Abstract
Several lines of evidence predict that specific pathways must exist in metazoans for the escorted movement of heme, an essential but cytotoxic iron-containing organic ring, within and between cells and tissues, but these pathways remain obscure. In Caenorhabditis elegans, embryonic development is inextricably dependent on both maternally-derived heme and environmentally-acquired heme. Here, we show that the multidrug resistance protein, MRP-5/ABCC5, likely acts as a heme exporter and targeted depletion of mrp-5 in the intestine causes embryonic lethality. Transient knockdown of mrp5 in zebrafish leads to morphological defects and failure to hemoglobinize red blood cells. MRP5 resides on the plasma membrane and endosomal compartments and regulates export of cytosolic heme. Together, our genetic studies in worms, yeast, zebrafish, and mammalian cells identify a conserved, physiological role for a multidrug resistance protein in regulating systemic heme homeostasis. We envision other MRP family members may play similar unanticipated physiological roles in animal development.
Heme is almost ubiquitously required by living organisms as a prosthetic group in proteins (Hamza and Dailey, 2012). Heme is synthesized in the mitochondrial matrix but must be trafficked to various subcellular compartments for incorporporation into hemoproteins in the cytoplasm, ER/Golgi, lysosomes, and peroxisomes (Severance and Hamza, 2009). However, unescorted movement of heme within a cell is inherently hazardous due to the reactivity of free heme. It follows that cells must have specific pathways for the directed movement of heme within and between cells and tissues but these intra- and intercellular pathways remain poorly defined (Hamza and Dailey, 2012; Severance and Hamza, 2009).
The existence of heme effluxers is all but certain, given that free heme is toxic to cells and must be escorted to various subcellular compartments. One such protein, the major facilitator superfamily member FLVCR1, has been identified (Keel et al., 2008; Quigley et al., 2004). FLVCR1 null mice lack effective erythropoiesis and die as embryos (Keel et al., 2008). Interestingly, mammalian Flvcr1 encodes two FLVCR isoforms: FLVCR1a localizes to the plasma membrane while FLVCR1b localizes to mitochondrial membranes (Chiabrando et al., 2012). The erythropoietic defect observed in FLVCR1 mutant mouse embryos has been attributed to the inability of FLVCR1b to export heme from the mitochondria into the cytosol. However, exactly how cytosolic heme reaches hemoproteins located within subcellular organelles remains undefined (Fleming and Hamza, 2012).
We have exploited Caenorhabditis elegans as a genetic model organism because this roundworm is a heme auxotroph (Rao et al., 2005). C. elegans is dependent on both maternally-derived heme for embryonic development, as well as heme acquired from the diet during larval growth (Rao et al., 2005). Heme is imported into the intestine via the conserved heme permease, HRG-1 and its paralog HRG-4 (Figure 1A) (Rajagopal et al., 2008). The intercellular heme-trafficking protein, HRG-3, is secreted from the intestine and carries heme to developing embryos (Chen et al., 2011). HRG-2 is an extra-intestinal, heme-binding membrane protein that facilitates heme utilization in the worm hypodermis (Chen et al., 2012). How does intestinal heme, derived from the environment, get delivered to hemoproteins in extra-intestinal tissues? Are these intercellular heme transport pathways found in vertebrates? Herein, we show that a multidrug resistance protein, MRP-5/ABCC5, likely acts as a cellular heme exporter and is essential for viability in C. elegans. This conclusion is supported by our genetic studies in yeast, C. elegans, zebrafish, and mammalian cell culture models which ascribe a physiological role for a multidrug resistance protein in regulating systemic heme homeostasis in metazoa.
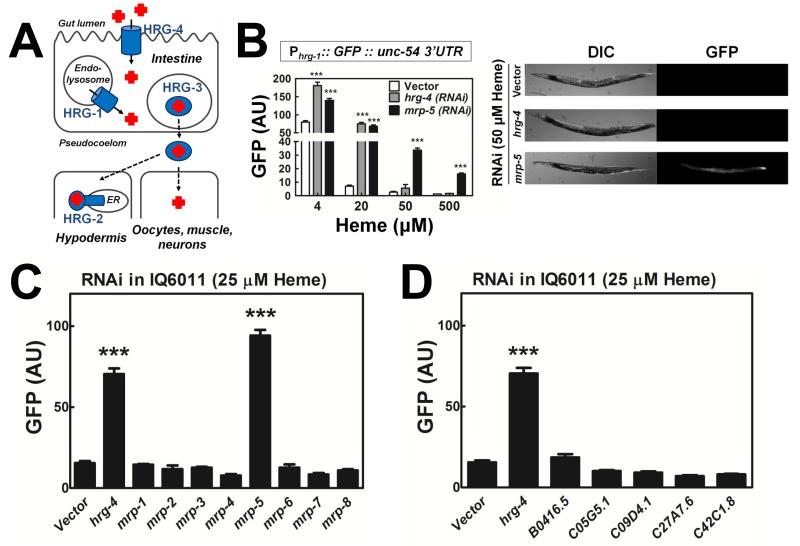
Figure 1. mrp-5 is an essential regulator of C. elegans heme homeostasis.
(A) Current model of heme homeostasis pathways in C. elegans. HRG-1/4 proteins import heme into the cytosol of intestinal cells, HRG-3 is secreted from the intestine for heme delivery to other tissues, and HRG-2 is a resident ER protein involved in heme utilization within the hypodermis. (B) Loss of mrp-5 results in a heme depletion signal that can be rescued by dietary heme. LEFT: GFP fluorescence (60-120 worms per treatment) quantified using COPAS BioSort in IQ6011 (Phrg-1::GFP::unc-54 3′ UTR; unc119(ed3); unc-119 rescue fragment) exposed to vector, hrg-4, or mrp-5 by feeding RNAi at varying heme concentrations. ***P<0.001 when compared to vector control under the same conditions (two-way ANOVA, Bonferroni post-test). RIGHT: Images of IQ6011 RNAi worms supplemented with 50 μM heme. (C) Loss of mrp-5, and no other mrp, results in a heme depletion signal in the heme sensor strain, IQ6011. GFP fluorescence (60-120 worms per treatment) quantified from the hrg-1 transcriptional fusion line (IQ6011) exposed to vector, or RNAi against an mrp gene at 25 μM heme. GFP was quantified using COPAS BioSort. ***P<0.001 when compared to vector control under the same conditions (one-way ANOVA, Bonferroni post-test). (D) RNAi of FLVCR1 homologs in C. elegans does not activate a heme depletion signal in IQ6011. GFP fluorescence in IQ6011 was measured as in Figures 1B and 1C. ***P<0.001 when compared to vector control under the same conditions (one-way ANOVA, Bonferroni post-test). See also Figure S1.
Results
HRG-3-independent pathway for heme transport
Our previous studies implicated HRG-3 in the directed trafficking of heme to extra-intestinal tissues, including embryos (Figure 1A). However, hrg-3 mutant embryos are viable unless subjected to severe maternal heme limitations in utero (Chen et al., 2011). In fact, when worms are grown in the presence of >6 μM heme, hrg-3 mRNA is undetectable (Chen et al., 2011). Thus, HRG-3 serves as an inducible mechanism for redirecting heme stores only under heme-limiting conditions. These results would also predict that, in the absence of HRG-3, an alternate pathway exist in C. elegans.
We postulated that membrane-bound heme transporters would be suitable candidates for regulating systemic heme homeostasis in the worm, and consequently impact the regulation of other heme-responsive genes. By individually depleting 288 heme-responsive genes, which included 41 genes encoding transmembrane-domain containing proteins, we uncovered mrp-5 (F14F4.5) as a potent regulator of the C. elegans transgenic heme sensor strain, IQ6011. The IQ6011 strain expresses GFP in the intestine from the heme-responsive hrg-1 promoter; GFP levels in this strain are inversely correlated with heme levels in the worm (Rajagopal et al., 2008; Sinclair and Hamza, 2010). Depletion of mrp-5 in IQ6011 by RNAi resulted in significantly greater GFP levels compared to control RNAi, indicating that loss of mrp-5 results in the animal sensing less heme (Figure 1B; and (Severance et al., 2010)). Importantly, this heme depletion signal could be rescued in a concentration-dependent manner by supplementation with dietary heme (Figure 1B). The increased GFP signal observed by knockdown of the intestinal heme importer, hrg-4, could be completely suppressed with 50 μM heme, while the mrp-5 RNAi signal persisted even at 500 μM, indicating a far more severe defect (Figure 1B). In addition, microarray analysis and qRT PCR studies show that mrp-5 is itself a heme responsive gene as its mRNA increased over 3-fold under low heme conditions (Severance et al., 2010). Indeed, in silico analysis of the putative mrp-5 promoter revealed the presence of a canonical 23-base pair heme response element, which we have previously shown is necessary and sufficient to mediate heme dependent regulation of hrg-1 in the worm intestine (Sinclair and Hamza, 2010).
