Abstract
We report a new case of p63/cytokeratin 7 (CK7) positive syringocystadenocarcinoma papilliferum (SCACP), on the shoulder of an 88-year-old man, with superficial dermal infiltration and squamoid differentiation. We describe the 24th case of SCACP, the malignant counterpart of syringocystadenoma papilliferum (SCAP). At the present, we do not know whether SCACP arises from eccrine or apocrine glands because of the contrasting opinions in the literature. Only few histochemical and ultrastructural studies have previously advised that SCACP could arise from pluripotent stem cells. Through our case, we wish to suggest the stem cell-like properties of the syringocystadenocarcinoma papilliferum. This rare neoplasm shows two different patterns of stem cell marker expression in the glandular and squamous components, respectively. For the double phenotype of SCACP, we propose it like an intriguing model to study histogenesis and stem cell properties for more wide-ranging epithelial tumors.
1. Introduction
Syringocystadenocarcinoma papilliferum (SCACP) is a rare malignant form of syringocystadenoma papilliferum (SCAP), cutaneous adnexal neoplasm defined by Stokes in 1917. Some authors are unsure about the malignant transformation in syringocystadenoma papilliferum but, recently, Hoekzema et al. [1] have presented a well-documented SCACP case arising from preexisting SCAP, which appeared to be an epidermal nevus. SCAP mostly occurs in the head and neck region and is associated with a nevus sebaceous (NS) of Jadassohn in 30–40% of cases, but may also appear de novo (without NS) on other parts of the body.
There is an increasing evidence for an apocrine histogenesis of SCAP and SCACP [2] but the possibility of an eccrine origin from sweat glands for a few cases seems likely. Another theory suggests the apoeccrine glands to be the origin of these tumors that could be skin hamartomas [1, 3]. Very few histochemical and ultrastructural studies propose that SCACP arises from pluripotent cells [1, 3, 4]. A little map of the different theories is shown in Table 1.
Table 1.
Histogenetic theories of syringocystadenocarcinoma papilliferum.
| Source, year | Eccrine and/or apocrine histogenesis |
|---|---|
| Dissanayake and Salm, 1980 [5] | Eccrine: in situ carcinoma |
|
| |
| Dissanayake and Salm, 1980 [5] | Eccrine |
|
| |
| Seco Navedo et al., 1982 [6] | Apocrine |
|
| |
| Numata et al., 1985 [7] | Apocrine |
|
| |
| Bondi and Urso, 1996 [8] | Eccrine |
|
| |
| Ishida-Yamamoto et al., 2001 [4] | In situ carcinoma, near the anal apocrine glands, small number of tumor cells diastase-resistant, origin from a pluripotential germinative cell |
|
| |
| Arai et al., 2003 [9] | In situ carcinoma, diastase-resistant PAS-positive |
|
| |
| Chi et al., 2004 [10] | Either pluripotential appendageal cells or primitive apocrine glands |
|
| |
| Woestenborghs et al., 2006 [11] | Eccrine: in situ carcinoma |
|
| |
| Kazakov et al., 2007 [12] | In situ carcinoma arising in association with SCAP and sebaceous carcinoma |
|
| |
| Park et al., 2007 [13] | SCACP originates from apocrine glands |
|
| |
| Langner and Ott, 2009 [14] | In situ carcinoma, luminal columnar cells with decapitation secretion |
|
| |
| Kazakov et al., 2010 [15] | Apocrine: 5 new cases: in situ carcinoma and invasive tumors; squamous differentiation |
|
| |
| Leeborg et al., 2010 [2] | Apocrine: in situ carcinoma and invasive tumor; squamous differentiation |
|
| |
| Sroa et al., 2010 [16] | Eccrine |
|
| |
| Aydin et al., 2011 [17] | Eccrine |
|
| |
| Hoekzema et al., 2011 [1] | Apocrine: from pluripotent cells |
|
| |
| Hoguet et al., 2012 [18] | Apocrine |
|
| |
| Zhang et al., 2012 [19] | Apocrine with squamous differentiation |
|
| |
| Present case, 2014 | Apocrine: from pluripotent cells by epithelial mesenchymal transition |
Histologically, the connection to the skin surface, by typical epidermal transition, the decapitation on the luminal surface of inner layer cells, the tumor connection to folliculosebaceous structures, the layering of apocrine glands in the underlying tissues, and positive reactions to diastase-resistance periodic acid-Schiff (PAS) provide evidence of apocrine differentiation of SCACP [1, 2].
Herein, we report a case of SCACP occurring on the left shoulder of an 88-year-old European male patient.
2. Materials and Methods
2.1. Case
An 88-year-old man presented with a single nodule on the left shoulder. He had had multiple lesions for 20 years of bowenoid actinic keratosis on the face, the forehead, and the left ear. In the last 13 years, the patient underwent prostatectomy for adenocarcinoma, score 8 (5+3) according to Gleason and squamous cell carcinoma on the left eyelid surgically treated. The man stated that the lesion began as a bean-sized papule and had increased gradually in size with time. It also became painful. Physical examination revealed a single, 1.5 × 1.5 cm, erythematous, dome-shaped, and firm tumor surrounded by normal skin on the left shoulder. Regional lymph nodes were not palpable, and the physical examination did not reveal any remarkable findings except for the mass. The patient had no clinical or radiographic evidence of a head and neck or lung primary tumors. He died after two years of old age.
