Summary
Intravital microscopy (IVM) is a powerful tool that enables imaging various biological processes in live animals. Here, we describe a series of procedures designed to image subcellular structures, such as endsosomes and secretory vesicles in the salivary glands (SGs) of live rats. To this aim, we used fluorescently labeled molecules and/or fluorescently-tagged proteins that were transiently transfected in the live animal.
Keywords: Intravital Microscopy, Salivary glands, Endocytosis, Exocytosis
1. Introduction
IVM has been utilized primarily to image live animals at the tissue or the single cell level (1, 2). Although endosomes have been imaged in the kidney of live rats for short period of times, the ability to perform sub-cellular imaging has been hindered by the motion artifacts due to the respiration and the heartbeat (3, 4). Recently, we have developed some strategies to image sub-cellular structures in live rodents, thus opening the door to study cell biology in physiological conditions (1, 2, 4–7). As a model organ, we used the SGs of live rats, which offer several advantages. First, they can be exposed by a relatively minor surgery and easily immobilized in order to minimize the motion artifacts that are detrimental to high-resolution imaging (5, 6). Second, SGs are amenable to both pharmacological and genetic manipulations making these organs ideal to study cellular processes at a molecular level. Indeed, molecules can be delivered to SGs through multiple routes: 1) via systemic injection, 2) via intra-organ injection, and 3) via the salivary ducts that can be accessed from the oral cavity (5, 7). Specifically, this last route has been utilized to deliver plasmid DNA, enabling us to transiently express fluorescently tagged proteins (7).
Since the most challenging part in IVM is the preparation of the animal, here we focus on the description of the basic surgical procedures that are utilized to expose and immobilize the SGs without compromising their function, and to deliver probes to label subcellular compartments such as endosomes, secretory granules and the Golgi apparatus. Our goal is to provide investigators who have minimal experience in surgical procedures and animal handling, with basic protocols to successfully perform IVM at a subcellular resolution.
2. Materials
2.1 Animals and surgical tools
Sprague-Dawley rats, 150–250 g body weight (Harlan Laboratories, Frederick, MD).
Anestethics: Isofluorane (Forane, 100 ml, Baxter, Deerfield, IL), Ketamine (Ketaved, 100 mg/ml, Fort Dodge Animal Health, Fort Dodge, IA), and Xylazine (Anased, 100 mg/ml Akorn, Decatur, IL) (see Note 1)
Vaporizer for isoflurane (Isolfuorane V 1.9) connected to a plexiglass restraining tube able to accommodate rats weighing up to 300 g (Braintree Scientific, Braintree, MA)
Eye lubricant: Neomycin/Polymyxin B (Bausch and Lomb, Tampa, Fl)
Downdraft table equipped with HEPA filter (Hazard Technology, Pasadena, MD)
Heat lamp, model HL1 (Braintree Scientific, Braintree, MA)
MicroTherma 2T Thermometer with RET-3 and IT-21 thermocouple probes (Braintree Scientific, Braintree, MA)
70% Ethanol and sterile filtered normal saline (Qualilty Biological, Gaithersburg, MD)
Optical coupling gel: 0.3% Carbomer-940 (Snowdrift Farm, Tucson, AZ), 54.7 mg/ml D-Sorbitol, and Trietanolamine (pH 7.4), (see Note 2)
Surgical instruments: Operating scissors (11.5 cm straight); two #7 curved tip tweezers (one with blunt and one with sharper tips), disposable scalpel tips, scalpel holder, microscissors (World Precision Instruments, Sarasota, FL) (see Note 3)
2 % Lidocaine (Hospira, Lake Forest, IL)
Black Braided Silk suture # 4.0 (Deknatel, Triangle Park, NC)
Gauze sponges 2"×2" (Tyco Healthcare, Gosport, UK)
2.2. Fluorescent probes
All fluorescent probes were purchased from Invitrogen, Carlsbad, CA. Stock concentrations and doses utilized are indicated in Table 1. The amount of probe delivered is adjusted for a 200 g rat and may need fine adjustments. Volumes of injections are: 500 µl for IV, 100 µl for intra-ductal and 1 ml for intra-stromal delivery.
Table 1.
