Abstract
Pluripotent embryonic stem cells (ESCs) are a potential source for cell-based tissue engineering and regenerative medicine applications, but their translation into clinical use will require efficient and robust methods for promoting differentiation. Fluid shear stress, which can be readily incorporated into scalable bioreactors, may be one solution for promoting endothelial and hematopoietic phenotypes from ESCs. Here we applied laminar shear stress to differentiating ESCs using a 2D adherent parallel plate configuration to systematically investigate the effects of several mechanical parameters. Treatment similarly promoted endothelial and hematopoietic differentiation for shear stress magnitudes ranging from 1.5 to 15 dyne/cm2 and for cells seeded on collagen-, fibronectin-, or laminin-coated surfaces. Extension of the treatment duration consistently induced an endothelial response, but application at later stages of differentiation was less effective at promoting hematopoietic phenotypes. Furthermore, inhibition of the FLK1 protein (a VEGF receptor) neutralized the effects of shear stress, implicating the membrane protein as a critical mediator of both endothelial and hematopoietic differentiation by applied shear. Using a systematic approach, studies such as these help elucidate the mechanisms involved in force-mediated stem cell differentiation and inform scalable bioprocesses for cellular therapies.
Keywords: pluripotent stem cells, endothelial, hematopoietic, bioreactor, shear stress, bioprocessing
INTRODUCTION
Differentiated phenotypes derived from pluripotent stem cells are potential cell sources for tissue engineering and therapeutic applications, but effective and robust methods for scale-up production have yet to be identified. Directed differentiation techniques can increase cell yield but remain fairly inefficient and often require exogenous growth factors, which are considered a costly resource for large-scale cell production. Increasing evidence suggests that applied physical forces, such as fluid shear stress, may be a useful tool for promoting differentiation of stem cells, including embryonic stem cells (ESCs) (Stolberg and McCloskey 2009). Thus, differentiation approaches to generate the clinically relevant cell numbers (107-109) (Kehoe et al. 2010; Kirouac and Zandstra 2008) may benefit from leveraging the shear stresses inherent to many production-scale bioreactors.
Scale-up bioreactors are typically based on stir-based suspension systems, which not only remove gradients of cytokines and metabolic byproducts caused by biological activity, but also decrease the medium to cell ratio by minimizing areas of stagnant volume. ESCs differentiated as cell aggregates, known as embryoid bodies (EBs), and cultured in these bioreactor systems have been found to have improved EB homogeneity (Carpenedo et al. 2007; Gerecht-Nir et al. 2004), differentiation towards cardiomyocytes (Niebruegge et al. 2009; Sargent et al. 2009), and maintenance of hematopoietic (Cameron et al. 2006) phenotypes. Even though these are macroscopically well-mixed environments, aggregation of 3D cell clusters (with some cells in an interior core and others forming outer layers) leads to an inherently varied microenvironment for the individual cells with potential limitations in diffusion (Azarin and Palecek 2010). This suggests an opportunity to further enhance for differentiation towards target phenotypes if inputs could be homogeneously presented to the entire cell population. Adherent culture of ESCs onto surfaces of microcarriers is one potential alternative that ameliorates many of the heterogeneity problems while preserving the use of scalable suspension culture (Abranches et al. 2007; Fernandes et al. 2007). Initial studies indicate, however, that cells cultured on microcarriers may be more sensitive to shear stress (Chisti 2001; Gregoriades et al. 2000; Hua et al. 1993; Mollet et al. 2004) and therefore motivate a more thorough understanding of the biological impact of shear stress parameters.
Shear stress effects are often difficult to interpret in scalable systems due to complex patterns of fluid flow. Consequently, single cells or cell aggregates in these systems are commonly exposed to shear stresses up to 20 dynes/cm2 that vary based on location within the bioreactor chamber, such as distance from the impeller (Vallejos et al. 2011; Venkat et al. 1996) or the chamber walls (Bilgen et al. 2006). Instead, use of simpler configurations which allow application of a well-defined shear stress can help decipher the effect of shear stress parameters. For example, the parallel plate system, which applies a uniform shear stress to cells attached to a surface, is a suitable surrogate to study the effect of shear stress magnitude and protein substrate for adherent cell culture on microcarrier surfaces.
