Abstract
Historical data in the 1950s suggests that 7%, 11%, 33%, and 87% of couples were infertile by ages 30, 35, 40 and 45, respectively. Up to 22.3% of infertile couples have unexplained infertility. Oxidative stress is associated with male and female infertility. However, there is insufficient evidence relating to the influence of oxidative stress on the maintenance of a viable pregnancy, including pregnancy complications and fetal development. Recently, we have established Tet-mev-1 conditional transgenic mice, which can express the doxycycline-induced mutant SDHCV69E transgene and experience mitochondrial respiratory chain dysfunction leading to intracellular oxidative stress. In this report, we demonstrate that this kind of abnormal mitochondrial respiratory chain-induced chronic oxidative stress affects fertility, pregnancy and delivery rates as well as causes recurrent abortions, occasionally resulting in maternal death. Despite this, spermatogenesis and early embryogenesis are completely normal, indicating the mutation's effects to be rather subtle. Female Tet-mev-1 mice exhibit thrombocytosis and splenomegaly in both non-pregnant and pregnant mice as well as placental angiodysplasia with reduced Flt-1 protein leading to hypoxic conditions, which could contribute to placental inflammation and fetal abnormal angiogenesis. Collectively these data strongly suggest that chronic oxidative stress caused by mitochondrial mutations provokes spontaneous abortions and recurrent miscarriage resulting in age-related female infertility.
Keywords: Mitochondria, SDHC, Infertility, Abortion, Oxidative stress
Abbreviations: ROS, reactive oxygen species; SDH, succinate dehydrogenase; SDHC, succinate dehydrogenase C subunit; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; NGF, non-growing follicles; VEGF, vascular endothelial growth factors; VEGFR-1, VEGF receptor-1; PLGF, placenta growth factor
Highlights
-
•
SDHC mutation induces oxidative stress in the female reproductive organs.
-
•
Early development is completely normal in Tet-mev-1 mice.
-
•
Non-pregnant and pregnant Tet-mev-1 mice exhibit thrombocytosis and splenomegaly.
-
•
Pregnant mice have placental angiodysplasia with decreased Flt-1/ VEGFR-1.
-
•
Habitual abortion is frequently caused with occasional maternal death in Tet-mev-1 mice.
Graphical abstract
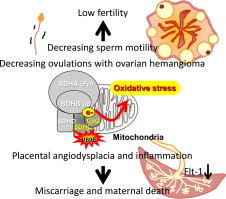
Introduction
Human historical data in the 1950s suggests that 7%, 11%, 33%, and 87% of couples were infertile by ages 30, 35, 40 and 45, respectively [1]. Under natural conditions, 75% of 30-year old women attempting to conceive will have a conception ending in a live birth within 1 year, 66% at age 35 years, and only 44% at age 40 [2]. Spontaneous abortion refers to the unintentional termination of a pregnancy before fetal viability at 20 weeks of gestation or when fetal weight is <500 g. The incidence of such abortions is 12–24% of all pregnancies [3]. The risk of a spontaneous abortion was 8.9% in women aged 20–24 years and 74.7% in those aged 45 years or older [4]. Unexplained infertility is defined as the inability to conceive after 12 months of unprotected intercourse in couples where known causes of infertility have been ruled out. Up to 22.3% of infertile couples have unexplained infertility [5].
Oxidative stress is associated with male and female infertility [6,7]. Evidence exists supporting the role of oxidative stress in male infertility, including decreased sperm motility, sperm number, and sperm–ovum fertilization [6,8]. However, there is insufficient evidence relating to the influence of oxidative stress for maintenance of a viable pregnancy, including pregnancy complications and fetal development. Oxidative stress occurs when the generation of reactive oxygen species (ROS) and other radical species exceeds the scavenging capacity by antioxidants due to excessive production of ROS and inadequate intake or increased utilization of antioxidants. Most ROS are formed as a consequence of the mitochondrial respiratory chain, but can also be formed by exogenous exposures such as smoke and environmental pollutants [6]. It has been reported that mitochondrial mutations leading to ROS production and excessive apoptosis in a mouse model results in male infertility, but not female infertility [9].
The mev-1 mutant of the nematode Caenorhabditis elegans was isolated based upon its hypersensitivity to the ROS-generating chemical methyl viologen [10]. In addition to its hypersensitivity to oxidative stress, short-lived mev-1 mutants age precociously under hyperoxia [11–13]. The mev-1(kn-1) mutation, which results in an amino acid substitution at the 71st position from glycine to glutamate (G71E), has been identified as residing in the putative gene cyt-1 (a human SDHC gene homologue), which is homologous to the succinate dehydrogenase (SDH) cytochrome b large subunit in complex II [14]. The biochemical pathologies of mev-1 include elevated ROS caused by the compromised complex II resulting in electron leakage from the electron transport system [15]. These mev-1 mutant hermaphrodites have low numbers of progeny and excessive apoptosis during fetal development [10,16].
A mev-1-mimic transgenic mouse was created that overexpressed the mutated SDHCV69E transgene [17]. The mutated SDHCV69E transgene results in the substitution of valine for glutamic acid at position 69 (V69E), which is equivalent to the kn-1 mutation of C. elegans mev-1 mutant [18,19]. This mouse had increased O2•- levels in the mitochondria of its various tissues as well as decreased body weight and locomotion. Unfortunately, this mev-1 transgenic mouse was infertile, which prevented propagation of the strain for further studies. Recently, we established mev-1-mimic (Tet-mev-1) conditional transgenic mice with the SDHCV69E transgene using our modified Tet-On/Off construct, so that the induced SDHCV69E was competitively expressed to the endogenous SDHC protein level [20]. Induction of the transgene with doxycycline led to increased mitochondrial oxidative stress that triggered excessive apoptosis, reductions in birth weight and significant delays in development in Tet-mev-1 mice, just as in the C. elegans mev-1 mutant [20].
In this report, we assessed the effects of the mitochondrial oxidative stress caused by the SDHC mutation on the male and female fertilities in mice. Specifically, we demonstrate that Tet-mev-1 mice exhibit the same oxidative stress-induced short-lived and low fertility phenotypes that characterize the nematode mutant. Thus, this mouse model can provide a powerful opportunity to study the effects of chronic oxidative stress on infertility and abortion with age as in humans.