List of the Fluorescent probes utilized to image membrane traffic and subcellular organelles in a live rat
| Fluorescent probe | Stock conc. |
Store At |
IV injection | Intra- ductal |
Intra- stromal |
|---|---|---|---|---|---|
| Hoechst 33342 | 10 mg/ml | 4°C | 0.02 mg/ml | - | - |
| FM1-43 | 20 mM | −20°C | - | 200 µM | 20 µM |
| Texas Red DHPE | 20 mM | −20°C | - | 100 µM | 20 µM |
| Cascade Blue 10 kDa dextran | 25 mg/ml | 4°C | - | 1 mg/ml | 50 µg/ml |
| Alexa 488 10 kDa dextran | 10 mg/ml | 4°C | - | 1 mg/ml | 50 µg/ml |
| Texas Red 10 kDa dextran | 25 mg/ml | 4°C | - | 1 mg/ml | 50 µg/ml |
| Alexa 647 10 kDa dextran | 10 mg/ml | 4°C | - | 1 mg/ml | 50 µg/ml |
| Oregon G. 488 70 kDa dextran | 10 mg/ml | 4°C | 0.4 mg/ml | - | 50 µg/ml |
| Texas Red 70 kDa dextran | 25 mg/ml | 4°C | 0.4 mg/ml | - | 50 µg/ml |
| FITC 500 kDa dextran | 10 mg/ml | 4°C | 1 mg/ml | - | - |
| FITC 2000 kDa dextran | 10 mg/ml | 4°C | 2 mg/ml | - | - |
| Tetramethylrhodamine 2000 kDa dextran | 10 mg/ml | 4°C | 2 mg/ml | - | - |
2.3 In vivo gene delivery to submandibular salivary gland
SZX7 Stereo microscope mounted on an adjustable arm (Olympus America, Center Valley, PA)
Custom-made stereotactic device for salivary duct cannulation (see Fig. 1A–C)
Polyethylene PE-5 cannula (Strategic Applications, Libertyville, IL) (see Note 4)
Histoacryl tissue adhesive (TissueSeal, Ann Arbor, MI)
1 ml plastic syringes
30G 1/2 inch needle (Exelint, Los Angeles, CA)
Plasmid DNA purified from maxi-prep kit (Qiagen, Valencia, CA)
In vivo-jet PEI polyethyleneimine (PEI) (Polyplus Transfection, New York, NY)
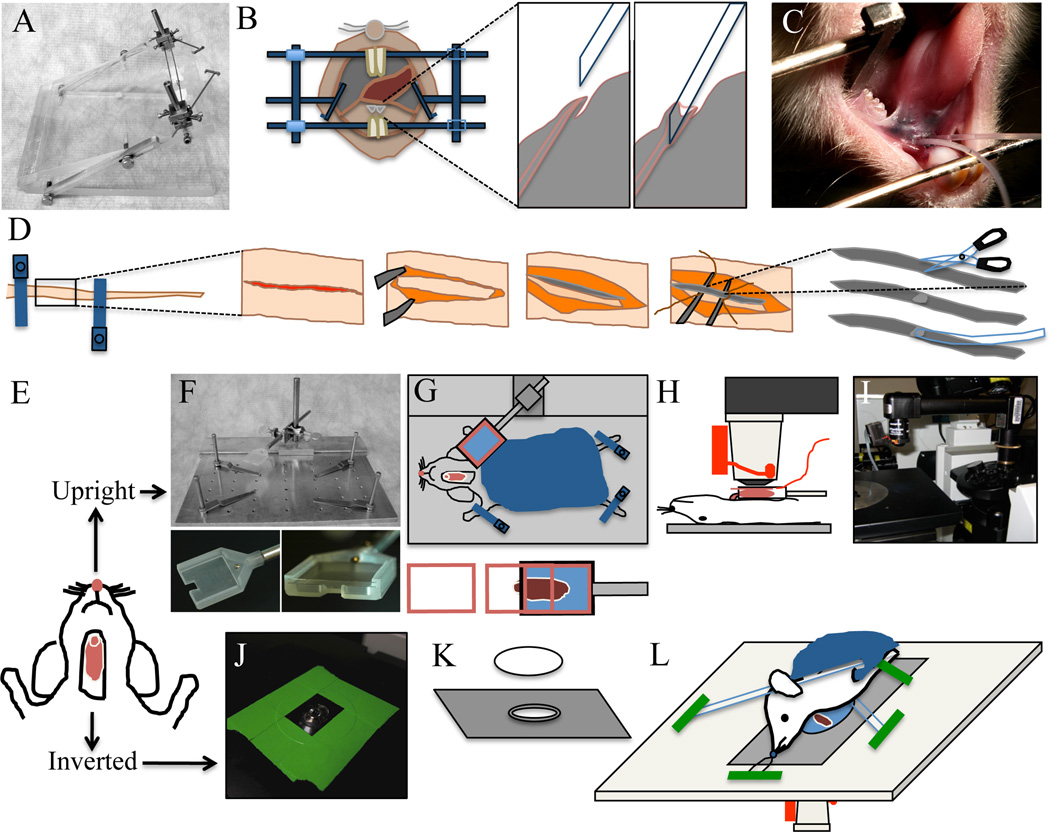
Figure 1. Animal preparation for intravital microscopy.