Although the C. elegans genome contains eight mrp genes (Figure S1, red boxes), mrp-5 is the only mrp family member that significantly alters GFP expression in IQ6011 (Figure 1C), and is the sole mrp that is transcriptionally responsive to heme (Severance et al., 2010). Moreover, systematically depleting each of the five FLVCR worm homologs had little or no effect on GFP levels in the heme sensor worm (Figure 1D) (Keel et al., 2008; Lipovich et al., 2002; Quigley et al., 2004).
MRP-5 is essential for embryonic and larval development
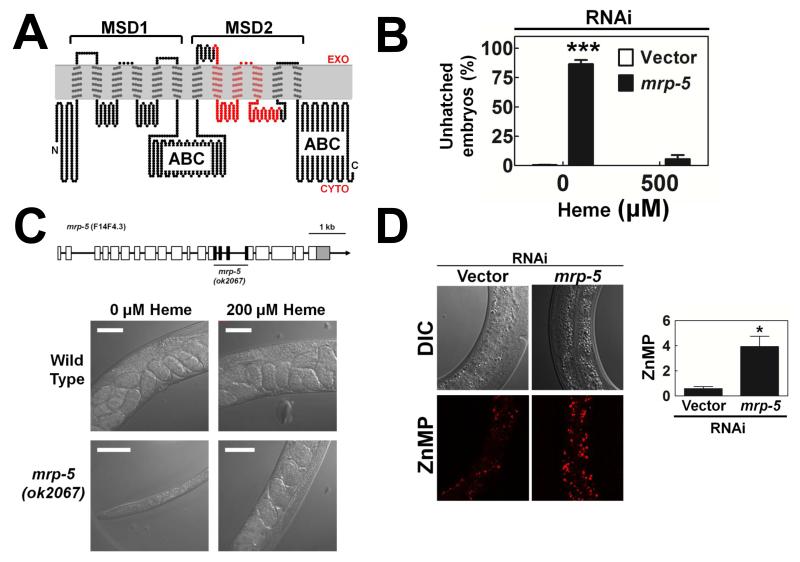
In worms, mrp-5 (multidrug resistance protein 5) encodes an ABC transporter of the MRP/ABCC family. C. elegans MRP-5 is predicted to include two membrane spanning domains (MSDs), each containing six transmembrane helices, and two intracellular ATP binding cassette (ABC) domains (Figure 2A) (Borst et al., 2000). Worms were analyzed for growth and developmental phenotypes after RNAi depletion of mrp-5. When synchronized larvae (P0) were fed mrp-5 RNAi bacteria, they showed no major developmental defects and were able to reach adult gravid stage and lay eggs. However, 80% of the F1 eggs laid by P0 worms failed to hatch, and the small number that did hatch arrested as L1 larvae (Figure 2B). This striking embryonic lethal phenotype could be rescued by supplementation of the bacterial food with exogenous heme; 95% of F1 progeny hatched and became adults in the presence of 500 μM heme. Together, these results indicate that mrp-5 is required for embryonic development in C. elegans.
Figure 2. Worm mrp-5 is essential for embryonic development and larval growth.
(A) MRP-5 membrane topology showing an N-terminal membrane spanning domain (MSD1) consisting of six TMDs, followed by a cytosolic ATP binding cassette (ABC) domain, a second MSD (MSD2) and a second ABC domain. (B) Dead progeny of vector control or mrp-5(RNAi) worms. ***P<0.001 when compared to vector control worms under identical conditions, n=3 (two-way ANOVA, Bonferroni post-test). (C) TOP: The C. elegans mrp-5 gene contains 20 exons across 7 kb of the X chromosome. mrp-5 mutants harbor a 1.2 kb deletion (ok2067) spanning exons 14 through 17. BOTTOM: wildtype and mrp-5 broodmates were grown to gravid adult stage at 200 μM heme. Their F1 progeny were placed as synchronized L1 larvae on plates seeded with OP50 bacteria with or without 200 μM added heme. Representative images of F1 worms 4 days post-hatching are shown. Scale bar, 20 μM. (D) ZnMP staining in vector control or mrp-5(RNAi) worms. TOP: Worms were exposed to RNAi from L1 to L4 larval stages, pulsed with 60 μM ZnMP for 3 hr, and imaged using confocal microscopy. BOTTOM: Quantification of ZnMP staining (mean ± SEM of 10 worms). *P<0.05 when compared to control worms (one-way ANOVA, Bonferroni post-test). See also Figure S2.
The strain VC1599 contains a deletion in mrp-5 (ok2067) located on the X chromosome, spanning exons 14 through 17 (Figure 2C, top panel, and Figure S2A), but is genetically balanced by a marked chromosomal translocation as mrp-5 mutant worms are embryonic lethal (Edgley et al., 2006). The mrp-5 (ok2067) deletion removed 176 amino acids, including three transmembrane helices (Figure 2A). Consequently, the predicted topology of the mutant protein will contain only nine transmembrane helices, resulting in a dysfunctional protein with the second ABC domain located on extracellular surface (Figure S2B). Although RT-PCR and sequencing analysis reveals the presence of mrp-5 mRNA in mutant worms (Figure S2C), depletion of mrp-5 by RNAi in the mrp-5(ok2067) mutants does not enhance or result in additional phenotypes suggesting that mrp-5(ok2067) is likely a null mutation (Figure S2D).
We tested whether supplementary heme could rescue the embryonic lethality of mrp-5 mutants and found that when VC1599 worms were crossed to wildtype N2 worms to eliminate the balancer, viable F2 homozygous mrp-5 mutant worms were obtained and easily propagated – but only when worms were grown on food supplemented with >200 μM heme. When grown on plates containing bacteria with no added heme, mrp-5(ok2067) homozygous mutant larva arrested at mid-larval stages, indicating that mrp-5 is required during larval development (Figure 2C, lower panel). To determine if the rescue of mrp-5 lethality by dietary heme was due to the presence of redundant mechanisms for heme transport, we depleted FLVCR homologs in the mrp-5(ok2067) background. Only one, B0416.5, showed a significantly enhanced phenotype in the mrp-5 mutant worms when depleted (Figure S2E); however, this effect could not be rescued in a dose-dependent manner by exogenous heme and is likely unrelated to heme export from the intestine. It is worth noting that this experiment does not rule out the presence of other low affinity transporters that can compensate for the loss of mrp-5, or the possibility that at such high dietary concentrations, heme, which can intercalate into membrane lipids, is traversing the membrane without the assistance of a transporter.
Since MRP-5 is a member of the ABCC/MRP transporter family, members of which function as exporters of lipophilic and organic compounds (Borst et al., 2007; Kos and Ford, 2009), we examined whether MRP-5 was involved in the regulation of heme homeostasis in C. elegans. Worms in which mrp-5 had been depleted showed significantly greater accumulation of zinc mesoporphyrin IX (ZnMP), a fluorescent heme analog, in the intestine compared to control worms (Figure 2D). Notably, VC1599 worms, which are heterozygous for mrp-5, exhibited haploinsufficiency phenotypes as they not only accumulated ZnMP in the intestine but were also resistant to the toxic heme analog, gallium protoporphyrin IX, indicating that heme analogs entered the intestine but were poorly accessible to extra-intestinal cells (Figure S2F and S2G).