2.2. Histology
The present skin tumor was clinically analyzed and then resected tumor specimens were fixed in 10% buffered formalin and embedded in paraffin. Sections of 5 μm were subjected to conventional microscopic histochemical and immunohistochemical observations.
PAS reaction, with and without diastase digestion, was also carried out.
For the immunostaining, we used an automatic and clinically validated instrument based on Ventana Benchmark Ultra systems from Roche Tissue Diagnostics. Immunohistochemistry was performed by means of a new enhanced sensitivity biotin-free multimer technology system, based on direct linkers between peroxidase and secondary antibodies (ultraView Universal DAB Detection Kit, Ventana Medical System). The following primary antibodies were used prediluted: antipancytokeratin (PCT), anticytokeratin 7 (CK7), anticytokeratin 5/6 (MES), anti-e-cadherin (epithelial) (e-cad), antiepithelial membrane antigen (EMA), anticarcinoembryonic antigen (CEA), anti-p63, anti-S100 by Ventana; anti-CD117 (c-kit) (Cell Marque), and anti-Ki-67 antigen (MIB-1) by DAKO.
For immunofluorescence, sections were dewaxed, rehydrated, as described above, and unmasked using a commercially available kit (Unmasker, Diapath). Slices were incubated with Immunological Sciences primary antibodies, mouse monoclonal anti-CD44 1 : 50, and rabbit polyclonal anti-CD133 4 μL/mL and then incubated with Molecular Probes secondary antibodies, 1 : 100, goat anti-mouse, green fluorescent Alexa Fluor 488-conjugated and goat anti-rabbit, and red fluorescent Alexa Fluor 594-conjugated and finally counterstained with 0.0001% DAPI (4′, 6-diamidino-2-phenylindole), in accordance with the preview published protocol [20].
3. Results
All the immunoprofiling data about the normal skin, the benign lesion, and the carcinomatous component are summarized in Table 2.
Table 2.
Antigen expression of syringocystadenocarcinoma papilliferum.
| Immunohistochemical studies | Normal skin epithelium | Syringocystoadenoma | Syringocystoadenocarcinoma |
|---|---|---|---|
| PCT | Completely positive | Completely positive | Positive outer layer of budding; heterogeneously stained glandular component with accentuation of the membranes |
|
| |||
| CK5-6 | Positive basal and spinous layers | Positive basal layer | Strongly stained transition area from stratified squamous epithelium to neoplasia; heterogeneously and moderately stained squamous solid component and glandular structures |
|
| |||
| CK7 | Negative | Negative | Positive transition area from stratified squamous epitelium to neoplasia; negative squamous solid neoplasia with weak speckled area; positive glandular budding and papillary components |
|
| |||
| e-cad | Positive epidermidis epithelium; negative keratin layer | Increased pericytoplasmic positivity of the transition area from stratified squamous epitelium to neoplasia with focally negative neoplastic cells | Decreased staining of the neoplastic glands and papillary component and negative infiltrative portion |
|
| |||
| EMA | Negative | Focally positive | Strongly stained transition area and glandular and papillary components |
|
| |||
| CEA | Negative | Focally positive | Negative |
|
| |||
| p63 | Almost positive throughout the whole epidermis epithelium, with the strongest expression in basal and suprabasal layers | Positive in discontinuous basal layer | Strongly stained transition area from stratified squamous epithelium to neoplasia; highly positive squamous neoplasm and basal layer of glandular component; moderately positive papillary neoplasia |
|
| |||
| c-kit | Positive basal layer of epidermis | Positive basal layer of neoplasia | Positive; highly positive basal layer of neoplastic budding; heterogeneously intense-moderate amount of staining of glandular and papillary structures; almost negative, limited occurrence and speckled staining of the squamous-solid component; some positive spindle cells in perineoplastic stroma |
|
| |||
| S100 | Focal positivity of basal layer | Focal positivity | A few positive cells in the squamous component and in perineoplastic stroma |
|
| |||
| K67 | Focal positivity of basal layer | Moderately positive | Completely positive |
|
| |||
| Nestin | Negative; positive-endothelial cells and hair follicles | Negative; positive-endothelial cells and hair follicles | A few positive cells in the squamous component and in perineoplastic stroma |
|
| |||
| CD133 | Almost negative; very rare positivity in the granular layer epidermis | Moderately positive | Aberrant cytoplasmic overexpression, more intense in the glandular and papillary components |
|
| |||
| CD44 | Transmembrane positivity with the strongest expression in basal layers | Faintly positive | Increasing of pericytoplasmic expression in the neoplastic squamous component; almost unstained glandular component; positivity in the stroma |
Hematoxylin and eosin staining of histological sections showed squamous cancer cells in the dermis to be gradually deepening. The squamous budding generated internal cavities giving rise to glandular papillary structures. These tumoral squamous-papillary glands were positively stained by anti-PCT antibody in the outer layer of budding; the glandular component was heterogeneously stained with accentuation of the membranes. Anti-CK5/6 strongly stained the transition area from stratified squamous epithelium to neoplasia; the squamous solid component and the glandular structures were moderately stained. The transition area from stratified squamous epithelium to neoplasia was strongly positive for CK7; the glandular budding and the papillary components were intensely stained by CK7 antibody; contemporaneously, the squamous solid neoplasia appeared negative. E-cad staining showed an increase of pericytoplasmic positivity in the transition area from stratified squamous epithelium to neoplasia but completely negative was the infiltrative portion. Neoplastic areas, in contrast with normal epidermis, resulted in totally stained EMA. Syringocystadenocarcinoma was completely negative for CEA and robustly positive with patchy nuclear for p63 staining. By anti-c-kit antibody, we obtained highly positive staining of basal layer of neoplastic budding; the glandular and papillary structures were heterogeneously positive but almost negative and/or speckled positive was the squamous solid component. The evident desmoplastic stromal reaction accompanied the budding and contained numerous plasma cells and lymphocytes. The epithelial transition and the decapitated secretion provided evidence of apocrine differentiation; the cytological atypia gave a precious suggestion of the neoplasm. An invasive squamous component coexisted with glandular elements typical for adenocarcinomas. The dual p63-CK7 immunohistochemical positivity was useful in confirming the diagnosis definitive of syringocystadenocarcinoma papilliferum.