Results
Function of testis and spermatogenesis
Oxidative stress is well known to affect germ-cell development and testes in male infertility [21,22]. We addressed this using Tet-mev-1, a mouse model characterized by chronic oxidative stress owing to the doxycycline-inducible expression of a defective complex II protein (SDHCV69E) involved in electron transport. SDHC protein levels were first measured in testes and female reproductive organs (ovaries and uteri) of doxycycline-treated Tet-mev-1 and wild-type C57BL/6j mice. Under these conditions, SDHC protein levels in Tet-mev-1 mice were increased from levels equal to wild-type C57BL/6j in un-induced mice to approximately twice that of wild-type with doxycycline induction. This is consistent with our previously reported measurements in other tissues [20,23]. Next, biochemical analyses were performed in the testes of doxycycline-treated Tet-mev-1 and wild-type C57BL/6j mice, with no detectable changes in oxidative stress and malonate-dependent cytochrome c oxidoreductase activity. In contrast, and unlike other tissues examined in Tet-mev-1 mice, succinate-cytochrome c oxidoreductase activity, from complex II and complex III activity was essentially similar to wild-type. The energy metabolism in testes as a whole results from many of the testicular cells such as spermatocytes, spermatids and spermatozoa, which are in various stages of meiosis. These cells are powered primarily by glycolysis rather than electron transport [24]. This leads to the suggestion that a mutation affecting electron transport, such as one in complex II SDHC mutation, should have modest effects on testicle functions. It has been reported that so-called mito-mice, which harbor mitochondrial DNA-deletions that severely reduce mitochondrial functions, suffer male infertility, abnormal spermatogenesis and decreasing sperm motility [9]. We therefore examined Tet-mev-1 mice, which also suffer chronically elevated levels of superoxide anion at a more modest rate than mito-mice, to see whether they have similar phenotypes. We observed excessive apoptosis in the spermatogonial cells of Tet-mev-1mice as revealed by TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining (Fig. 1A and B). However, the number of spermatozoa in Tet-mev-1 parorchis was experimentally identical to that of wild-type C57BL/6j mice, and there were no detectible morphological abnormalities (Fig. 1C). However, spermatozoon motility was slightly decreased in Tet-mev-1mice compared to wild-type C57BL/6j mice (Fig. 1D).
Fig. 1.
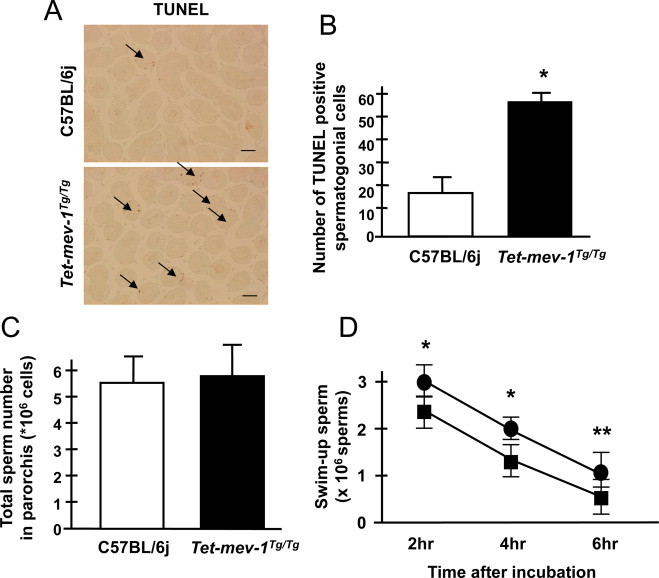
Testicle conditions, spermatogenesis and sperm motilities. (A) Cross sections of testis, showing TUNEL-stained cells (brown), which are undergoing apoptosis. Scale bar = 100 µm. (B) Statistical analysis of apoptosis in testis, using data such as portrayed in (A). Results are expressed as mean ± SD; *P < 0.001; n = 4 in each group. (C) The chart indicates the total sperm number in parorchis. Results are expressed as mean ± SD; n = 7 in each group. (D) The line chart indicates the sperm motility that was measured as the swim-up sperm number after 2, 4 and 6 h incubation in HTF medium. Circles and squares indicate wild-type C57BL/6j and Tet-mev-1 mice, respectively. Results are expressed as mean ± SD; *P < 0.01, **P < 0.05; n = 7 in each group.
Function of ovaries, oogenesis, in vitro fertilization ability and early embryogenesis
Unlike in the testes, mitochondrial ROS production and carbonylated protein levels were significantly increased in the ovaries of Tet-mev-1 mice compared to wild-type C57BL/6j mice (Fig. 2A and B). Thus, Tet-mev-1 mice show excessive intracellular oxidative stress, presumably because they are chronically subject to elevated levels of superoxide anion from mitochondrial complex II owing to the SDHCV69E mutation. Given this, it is not surprising that the Tet-mev-1 ovaries were swollen and vacuolated with increased angiogenesis such as ovarian hemangiomas [25], as well as increased apoptosis detected as TUNEL-positive cells in ovarian interstitial cells (Fig. 2C). In addition, the follicle maturations were not synchronously developed in Tet-mev-1mice (Fig. 2C). Moreover, the number of ovulations was significantly decreased in Tet-mev-1mice (Fig. 2D). These results are consistent with the fact that the number of non-growing follicles (NGF) declines with age as in humans. It is estimated that only 12% of the maximum pre-birth NGF population is present in 30-year old women and by the age of 40 years only 3% remain [26].
Fig. 2.
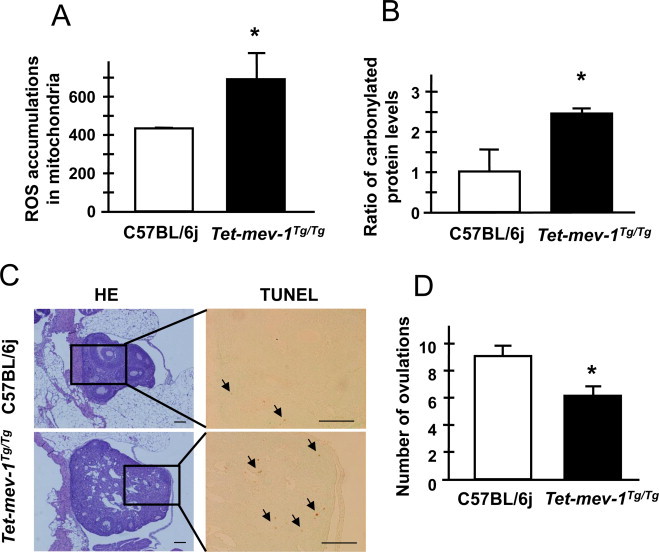
Ovary conditions and number of ovulations. (A) ROS accumulation levels in mitochondria from ovaries taken from Tet-mev-1 or wild-type C75BL/6j mice. Results are expressed as mean ± SD; *P < 0.01; n = 4 in each group. (B) The carbonylated protein levels in the membrane fraction protein lysate from ovaries taken from Tet-mev-1 or wild-type C75BL/6j mice. Results are expressed as mean ± SD as the ratio compared to the levels of wild-type C75BL/6j mice under the calibration curve method with standard oxidized protein; *P < 0.01; n = 4 in each group. (C) Micrographs of hematoxylin-eosin- and TUNEL-stained ovaries. The blocked areas feature TUNEL-positive cells that are undergoing apoptosis. Scale bar = 100 µm. (D) The number of ovulations in Tet-mev-1 or wild-type C75BL/6j mice. Results are expressed as mean ± SD; *P < 0.001; n = 4 in each group.