Adherent model systems have shown that shear stress promotes an endothelial phenotype when applied to endothelial progenitor cells (Obi et al. 2009; Yamamoto et al. 2003) or to embryonic stem cell (ESC)-derived populations sorted for FLK1 (Yamamoto et al. 2005) or CD31 (Nikmanesh et al. 2012). Separate studies which sorted for CD41+ cells found that shear stress can promote the maturation of cells in the hematopoietic compartment (Adamo et al. 2009). Without extensive differentiation or cell sorting, it has been found that the sustained application of shear stress during earlier stages of ESC differentiation can be used to promote the mesodermal lineage and even inhibit endodermal specification (Wolfe et al. 2012). Furthermore, shear stress applied under those same conditions promoted an endothelial phenotype (Ahsan and Nerem 2010; Zeng et al. 2006). It has yet to be determined, however, if shear stress applied during early ESC differentiation, prior to hematopoietic commitment, can also be used to generate hematopoietic cells.
Studies in development have long observed that hematopoietic and endothelial cells arise with spatial and temporal proximity in the embryo (Murray 1932; Sabin 1920), suggesting that both phenotypes emerge in similar microenvironments in vivo. Recent in vitro studies using ESCs have shown that endothelial and hematopoietic cells are derived from a common progenitor that expresses the VEGF receptor FLK1 (Eilken et al. 2009; Lancrin et al. 2009), which is now known to be expressed by progenitors of several mesodermal phenotypes (Bautch 2006; Ema et al. 2006). Taken together, this implies that identical culture conditions may drive differentiation towards both endothelial and hematopoietic phenotypes through common stages of early specification. Thus, a simultaneous analysis of both endothelial and hematopoietic differentiation may help elucidate the shear-mediated mechanism present during early ESC differentiation.
The objective of this study was to determine if the application of fluid shear stress is a robust method for generating endothelial and hematopoietic phenotypes from pluripotent cells. Using an in vitro 2D parallel plate model system, we found that during early ESC differentiation shear stress promoted both endothelial and hematopoietic phenotypes for a range of shear stress magnitudes and underlying protein substrates. The response was amplified with increasing durations of shear stress, although delayed application of treatment was less effective at promoting hematopoietic phenotypes. Inhibition with SU1498 nullified the effects of stress treatment, suggesting this process is mediated by activation of the FLK1 membrane protein. Together these results elucidate the mechanisms behind force-mediated stem cell differentiation and provide insight for improving scale-up bioreactor design which can help realize the promising impact of stem cells on public health.
MATERIALS AND METHODS
Expansion of Mouse Embryonic Stem Cells
Mouse D3 embryonic stem cells (ESCs) and embryonic fibroblasts (MEFs) were purchased from ATCC and cultured as previously described (Ahsan and Nerem 2010). Briefly, ESCs were expanded on mitotically-inactivated MEFs and then stored in liquid nitrogen. Prior to experiments, ESCs were thawed and expanded for one passage on gelatin-coated tissue culture plastic. Culture medium consisted of Dulbecco's Modification of Eagles Medium with 15% ES-qualified fetal bovine serum (Invitrogen), 2mM L-glutamine, 0.1mM non-essential amino acids, 1000 U/ml Leukemia Inhibitory Factor (LIF; ESGRO® from EMD Millipore) and antibiotics.
Application of Shear Stress
ESCs were differentiated without LIF on protein-coated glass slides to promote cell attachment before shear stress treatment, as described previously (Ahsan and Nerem 2010). Briefly, either collagen type IV (COL: BD Biosciences), fibronectin (FN: BD Biosciences), or laminin (LM: Sigma-Aldrich) was adsorbed onto glass slides for one hour at a concentration of 3.5μg/cm2. ESCs were then seeded onto slides (10,000 cells/cm2) and cultured in an incubator (37°C, 5% CO2) for up to three days in differentiation medium, consisting of Minimum Essential Alpha Media supplemented with 10% fetal bovine serum, 0.1mM beta-mercaptoethanol, and antibiotics.
Fluid shear stress was applied using a parallel plate bioreactor system (Ahsan and Nerem 2010; Levesque and Nerem 1985) to cells attached to glass slides as described above. A media reservoir and peristaltic pump (both from Cole-Parmer) were connected in series with a dampener and flow block. Slides were placed in the flow block such that τ=6Qμ/(bh2), where the shear stress (τ) depended on the flow rate (Q), viscosity of the media (μ), as well as the width (b) and height (h) of the flow channel. Using this system we applied steady laminar shear stress at 15, 5.0 or 1.5 dyne/cm2 for up to four days (SHEAR samples). These magnitudes are comparable to the physiological levels observed in the adult (15 dyne/cm2) and embryonic (5.0 dyne/cm2) aortas (Adamo et al. 2009), in addition to lower magnitudes (1.5 dyne/cm2) which are known to effect adult phenotypes including hemo-vascular cells (Fukuda and Schmid-Schonbein 2003; Gerszten et al. 1998). Time matched controls were cultured under static conditions by placing slides in petri dishes (STATIC samples). Samples from both groups were cultured with 125 ml of differentiation medium at 37°C and 5% CO2. For FLK1 inhibition studies, medium was supplemented with 4.5μg/ml SU1498 (Calbiochem® from EMD Millipore) during the two day STATIC or SHEAR treatment.