Nestin, CD44, and CD133 antigens, markers of stem cell properties, were overexpressed in the neoplasm and could suggest a tumor origin by pluripotent stem cells.
4. Discussion
We report a second case of syringocystadenocarcinoma with some unique histologic features; preview described by Leeborg et al. [2] demonstrates the p63 positive-gradual transition area from keratinizing squamous epithelium to neoplasm (Figures 2(a) and 2(b)). Syringocystadenocarcinoma papilliferum is a rare neoplasm of doubtful (apocrine versus eccrine) origin and is the malignant counterpart of the more common benign syringocystadenoma papilliferum. Histologically, syringocystadenocarcinoma papilliferum resembles syringocystadenoma papilliferum and solid areas of tumor may be present, in addition to the glandular, cystic, and papillary areas that deepen like dermal cleft by a squamous transition epithelium (Figures 1(a) and 1(b)). The glandular epithelial cells show malignant cytological features, such as high nuclear to cytoplasmic ratios and nuclear irregularity, and, in particular, they are PAS diastase positive, with decapitated cytoplasm, confirming the apocrine origin. Our case is distinctive in several aspects. It is the second report, to our knowledge, to document the presence of a high-grade cell component originally considered to be a high-grade squamous cell carcinoma and then demonstrated adenocarcinoma [2]. It is the first case to provide immunohistochemical evidence of stem cell features and epithelial-mesenchymal transition- (EMT-) like properties.
Figure 2.
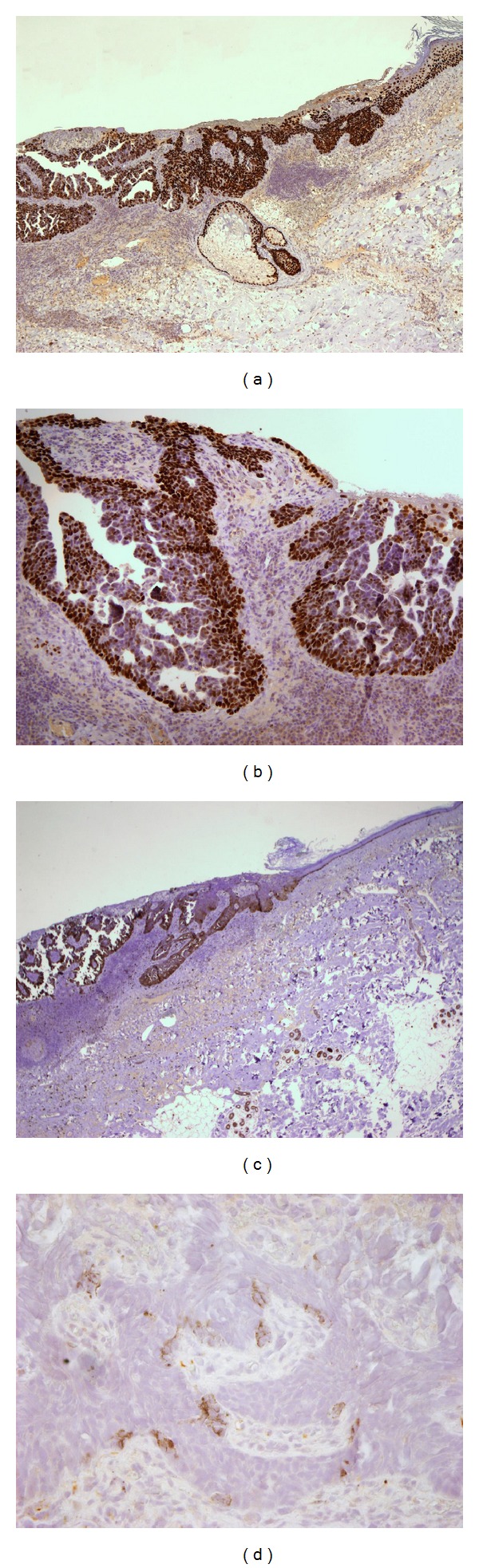
Analysis of a skin syringocystadenocarcinoma papilliferum specimen. (a) p63 antibody stains the transition area from epidermis epithelium to the squamous neoplasm and the basal layer of glandular component (original magnification ×40). (b) The papillary structures are moderately positive and/or negative for p63 (original magnification ×100). (c) Immunohistochemical staining demonstrates c-kit at the basal layer of neoplastic budding; almost negative, only with speckled positive cells, is the squamous solid component (original magnification ×20). (d) A few positive cells of the squamous neoplasia are stained by Nestin antibody, stemness marker (original magnification ×400).
