multicomponent synthesis of aminoindolizines from aldehydes, amines, and alkynes under solvent-free conditions or in water. Org. Lett. 2007;9:4323–4326. doi: 10.1021/ol701886e. [DOI] [PubMed] [Google Scholar]
- Yang Q., Wang S.H., Fan P.W., Wang L.F., Di Y., Lin K.F., Xiao F.S. pH-responsive carrier system based on carboxylic acid modified mesoporous silica and polyelectrolyte for drug delivery. Chem. Mater. 2005;17:5999–6003. [Google Scholar]
- Yao X., Li C.-J. Water-triggered and gold(I)-catalyzed cascade addition/cyclization of terminal alkynes with ortho-alkynylaryl aldehyde. Org. Lett. 2006;8:1953–1955. doi: 10.1021/ol060645p. [DOI] [PubMed] [Google Scholar]
- Yguerabide J., Yguerabide E.E. Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications. Anal. Biochem. 1998;262:137–156. doi: 10.1006/abio.1998.2759. [DOI] [PubMed] [Google Scholar]
- Ying Z., Ruo Y., Yaqin C., Dianping T., Ying Z., Na W., Xuelian L., Qiang Z. A reagentless amperometric immunosensor based on gold nanoparticles/thionine/nafion-membrane-modified gold electrode for determination of α-1-fetoprotein. Electrochem. Commun. 2005;7:355–360. [Google Scholar]
- Zhao R., Ji D., Lv G., Qian G., Yan L., Wang X., Suo J. A highly efficient oxidation of cyclohexane over Au/ZSM-5 molecular sieve catalyst with oxygen as oxidant. Chem. Commun. 2004;7:904–905. doi: 10.1039/b315098d. [DOI] [PubMed] [Google Scholar]
- Zhuo Y., Yuan R., Chai Y., Zhang Y., Wang N., Li X., Zhu Q., Wang N. An amperometric immunosensor based on immobilization of hepatitis B surface antibody on gold electrode modified gold nanoparticles and horseradish peroxidase. Anal. Chim. Acta. 2005;548:205–210. [Google Scholar]