A–C - Cannulation of the Wharton’s duct. A - Custom made stereotactic device for cannulation. B - Diagram showing the Wharton’s duct cannulation procedure. C - Picture of an anesthetized rat, immobilized on the stereotactic device. Two PE-5 cannulae were inserted in the ducts. D - Diagram of tail artery catheterization procedure. The tail artery is isolated, an incision is made and PE-5 tubing is inserted. E - Diagram showing the rat after SGs externalization surgery. The animal can be positioned for imaging either for upright or inverted configuration. F–I - Setup for the upright configuration. F - Custom made animal stabilization stage with salivary gland holder. Lower panels show the details of SG holder. G - diagram showing the positioning of the animal for the upright configuration. Lowe panel shows the insertion of the coverglass. H - Diagram showing the animal in a side view on the stage. The objective equipped with a heater is brought on top of the coverslip covering the SG. I - Picture showing the microscope in the upright configuration. Objective and heater are mounted on to an objective inverter. J–L - Setup for the inverted configuration. J - A round 40 mm cover slip secured to a 35 mm inverted dish holder stage insert is pictured. Plan-Apo 60× water-immersion objective is visible. K - Diagram showing the 35 mm dish holder stage insert and a coverslip. L - Diagram showing the positioning of the animal for the inverted configuration. The animal is stabilized by a suture holding the incisors and several bars holding down the neck and the thorax areas. These stabilization devices are held in place by masking tape.
2.4 Animal holders and positioning devices
Custom-made immobilization stage (Fig. 1F). Alternatively, the stage can be assembled from components that are commercially available (Thorlabs, Newton, NJ): Aluminum Breadboard, 8"×8"×1/2", ¼-20 Threaded (catalog # MB8), Mini-Series Swivel Post Clamp (catalog # MSWC), Cage Assembly Rods, 4" Long, Ø6 mm (catalog # ER4), Thread Adapter, 1/4"-20 to #4–40 (catalog # MSA25) (see Note 5).
Custom-made submandibular salivary gland holder (see Fig. 1G and Note 5)
22 mm × 22 mm glass coverslips, # 1.5 and # 0
Stage insert for 35 mm dishes to accommodate a 40 mm glass coverslip (Olympus America, Center Valley, PA) (Fig. 1 J–L) (see Note 5)
Glass 40 mm round coverslips, # 1.5 (Bioptechs, Butler, PA)
Custom made salivary gland stabilizers for inverted configuration
Objective warmer system (Bioptechs, Butler, PA)
Gauze sponges 4"×4" (Tyco Healthcare, Gosport, UK) to be used as blankets
Disposable Foot Warmers (Heat Factory, Vista, CA)
2.5 Microscope
3. Methods
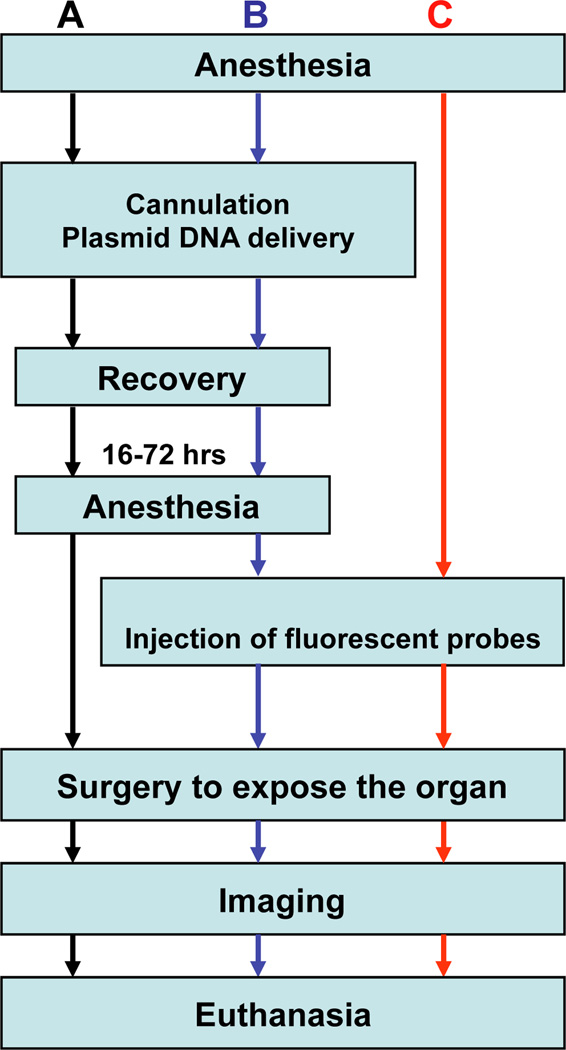
Subcellular structures can be labeled using different strategies such as systemic delivery of fluorescent probes, transfection of fluorescently labeled proteins, or a combination of both. A flow chart of the procedures described hereafter is provided in Fig. 2. Salivary glands can be imaged using either an upright or an inverted microscope. The choice depends on the characteristics of the available microscope. The upright configuration requires the use of custom-made holders, whereas the inverted configuration requires a particular care in immobilizing the animal.