An in vitro fertilization assay revealed that egg fertilization was significantly decreased when using either Tet-mev-1 sperm or eggs or both (Fig. 3A). In contrast, the percentage of abnormally fertilized eggs, e.g., polyspermy eggs and developmental arrested eggs before 2-cell embryo, was normal in Tet-mev-1mice compared to wild-type C57BL/6j mice (Fig. 3B). Similarly, early embryogenesis through the blastocyst stage was no different between Tet-mev-1 and wild type mice (Fig. 3C). This suggests that the mitochondrial complex II SDHCV69E mutation did not affect fertilization and early embryogenesis but rather exerted its effects later in development. This is logical given that energy metabolism is mainly from glycolysis in the enclosed environment during the blastomere stage [27].
Fig. 3.
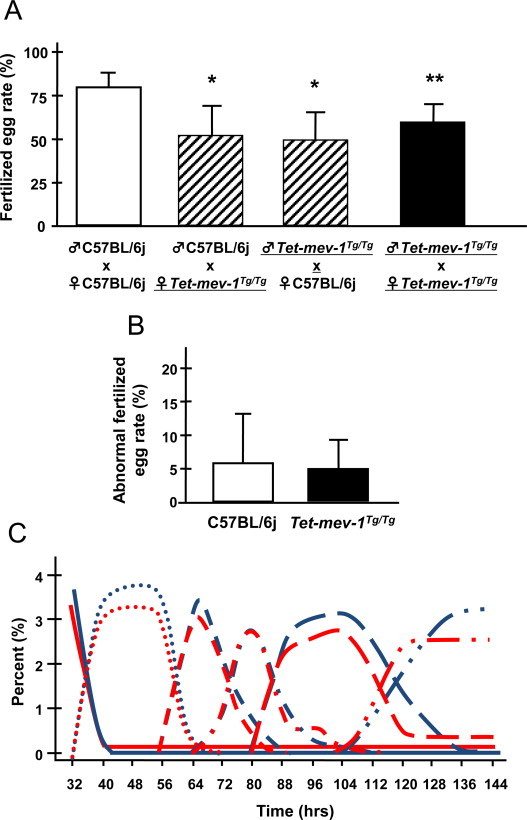
Fertility and early embryogenesis in vitro fertilization assay. (A) The chart indicates the fertilized egg rates using eggs and sperm from the indicated strain. Results are expressed as mean ± SD; *P < 0.01; **P < 0.05; n = 5 in each group. (B) Rates of abnormal fertilization. Results are expressed as mean ± SD; n = 100 in each group. (C) The progress of early embryogenesis through the blastocyst stage in in vitro fertilized animals. Blue or red lines indicate the developmental stage rates of C57BL/6j or Tet-mev-1 mice. Solid lines, 1-cell embryo stage; dotted lines, 2-cell embryo stage; dashed lines, 3–4-cell embryo stage; 1-dot dashed lines, 5–8-cell embryo stage; long dashed lines, morula stage; 2-dot long dashed lines, blastocyst stage. n = 214 in wild-type C57BL/6j and Tet-mev-1 mice group.
Decreasing pregnancy and delivery rates with thrombocytosis and splenomegaly
The pregnancy rate before first experience of safe delivery (primiparity) was decreased by about 50% in Tet-mev-1mice allowed to mate and conceive naturally (Fig. 4A). Similarly, the delivery rate was also significantly decreased in pregnant Tet-mev-1mice (Fig. 4B). These results indicate that the C. elegans short-lived mev-1 mutant-mimics Tet-mev-1 mice's accelerated age-related infertility. It is also the same as conditions of human age-related pregnancy rate decline. Given this, it was expected that the Tet-mev-1mice could demonstrate a similar unexplained age-related female infertility. Indeed, fetal loss and placental inflammation were frequently observed in 13.5-day pregnant Tet-mev-1 mice (Fig. 4C). In humans, spontaneous abortion refers to the unintentional termination of a pregnancy before fetal viability at 20 weeks of gestation or when fetal weight is <500 g. The incidence of abortion is 12–24% of all pregnancies [3]. The risk of a spontaneous abortion was 8.9% in women aged 20–24 years and 74.7% in those aged 45 years or older [4]. Similarly, it is not surprising that the incidence of maternal death was increased at first delivery in Tet-mev-1 mice (Fig. 4D), suggesting that reduced blood flow to the placenta contributed to these phenomena. It has been reported that thrombocytosis becomes a risk factor to both the fetus and mother during pregnancy [28]. To explore this possibility, a peripheral blood assay was performed in non-pregnant mice using a Sysmex XT-2000iV hematology analyzer, because intracellular oxidative stress activates megakaryocytic maturation by Nrf-2 activation leading to increasing platelet number in peripheral blood [29]. An increased number of platelets (PLT-I and PLT-O), an abnormally large platelet ratio (P-LCR), and an increased platelet density (PCT) all characterized the peripheral blood of non-pregnant Tet-mev-1mice compared to wild-type C57BL/6j mice (Fig. 5A). In addition, the spleen weight, which is marker of inflammation (one risk factor for recurrent abortion), was dramatically increased in non-pregnant Tet-mev-1mice (Fig. 5B). These results suggest that the mev-1 mutation (SDHCV69E) caused thrombocytosis and splenomegaly, contributing in a low delivery rate. These conditions were not changed in pregnant mice.
Fig. 4.