Analysis for mRNA Expression
Gene expression of mesodermal, endothelial and hematopoietic markers were evaluated using real-time RT-PCR. Samples were lysed and RNA isolated using QIAshredders and the RNeasy kit (Qiagen). RNA concentrations were quantified using a Nanodrop® spectrophotometer and 1ug of RNA from each sample was converted into cDNA (Invitrogen Superscript® III First-strand synthesis). SYBR® Green (Applied Biosystems) and a StepOnePlus™ PCR system was used to quantify mRNA expression using the standard curve method where flk1, tie2, and runx1 were evaluated as early markers of mesodermal, endothelial, and hematopoietic differentiation, respectively. Downstream endothelial (pecam1 and klf2) and hematopoietic (cd41, c-kit, cd34, cd133, scl, and vav1) genes were also assessed. All gene expression levels were normalized to the housekeeping gene gapdh. Descriptions and primer sequences for individual genes are listed in Supplemental Table 1.
Analysis for Protein Expression
Flow cytometry was used to quantitate changes in protein expression due to shear stress treatment, as done previously (Ahsan and Nerem 2010). StemPro® Accutase® (Invitrogen) was used to generate single cell suspensions that were fixed in 4% formaldehyde for 15 min and then stored in a buffer solution consisting of PBS with 0.3% BSA and 0.001% TWEEN®20 (Sigma-Aldrich). Cells were permeabalized using 0.5% triton-X (Sigma-Aldrich), blocked with 10% serum, and then stained with primary and secondary antibodies. Dilutions for primary and secondary (as needed, AF488 Invitrogen) antibodies were 1:100 and was 1:200, respectively. Antibodies used were FLK1 (Fitzgerald, Acton, MA), PE conjugated-TIE2 (Abcam, Cambridge, MA), as well as RUNX1, PECAM1, vWF, eNOS, and CD41 (all from Santa Cruz Biotechnology, Santa Cruz, CA). Fluorescence was detected using a BD FACSCanto II (Supplemental Figures 1-3). For each sample, the positive-expressing cells were defined as being above the 98% level of the flow cytometry controls. Characterization of certain downstream markers used an upper quartile percentage (i.e. the percent of cells above the 75% level of flow cytometry controls) to detect more subtle differences in expression level. Values of samples from the same group were averaged for statistical analysis.
Hematopoietic Colony Assessments
Quantitative analysis of colony formation was evaluated to determine hematopoietic potential. At the end of STATIC and SHEAR culture, individual samples were treated with Accutase® and single cell solutions were put into suspension culture on an orbital shaker system (40rpm) to form aggregates. After seven days, cell clusters were dissociated and assessed for hematopoietic potential using MethoCult® GF M3434 (STEMCELL Technologies). Following the manufacturer's instructions, cells were placed into low adherence 35mm petri dishes containing the provided methylcellulose medium with the number of colonies in the entire dish counted after 10 days.
Statistical Analysis
All quantitative data are presented as mean±SEM. Student paired t-tests were used to detect differences between SHEAR samples and time- and condition-matched STATIC controls. For all statistical comparisons, p-values less than 0.05 were considered significant.