MRP-5 deficiency prevents heme export from the intestine
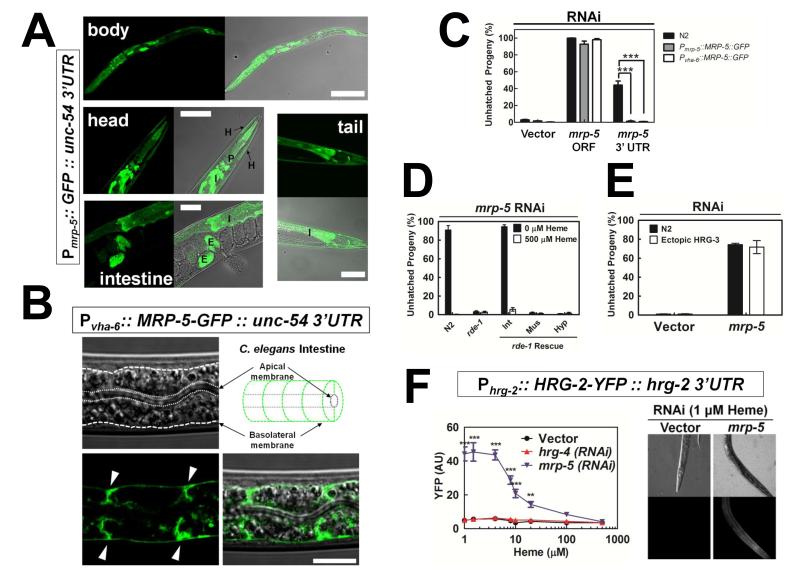
A transcriptional reporter was generated using the putative promoter region of mrp-5 (3 kb upstream of the ATG start codon) fused to a GFP reporter. Pmrp-5::GFP was expressed at all developmental stages, with low levels in the hypodermis and in some neurons, and was consistently highly expressed in the intestine and pharynx (Figure 3A), confirming published intestinal and pharyngeal in silico gene expression analysis (Contrino et al., 2012; McGhee et al., 2007).
Figure 3. Worm mrp-5 encodes a putative intestinal heme exporter.
(A) GFP expression in IQ5051 (Pmrp-5::GFP::unc-54 3′UTR; unc-119(ed3); unc-119 rescue fragment) as determined using confocal microscopy. mrp-5 is expressed in the hypodermis and some neurons, and at higher levels in the pharynx and intestine. P, pharynx, I, intestine, H, hypodermis, E, embryo. Scale bars, 20 μM. (B) Transgenic IQ5351 worms (Pvha-6::MRP-5:GFP::unc-54 3′UTR; unc-119(ed3); unc-119 rescue fragment) expressing an mrp-5 translational reporter were imaged by confocal microscopy. Dotted lines indicate apical membrane, dashed lines indicate basolateral membrane, and arrowheads indicate lateral membranes between adjacent intestinal cells. Scale bar, 50 uM. (C) The MRP-5::GFP fusion gene can rescue the embryonic lethality of mrp-5 RNAi. RNAi targeting the mrp-5 ORF causes embryonic lethality in both wildtype N2 and transgenic worms. RNAi against the mrp-5 3′ UTR results in a less severe embryonic lethal phenotype in N2 worms, but this lethality is significantly rescued by expression of the MRP-5::GFP transgene from either the mrp-5 or the intestinal vha-6 promoter. ***P<0.001 when compared to wildtype N2 worms under identical conditions, n=3 (two-way ANOVA, Bonferroni post-test). (D) Intestinal RNAi of mrp-5 recapitulates the embryonic lethality of whole animal mrp-5 RNAi. Wildtype N2 worms and the tissue specific RNAi strains were grown on RNAi plates with no added heme. Int, intestinal RNAi, Mus, muscle RNAi, Hyp, hypodermal RNAi, n=2. See Results section and Figure S2 for further strain information. (E) Ectopic expression of hrg-3 does not rescue the embryonic lethality of whole animal mrp-5 RNAi. Experiment was performed as in Figure 1C using wildtype N2 worms and worms ectopically expressing HRG-3 and GFP separated by the SL2 intercistronic sequence (hrg-3(tm2468); Pvha-6::HRG-3::ICS::GFP, unc-119(ed3); unc-119 rescue fragment) grown on RNAi plates with no added heme. (F) Loss of mrp-5 activates an extra-intestinal heme depletion signal. LEFT: YFP fluorescence (60-100 worms per treatment) quantified using COPAS BioSort in the hrg-2 translational fusion line, IQ8122 (Phrg-2::HRG-2:YFP::hrg-2 3′ UTR, unc-119(ed3); unc-119 rescue fragment) exposed to vector, hrg-4, or mrp-5 RNAi at varying heme concentrations. ***P<0.001, **P<0.01 when compared to vector control worms (two-way ANOVA, Bonferroni post-test). RIGHT: Representative images of worms grown at 1 μM heme from left panel. See also Figure S3.
To delineate the subcellular localization of MRP-5, GFP was fused to the C-terminus of MRP-5 and expressed from the intestinal vha-6 promoter (Oka et al., 2001). In the polarized worm intestinal cells, Pvha-6::MRP-5::GFP localized to basolateral membranes and to intracellular compartments, reminiscent of basolateral sorting vesicles in C. elegans (Chen et al., 2010) (Figure 3B and Figures S3A and S3B). Similar localization was observed for MRP- 5::GFP expressed from the endogenous mrp-5 promoter (not shown). We next determined if the transgene was capable of rescuing the embryonic lethal phenotype induced by mrp-5 deficiency. RNAi directed against the 3′ untranslated region (UTR) of mrp-5 depleted endogenous mrp-5, whereas the MRP-5::GFP transgene, which is expressed with the generic unc-54 3′ UTR, was left intact, as confirmed visually by GFP fluorescence (not shown). RNAi against the mrp-5 3′ UTR resulted in a significant reduction in embryonic lethality in MRP-5::GFP transgenic worms, indicatating that MRP-5::GFP is a functional protein (Figure 3C).
As MRP-5 is expressed in multiple tissues, it is conceivable that mrp-5 depletion in any or all of the tissues may contribute to the embryonic lethal phenotype of mrp-5 mutant worms. To address the contribution of each tissue to the mrp-5 phenotype, we utilized tissue-specific RNAi worm strains. Worms carrying the rde-1 mutation are resistant to RNAi; ectopic expression of rde-1 from a tissue-specific promoter in the rde-1 mutant background results in RNAi only in that tissue (Qadota et al., 2007). We depleted mrp-5 in the VP303 (rde-1 rescue from the intestinal nhx-2 promoter), WM118 (rde-1 rescue from the muscle myo-3 promoter), and NR222 (rde-1 rescue from the hypodermal lin-26 promoter) transgenic worm lines. Depletion of mrp-5 in the intestine fully recapitulated the F1 embryonic lethality of whole animal RNAi, while depletion of mrp-5 either in the hypodermis or muscle had no effect on F1 viability (Figure 3D). The lethality caused by RNAi in the VP303 strain could be rescued by supplementation with 500 μM heme in the diet. Thus, the lethality of mrp-5 mutants can be attributed to loss of functional MRP-5 specifically in the intestine, even though mrp-5 is expressed in extra-intestinal tissues. This is further supported by the fact that the MRP-5::GFP transgene expressed exclusively in the intestine (Pvha-6::MRP-5::GFP) is capable of rescuing the hatching phenotype associated with depletion of endogenous mrp-5 (Figure 3C).
We next determined whether MRP-5 deficiency phenotypes could be overcome by constitutively expressing HRG-3, the intercellular heme delivery protein, from the intestine. Ectopic expression of hrg-3 from the intestinal vha-6 promoter was unable to rescue the embryonic lethality of mrp-5 RNAi (Figure 3E). This was not due to impaired secretion of HRG-3 from the intestine, as HRG-3::mCherry still accumulated in the extraintestinal coelomocytes when mrp-5 was depleted by RNAi (Figure S3C).
To evaluate the heme status in an extra-intestinal tissue when mrp-5 is depleted, we utilized Phrg-2::HRG-2::YFP transgenic worms. The hrg-2 promoter is active only in the hypodermis and Phrg-2::HRG-2::YFP is induced in the hypodermis when heme levels are limiting in that tissue (Chen et al., 2012). Depletion of mrp-5 resulted in a striking increase in HRG-2::YFP levels and its expression was not fully suppressed until worms were fed 500 μM heme (Figure 3F). By contrast, worms in which hrg-4, the intestinal heme importer, was depleted did not upregulate Phrg-2::HRG-2::YFP, indicating that loss of this particular transporter does not result in limiting heme levels in the hypodermis. Taken together, these results provide strong evidence that MRP-5 is the major intestinal heme exporter.