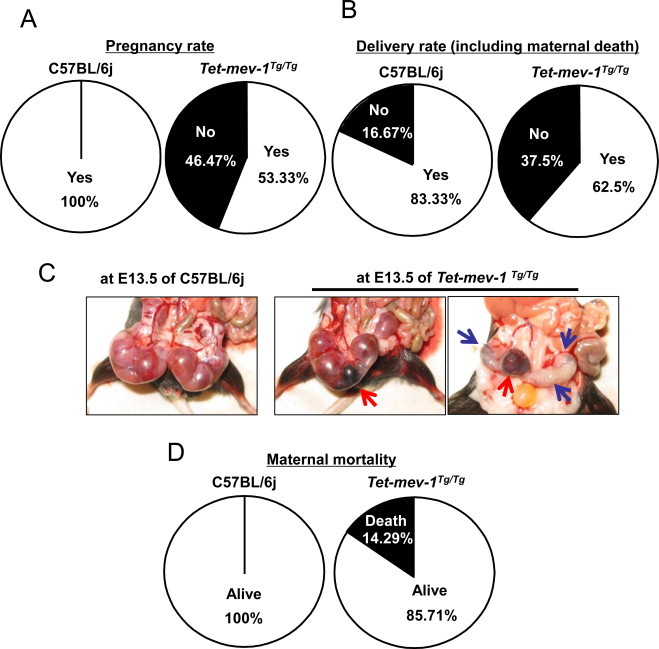
Pregnancy and delivery rates, intrauterine conditions and maternal mortality at first delivery. (A and B) Pregnancy (A) and delivery (B) rates after natural mating in each strain. The comparisons of rates between C57BL/6j and Tet-mev-1 mice; P < 0.05; n = 15 in each group. (C) Uterine conditions at 13.5-day pregnant mice. The red and blue allows indicate the placental inflammation and fetal loss, respectively. (D) Maternal mortality at first delivery in each strain. The comparison of maternal mortality at first delivery between C57BL/6j and Tet-mev-1 mice; P < 0.05; n = 30 in each group.
Fig. 5.
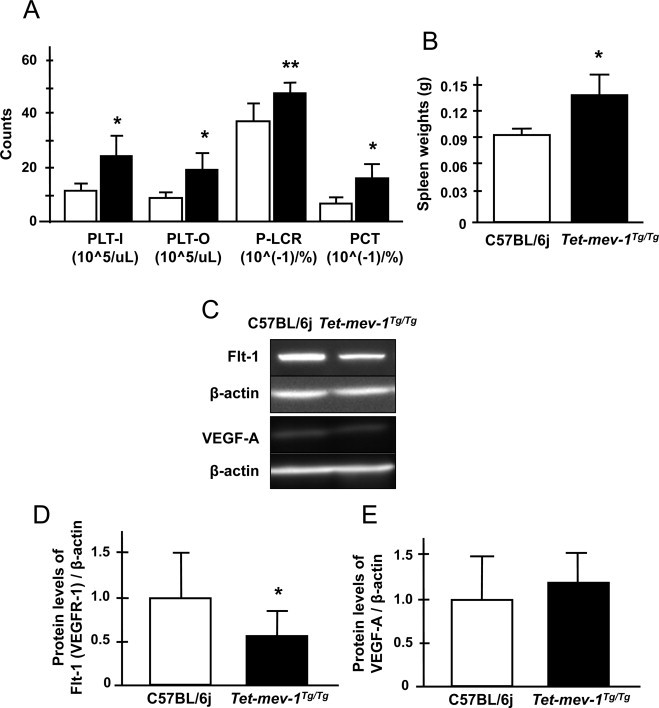
Conditions of peripheral blood and spleen weight in non-pregnant mice and condition of normal morphological placentas at 13.5-day pregnant mice. (A) The number of platelets (PLT-I indicates the impedance method used to estimate platelet counts and PLT-O indicates the optical method used to measure platelet counts.), the ratio of large platelet (P-LCR) and platelet density (PCT) derived from peripheral blood taken from non-pregnant mice using a Sysmex XT-2000iV hematology analyzer. White and black bars indicate the wild-type C57BL/6j and Tet-mev-1 mice. Results are expressed as mean ± SD; *P < 0.01; **P < 0.05; n = 5 in each group. (B) The chart indicates the spleen weight. Results are expressed as mean ± SD; *P < 0.01; n = 5 in each group. (C) The results of western blot analysis using anti-Flt-1 (VEGFR-1), VEGF-A, and ß-actin antibodies. (D and E) Quantification values of Flt-1 (D) and VEGF-A (E) protein levels derived from western blots. Results are expressed as mean ± SD; *P = 0.01; n = 8 in each group.
Angiodysplasia with decreasing Flt-1 protein in placenta
Next, placentas were examined from pregnant mice. The vascular endothelial growth factors (VEGF) signaling is important in regulating vascular cell recruitment and proliferation for the placental formation [30,31]. It is well known that the most abundant and active member of the VEGF receptors is VEGF receptor-1 (VEGFR-1), also known as Flt-1. This binds to VEGF-A and PLGF-1 as a key factor promoting angiogenesis in the placenta. Disruptions contribute to the pathogenesis of female infertilities such as preeclampsia [32]. Flt-1 protein levels were dramatically decreased in morphologically normal placentas from 13.5-day pregnant Tet-mev-1 mice, even though VEGF-A, estrogen receptor (ER)-a and ß protein levels were normal compared to control placentas of C57BL/6j mice (Figs. 5C–E and S1A and B). Additionally, the inflammatory cytokine levels, IFN?, IL-1ß and IL-6, were not changed in these placentas from 13.5-day pregnant Tet-mev-1mice (Fig. S1C–E). Given this, it is not surprising that developmental arrest and abnormal angiogenesis were observed at the 13.5-day-embryonic age in Tet-mev-1mice (Fig. 6A). As well, the number of progeny was significantly decreased in Tet-mev-1 mice (Fig. 6B). The neonatal mortality was increased, thus the number of weaning mice was dramatically decreased at first delivery in Tet-mev-1mice (Fig. 6C,D). Surprisingly, the Tet-mev-1 mice that successfully delivered, nurtured and weaned offspring had statistically normal numbers of progeny, neonatal mortality and weaning mice in ensuing pregnancies (Fig. S2A and B). Thus, the endogenous oxidative stress in these mice profoundly affected the first pregnancy but not subsequent ones.
Fig. 6.