Figure 2. Flowchart of common experimental procedures for intravital microscopy.
A - Workflow for imaging only the transfected cells in vivo. B - Procedures for imaging a combination of expressed fluorescent proteins and injected fluorescent probes. C - Workflow for visualizing fluorescent probes introduced into the SG tissue. Note, that the injection of the fluorescent probes can be performed while imaging.
3.1 Animals and Anesthesia
Rats are housed in a controlled environment in the local animal facility and one week prior to the experiments are moved and housed into the lab to acclimate (9). Water and food are provided ad libitum.
Weigh the animal prior to anesthesia.
Gently place the animal into the vaporizer chamber, and turn on the oxygen flow (800–900 mmHg). Set the Isofluorane level to 3% to proceed with the anesthesia induction (seeNote 8).
Once the animal is unconscious inject a mixture of 100 mg/kg ketamine and 20 mg/kg xylazine (see Note 1) into the peritoneal cavity (25-gauge needle)
Observe the animal for signs of wakefulness and inject more anesthetic mixture as needed (75 % of the initial dose within 45 min after the first injection and 50 % every hour thereafter (see Note 9).
Apply eye lubricant to prevent eye dryness.
Place the animal under the heat lamp or on a Foot Warmer pad.
Monitor the body temperature of the animals with the MicroTherma 2T Thermometer equipped with a RET-3 rectal temperature probe (see Note 10).
3.2 In vivo gene delivery to submandibular salivary gland
Secure the anesthetized animal into the stereotactic device with the mandibles wide open and the cheeks extended to the sides (Fig. 1B). The tongue should be folded towards the back of the mouth to expose the ductal orifices without obstructing the airways.
Incline the stereotactic device at about 45 degrees and adjust the stereo-microscope to visualize the area below the tongue.
Focus on the two orifices of the Wharton’s ducts. They will appear as two small flaps of tissue in the center of the area under the tongue (Fig. 1B).
Use bent sharp #7 tweezers to grab the PE-5 cannula close to the tip (see Note 4). Gently push the orifice with the tip of the cannula until it gets inserted into the duct (see Fig. 1B and Note 11)
Once the Wharton’s duct is cannulated, apply a small drop of histoacryl tissue glue to the orifice, and let it dry.
Prepare the transfection mixture according to the PEI manufacturer’s instructions. Typically, efficient transfection is achieved by using 12–24 µg of DNA/gland in a final volume of 100 µl (see Note 12).
Aspirate the transfection mixture with a syringe (30-gauge needle) and make sure that no air bubbles are released when injecting the fluid.
Carefully connect the needle to the cannula without piercing the tubing.
Inject the transfection mixture gradually over a 5 min time period.
Remove the cannula and the syringe from the mouth and allow the animal to recover in the cage. A warm environment should be provided to facilitate the recovery and the animal should be monitored for at least two hours.
Transfected cells can be visualized after 12 to 48 hours either by excising the glands from euthanized animals or by performing intravital microscopy (see Section 3.3–3.4 and Note 12)
The cannula can be also used to introduce fluorescent probes or pharmacological agents into the apical side of the epithelium of the SGs (see Note 13) (see Section 2.2 for probes and dilutions).
3.3 Insertion of the catheter in the tail artery
This procedure should be performed before preparing the animal for imaging and requires minimal surgical procedures when compared with the catheterization of the femoral or the jugular vein.
Place the anesthetized animal on its back into the custom-made immobilization stage (Fig 1G).
Secure the tail by clamping it down with two screw-down clamps - one at the very base of the tail and another 4–5 cm further down the tail (Fig. 1D).
Clean the area with 70 % ethanol. Carefully make a 3 cm incision along the midline of the tail with a scalpel, without cutting the underlying arteries or veins. The cut should be deep enough to reach the connective tissue. Clean any bleeding with gauze and saline as needed.
With a sharp scalpel carefully cut the thin layer of white connective tissue covering the tail artery. This is best accomplished by sliding the edge of the scalpel underneath the connective tissue sheath and cutting it from below. Be careful not to damage the artery.
Now that the artery is accessible, use blunt bent tweezers to isolate the artery from the rest of the tail. This is accomplished by placing the tips of the tweezer under the artery and sliding them up and down.
Using a suture, tie off the lower part of the artery. This suture will be used to provide tension on the artery.
Prepare a 1 ml syringe with normal saline connected to a 20 cm PE-5 tubing (via a 30-gauge needle). The end of the catheter should be cut at a 45 degrees angle.