Mrp5 is essential for erythropoiesis in zebrafish
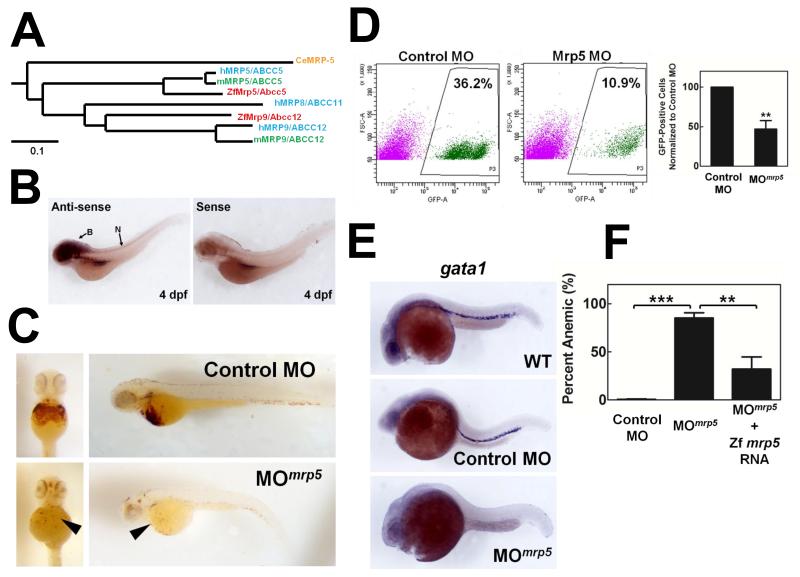
Vertebrate ABCC5/MRP5 is ≈38% identical to worm MRP-5 with similar overall membrane topology. Within the ABCC/MRP family, the lack of an additional amino-terminal MSD (called MSD0) places ABCC5/MRP5 in a distinct group containing the ABCC4/MRP4, ABCC7 (CFTR), and ABCC12/MRP9 proteins (Figure S1, blue box) (Toyoda et al., 2008). Although C. elegans contains a single mrp-5, the gene has undergone a duplication event and two MRP5 paralogs are found in most vertebrates (Figure 4A). Interestingly, the human genome contains three paralogs; in addition to ABCC5/MRP5 on chromosome 3, ABCC11/MRP8 and ABCC12/MRP9 are located in tandem on chromosome 16 (Yabuuchi et al., 2001). Orthologs of ABCC11/MRP8 can be found in other eutherians, including primates, dogs, and cows, but are not found in rodent genomes. In all analyzed vertebrate species, the closest homolog of C. elegans mrp-5 is the vertebrate ABCC5/MRP5.
Figure 4. mrp5 is required for zebrafish erythropoiesis.
(A) Phylogenetic analysis of MRP5/ABCC5 clade in C. elegans (orange), zebrafish (red), mice (green), and humans (blue). Sequences were aligned using ClustalW and a phylogenetic tree was generated using the Neighbor-Joining method in MEGA5. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances are in the units of the number of amino acid substitutions per site. (B) Lateral view of zebrafish mrp5 expression by whole mount in situ hybridization using anti-sense probe, 4 days post-fertilization. Anterior is to the left. Sense probe image is shown to indicate background staining. B, brain, N, neural tube. (C) Knockdown of zebrafish mrp5 using morpholinos (MOmrp5) results in severe anemia, as indicated by reduced staining of o-dianisidine-positive red cells, as indicated by black arrowheads. (D) Knockdown of zebrafish mrp-5 using MOmrp5 results in reduced red cell formation. Transgenic embryos expressing GFP from the globin locus control region (LCR-GFP) were injected with control MO or MOmrp5. LEFT: On day 2 post-fertilization, percent GFP-positive RBCs was analyzed by FACS. X and Y axes measure GFP and forward scatter, respectively; boxed area indicates gate for RBCs. RIGHT: Quantification of morphants shown at left. For MOmrp5 injection, n=4. **P<0.01 for MOmrp5 morphants compared to control morphants under identical conditions. (One-way ANOVA, Bonferroni post-test.) (E) Lateral view of zebrafish gata1 expression in wildtype, control MO, and MOmrp5 morphants by whole mount in situ hybridization using anti-sense probe, 24 hpf. Anterior is to the left. (F) Quantification of anemia rescue in zebrafish coinjected with mrp5 cRNA. ***P<0.001 for MOmrp5 morphants compared to control morphants under identical conditions, n=4. **P<0.01 for mrp5 morphants co-injected with rescue cRNA when compared to mrp5 morphants with no rescue cRNA under identical conditions, n=3 (One-way ANOVA, Bonferroni post-test.) See also Figure S4 and Table S1.
Previous studies with HRG-1 have shown that even though zebrafish and worm HRG-1 proteins are only ≈20% identical, they are functional orthologs (Rajagopal et al., 2008). Because zebrafish embryos provide a vertebrate animal model to interrogate hematological changes as a function of aberrant heme homeostasis (Shafizadeh and Paw, 2004), we analyzed the expression and function of mrp5/abcc5 in zebrafish. Whole mount in situ hybridization revealed that mrp5/abcc5 is widely expressed throughout the embryo, with the greatest expression in the developing central nervous system (Figure 4B and Figure S4).
To knockdown mrp5/abcc5, we injected fish embryos with morpholinos (MO) specifically targeted against the ATG start codon of mrp5/abcc5 (MOmrp5) mRNA. Embryos injected with MOmrp5 showed severe anemia with very few o-dianisidine-positive red blood cells (RBCs) compared to embryos injected with control MO (Figure 4C). MOmrp5 morphants also exhibited developmental malformations including body axis curvature defects and enlarged hearts. The anemia phenotype was reproducible using splice junction MO which targeted specific exon-intron junctions (not shown) resulting in a mutant form of MRP5 that would be functionally equivalent to mutation at the corresponding position in worm mrp-5. (Figure 2C and Figure S2A).
To quantify the severe anemia phenotype, we analyzed levels of globin-expressing RBCs in the morphant fish. Transgenic zebrafish expressing GFP from the globin locus control region (LCR-GFP) were injected with control and mrp5 morpholinos, and morphant blood was analyzed two days post-fertilization for GFP expression. Fish injected with MOmrp5 showed significantly decreased GFP-positive RBCs compared to control MO fish (Figure 4D). Correspondingly, gata1, a transcription factor required for primitive erythropoiesis (Paik and Zon, 2010) was robustly expressed in wildtype and control MO embryos, but little or no gata1 staining was observed in MOmrp5 morphants (Figure 4E). The MOmrp5 anemia was indeed due to MRP5/ABCC5 deficiency, as co-injecting zebrafish with cRNA encoding MRP5/ABCC5 significantly corrected the anemia phenotype (Figure 4F and Table S1). Taken together, these data indicate that mrp5 is critical for zebrafish erythropoiesis, and that MRP-5 regulation of systemic heme homeostasis is likely conserved from worms to vertebrates.
MRP5 is a putative heme transporter
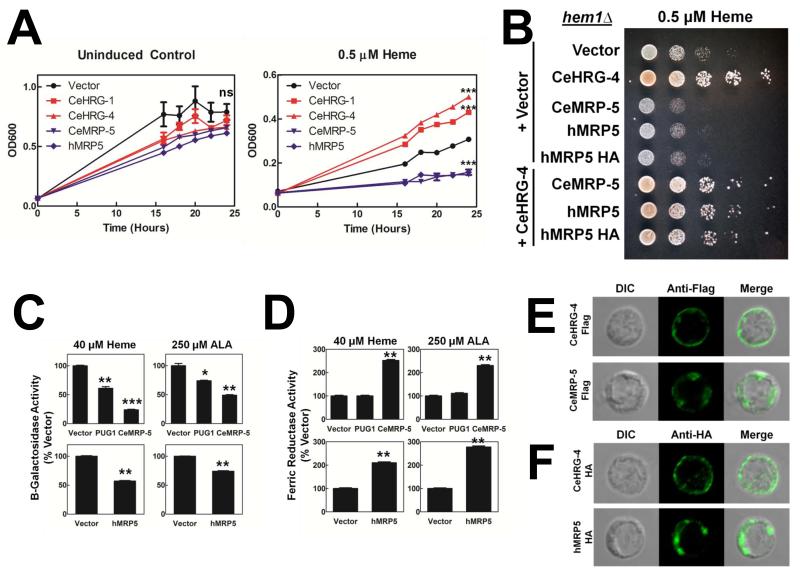
To determine whether MRP5 likely transports heme, we exploited previously established assays in yeast and mammalian cells. Saccharomyces cerevisiae hem1 mutants are unable to synthesize δ-aminolevulinic acid (ALA), a precursor for heme synthesis, and grow poorly even in the presence of exogenous heme due to an inefficient heme uptake system (Protchenko et al., 2006; Protchenko et al., 2008). This inadequate growth of hem1Δ can be greatly improved by either expression of a heme importer in the presence of heme or supplementation of ALA to the growth medium (Yuan et al., 2012). When hem1Δ yeast express the C. elegans heme importer HRG-4 (CeHRG-4), they show significantly improved growth in medium supplemented with 0.5 μM heme when compared to uninduced controls (Figure 5A) (Yuan et al., 2012). However, yeast expressing C. elegans MRP-5 or human MRP5 showed significantly reduced growth. Indeed, hem1Δ yeast expressing CeMRP-5 or hMRP5 showed a reproducible growth defect in dilution spot assays on agar plates (Figure 5B). The reduced growth of yeast expressing CeMRP-5 or hMrp5 was not due to cell toxicity associated with overexpression of a large polytopic membrane protein, as growth was restored when cells were cotransformed with the heme importer CeHRG-4 (Figure 5B, bottom three rows).