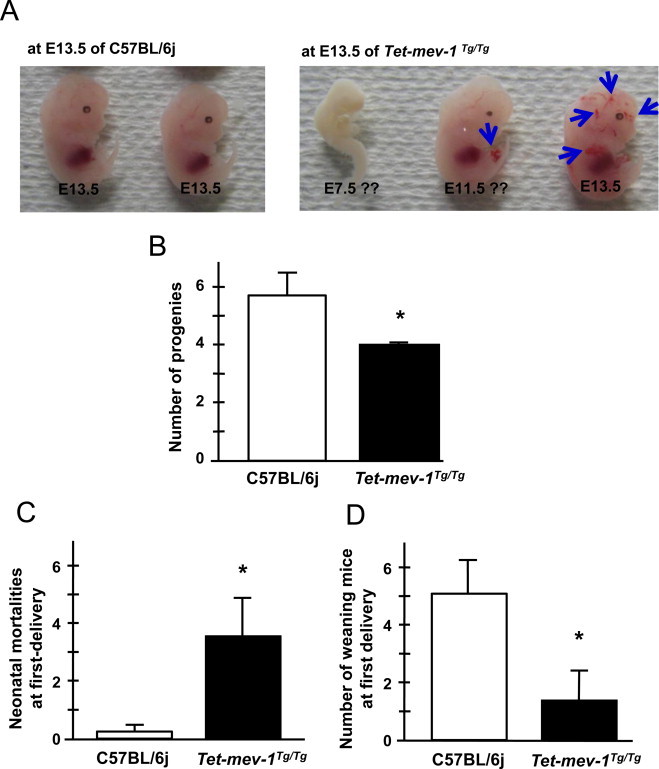
Neonatal mortalities as well as the number of progeny and weaning mice. (A) Mouse fetuses at 13.5-days of embryogenesis. The blue arrows indicate abnormal angiogenesis. (B) The number of progenies at first delivery. Results are expressed as mean ± SD; *P = 0.01; n = 8 in C57BL/6j, n = 11 in Tet-mev-1 mice. (C) Neonatal mortalities in the first delivery. Results are expressed as mean ± SD; *P = 0.001; n = 8 in C57BL/6j, n = 11 in Tet-mev-1 mice. (D) The number of weaning mice at first delivery. Results are expressed as mean ± SD; *P = 0.001; n = 8 in C57BL/6j, n = 11 in Tet-mev-1 mice.
Discussion
We have demonstrated that a mitochondrial defect resulting from the SDHCV69E mutation causes mitochondrial and intracellular oxidative stress in the nematode C. elegans mev-1[15], a mev-1-mimic transgenic Drosophila model [33] and mouse embryonic fibroblast SDHC E69 cells [18]. Recently, we have reported that Tet-mev-1 mice expressing the SDHCV69E transgene exhibit much the same effects [20]. The mutation site at the 69th position is located in a ubiquinone-binding region, changing the neutral and hydrophobic valine to an acidic and hydrophilic glutamate. This mutation causes decreased affinity between complex II and ubiquinone, leading to defects in electron transport. The leaked electrons react with nearby oxygen in mitochondria leading to mitochondrial and intracellular reactive oxygen species such as superoxide anion (O2•-), hydrogen peroxide (H2O2) and hydroxyl radical (•OH). The resultant intracellular oxidative stress causes excessive apoptosis during the mitotic cell divisions inherent to embryogenesis and neonatal development, resulting in low birth-weight infants and growth retardation in Tet-mev-1 mice [20]. Tet-mev-1 mice have normal food intake and water consumption. They recover body size and weight about 12 weeks after birth. Moreover, Tet-mev-1 mice exhibit accelerated age-dependent corneal physiological and pathological phenotypes such as Fuchs's corneal dystrophy, keratoconus and dry eyes with keratitis and lacrimal gland inflammation, especially in male mice [23,34]. In this report, we have documented that mouse infertility also results from the mev-1 mutation (SDHCV69E) and have identified its likely causation.
Male Tet-mev-1 mice produce sperm in normal numbers and of wild-type morphology. This was initially surprising, given that mitochondrial-DNA deleted mouse models (mito-mice) result in male infertility owing to abnormal spermatogenesis and sperm morphology [9]. However, mito-mice likely have more severe phenotypes since the mitochondrial DNA deletions completely abolish the mitochondrial ATP production. In addition, these mice are reported to have mitochondrial structural defects that might impact spermatogenesis [9]. In contrast, the SDHCV69E mutation coding transgene expression level has been tailored such that its expression equals that of endogenous wild-type SDHC protein. Consequently, Tet-mev-1 mice are not influenced by the mitochondrial complex II weak dysfunctions during spermatogenesis except for sperm motility. The decreased mobility could be due to either changes in glucose and pyruvate metabolism or metabolic stores with chronic oxidative stress in the Tet-mev-1 sperm.
Ovaries of Tet-mev-1 mice showed considerable mitochondrial oxidative stress. They were swollen and vacuolated in ovarian interstitial cells. Moreover, the follicle maturations were not synchronously developed, leading to slightly decreasing ovulations in Tet-mev-1 mice. In the in vitro fertilization assay, early embryogenesis through the blastocyst stage was completely normal; however, the fertilization rate was low, which was likely caused by seemingly healthy but defective unfertilized eggs and low sperm motility in Tet-mev-1 mice. On the other hand, the fertilization rate was lower between cross-species gametes of C57BL/6j and Tet-mev-1 conditional transgenic mice, respectively. This result likely reflects the fact that ATP synthesis is mainly through glycolysis in these blastomere developmental phases [27]. SDHCV69E is one of complex II subunits, which works in conjunction with the citric cycle, and would therefore be expected to have minimal effects on early embryogenesis.
Non-pregnant Tet-mev-1 mice exhibit an increased number of platelets (PLT-I and PLT-O), an abnormally large platelet ratio (P-LCR) and an increased platelet density (PCT). These thrombocytosis factors could contribute to decreased pregnancy and delivery rates. In addition, non-pregnant Tet-mev-1 mice showed splenomegaly. This is consistent with the fact that 13.5-day pregnant Tet-mev-1 mice had significant fetal loss and placental inflammation. In keeping with this, Flt-1 (VEGFR-1) was dramatically decreased in the morphologically-normal placentas of Tet-mev-1 mice. These placental phenotypes lead to hypoxic conditions that partially explain the abnormal angiogenesis in embryos (Fig. 6A). On the other hand, VEGF-A, estrogen receptors a and ß, and inflammatory cytokine levels such as IFN?, IL-1ß and IL-6, which are also known as risk factors of contributing to abortion, were not changed in Tet-mev-1 mice.