Apply extra pressure to the clamp at the base the tail to stop the blood flow to the artery. Extend the artery by pulling slightly on the suture and make a 45 degree angle incision, about 1/3 into the artery.
Apply several drops of 2 % lidocaine solution to the area to dilate the artery.
Insert the catheter into the artery for about 1 cm and tie it with the suture. Make sure that the catheter is stable and well sutured to the tail, as some accidental pull on the catheter may dislodge it.
Release the clamps and inject 100–200 µl of saline. Saline should be injected every 30 minutes to ensure that the artery does not clog.
Now any fluorescent probe can be delivered to the systemic circulation (see Section 2.2 for probes and dilutions).
3.3 Animal surgery and positioning for intravital microscopy
Wipe down the ventral side of the neck area of the anesthetized animal with a gauze sponge soaked in 70 % ethanol. Dry the excess of ethanol with a Kim wipe.
With dull tweezers lift up a small piece of skin at the midline, approximately at the lower side end of the masseter muscles and make a small incision (see Note 14).
Insert the scissors into the opening and separate the skin away from the underlying tissue by opening the scissor blades. Avoid damaging the underlying glandular tissue. Repeat this step several times until the scissors are free to move under the skin.
Using a pair of scissors excise a strip of loosened skin about 0.5 cm wide and 2 cm long.
With a syringe apply the optical coupling gel into the middle of the cut and wipe it towards the outside with sterile gauze. Use new gauze for each wipe to prevent introduction of hair or blood onto the glands. Repeat this procedure until all loose hairs are removed and bleeding has stopped.
Using #7 tweezers separate the connective tissue away from the submandibular gland all the way to the base of the gland (see Note 15).
If intra-stromal delivery of fluorescent probes is desired, the glands can be bathed in situ with pre-warmed probe solution (see section 2.2 for probes and dilutions)
If setting up for intravital imaging in the upright configuration, follow the instructions below, otherwise skip to point #14.
Place a layer of gauze onto the custom-made immobilization stage. Cut the ends of a Foot Warmer packet to allow the air in. Place the packet onto the gauze and cover it with another piece of thin gauze (see Note 16).
Position the animal on its back on the stage and secure the salivary gland holder (Fig. 1F and 1G). Add some optical coupling gel inside the holder.
Position the body of the animal and the holder to minimize the angle between the body and the gland. This will prevent a reduction in the vascular flow. Place the gland into the holder with the ventral side of the gland facing up.
Insert the glass coverslip into the holder with gentle pressure so that the gland is stabilized. Avoid compressing the gland, which may result in restricting the blood flow (see Note 17).
Cover the animal with another layer of gauze and move the whole stage assembly on the upright microscope stage (Fig. 1G–I).
For imaging in the inverted configuration, place the gauze and the Foot Warmer pack directly onto the microscope stage fitted with an 35 mm dish insert and a 40 mm coverslip (Fig. 1J and 1K). Cover the warming pack with a thin piece of gauze.
Place the animal on its side and onto the gauze with the warming pack. Position the animal with the submandibular salivary gland placed in the middle of the coverslip.
The animal has to be secured and stabilized in this position before immobilizing the gland (see Fig. 1L and note 18).
Place a small piece of lens cleaning tissue over the gland. Extend the gland slightly and place a customized stabilizer over the gland. Tightly couple the stabilizer to the stage with masking tape (see Note 19).
Cover the animal with a gauze and image.
3.4 Imaging parameters
The modality of imaging (inverted vs. upright and two-photon vs. confocal) should be chosen in advance.
Using epifluorescence illumination focus on the surface of the gland. If no fluorophores were injected the glands could be visualized using a DAPI filter set to reveal the autofluorescence of the tissue.
Assess the health status of the tissue by visualizing the blood flow in the capillaries and then the overall morphology of the tissue. Signs of tissue damage include membrane blebbing, improper organelle aggregation, and sluggish motility of organelles or cells.
Assess the stability of the tissue either via epifluorescence or pre-scanning the selected area. A stable preparation is essential, since most of the motion artifacts cannot be removed or corrected for in post-processing data analysis. If the preparation is not stable, adjustments are required.
Determine the scan speed (temporal resolution) and the duration of the time-lapse acquisition (see Note 20).
Determine the number of pixels acquired vs. the scan area zoom (spatial resolution) (see Note 20)
Determine the slice thickness by adjusting the pinhole (for confocal acquisition only). The idea is to find an acceptable balance between a good confocality effect and enough signal to form a clear image. Depending on the sample, we normally image using a theoretical optical slice thickness between 0.8 and 1.2 µm.
Determine the excitation laser power (see Note 21).
Adjust the detector settings to make sure that the intensities of the structures of interest fall within the dynamic range of the detectors.
Once all these parameters are set, imaging can be started. While imaging, drifting of the tissue in XY and Z may occur. This can be manually compensated by visually assessing the area and adjusting the scan area and Z position. The adjustments should be made gradually and by small increments to avoid motion artifacts.