Figure 5. Ectopic MRP5 expression in yeast suggests the protein plays a role in heme transport.
(A) The hem1Δ yeast strain was transformed with indicated vectors, grown for 12 hours without added heme or ALA, and then grown for 24 hours under the indicated conditions. Yeast growth was assessed by measuring OD600. LEFT: Uninduced yeast which did not express the transgenes showed no difference in growth after 24 hours in the presence of 250 μM ALA. RIGHT: Yeast expressing heme importers HRG-1 and HRG-4 grow significantly better than control yeast. Yeast expressing MRP-5 grow significantly worse. ***P<0.001 compared to vector control after 24 hours, n=3 (two-way ANOVA, Bonferroni post-test). (B) The hem1Δ yeast strain was transformed, grown overnight without added heme or ALA and spotted on plates supplemented with 0.5 μM heme. Plates were incubated at 30°C for 72 h. (C) Heme-dependent beta-galactosidase activity. The hem1Δ yeast strain was transformed with pCYC1-LacZ, as well as empty vector, ScPUG1, CeMRP-5, or hMRP5 and grown with the indicated amount of heme or ALA. Cell lysates were then analyzed for β-galactosidase activity, normalized to vector. ***P<0.001, **P<0.01, *P<0.05 when compared to yeast expressing empty vector under identical conditions, n=2 (one-way ANOVA, Bonferroni post-test). (D) Heme-dependent ferric reductase activity. The hem1Δfre1Δfre2ΔPGK1-FRE1 yeast strain was transformed with indicated vectors and grown with the indicated amount of heme or ALA. Ferric reductase activity from whole cells was analyzed. **P<0.01, *P<0.05 when compared to yeast expressing empty vector under identical conditions, n=2 (one-way ANOVA, Bonferroni post-test). (E) and (F) The hem1Δ yeast strain expressing (E) CeHRG-4-flag or CeMRP-5-flag or (F) CeHRG-4-HA or hMRP5-HA was subjected to indirect immunofluorescence microscopy using anti-flag or anti-HA antibodies and imaged by confocal microscopy.
To assess whether MRP5 expression could alter heme levels in the yeast, we measured the activity of beta-galactosidase derived from the lacZ reporter under control of the CYC1 promoter. This promoter is activated by Hap1, a transcription factor positively regulated by cytosolic heme levels (Hon et al., 2003). Yeast expressing CeMRP-5 or hMRP5 showed decreased beta-galactosidase activity (Figure 5C), a result consistent with the poor growth phenotype in the spot assay. This result was reproducible in yeast grown in the presence of ALA, indicating that MRP5 can also affect availability of endogenously synthesized heme (Figure 5C, right panels). Interestingly, yeast expressing MRP5 showed lower beta-galactosidase activity than cells expressing the yeast heme effluxer Pug1p (Protchenko et al., 2008).
To evaluate heme availability in the yeast secretory compartment, we measured ferric reductase activity, as Fre1p acquires a heme cofactor needed for enzymatic activity in the secretory pathway (Dancis et al., 1990). While Pug1p expression had minimal effect, yeast expressing CeMRP-5 or hMRP5 showed significantly greater ferric reductase activity in the presence of heme and ALA (Figure 5D). Although most MRP transporters expressed in yeast localize to the vacuolar membranes (Paumi et al., 2009), indirect immunofluorescence microscopy localized CeMRP-5 and hMRP5 primarily to intracellular compartments that are distinct from the plasma membrane and the vacuole (Figures 5E and 5F). These results indicate that when expressed in yeast, MRP5 proteins are likely capable of exporting heme from the cytosol into intracellular organelles for delivery to hemoproteins such as Fre1p.
MRP5 alters heme levels in the secretory compartment
In mammals, MRP5 is expressed almost ubiquitously (Borst et al., 2007; McAleer et al., 1999; Suzuki et al., 2000), and Mrp5 knockout mice, previously generated in the FVB genetic background, are viable with no overt phenotypes (de Wolf et al., 2007). To determine how loss of Mrp5 affected heme homeostasis in a mammalian cell model, we generated mouse embryonic fibroblasts (MEFs) from Mrp5+/+ and Mrp5−/− embryos.
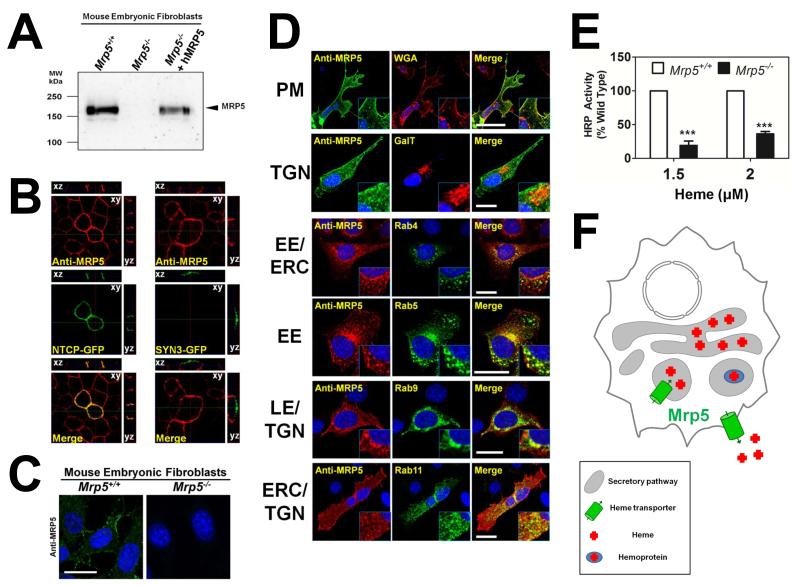
Probing MEF lysates with M5I-10, a monoclonal antibody generated against the first 38 amino acids of the mouse Mrp5, revealed a band of the expected molecular weight by immunoblotting (Figure 6A) (Scheffer et al., 2000). The antibody recognized endogenous Mrp5 in cell lysates from Mrp5+/+ mouse embryonic fibroblasts (MEFs), as well as human MRP5 ectopically expressed in Mrp5−/− MEFs. Human MRP5 colocalized with the basolateral membrane marker, Na+-taurochlorate co-transporting polypeptide (NTCP), and not with the apical membrane marker, syntaxin 3, in polarized MDCKII cells (Figure 6B). In Mrp5+/+ MEFs, endogenous Mrp5 was found in punctuate intracellular vesicles throughout the cytoplasm, with some protein on the cell periphery (Figure 6C). Confocal microscopy studies in Mrp5−/− MEFs colocalized MRP5 not only with the plasma membrane (WGA), but also partially with the Golgi (galactosyltransferase) and endosomal recycling organelles (Rab4, Rab5, Rab9, Rab11) (Figure 6D).
Figure 6. MRP5 localizes to the secretory pathway and alters heme levels in this compartment.