We have documented that female Tet-mev-1 mice exhibit thrombocytosis, splenomegaly and placental angiodysplasia, which may constitute risk factors predisposing towards spontaneous abortion and recurrent miscarriage. In addition, fetal and neonatal Tet-mev-1 mice frequently died by developmental arrest with an excessive apoptosis [20] and abnormal angiogenesis under the hypoxic condition through placental dysfunctions with decreased Flt-1 protein helps explain this. Interestingly, Tet-mev-1 mice that were successful in one pregnancy had second deliveries at almost wild-type rates. This is worth exploring and is just one aspect that we feel makes Tet-mev-1 mice a compelling animal model to study recurrent abortion.
Oxidative stress is present in most organs exposed to high oxygen metabolism such as the placenta. There is an emerging confluence of opinion suggesting that oxidative stress is one of the main underlying mechanisms in the pathogenesis of a continuum of disease processes such as spontaneous abortion and eclampsia. Oxidative stress and ROS-induced damage may be important missing pieces of the puzzle explaining abortions and recurrent pregnancy loss of unexplained etiology. Our study using Tet-mev-1 mice clearly demonstrates that oxidative stress from mitochondria leads to spontaneous abortion and recurrent miscarriage with placental inflammation and embryonic developmental arrest resulting in fetal loss under the hypoxic conditions caused by abnormal angiogenesis in placenta. Human recurrent miscarriage in the United Kingdon is defined as three or more consecutive abortions during the first and second trimesters. A human abortion does not necessarily indicate a recurrent miscarriage is caused by maternal and fetal conditions in the first and second trimesters. Tet-mev-1 mice have an embryonic arrest leading to fetal loss at early pregnancy and a placental inflammation at later-term pregnancy, thus mimicking the human conditions of miscarriage during the first or second trimesters. We have not yet clearly demonstrated why Tet-mev-1 mice that experience a successful pregnancy are seemingly refractory to subsequent recurrent miscarriages, but we are actively exploring this interesting question. In conclusion, Tet-mev-1 mice provide a powerful opportunity to study recurrent abortion with oxidative stress. Thus, reducing oxidative stress might be one possible treatment to combat spontaneous abortion and recurrent miscarriage.
Materials and methods
Animals
Wild-type C57BL/6j and Tet-mev-1 conditional transgenic C57BL/6j background mice were treated with doxycycline hyclate (0.1 mg/ml doxycycline with 0.01 mg/ml saccharine or 2 mg/ml with 0.2% saccharine in drinking water) throughout prenatal development to adult. Tet-mev-1 mice were constructed with the Tetracycline system that was uniquely developed by both rTetR (recombinant tetracycline receptor)-VP16 (reverse tetracycline-dependent transcriptional activator) and TetR-KRAB (tetracycline-dependent transcriptional repressor) as previously described [20]. This mouse model ubiquitously expresses the SDHCV69E mutation-coding transgene in various tissues with doxycycline reagent to competitively and equally induce SDHCV69E mutant protein to the endogenous SDHC protein level [18,20]. While suffering initial growth retardation, animals are of normal size after 12 weeks [20]. After 3 months, male and female Tet-mev-1 mice show accelerated corneal dysfunctions with age, i.e., delayed epithelialization with keratitis, decreasing endothelial cells such as Fucks corneal dystrophy, and thickened Descemet's membrane [23]. Male Tet-mev-1 mice also display lacrimal gland inflammation [34]. All animals were maintained on a 12 h light/dark cycle under an SPF condition: 22 ± 1 °C, approximately 40% humidity, 12/12-h light/dark cycle, a standard diet CE2 (9.3% water, 25.8% crude protein, 4.5% crude fat, 4.5% crude fiber, and 6.7% crude ash) (CLEA Japan, Inc.) and sterile water ad libitum. All protocols complied with the Guidelines for Animal Experimentation of Tokai University and were approved by the Tokai University Animal Care Committees.
Mitochondrial reactive oxygen species measurement
The basal reactive oxygen species (ROS) levels in mitochondrial fraction proteins of tissues were measured in intact mitochondrial fraction proteins as previously described [20]. Basal ROS levels in mitochondrial fraction proteins were measured by the photon counter with an AB-2200 type Luminescencer-PSN (ATTO, Tokyo, Japan) at 37 °C using the chemiluminescent probe MPEC (2-methyl-6-p-methoxyphenylethynyl-imidazopyrazinone) (ATTO Co., Tokyo, Japan). The rates of ROS were expressed as counts per second and the amounts were calculated by subtracting the optical density of samples in the presence of 10 µg/ml bovine Cu, Zn-superoxide dismutase from that in the absence of the enzyme. Cu, Zn-superoxide dismutase was used as the compensation for only mitochondrial O2•- level measurements in all cases.
Carbonylated protein measurement
The mouse tissue protein extracts were prepared by the same protocol in western blot analysis. The DNPH-treated 200 ng proteins quantified protein carbonyls contents by enzyme-linked immunosorbent assay (ELISA) using 96-well plate (Corning Coster, Cambridge, MA). DNPH treatment methodology was as previously described [20]. The plates were incubated with anti-DNPH antibody in PBS containing 0.1% BSA, 0.1% Gelatin, 0.1% NaN3 , 1 mM MgCl2. After washing with PBS, the plates were incubated with HRP-conjugated rabbit IgG antibody, and then o-phenylenediamine (OPD) (Sigma Co., Tokyo, Japan) - H2O2 solutions were added. Finally, peroxidase activities were measured at 492 nm on SpectraMax 250 (Molecular Devices Co., Sunnyvale, CA) after 30-min incubation at room temperature followed by the addition of 1 M H2SO4.
TUNEL staining
The segments of the tissues of 3 month-old mice were perfused and fixed with 4% paraformaldehyde in PBS under physiological pressure, and embedded in paraffin and sectioned at a thickness of 2–3 µm. TUNEL reactions were performed using TUNEL Enzyme, TUNEL Dilution Buffer and Biotin-16-dUTP (Roche Diagnostics Corp., Tokyo, Japan) according to the manufacturer's instructions. Sections were then incubated with anti-streptAvidin-HRP conjugated antibody (1:40 dilution), developed using diaminobenzidine [20 mg DAB, 65 mg NaN3, 17 µl H2O2 in 100 ml 0.05 M Tris–HCl buffer], and treated in methyl green nucleus staining.