Figure 1.
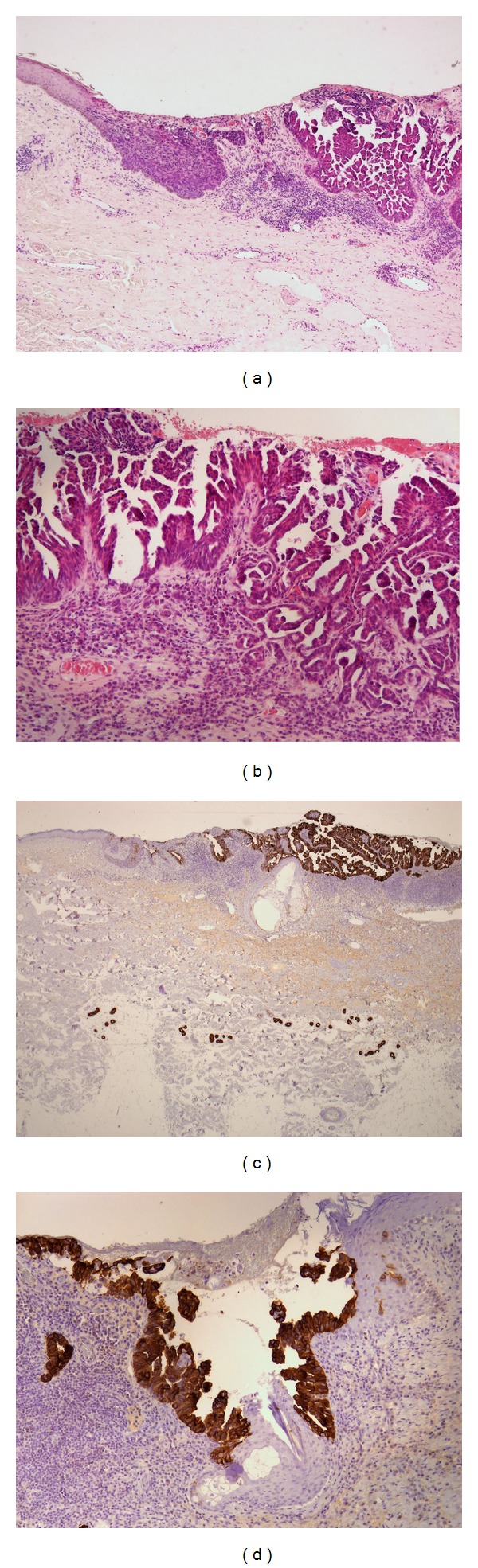
Analysis of a skin syringocystadenocarcinoma papilliferum specimen. (a) Specimen shows squamous cancer cells in the dermis gradually deepening. The squamous budding generates internal cavities giving rise to glandular papillary structures (hematoxylin-eosin [H&E], original magnification ×40). (b) Note the adenopapillary infiltrative structures ([H&E], original magnification ×100). (c) Immunohistochemical staining demonstrates CK7 at the glandular budding forming the papillary components (original magnification ×40). (d) The squamous solid neoplasia persists negative for CK7 (original magnification ×100).
To analyze the biological and carcinogenetic property of our tumor case, we have used a series of antibodies fit for recognizing epithelial expression, proliferation index, and stem cell properties.
Like Leeborg et al. suggested [2], we have found CK7 and p63 positive-cells in continuous stream from normal epithelium to neoplastic adenocarcinoma clefts, that is, the squamous-glandular transdifferentiation of the tumor (Figures 1(c), 1(d), 2(a), and 2(b)). Furthermore p63 positivity supports the primary origin of the squamous and/or cutaneous-adnexal tumor and distinguishes it from metastatic adenocarcinoma [21]. Simultaneously, we have demonstrated, for the first time, a possible staminal origin of syringocystadenocarcinoma papilliferum by c-kit, nestin, CD44, and CD133 immunopositivity.
The transmembrane tyrosine kinase receptor c-kit (CD117) is a 145–165-kD proto-oncogene, related to platelet-derived factor receptor and the colony stimulatory factor receptor. The primary ligand for c-kit is stem cell factor (SCF) or also named mast cell growth factor and steel factor. Evidence supports [22] the existence of CD117 as putative marker of mesenchymal stem cells and/or cancer stem cells. Overexpression of c-kit has been demonstrated in several human tumors such as gastrointestinal stromal tumor (GIST), small-cell lung cancer, colorectal cancer, Ewing's tumor, and chronic myelogenous leukemia (CML). Other c-kit positive-normal cells include epithelial cells in skin adnexa, breast, and subsets of cerebellar neurons.