(A) Immunoblot analysis of Mrp5 expression in MEFs generated from Mrp5+/+ and Mrp5−/− FVB mice. Cell lysates were resolved on SDS/PAGE and blotted to nitrocellulose membranes for probing with a monoclonal anti-MRP5 antibody. (B) MDCKII cells stably expressing human MRP5 were transfected with the basolateral marker NTCP-GFP or the apical marker Syntaxin3-GFP and grown to confluency on transwell filters. Polarization of the monolayer was determined after measuring a transient spike in trans-epithelial electrical resistance, which remained above baseline level. Cells were fixed and probed with monoclonal anti-MRP5, followed by Alexa 568-conjugated secondary antibody and imaged using confocal microscopy. A single confocal section (xy) is depicted along with composite stacks in side views (yz, xz). (C) Immunohistochemistry of endogenous MRP5 in Mrp5+/+ and Mrp5−/− mouse MEFs. MRP5 staining was performed as in Figure 6B, using an Alexa 488-conjugated secondary antibody. Scale bar, 20 μM. (D) Immunolocalization of human MRP5 overexpressed in MEFs by confocal microscopy. WGA is used as a plasma membrane (PM) marker, RFP-GalT as a trans-Golgi (TGN) marker, Rab4YFP marks early endosomes (EE) and the endocytic recycling compartment (ERC), Rab5YFP marks EEs, Rab9YFP marks late endosomes (LE) and the TGN, Rab11YFP marks the ERC and TGN. Scale bar, 20 μM. (E) Heme-dependent horseradish peroxidase activity in Mrp5+/+ or Mrp5−/− MEFs. Cells were transfected with GolgiHRP and then grown for 24 hr in heme-depleted media plus succinyl acetone (HD+SA) for complete heme depletion. Indicated amounts of heme were added back and cells were incubated for a further 24 h. Cell lysates were harvested and analyzed for peroxidase activity, which was normalized to peroxidase activity from samples not expressing GolgiHRP and then to the protein concentration of each sample. ***P<0.001 for knockout MEFs when compared to wildtype MEFs under identical conditions, n=3 (two-way ANOVA, Bonferonni post-test). (F) Proposed model for heme transport by MRP-5: in this composite model, based on results from genetic, biochemical, and localization studies in worm and mammalian systems, MRP-5 can localize to the plasma membrane for heme export as well as to the secretory pathway for heme delivery to luminal hemoproteins.
To verify the yeast results, we transfected Mrp5+/+ and Mrp5−/− MEFs with an engineered horseradish peroxidase (HRP) that was confined to the Golgi with a targeting sequence (White et al., 2013). Because holo-HRP requires heme as a cofactor, HRP activity reflects heme availability in the Golgi compartment (White et al., 2013). When heme-depleted MEFs expressing HRP were supplemented with heme in the growth medium, robust HRP activity was detected in Mrp5+/+ cells but not Mrp5−/− cells; HRP activity was significantly suppressed by 65 to 80% in Mrp5−/− cells (Figure 6E). Together, these results in yeast and mouse cells support a composite model in which MRP5 is a heme exporter that transports heme from the cytosol into the lumen of the secretory pathway (Figure 6F).
Discussion
The nematode C. elegans is unable to synthesize heme and therefore is innately dependent on a network of heme sensing, trafficking, and transporting molecules to import environmental heme into the intestine and then export this heme to different tissues and subcellular compartments. In the current study, we show that MRP5 plays an essential role in C. elegans heme homeostasis and that heme is potentially the physiologically relevant substrate of MRP5 across metazoans. For almost two decades, MRP5 has been studied as an exporter of cancer drugs, organic anions and nucleoside monophosphates, although none of these studies provided direct genetic evidence for a physiological role for MRP5 in growth and development (Borst et al., 2007; Kool et al., 1997; Wijnholds et al., 2000). Our conclusions about MRP-5 function are supported by the following findings: 1) targeted mrp-5 deficiency in the intestine causes embryonic lethality; 2) MRP-5 primarily localizes to the basolateral plasma membrane and MRP-5 deficiency results in ZnMP accumulation in the worm intestine; 3) mrp-5 is expressed during all developmental stages, and over a wide range of heme concentrations; and 4) functional heme transport assays in yeast suggest that MRP-5 has the capability to export heme. Altogether, these results suggest that MRP-5 is an important membrane-bound heme exporter in C. elegans.
An unanticipated consequence of MRP-5 deficiency in worms is the apparent disconnect between heme levels in the intestine and levels in extra-intestinal tissues. In the absence of an intestinal heme exporter, it would be expected that heme will accumulate in the intestine and extra-intestinal tissues will be heme-deprived, as seen in the HRG-2::YFP reporter strain. However, depletion of mrp-5 also results in robust expression of the Phrg-1::GFP intestinal heme reporter, and this occurs when heme is accumulating within the intestine, a condition when such transporters are not normally expressed. If the hrg-1 promoter was solely regulated by intestinal heme levels, we would expect intestinal GFP to be suppressed. It could be that mrp-5 depletion causes compartmentalization of accumulated heme in the intestine such that this heme can no longer be detected by the hrg-1 promoter. However, another plausible interpretation of this paradox is that intestinal heme levels are integrated with and regulated by “heme signals” from extra-intestinal tissues. That low extra-intestinal heme levels activate a depletion signal within a heme-loaded intestine implies the existence of a network for communicating heme status between extra-intestinal tissues and their sole source of heme, the intestine. We envisage that cellular heme levels in C. elegans, and plausibly vertebrates, are not solely regulated by internal heme content (cell autonomous), but also by distally located proteins which signal systemic heme requirements to an inter-tissue heme trafficking network (cell non-autonomous). This prediction is further supported by our findings that depletion of either a heme exporter (mrp-5) or a heme importer (hrg-1) produces similar, overlapping phenotypes in worms and zebrafish i.e. that mobilization of heme in and out of tissues is as important as endogenous heme synthesis (Rajagopal et al., 2008; White et al., 2013; Yuan et al., 2012). Although both heme importers and exporters are obviously essential for survival of a heme auxotroph, these proteins also play an important role in vertebrates as demonstrated by developmental and blood defects in zebrafish.
Given the severe phenotypes associated with mrp-5 deficiency in worms and zebrafish, why do Mrp5 null mice not exhibit any overt hematological phenotypes? Clearly, worm and human MRP5 have similar phenotypes in yeast. One plausible explanation could be that, in mammals, FLVCR1 isoforms play a prominent role in heme export, while MRP5 performs a more specialized role (Chiabrando et al., 2012; Fleming and Hamza, 2012; Keel et al., 2008). It is notable that worms in which each of the five FLVCR homologs were depleted exhibited no heme-dependent phenotypes signifying that, at least in lower metazoans, MRP5 plays a definitive and essential role in heme homeotasis. A second explanation is the influence of the FVB genetic background of the Mrp5 mutant mouse (de Wolf et al., 2007). Inherently, the FVB strain has high liver and spleen iron content and therefore ill-suited for studies of systemic iron homeostasis. This is in contrast to the C57BL/6 strain which has a much lower liver and spleen iron content and, therefore, presents a mouse model that is more sensitive to perturbation in iron metabolism (Wang et al., 2007). For example, mutations in the iron transporter DMT1 are viable in certain mouse strains, but become lethal when backcrossed into the C57BL/6 background (Mark Fleming, Boston Children’s Hospital, personal communication.) Lastly, in vivo compensatory pathways may exist to overcome heme or iron metabolism defects in Mrp5−/− mice. It is noteworthy that in humans and other placental mammals, MRP5/ABCC5 has two recently described paralogs – ABCC11 and ABCC12; mice and zebrafish genomes contain only ABCC12 (Kruh et al., 2007; Tammur et al., 2001; Yabuuchi et al., 2001). Although it has been reported that MRP9/ABCC12 also localizes to the secretory pathway in unpolarized mammalian cells (Ono et al., 2007), the functions of ABCC11 and ABCC12 are largely unknown.
In worms, yeast, and mammalian cells, MRP5 localizes to both the plasma membrane and intracellular vesicles. The cell surface localization of MRP5 could be reconciled by the well-studied function of ABCC transporters to efflux substrates into the extracellular milieu, consistent with the expected role of exporting heme from the C. elegans intestine into the worm’s circulatory system. However, MRP5 is also found in the secretory pathway as part of the endocytic recycling compartment. While we do not show that MRP5 has heme export activity by direct biochemical assays or that it can deliver heme to an endogenously-expressed mammalian hemoprotein, our genetic and cell biological results in yeast and MEFs support a model in which heme transported into the secretory pathway by MRP5 is incorporated into luminal hemoproteins (Figure 6F). The endosomal trafficking of MRP5 could be mediated by an acidic-dileucine based sorting signal located in the cytoplasmic carboxy termini of vertebrate MRP-5 proteins (Bonifacino and Traub, 2003). It is noteworthy that ATP7A, a copper-transporting P-type ATPase, pumps copper into the secretory compartment for metallation of essential cuproproteins in the Golgi, as well as export copper across the plasma membrane to regulate body copper stores (Lutsenko and Petris, 2003). Conceivably, MRP5 may perform a similar dual function as a heme transporter.