Total sperm number, sperm motility, and in vitro fertilization assays
Sperm were collected from the incubated cauda epididymidis in 1 ml of HTF medium, and the total sperm number was counted. These sperm samples were also used for the motility and in vitro fertilization assays. After 2 h incubation in HTF medium, the activated sperm could swim up to the upper layer of the medium within the incubation time, the swim-up sperm were counted as the number of motile sperm in 1.5 ml HTF medium after 2, 4 and 6 h of incubation. Female mice were induced to superovulate by consecutive injections of pregnant mare serum gonadotropin and human chorionic gonadotropin (hCG) with an interval of 48 h between injections. Unfertilized oocytes were collected from the oviducts 15 h after the hCG injection. The in vitro fertilization was carried out by using activated sperm collected from cauda epididymidis in HTF medium. The developmental frequency of embryos was measured in KSOM medium in a 37 °C incubator.
Western blot analysis
Forty micrograms of total protein extract denaturalized by boiling after the addition of 2 × SDS-PAGE sample buffer [0.125 M Tris–HCl (pH 6.8), 10% 2-mercaptoethanol, 4% SDS, 10% sucrose and 0.004% bromophenol blue]/lane were resolved using 10–20% SDS-PAGE gradient gel and analyzed by Western blots. After electrophoresis, the proteins were transferred to PVDF (polyvinylidene difluoride) membrane Clearblot membrane (ATTO) using a Semi-dry blotting machine AE-6677 (ATTO). To block nonspecific protein binding, membranes were treated for 2–8 h at 20–25 °C with 5% bovine serum albumin (EQUITECH-BIO, Inc: #BAH64), 0.1% Tween-20 in TBS [0.02 M TRIZMA BASE, 0.137 M NaCl (pH 7.6)]. Primary antibodies [Rabbit monoclonal [Y103] to VEGF Receptor 1 (abcam: ab32152), Rabbit anti-mouse Vascular Endothelial Growth Factor (Millipore: ab1876), Rabbit monoclonal antibody [E115] to Estrogen Receptor alpha (GeneTex: GTX61047), Rabbit polyclonal Estrogen receptor beta antibody (abcam: ab3576), Rat monoclonal [RMMG-1] to Interferon gamma antibody (abcam: ab24979), Rabbit polyclonal to IL-1 beta antibody (abcam: ab9722), Rabbit polyclonal to IL-6 antibody (abcam: ab6672), Goat polyclonal to beta Actin antibody (abcam: ab8229), and Rabbit polyclonal antibody to Beta-Actin (GeneTex: GTX110564)] were used in TBS containing 5% bovine serum albumin in each condition. Actin was used as an internal control protein for loading normalization of the quantification analysis. Horseradish peroxidase-coupled specific secondary antibodies [anti-rabbit IgG, HRP-linked antibody (Cell Signaling Technology: #7074) or polyclonal rabbit anti-goat immunoglobulines/HRP antibody (Dako: P0449)] were incubated in TBS containing 5% bovine serum albumin for 2 h at room temperature in each condition. The detection system used was ECL-plus Western Blotting Detection Reagent (GE Healthcare, UK). The chemiluminescence signals were visualized under LAS3000 mini (Fuji Photo Film Co., Japan). Quantitative densitometric analysis was done using Multi Gauge Ver3.0 (Fuji Photo Film Co., Japan).
Data analyses
Statistically measurements were analyzed by one-way ANOVA, Bonferroni post hoc comparisons and a two-tailed, unpaired student's t-test. All values in the figures and figure legends are means ± SD.
Acknowledgments
We are very thankful to the member of the Education and Research Support Center and Animal Care Center in Tokai University. We also thank the undergraduate students of laboratory member for assisting animal care. This work was supported by the KAKENHI (22689012) in Grant-in-Aid for Young Scientists(A) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and Japan Society for the Promotion of Science (JSPS) and the Tokai University School of Medicine Research Aid.
Contributor Information
Takamasa Ishii, Email: 1amfd001@is.icc.u-tokai.ac.jp.
Naoaki Ishii, Email: nishii@is.icc.u-tokai.ac.jp.
Appendix A. Supplemental Materials
Supplementary material associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.redox.2014.05.001
Appendix A. Supplementary Materials
Supplementary Materials for Genetically induced oxidative stress in mice causes thrombocytosis, splenomegaly and placental angiodysplasia that leads to recurrent abortion.
References
- 1.Tietze C. Reproductive span and rate of reproduction among Hutterite women. Fertility and Sterility. 1957;8(1):89–97. doi: 10.1016/s0015-0282(16)32587-0. 13405050 [DOI] [PubMed] [Google Scholar]
- 2.Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Human Reproduction (Oxford, England) 2004;19(7):1548–1553. doi: 10.1093/humrep/deh304. 15205397 [DOI] [PubMed] [Google Scholar]
- 3.Jurkovic D., Overton C., Bender-Atik R. Diagnosis and management of first trimester miscarriage. British Medical Journal (Clinical Research Ed.) 2013;346:f3676. doi: 10.1136/bmj.f3676. 23783355 [DOI] [PubMed] [Google Scholar]
- 4.Nybo Andersen A.M., Wohlfahrt J., Christens P., Olsen J., Melbye M. Maternal age and fetal loss: population based register linkage study. British Medical Journal (Clinical Research Ed.) 2000;320(7251):1708–1712. doi: 10.1136/bmj.320.7251.1708. 10864550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins J.A., Rowe T.C. Age of the female partner is a prognostic factor in prolonged unexplained infertility: a multicenter study. Fertility and Sterility. 1989;52(1):15–20. doi: 10.1016/s0015-0282(16)60781-1. 2526029 [DOI] [PubMed] [Google Scholar]
- 6.Lavranos G., Balla M., Tzortzopoulou A., Syriou V., Angelopoulou R. Investigating ROS sources in male infertility: a common end for numerous pathways. Reproductive Toxicology (Elmsford, N.Y.) 2012;34(3):298–307. doi: 10.1016/j.reprotox.2012.06.007. 22749934 [DOI] [PubMed] [Google Scholar]
- 7.Agarwal A., Aponte-Mellado A., Premkumar B.J., Shaman A., Gupta S. The effects of oxidative stress on female reproduction: a review. Reproductive Biology and Endocrinology. 2012;10:49. doi: 10.1186/1477-7827-10-49. 22748101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tremellen K. Oxidative stress and male infertility—a clinical perspective. Human Reproduction Update. 2008;14(3):243–258. doi: 10.1093/humupd/dmn004. 18281241 [DOI] [PubMed] [Google Scholar]