In our case, we observed c-kit positive-epidermal epithelium basal layer connected with neoplastic budding by c-kit positive-tumor cells that presented the same pattern of positivity for CK7 and p63, too (Figures 2(c), 1(c), and 2(a)). The positivity for c-kit was robust and more intense in the basal layer of the glandular neoplastic budding, in continuity with the c-kit positive-normal basal layer of epithelium. We observed heterogeneously intense-moderate amount of staining of glandular and papillary structures; almost negative, with limited occurrence and speckled positivity appeared the squamous solid component. Interestingly, we found some positive spindle cells in perineoplastic stroma (see Figure S1 in Supplementary Material available online at http://dx.doi.org/10.1155/2014/453874). Recently, it has been reported that c-kit plays a critical role in the invasion and metastasis of salivary adenoid cystic carcinoma (ACC) and may participate in EMT of salivary ACC [23]. Furthermore e-cad staining was interestingly expressed (data not shown). We found, as recent literature has proposed [24], a different level of this central component of cell junctions-adhesion molecule: plasmalemmal positivity in the normal tissue and in the transition area from stratified squamous epithelium to neoplastic budding with occasionally negative neoplastic cells; we observed a gradual decreasing of e-cad positivity into neoplasia, with focally negative neoplastic glands. Totally unstained appeared the invasive papillary component. All of these data together either with c-kit pattern or with the spindle cell morphology in the front of infiltration and the surrounding desmoplastic stromal reaction (Figures 2(c), S1, and 1(b)) could be convincing features of EMT-like process [25, 26]. The EMT paradigm is a developmental pathway by which epithelial cells are transdifferentiated to mesenchymal cells during embryogenesis, tissue remodelling, and wound healing. Recently, an interesting hypothesis considers EMT as a more general event that has been implicated in all types of carcinoma and provides them with an additional survival advantage. By means of EMT, the epithelial tumor cells would transdifferentiate into myofibroblasts, losing their malignant phenotype but producing the desmoplastic stroma which is essential for tumor growth, invasion, and metastasis. In human cancer, the phenomenon is rarely demonstrated because, after the infiltrative spreading, it is fast reverted by mesenchymal epithelial transition (MET) program, in cancerous epithelial phenotype [27–29]. It has been observed that, during the EMT process, cancer epithelial cells acquire stem cell-like traits and appear positive for putative cancer stem cell (CSC) markers, resulting in a migratory cell phenotype [30, 31]. So EMT-like properties of carcinoma are more persuasive, when they are associated with an increase of putative stemness marker expression. For this reason, we have analyzed nestin, CD44, and CD133 antigens [21, 24, 32–34].
Nestin, VI intermediate filament protein and marker of precursor cells, is also expressed in the stem cells of the hair follicle and in the endothelial cells; moreover, nestin is expressed in the peritumoral stroma of basal cell carcinomas [21, 33]. In our case, we found in bulge squamous area of neoplasia some nests of nestin-positive stroma cells with mesenchymal phenotype (Figure S2) but also convincing clusters of nestin positive-cancer stem cells in the epithelial neoplasia (Figure 2(d)).
The CD44 antigen is a cell-surface glycoprotein involved in cell-cell interactions and cell adhesion and migration, for these properties participate in a wide variety of cellular functions including lymphocyte activation, recirculation and homing, hematopoiesis, and tumor metastasis. Recently CD44 has been recognized as a marker of putative cancer stem cells [35], in particular of squamous phenotype; CD44 high expression plays a crucial key role in initiation, malignant transformation, and EMT-like program [24, 32, 36]. An interesting aspect of our case consists of evident overexpression of CD44 in squamous component of neoplasia, with spreading positivity in the perineoplastic stroma cells(Figure S3). Almost completely negative for CD44 appeared the glandular and papillary components (Figure 3).
Figure 3.
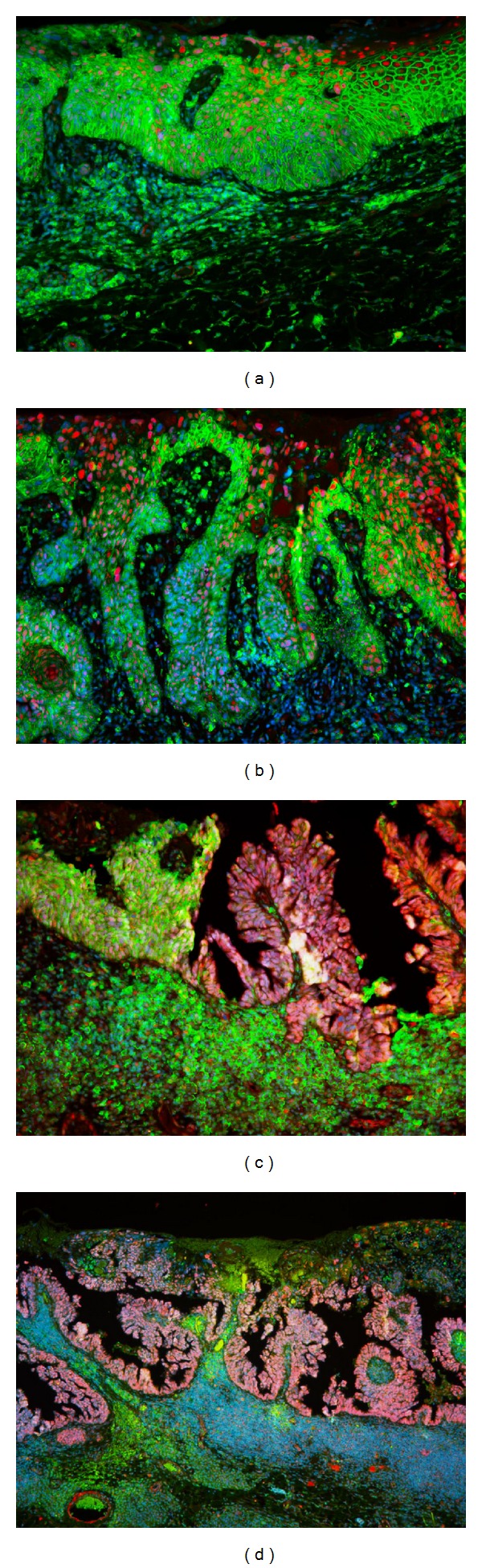
Fluorescence microscopy analysis of the staminal property of syringocystadenocarcinoma papilliferum. Immunofluorescent staining demonstrates anti-CD133 (in red) and anti-CD44 (in green) antibodies; the nuclei are contrasted by DAPI (in blue). (a) The tumoral squamous portion shows membranous CD44 overexpression (original magnification ×200). (b) The transition area presents squamous budding with decreasing of CD44 positivity but increasing of nuclear-cytoplasmic CD133 expression (original magnification ×200). (c) Bifront-like staining showing CD44 more represented in the squamous area and CD133 stronger in the papillary component (original magnification ×200). (d) Overview of CD133 positive-cystic and papillary structures (original magnification ×100).