Fluorescent probes can be introduced either into the circulation via tail artery catheter or into the ductal system via the cannulation of the Wharton’s duct as described earlier.
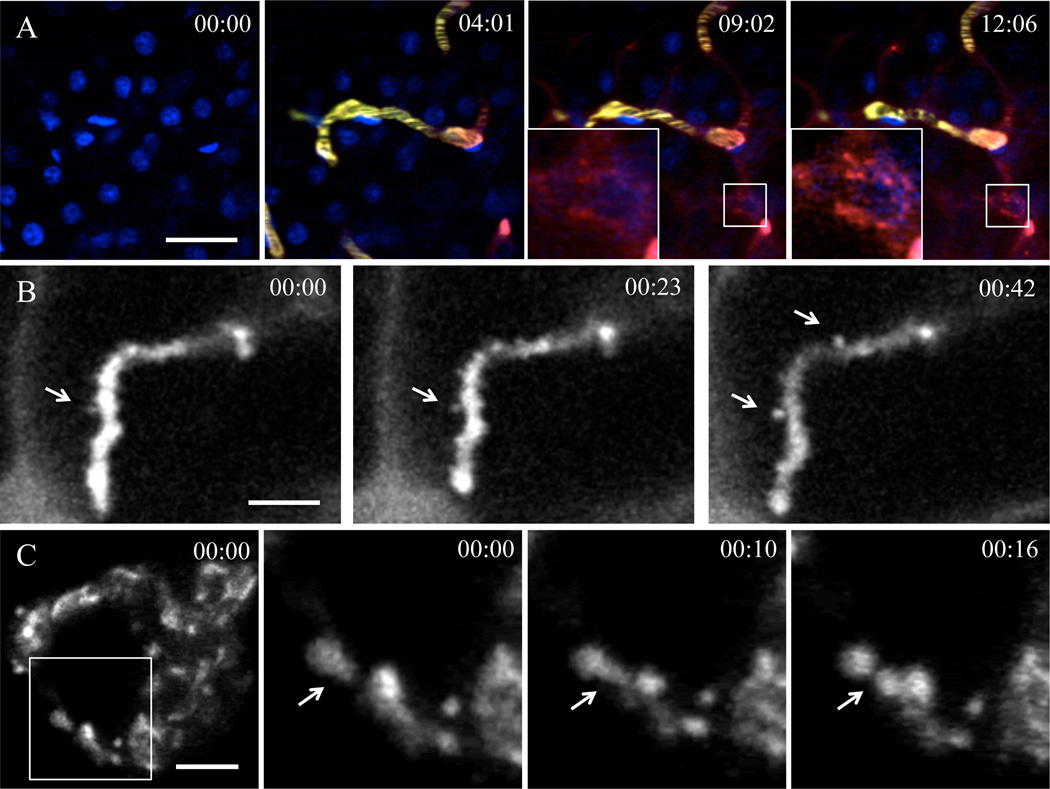
We have successfully observed a number of membrane trafficking events with this setup. For example, we followed endocytosis and endocytic vesicle fusion and probe trafficking trough endosomal pathways (Fig. 3A) (2). In addition, compensatory endocytosis can be visualized by labeling the apical plasma membranes or the fluid phase (4). Exocytosis of the secretory granules can also be observed in the acinar cells upon stimulation with adrenergic agonists (Fig. 3B) (6). Lastly, a number of traficking events, such as the dynamics of the TGN can be followed by transfecting the acinar cells with plasmid DNA (Fig. 3C).
Figure 3. Imaging subcellular structures in live animals.
A - Endocytosis of fluorescently labeled dextran visualized in the salivary glands of live rats. Still images from a timelapse are shown. Tail artery of an anesthetized rat was cannulated and connected to a syringe. First, the animal was injected with Hoechst dye to label the nuclei (time 00:00), and imaged in time-lapse mode by using multiphoton microscopy. After 4:00 min a Texas-red 70 kDa dextran was injected (time 04:01) to image the endocytic process. Endocytic structures containing Texas-red 70 kDa dextran appeared within minutes after the injection and they increased in number and in size over time. Excitation wavelength 820 nm. Acquisition speed was 2 s per frame. Time is shown in minutes : seconds. Scale bar - 20 µm. B - Exocytosis of salivary protein containing granules visualized in acinar cells of live rats. The Wharton’s duct of an anesthetized rat was cannulated and filled with Alexa 647-10 kDa dextran to label the acinar canaliculi (apical space in between the acinar epithelial cells). 0.1 mg/kg of Isoproterenol was injected subcutaneously into the animal to stimulate salivary secretion and one minute later the time lapse acquisition was started (time 00:00). The exocytic granules fused with the plasma membrane were filled with dextran (arrows). Excitation wavelength 633 nm. Acquisition speed was 220 ms per frame. Time is shown in minutes : seconds. Scale bar - 5 µm. C - Dynamics of a the TGN in salivary glands of a live rat. The Wharton’s duct of an anesthetized rat was cannulated and the glands were transfected with plasmid DNA encoding TGN38-RFP, a marker for the TGN. The next day the salivary glands were imaged by confocal time-lapse mode. Fission events of TGN38-RFP containing membranes can be observed. Excitation wavelength 561 nm. Acquisition speed was 2 s per frame. Time is shown in minutes : seconds. Scale bar - 5 µm.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute of Dental and Craniofacial Research.