Experimental Procedures
Strains, vertebrate experiments
All vertebrate animal experiments were approved by the University of Maryland Institutional Animal Care and Use Committee. Wildtype zebrafish were obtained from the Zebrafish International Resource Center and were staged, raised, and maintained as described (Kimmel et al., 1995; Westerfield, 2000). Some worm strains were obtained from the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). All worm strains used in this study are listed in Table S2. Worms were maintained either in liquid mCeHR2 or on Nematode Growth Medium agar plates (Nass and Hamza, 2007). C. elegans transcriptional and translational reporters were generated using Multisite Gateway recombination (Invitrogen) and introduced into unc-119 worms using the PDS-1000 particle delivery system (BioRad) (Chen et al., 2011).
Worm Sorting and Imaging
Worms were grown from the L1 larval stage to the early adult stage on RNAi plates. Worms for each condition were analyzed for time of flight (length) and extinction (optical density) using a COPAS BioSort (Union Biometrica, Holliston, MA) with gating parameters for mixed worm populations as in Chen et al (Chen et al., 2011). GFP, YFP, and ZnMP fluorescence in worms was imaged using a DMIRE2 epifluorescence microscope (Leica) connected to a Retiga 1300 cooled mono 12-bit camera or using a laser scanning confocal microscope (LSM710) (Zeiss).
Zebrafish experiments
Zebrafish knockdowns were performed using ~1.4 nl/embryo of ~0.5 M of MO injected into 1-cell stage embryos (Rajagopal et al., 2008). Embryos were analyzed at 24-72 hpf by o-dianisidine staining for hemoglobinization, for LCR-GFP expression, and for gata1 expression using standard procedures (Ganis et al., 2012). Pools of ~50 embryos were analyzed by fluorescence-activated cell sorting (FACS) from wild type, control MO and mrp5 morpholino-injected LCR-GFP fish. Whole mount ISH was performed on wild type embryos with an mrp5 cDNA probe at 1-cell to 5 dpf, as well as on wild type, control and mrp5 morphants with a gata1 cDNA probe using standard procedures at 24 hpf (Hauptmann, 1999). Rescue injections were performed using 175 pg of rescue construct.
Yeast assays
S. cerevisiae strains were grown and assays performed as described previously (Yuan et al., 2012). Plasmids encoding potential heme transporters were transformed into yeast using the lithium method. Before each assay, yeast were heme-starved by growth in 2% w/v raffinose SC (-Ura) liquid medium. Please see extended experimental procedures for information regarding the growth assays, the β-galactosidase assay, and the ferric reductase assay.
Mammalian cell culture and HRP assays
Mammalian cell lines were cultured in growth medium consisting of DMEM, 10% FBS, and 1% PSG (100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM L-glutamine Golgi-targeted HRP was transfected into MEFs using the Lipofectamine transfection reagent (Invitrogen). Following overnight transfection, MEFs were incubated in heme-depleted media (DMEM with 10% heme-depleted FBS and 0.5 mM succinylacetone) for 24 hours. Following heme depletion, cells were switched to heme-depleted media with added heme for 24 hours and harvested for peroxidase activity (White et al., 2013).
Bioinformatics and Statistics
ClustalW and MEGA5 were used to generate a phylogenetic tree for the full length sequences of all human, mouse, zebrafish, and C. elegans MRP/ABCC proteins (Larkin et al., 2007; Tamura et al., 2011). Membrane protein topologies were generated using TMHMM and drawn using TOPO2 (Johns; Krogh et al., 2001). All data are presented as mean ± the standard error of the mean. Statistical significance was determined using one-way or two-way ANOVA with Bonferroni post-tests in GraphPad Prism, version 5.00 (GraphPad Software, Inc). Characters with spaces: 3,867
Supplementary Material
Research highlights.
Pathways for subcellular heme trafficking are critical but poorly understood
C. elegans mrp-5 is required for export of intestinal heme to extraintestinal tissues
Loss of MRP5 in C. elegans and zebrafish causes lethality and anemia, respectively
In mammals, MRP5 regulates export of cytoplasmic heme into the secretory pathway
Acknowledgements
We thank Piet Borst, Koen van de Wetering, Harry Dailey, Mike Krause, John Phillips, and Carine White for critical discussions and reading of the manuscript; Piet Borst for the MRP5 cDNA, cell lines, and mice; Barry Paw for the globin LCR-GFP zebrafish strain; and John Hanover for use of the COPAS BioSort. This work was supported by funding from the National Institutes of Health DK85035 and DK74797 (I.H.); and the Roche Foundation for Anemia Research (I.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental information. Supplemental Information includes Extended Experimental Procedures, 4 figures, and 2 tables.
Additional material available in the Extended Experimental Procedures
Author Contributions. Experimental design and execution were as follows: C. elegans experiments T.K., S.B., I.H.; zebrafish experiments J.Z., I.H.; mammalian cell experiments T.K., I.H.; MRP5 antibody G.L.S.; T.K. and I.H. wrote the manuscript. All authors discussed the results and commented on the manuscript.
References
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 2007;453:661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- Chen B, Jiang Y, Zeng S, Yan J, Li X, Zhang Y, Zou W, Wang X. Endocytic sorting and recycling require membrane phosphatidylserine asymmetry maintained by TAT-1/CHAT-1. PLoS Genet. 2010;6:e1001235. doi: 10.1371/journal.pgen.1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Samuel TK, Krause M, Dailey HA, Hamza I. Heme utilization in the Caenorhabditis elegans hypodermal cells is facilitated by heme-responsive gene-2. J Biol Chem. 2012;287:9601–9612. doi: 10.1074/jbc.M111.307694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Samuel TK, Sinclair J, Dailey HA, Hamza I. An intercellular heme-trafficking protein delivers maternal heme to the embryo during development in C. elegans. Cell. 2011;145:720–731. doi: 10.1016/j.cell.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiabrando D, Marro S, Mercurio S, Giorgi C, Petrillo S, Vinchi F, Fiorito V, Fagoonee S, Camporeale A, Turco E, et al. The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation. J Clin Invest. 2012;122:4569–4579. doi: 10.1172/JCI62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contrino S, Smith RN, Butano D, Carr A, Hu F, Lyne R, Rutherford K, Kalderimis A, Sullivan J, Carbon S, et al. modMine: flexible access to modENCODE data. Nucleic Acids Res. 2012;40:D1082–1088. doi: 10.1093/nar/gkr921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis A, Klausner RD, Hinnebusch AG, Barriocanal JG. Genetic evidence that ferric reductase is required for iron uptake in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:2294–2301. doi: 10.1128/mcb.10.5.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wolf CJ, Yamaguchi H, van der Heijden I, Wielinga PR, Hundscheid SL, Ono N, Scheffer GL, de Haas M, Schuetz JD, Wijnholds J, et al. cGMP transport by vesicles from human and mouse erythrocytes. FEBS J. 2007;274:439–450. doi: 10.1111/j.1742-4658.2006.05591.x. [DOI] [PubMed] [Google Scholar]
- Edgley ML, Baillie DL, Riddle DL, Rose AM. Genetic balancers. WormBook; 2006. pp. 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MD, Hamza I. Mitochondrial heme: an exit strategy at last. J Clin Invest. 2012;122:4328–4330. doi: 10.1172/JCI66607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganis JJ, Hsia N, Trompouki E, de Jong JL, DiBiase A, Lambert JS, Jia Z, Sabo PJ, Weaver M, Sandstrom R, et al. Zebrafish globin switching occurs in two developmental stages and is controlled by the LCR. Dev Biol. 2012;366:185–194. doi: 10.1016/j.ydbio.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza I, Dailey HA. One ring to rule them all: trafficking of heme and heme synthesis intermediates in the metazoans. Biochim Biophys Acta. 2012;1823:1617–1632. doi: 10.1016/j.bbamcr.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon T, Dodd A, Dirmeier R, Gorman N, Sinclair PR, Zhang L, Poyton RO. A mechanism of oxygen sensing in yeast. Multiple oxygen-responsive steps in the heme biosynthetic pathway affect Hap1 activity. J Biol Chem. 2003;278:50771–50780. doi: 10.1074/jbc.M303677200. [DOI] [PubMed] [Google Scholar]