CD133, prominin 1, is pentaspan 120-kDa transmembrane glycoprotein and considered to be a hematopoietic and ubiquitarian stem cell marker, although its biological function remains largely unknown. However, this protein has been identified as a potential cancer stem cell marker in the brain, colon, prostate, pancreas, and, recently, the skin [37]. Until now, the correlation between CD133 expression and clinicopathological features of tumors, like increased tumorigenic and patient survival, persist undemonstrated. Only few data in the literature describe the clinical significance of the expression patterns of CD133 (membranous and/or cytoplasmic) [38] but it is clear that aberrant overexpression is pathognomonic for neoplasia. In particular, we found as a strong uniform pattern of nuclear-cytoplasmic CD133 overexpression in the adenocomponent that had lost the CD44 membranous positivity during the glandular neoplastic transdifferentiation (Figure 3).
By PCT immunostaining, we demonstrated the epithelial origin of the tumor because PCT immunopositive-cells appeared in normal epithelium and in continuity with the external layer of the neoplasm. This suggests the primary epithelial budding of adenocarcinoma. (data not shown). CD133, prominin, increased in squamous carcinoma but more prominently in cystic-glandular neoplasia. This pattern, associated with specular behavior of CD44, can suggest the development and evolution of glandular part from squamous component.
By intense immunopositivity for CK7, p63, and c-kit and the particular neoplastic stromal reactivity, we hypothesized that this tumor could have EMT-like properties. Furthermore, the p63/CK7 staining has also highlighted the squamous transdifferentiation of this adnexal-like neoplasm. Since the normal basal layer of epithelium was c-kit positive like the neoplastic component, we suggest that this tumor could derive from pluripotent-like cells [39], also with the support of high positivity for nestin, CD133, and CD44. Typical PCT immunostaining demonstrated the ontogenetic behavior of epithelial-adnexal neoplastic budding. In front of these original data, we suggest to reanalyze the syringocystadenocarcinoma papilliferum cases in the light of new evidences regarding histogenesis and stem cell-like properties. Syringocystadenocarcinoma papilliferum is a very infrequent skin tumor but could be a good model to verify the existence of the cancer stem cells and their transdifferentiable properties in skin neoplasms.
Supplementary Material
EMT represents a general physiopathologic event implicated in all types of carcinoma and provides them an additional survival advantage. By means of EMT the epithelial tumor cells can transdifferentiate into myofibroblasts, producing the desmoplastic stroma which is essential for tumor growth, invasion and metastasis. During the EMT process, cancer epithelial cells acquire stem cell-like traits and appear positive for putative cancer stem cell (CSC) markers, resulting in a migratory cell phenotype. So EMT- like properties of carcinoma, showed by c- kit staining, are more persuasive when they are associated with an increase of putative stemness marker expression. For this reason we have analyzed nestin, CD44 and CD133 antigens.
Acknowledgments
The authors are grateful to Professor Vincenzo Eusebi (University of Bologna) for helpful discussions. They are indebted to the technician staff of Anatomic Pathology Institute, S. Anna University Hospital, for valuable technical assistance: Cristina Zampini, Patrizia Raisi, Maura Masiero, Maria Novi, Rosaria Morelli, Anna Cherubino, Elisa Zaffoni, Chiara Lamberti, and Marilena Lazzari. They thank Doctor Fulvio Chiesa, Customer Application Specialist at Roche (Italy) for his technical advice.
Conflict of Interests
The authors received no financial support for the research, authorship, and/or publication of this paper.