Footnotes
Anesthetics are prepared fresh by diluting 100 mg/ml Xylazine to 20 mg/ml in normal saline and then mixing it with Ketamine. The amounts in the mixture are determined by the weight of the animal.
0.3% Carbomer-940 gel is prepared by warming up 100 ml of ultra-pure water and dissolving 5.47 g of D-Sorbitol. Then 0.3 g of Carbomer-940 is added. The beaker should be covered and placed on stirring hot plate (lowest heat setting) for several hours or until all the carbomer clumps have completely dissolved. Lastly, Trietanolamine is added drop-wise while stirring gently until a pH of about 7.4 is reached. The gel is stored at 4°C (10).
Instruments should be cleaned and sterilized before use.
The cannulae should be cut at about 5 cm length with the tip beveled at a 45-degree angle.
The salivary gland holder should be mounted onto the immobilization stage. This setup is used for upright imaging configuration whereas a 35 mm dish stage insert is used for inverted configuration.
If the cells to be imaged are located within the first 20–30 µm from the surface, use confocal laser-scanning microscopy, which achieve higher resolution than multiphoton microscopy (11). Multiphoton microscopy is used for imaging deeper in the tissue or to excite endogenous fluorescence (2, 5, 12)
High NA objectives have to be used for high-resolution imaging. A Plan-Apo 60×, 1.2 NA, water-immersion objective works exceptionally well in both confocal and multiphoton modalities. We also use a Plan-Apo 60× / 1.42 NA oil objective for confocal imaging at depths of up to 10 microns.
We find that the stress induced by administration of injectable anesthetics may induce some undesired effects, such as salivation. Therefore, we first induce anesthesia with isoflurane and then switch to injectable anesthetics.
Animals should be carefully monitored for signs for wakefulness, which may manifest as slight whisker movements and sensitivity to touch by a sharp object. To perform the sharp object test, extend one of the legs of the animal and touch the paw with light pressure using sharp tweezers. Muscle contraction would indicate that anesthesia has worn out.
Anesthesia induces hypothermia, which can adversely affect animal health, survival and reproducibility of experiments. Therefore, the body temperature should be monitored and heat should be provided to the animal as needed. The temperature is measured using a rectal probe and should be maintained around 37 ± 1 °C by adjusting the heat provided.
The opening of the duct is roughly at the tip of the orifice. The tip should be probed gently to invert the orifice outside-in into the underlying mucosa. The beveled tip direction of the cannula can be rotated every few minutes, so that the orifice gets stretched evenly from different sides. Be careful not to apply too much pressure at once, as the mucosa can be ruptured damaging the duct. Normally if the duct is damaged, bleeding will be observed from the injured mucosa.
As reported in (2), plasmid expression peaks at 12 to 24 hours after transfection. Cells can be imaged in situ by intravital microscopy or alternatively transfection can be tested in excised tissue. Freshly excised tissue is stable for about 30 minutes post-excision. This is especially useful for 3D stack acquisitions. Imaging time can be extended if the tissue is cooled to 4 °C.
Longer cannulae (30 cm) can be used to deliver fluorescent markers or pharmacological agents.. The infusion is driven by gravity with the syringe raised approximately 25 cm above the animal and opened to the atmosphere. For fluorescent probes, a solution of 100 µl is prepared at the concentrations listed in Table 1.
The skin should be cut so that the final opening starts near the base of the submandibular gland where the nerve, the duct and the blood vessels connect the gland to the rest of the body. The resultant opening after cleaning the wound area should be oval in shape and measure roughly 1.5×2.5 cm. Both submandibular glands should be visible in the opening.
Using two bent #7 tweezers find the tip of the submandibular gland and grab the connective tissue few millimeters away from the tip. Tear the tissue around the gland. Once the gland is exposed, gently separate the connective tissue from the gland. If bleeding occurs, wash the blood away immediately with normal saline. Make sure that all the connective tissues are separated and the gland is exposed fully. The degree of freedom will be important for the stabilization step. Do not allow the surface of the gland to dry by applying optical coupling gel.