- Johns SJ. TOPO2, Transmembrane protein display software. [Google Scholar]
- Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, De Domenico I, Vaughn MB, Kaplan J, et al. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, Juijn JA, Baas F, Borst P. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57:3537–3547. [PubMed] [Google Scholar]
- Kos V, Ford RC. The ATP-binding cassette family: a structural perspective. Cell Mol Life Sci. 2009;66:3111–3126. doi: 10.1007/s00018-009-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Kruh GD, Guo Y, Hopper-Borge E, Belinsky MG, Chen ZS. ABCC10, ABCC11, and ABCC12. Pflugers Arch. 2007;453:675–684. doi: 10.1007/s00424-006-0114-1. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lipovich L, Hughes AL, King MC, Abkowitz JL, Quigley JG. Genomic structure and evolutionary context of the human feline leukemia virus subgroup C receptor (hFLVCR) gene: evidence for block duplications and de novo gene formation within duplicons of the hFLVCR locus. Gene. 2002;286:203–213. doi: 10.1016/s0378-1119(02)00457-2. [DOI] [PubMed] [Google Scholar]
- Lutsenko S, Petris MJ. Function and regulation of the mammalian copper-transporting ATPases: insights from biochemical and cell biological approaches. J Membr Biol. 2003;191:1–12. doi: 10.1007/s00232-002-1040-6. [DOI] [PubMed] [Google Scholar]
- McAleer MA, Breen MA, White NL, Matthews N. pABC11 (also known as MOAT-C and MRP5), a member of the ABC family of proteins, has anion transporter activity but does not confer multidrug resistance when overexpressed in human embryonic kidney 293 cells. J Biol Chem. 1999;274:23541–23548. doi: 10.1074/jbc.274.33.23541. [DOI] [PubMed] [Google Scholar]
- McGhee JD, Sleumer MC, Bilenky M, Wong K, McKay SJ, Goszczynski B, Tian H, Krich ND, Khattra J, Holt RA, et al. The ELT-2 GATA-factor and the global regulation of transcription in the C. elegans intestine. Dev Biol. 2007;302:627–645. doi: 10.1016/j.ydbio.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Nass R, Hamza I. The nematode C. elegans as an animal model to explore toxicology in vivo: solid and axenic growth culture conditions and compound exposure parameters. Curr Protoc Toxicol. 2007 doi: 10.1002/0471140856.tx0109s31. Chapter 1, Unit1 9. [DOI] [PubMed] [Google Scholar]
- Oka T, Toyomura T, Honjo K, Wada Y, Futai M. Four subunit a isoforms of Caenorhabditis elegans vacuolar H+-ATPase. Cell-specific expression during development. J Biol Chem. 2001;276:33079–33085. doi: 10.1074/jbc.M101652200. [DOI] [PubMed] [Google Scholar]
- Ono N, Van der Heijden I, Scheffer GL, Van de Wetering K, Van Deemter E, De Haas M, Boerke A, Gadella BM, De Rooij DG, Neefjes JJ, et al. Multidrug resistance-associated protein 9 (ABCC12) is present in mouse and boar sperm. Biochem J. 2007;406:31–40. doi: 10.1042/BJ20070292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik EJ, Zon LI. Hematopoietic development in the zebrafish. Int J Dev Biol. 2010;54:1127–1137. doi: 10.1387/ijdb.093042ep. [DOI] [PubMed] [Google Scholar]
- Paumi CM, Chuk M, Snider J, Stagljar I, Michaelis S. ABC transporters in Saccharomyces cerevisiae and their interactors: new technology advances the biology of the ABCC (MRP) subfamily. Microbiol Mol Biol Rev. 2009;73:577–593. doi: 10.1128/MMBR.00020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protchenko O, Rodriguez-Suarez R, Androphy R, Bussey H, Philpott CC. A screen for genes of heme uptake identifies the FLC family required for import of FAD into the endoplasmic reticulum. J Biol Chem. 2006;281:21445–21457. doi: 10.1074/jbc.M512812200. [DOI] [PubMed] [Google Scholar]
- Protchenko O, Shakoury-Elizeh M, Keane P, Storey J, Androphy R, Philpott CC. Role of PUG1 in inducible porphyrin and heme transport in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7:859–871. doi: 10.1128/EC.00414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadota H, Inoue M, Hikita T, Koppen M, Hardin JD, Amano M, Moerman DG, Kaibuchi K. Establishment of a tissue-specific RNAi system in C. elegans. Gene. 2007;400:166–173. doi: 10.1016/j.gene.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley JG, Yang Z, Worthington MT, Phillips JD, Sabo KM, Sabath DE, Berg CL, Sassa S, Wood BL, Abkowitz JL. Identification of a human heme exporter that is essential for erythropoiesis. Cell. 2004;118:757–766. doi: 10.1016/j.cell.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Rajagopal A, Rao AU, Amigo J, Tian M, Upadhyay SK, Hall C, Uhm S, Mathew MK, Fleming MD, Paw BH, et al. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature. 2008;453:1127–1131. doi: 10.1038/nature06934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AU, Carta LK, Lesuisse E, Hamza I. Lack of heme synthesis in a free-living eukaryote. Proc Natl Acad Sci U S A. 2005;102:4270–4275. doi: 10.1073/pnas.0500877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer GL, Kool M, Heijn M, de Haas M, Pijnenborg AC, Wijnholds J, van Helvoort A, de Jong MC, Hooijberg JH, Mol CA, et al. Specific detection of multidrug resistance proteins MRP1, MRP2, MRP3, MRP5, and MDR3 P-glycoprotein with a panel of monoclonal antibodies. Cancer Res. 2000;60:5269–5277. [PubMed] [Google Scholar]
- Severance S, Hamza I. Trafficking of heme and porphyrins in metazoa. Chem Rev. 2009;109:4596–4616. doi: 10.1021/cr9001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance S, Rajagopal A, Rao AU, Cerqueira GC, Mitreva M, El-Sayed NM, Krause M, Hamza I. Genome-wide analysis reveals novel genes essential for heme homeostasis in Caenorhabditis elegans. PLoS Genet. 2010;6:e1001044. doi: 10.1371/journal.pgen.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafizadeh E, Paw BH. Zebrafish as a model of human hematologic disorders. Curr Opin Hematol. 2004;11:255–261. doi: 10.1097/01.moh.0000138686.15806.71. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Hamza I. A novel heme response element mediates transcriptional regulation in Caenorhabditis elegans. J Biol Chem. 2010 doi: 10.1074/jbc.M110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Sasaki H, Kuh HJ, Agui M, Tatsumi Y, Tanabe S, Terada M, Saijo N, Nishio K. Detailed structural analysis on both human MRP5 and mouse mrp5 transcripts. Gene. 2000;242:167–173. doi: 10.1016/s0378-1119(99)00529-6. [DOI] [PubMed] [Google Scholar]
- Tammur J, Prades C, Arnould I, Rzhetsky A, Hutchinson A, Adachi M, Schuetz JD, Swoboda KJ, Ptacek LJ, Rosier M, et al. Two new genes from the human ATP-binding cassette transporter superfamily, ABCC11 and ABCC12, tandemly duplicated on chromosome 16q12. Gene. 2001;273:89–96. doi: 10.1016/s0378-1119(01)00572-8. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda Y, Hagiya Y, Adachi T, Hoshijima K, Kuo MT, Ishikawa T. MRP class of human ATP binding cassette (ABC) transporters: historical background and new research directions. Xenobiotica. 2008;38:833–862. doi: 10.1080/00498250701883514. [DOI] [PubMed] [Google Scholar]
- Wang F, Paradkar PN, Custodio AO, McVey Ward D, Fleming MD, Campagna D, Roberts KA, Boyartchuk V, Dietrich WF, Kaplan J, et al. Genetic variation in Mon1a affects protein trafficking and modifies macrophage iron loading in mice. Nat Genet. 2007;39:1025–1032. doi: 10.1038/ng2059. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio) Univ. of Oregon Press; Eugene: 2000. [Google Scholar]
- White C, Yuan X, Schmidt PJ, Bresciani E, Samuel TK, Campagna D, Hall C, Bishop K, Calicchio ML, Lapierre A, et al. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab. 2013;17:261–270. doi: 10.1016/j.cmet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, Beijnen JH, Scheper RJ, Hatse S, De Clercq E, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A. 2000;97:7476–7481. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi H, Shimizu H, Takayanagi S, Ishikawa T. Multiple splicing variants of two new human ATP-binding cassette transporters, ABCC11 and ABCC12. Biochem Biophys Res Commun. 2001;288:933–939. doi: 10.1006/bbrc.2001.5865. [DOI] [PubMed] [Google Scholar]
- Yuan X, Protchenko O, Philpott CC, Hamza I. Topologically conserved residues direct heme transport in HRG-1-related proteins. J Biol Chem. 2012;287:4914–4924. doi: 10.1074/jbc.M111.326785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.