References
- 1.Hoekzema R, Leenarts MFE, Nijhuis EWP. Syringocystadenocarcinoma papilliferum in a linear nevus verrucosus. Journal of Cutaneous Pathology. 2011;38(2):246–250. doi: 10.1111/j.1600-0560.2009.01419.x. [DOI] [PubMed] [Google Scholar]
- 2.Leeborg N, Thompson M, Rossmiller S, Gross N, White C, Gatter K. Diagnostic pitfalls in syringocystadenocarcinoma papilliferum: case report and review of the literature. Archives of Pathology and Laboratory Medicine. 2010;134(8):1205–1209. doi: 10.5858/2009-0399-CR.1. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto O, Doi Y, Hamada T, Hisaoka M, Sasaguri Y. An immunohistochemical and ultrastructural study of syringocystadenoma papilliferum. The British Journal of Dermatology. 2002;147(5):936–945. doi: 10.1046/j.1365-2133.2002.05027.x. [DOI] [PubMed] [Google Scholar]
- 4.Ishida-Yamamoto A, Sato K, Wada T, Takahashi H, Iizuka H. Syringocystadenocarcinoma papilliferum: case report and immunohistochemical comparison with its benign counterpart. Journal of the American Academy of Dermatology. 2001;45(5):755–759. doi: 10.1067/mjd.2001.117723. [DOI] [PubMed] [Google Scholar]
- 5.Dissanayake RV, Salm R. Sweat-gland carcinomas: prognosis related to histological type. Histopathology. 1980;4(4):445–466. doi: 10.1111/j.1365-2559.1980.tb02939.x. [DOI] [PubMed] [Google Scholar]
- 6.Seco Navedo MA, Fresno Forcelledo M, Ordu?a Domingo A, Junco Petrement P, Soler Sanchez T. Syringocytadenoma papilliferum with malignant evolution. Presentation of a case. Annales De Dermatologie Et De Venereology. 1982;109(8):685–689. [PubMed] [Google Scholar]
- 7.Numata M, Hosoe S, Itoh N, Munakata Y, Hayashi S, Maruyama Y. Syringadenocarcinoma papilliferum. Journal of Cutaneous Pathology. 1985;12(1):3–7. doi: 10.1111/j.1600-0560.1985.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 8.Bondi R, Urso C. Syringocystadenocarcinoma papilliferum. Histopathology. 1996;28(5):475–477. doi: 10.1046/j.1365-2559.1996.t01-4-297345.x. [DOI] [PubMed] [Google Scholar]
- 9.Arai Y, Kusakabe H, Kiyokane K. A case of syringocystadenocarcinoma papilliferum in situ occurring partially in syringocystadenoma papilliferum. The Journal of Dermatology. 2003;30(2):146–150. doi: 10.1111/j.1346-8138.2003.tb00363.x. [DOI] [PubMed] [Google Scholar]
- 10.Chi CC, Tsai RY, Wang SH. Syringocystadenocarcinoma papilliferum: successfully treated with Mohs micrographic surgery. Dermatologic Surgery. 2004;30(3):468–471. doi: 10.1111/j.1524-4725.2004.30023.x. [DOI] [PubMed] [Google Scholar]
- 11.Woestenborghs H, Van Eyken P, Dans A. Syringocystadenocarcinoma papilliferum in situ with pagetoid spread: a case report. Histopathology. 2006;48(7):869–870. doi: 10.1111/j.1365-2559.2006.02421.x. [DOI] [PubMed] [Google Scholar]
- 12.Kazakov DV, Calonje E, Zelger B, et al. Sebaceous carcinoma arising in nevus sebaceus of Jadassohn: a clinicopathological study of five cases. The American Journal of Dermatopathology. 2007;29(3):242–248. doi: 10.1097/DAD.0b013e3180339528. [DOI] [PubMed] [Google Scholar]
- 13.Park SH, Shin YM, Shin DH, Choi JS, Kim KH. Syringocystadenocarcinoma papilliferum: a case report. Journal of Korean Medical Science. 2007;22(4):762–765. doi: 10.3346/jkms.2007.22.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langner C, Ott A. Syringocystadenocarcinoma papilliferum in situ originating from the perianal skin. Acta Pathologica, Microbiologica, et Immunologica Scandinavica. 2009;117(2):148–150. doi: 10.1111/j.1600-0463.2008.00027.x. [DOI] [PubMed] [Google Scholar]
- 15.Kazakov DV, Requena L, Kutzner H, et al. Morphologic diversity of syringocystadenocarcinoma papilliferum based on a clinicopathologic study of 6 cases and review of the literature. The American Journal of Dermatopathology. 2010;32(4):340–347. doi: 10.1097/DAD.0b013e3181b96c0c. [DOI] [PubMed] [Google Scholar]
- 16.Sroa N, Sroa N, Zirwas M. Syringocystadenocarcinoma papilliferum. The American Journal of Dermatopathology. 2010;36(2):261–263. doi: 10.1111/j.1524-4725.2009.01403.x. [DOI] [PubMed] [Google Scholar]
- 17.Aydin OE, Sahin B, Ozkan HS, Gore O. A rare tumor: syringocystadenocarcinoma papilliferum. Dermatologic Surgery. 2011;37(2):271–274. doi: 10.1111/j.1524-4725.2011.01872.x. [DOI] [PubMed] [Google Scholar]
- 18.Hoguet AS, Dolphin K, McCormick SA, Milman T. Syringocystadenocarcinoma papilliferum of the eyelid. Ophthalmic Plastic and Reconstructive Surgery. 2012;28(1):e27–e29. doi: 10.1097/IOP.0b013e318216366a. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YH, Wang WL, Rapini RP, Torres-Cabala C, Prieto VG, Curry JL. Syringocystadenocarcinoma papilliferum with transition to areas of squamous differentiation: a case report and review of the literature. The American Journal of Dermatopathology. 2012;34(4):428–433. doi: 10.1097/DAD.0b013e318235dd34. [DOI] [PubMed] [Google Scholar]
- 20.Paradiso B, Marconi P, Zucchini S, et al. Localized delivery of fibroblast growth factor-2 and brain-derived neurotrophic factor reduces spontaneous seizures in an epilepsy model. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(17):7191–7196. doi: 10.1073/pnas.0810710106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahalingam M, Nguyen LP, Richards JE, Muzikansky A, Hoang MP. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Modern Pathology. 2010;23(5):713–719. doi: 10.1038/modpathol.2010.46. [DOI] [PubMed] [Google Scholar]