The heat intensity and duration of the Foot Warmer packs depends on the exposure to air oxygen. Cutting both ends and one side of the packet is sufficient to provide heat to the animal for several hours at reasonable intensity. The temperature of the animal should be monitored until results are reproducible.
The connection of the gland to the body should be loose and flexible, without much strain or harsh angles. The holder should be straight and tightly coupled to the immobilization stage to stabilize the glandular tissue. The stability may be improved by additional immobilization of the head and the thorax by stabilizing bars coupled to the stage. Blood flow should be assessed after placing the coverslip by examining the gland visually (it should retain pink coloration).
Stabilization of the animal for inverted configuration is more challenging and requires several custom-made accessories. Most of them are modified from common labware. The accessories are coupled to the stage by masking tape resulting in semi-rigid connections. These “devices” then are then gently pressed against the most problematic areas of the body, such as the head and the thorax (Fig. 1L).
A Gland stabilizer is cut from a piece of plastic and fitted with four spacers to accommodate the thickness of the submandibular gland (2–3 mm). Similarly to the upright configuration, this stabilization should avoid inducing any sharp angles or tensions onto the gland. The blood flow should be inspected via epi-fluorescence immediately after stabilization to ensure viability of the tissue.
The settings for the spatial resolution are closely tied to the temporal resolution. Most membrane trafficking processes occur within a second (for example, endosomal fusion) requiring the highest possible scanning speed. We mostly use scanning speeds ranging from 200 ms up to 1 s per frame. We acquire about 10 pixels per micron in the sample, which yields about 100 nm pixels. This is well beyond the diffraction limit of the optics, and we find that some oversampling improves the final quality of the images. When high spatial and temporal resolution scans are required, high zoom of the scan area is used. For example, using a 60× objective, a 16× scan area zoom, and 128×128 pixel image will yield 0.103 µm/pixel spatial scale. With these settings, when pixel dwell time is set to 4 µs/pixel the resultant scan speed is close to 200 ms per frame,.
The optimal laser power has to be empirically determined by performing a test acquisition for the required length. If signs of phototoxicity or photo-bleaching are observed the power should be reduced.
References
- 1.Amornphimoltham P, Masedunskas A, Weigert R. Intravital microscopy as a tool to study drug delivery in preclinical studies. Adv Drug Deliv Rev. 2011;63:119–128. doi: 10.1016/j.addr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weigert R, Sramkova M, Parente L, Amornphimoltham P, Masedunskas A. Intravital microscopy: a novel tool to study cell biology in living animals. Histochem Cell Biol. 2010;133:481–491. doi: 10.1007/s00418-010-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol. 2002;283:C905–C916. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- 4.Sandoval RM, Kennedy MD, Low PS, Molitoris BA. Uptake and trafficking of fluorescent conjugates of folic acid in intact kidney determined using intravital two-photon microscopy. Am J Physiol Cell Physiol. 2004;287:C517–C526. doi: 10.1152/ajpcell.00006.2004. [DOI] [PubMed] [Google Scholar]
- 5.Masedunskas A, Weigert R. Intravital two-photon microscopy for studying the uptake and trafficking of fluorescently conjugated molecules in live rodents. Traffic. 2008;9:1801–1810. doi: 10.1111/j.1600-0854.2008.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masedunskas A, Sramkova M, Parente L, Sales KU, Amornphimoltham P, Bugge TH, Weigert R. Role for the acto-myosin complex in regulated exocytosis revealed by intravital microscopy. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1016778108. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sramkova M, Masedunskas A, Parente L, Molinolo A, Weigert R. Expression of plasmid DNA in the salivary gland epithelium: novel approaches to study dynamic cellular processes in live animals. Am J Physiol Cell Physiol. 2009;297:C1347–C1357. doi: 10.1152/ajpcell.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masedunskas A, Weigert R. Internalization of fluorescent dextrans in the submandibular salivary glands of live animals: a study combining intravital two photon microscopy and second harmonic generation. Progress in Biomedical Optics and Imaging - Proceedings of SPIE. 2008;6860:1605–7422. [Google Scholar]
- 9.Peter B, Van Waarde MA, Vissink A, s-Gravenmade EJ, Konings AW. Degranulation of rat salivary glands following treatment with receptor-selective agonists. Clin Exp Pharmacol Physiol. 1995;22:330–336. doi: 10.1111/j.1440-1681.1995.tb02010.x. [DOI] [PubMed] [Google Scholar]
- 10.Rothstein EC, Nauman M, Chesnick S, Balaban RS. Multi-photon excitation microscopy in intact animals. J Microsc. 2006;222:58–64. doi: 10.1111/j.1365-2818.2006.01570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaspro A, Shepard CJR. Two-Photon Microscopy: Basic Principles and Architectures. 2002 [Google Scholar]
- 12.Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci U S A. 2003;100:7075–7080. doi: 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]