- 9.Nakada K. Mitochondria-related male infertility. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(41):15148–15153. doi: 10.1073/pnas.0604641103. 17005726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii N. A methyl viologen-sensitive mutant of the nematode Caenorhabditis elegans. Mutation Research. 1990;237(3–4):165–171. doi: 10.1016/0921-8734(90)90022-j. 2233820 [DOI] [PubMed] [Google Scholar]
- 11.Honda S., Ishii N., Suzuki K., Matsuo M. Oxygen-dependent perturbation of life span and aging rate in the nematode. Journal of Gerontology. 1993;48(2):B57–B61. doi: 10.1093/geronj/48.2.b57. 8473689 [DOI] [PubMed] [Google Scholar]
- 12.Hosokawa H. Rapid accumulation of fluorescent material with aging in an oxygen-sensitive mutant mev-1 of Caenorhabditis elegans. Mechanisms of Ageing and Development. 1994;74(3):161–170. doi: 10.1016/0047-6374(94)90087-6. 7934213 [DOI] [PubMed] [Google Scholar]
- 13.Adachi H., Fujiwara Y., Ishii N. Effects of oxygen on protein carbonyl and aging in Caenorhabditis elegans mutants with long (age-1) and short (mev-1) life spans. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1998;53(4):B240–B244. doi: 10.1093/gerona/53a.4.b240. 18314552 [DOI] [PubMed] [Google Scholar]
- 14.Ishii N. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature. 1998;394(6694):694–697. doi: 10.1038/29331. 9716135 [DOI] [PubMed] [Google Scholar]
- 15.Senoo-Matsuda N. A defect in the cytochrome b large subunit in complex II causes both superoxide anion overproduction and abnormal energy metabolism in Caenorhabditis elegans. Journal of Biological Chemistry. 2001;276(45):41553–41558. doi: 10.1074/jbc.M104718200. 11527963 [DOI] [PubMed] [Google Scholar]
- 16.Senoo-Matsuda N. A complex II defect affects mitochondrial structure, leading to ced-3- and ced-4-dependent apoptosis and aging. Journal of Biological Chemistry. 2003;278(24):22031–22036. doi: 10.1074/jbc.M211377200. 12672828 [DOI] [PubMed] [Google Scholar]
- 17.Ishii T., Miyazawa M., Hartman P.S., Ishii N. Mitochondrial superoxide anion (O2- ) inducible “mev-1” animal models for aging research. BMB Reports. 2011;44(5):298–305. doi: 10.5483/BMBRep.2011.44.5.298. [DOI] [PubMed] [Google Scholar]
- 18.Ishii T. A mutation in the SDHC gene of complex II increases oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Research. 2005;65(1):203–209. 15665296 [PubMed] [Google Scholar]
- 19.Ishii T. Model animals for the study of oxidative stress from complex II. Biochimica et Biophysica Acta. 2013;1827(5):588–597. doi: 10.1016/j.bbabio.2012.10.016. 23142169 [DOI] [PubMed] [Google Scholar]
- 20.Ishii T. Mitochondrial reactive oxygen species generation by the SDHC V69E mutation causes low birth weight and neonatal growth retardation. Mitochondrion. 2011;11(1):155–165. doi: 10.1016/j.mito.2010.09.006. 20870041 [DOI] [PubMed] [Google Scholar]
- 21.Gharagozloo P., Aitken R.J. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Human Reproduction (Oxford, England) 2011;26(7):1628–1640. doi: 10.1093/humrep/der132. 21546386 [DOI] [PubMed] [Google Scholar]
- 22.Wong E.W., Cheng C.Y. Impacts of environmental toxicants on male reproductive dysfunction. Trends in Pharmacological Sciences. 2011;32(5):290–299. doi: 10.1016/j.tips.2011.01.001. 21324536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onouchi H. Mitochondrial superoxide anion overproduction in Tet-mev-1 transgenic mice accelerates age-dependent corneal cell dysfunctions. Investigative Ophthalmology and Visual Science. 2012;53(9):5780–5787. doi: 10.1167/iovs.12-9573. 22786907 [DOI] [PubMed] [Google Scholar]
- 24.Miki K. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(47):16501–16506. doi: 10.1073/pnas.0407708101. 15546993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uppal S., Heller D.S., Majmudar B. Ovarian hemangioma—report of three cases and review of the literature. Archives of Gynecology and Obstetrics. 2004;270(1):1–5. doi: 10.1007/s00404-003-0584-8. 15205976 [DOI] [PubMed] [Google Scholar]
- 26.Wallace W.H., Kelsey T.W. Human ovarian reserve from conception to the menopause. PloS One. 2010;5(1):e8772. doi: 10.1371/journal.pone.0008772. 20111701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leese H.J., Barton A.M. Pyruvate and glucose uptake by mouse ova and preimplantation embryos. Journal of Reproduction and Fertility. 1984;72(1):9–13. doi: 10.1530/jrf.0.0720009. 6540809 [DOI] [PubMed] [Google Scholar]
- 28.Elliott M.A., Tefferi A. Thrombocythaemia and pregnancy. Best Practice and Research: Clinical Haematology. 2003;16(2):227–242. doi: 10.1016/s1521-6926(03)00019-7. 12763489 [DOI] [PubMed] [Google Scholar]
- 29.Motohashi H. NF-E2 domination over Nrf2 promotes ROS accumulation and megakaryocytic maturation. Blood. 2010;115(3):677–686. doi: 10.1182/blood-2009-05-223107. 19901266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vuorela P., Carpén O., Tulppala M., Halmesmäki E. VEGF, its receptors and the tie receptors in recurrent miscarriage. Molecular Human Reproduction. 2000;6(3):276–282. doi: 10.1093/molehr/6.3.276. 10694277 [DOI] [PubMed] [Google Scholar]
- 31.De Falco S. The discovery of placenta growth factor and its biological activity. Experimental and Molecular Medicine. 2012;44(1):1–9. doi: 10.3858/emm.2012.44.1.025. 22228176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kita N., Mitsushita J. A possible placental factor for preeclampsia: sFlt-1. Current Medicinal Chemistry. 2008;15(7):711–715. doi: 10.2174/092986708783885309. 18336285 [DOI] [PubMed] [Google Scholar]
- 33.Tsuda M. A mev-1-like dominant-negative SdhC increases oxidative stress and reduces lifespan in Drosophila. Biochemical and Biophysical Research Communications. 2007;363(2):342–346. doi: 10.1016/j.bbrc.2007.08.168. 17854771 [DOI] [PubMed] [Google Scholar]
- 34.Uchino Y. Oxidative stress induced inflammation initiates functional decline of tear production. PloS One. 2012;7(10):e45805. doi: 10.1371/journal.pone.0045805. 23071526 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials for Genetically induced oxidative stress in mice causes thrombocytosis, splenomegaly and placental angiodysplasia that leads to recurrent abortion.