- 22.Adhikari AS, Agarwal N, Wood BM, et al. CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Research. 2010;70(11):4602–4612. doi: 10.1158/0008-5472.CAN-09-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Y, Liang X, Zheng M, et al. Expression of c-kit and Slug correlates with invasion and metastasis of salivary adenoid cystic carcinoma. Oral Oncology. 2010;46(4):311–316. doi: 10.1016/j.oraloncology.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Geng S, Guo Y, Wang Q, Li L, Wang J. Cancer stem-like cells enriched with CD29 and CD44 markers exhibit molecular characteristics with epithelial-mesenchymal transition in squamous cell carcinoma. Archives of Dermatological Research. 2013;305(1):35–47. doi: 10.1007/s00403-012-1260-2. [DOI] [PubMed] [Google Scholar]
- 25.Bakhshi GD, Wankhede KR, Tayade MB, Shenoy SS, Gore ST, Valand AG. Carcino-sarcoma in a case of syringocystadenoma papilliferum: a rare entity. Clinics & Practice. 2012;2(3) doi: 10.4081/cp.2012.e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Marck VL, Bracke ME. Chapter 9: epithelial-mesenchymal transitions in human cancer. In: Savagner P, editor. Rise and Fall of Epithelial Phenotype: Concept of Epithelial Mesenchymal Transition. Georgetown, Tex, USA: Landes Bioscience; 2005. pp. 135–159. [Google Scholar]
- 27.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Tiwari A Gheldof N, Tatari M, Christofori G. EMT as the ultimate survival mechanism of cancer cells. Seminars in Cancer Biology. 22(3):194–207. doi: 10.1016/j.semcancer.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Yanjia H, Xinchun J. The role of epithelial-mesenchymal transition in oral squamous cell carcinoma and oral submucous fibrosis. Clinica Chimica Acta. 2007;383(1-2):51–56. doi: 10.1016/j.cca.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Li Y, Wang K, Wang Y, Yin W, Li L. P38/NF-kB/snail pathway is involved in caffeic acid-induced inhibition of cancer stem cells-like properties and migratory capacity in malignant human keratinocyte. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0058915.e58915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C, Zimmermann M, Tinhofer I, Kaufmann AM, Albers AE. Epithelial-to-mesenchymal transition and cancer stem(-like) cells in head and neck squamous cell carcinoma. Cancer Letters. 2013;338(1):47–56. doi: 10.1016/j.canlet.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Biddle A, Liang X, Gammon L, et al. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Research. 2011;71(15):5317–5326. doi: 10.1158/0008-5472.CAN-11-1059. [DOI] [PubMed] [Google Scholar]
- 33.Kanoh M, Amoh Y, Sato Y, Katsuoka K. Expression of the hair stem cell-specific marker nestin in epidermal and follicular tumors. European Journal of Dermatology. 2008;18(5):518–523. doi: 10.1684/ejd.2008.0485. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Brown RE. Immunohistochemical detection of epithelialmesenchymal transition associated with stemness phenotype in anaplastic thyroid carcinoma. International Journal of Clinical and Experimental Pathology. 2010;3(8):755–762. [PMC free article] [PubMed] [Google Scholar]
- 35.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang SJ, Wong G, de Heer A-M, Xia W, Bourguignon LYW. CD44 Variant isoforms in head and neck squamous cell carcinoma progression. Laryngoscope. 2009;119(8):1518–1530. doi: 10.1002/lary.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, Shi S, Yen Y, Brown J, Ta JQ, Le AD. A subpopulation of CD133+ cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Letters. 2010;289(2):151–160. doi: 10.1016/j.canlet.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Zhao P, Li Y, Lu Y. Aberrant expression of CD133 protein correlates with Ki-67 expression and is a prognostic marker in gastric adenocarcinoma. BMC Cancer. 2010;10, article 218 doi: 10.1186/1471-2407-10-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pietra G, Manzini C, Vitale M, et al. Natural killer cells kill human melanoma cells with characteristics of cancer stem cells. International Immunology. 2009;21(7):793–801. doi: 10.1093/intimm/dxp047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EMT represents a general physiopathologic event implicated in all types of carcinoma and provides them an additional survival advantage. By means of EMT the epithelial tumor cells can transdifferentiate into myofibroblasts, producing the desmoplastic stroma which is essential for tumor growth, invasion and metastasis. During the EMT process, cancer epithelial cells acquire stem cell-like traits and appear positive for putative cancer stem cell (CSC) markers, resulting in a migratory cell phenotype. So EMT- like properties of carcinoma, showed by c- kit staining, are more persuasive when they are associated with an increase of putative stemness marker expression. For this reason we have analyzed nestin, CD44 and CD133 antigens.