RESULTS
Shear Stress Promotes Early Differentiation
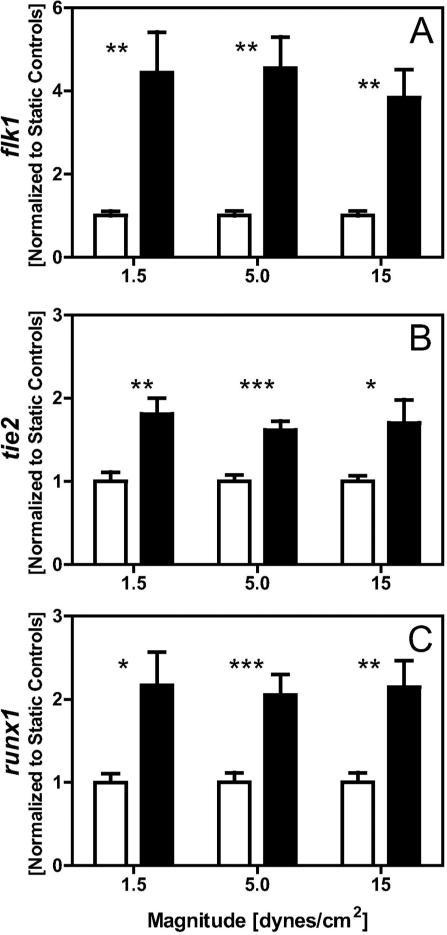
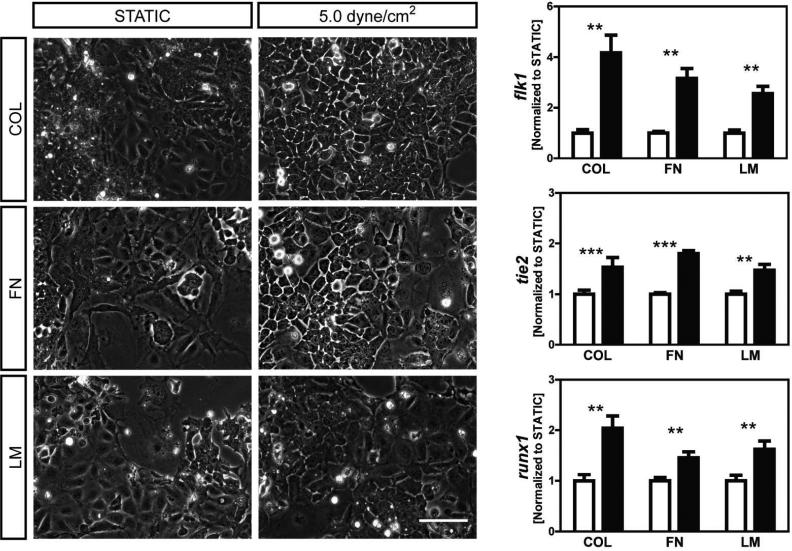
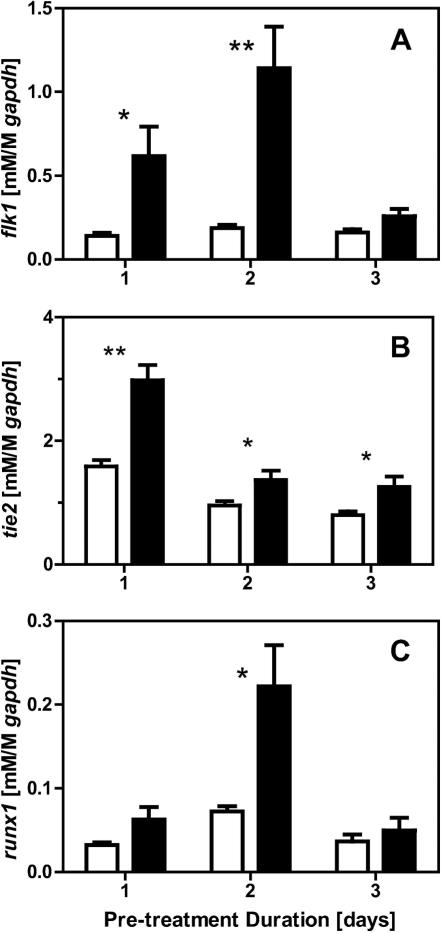
Initial studies screened for the effects of shear stress treatment on a variety of protein substrates and for a range of stress magnitudes. Allowing for two days of initial differentiation followed by two days of treatment (STATIC vs SHEAR), we evaluated gene changes for early differentiation markers flk1 (mesoderm), tie2 (endothelial), and runx1 (hematopoietic) for cells seeded on COL-, FN-, or LM-coated slides and exposed to 1.5, 5.0 or 15 dyne/cm2 shear stress. We have previously shown (Ahsan and Nerem 2010) that protein expression of mesodermal and endothelial markers were upregulated in response to 15 dyne/cm2 on COL-coated slides. Under these same conditions, here we found significant (p<0.05) gene upregulation for flk1 (>2.6 fold), as well as tie2 (>1.5 fold) and runx1 (>1.5 fold) as compared to STATIC controls (Figure 1). Here we further established that regulation of these genes was not found to be stress magnitude dependent, as levels of upregulation were similar at 1.5, 5.0, and 15 dyne/cm2. To instead look at the effects of protein coating, cells were seeded onto slides coated with either FN or LM and then exposed to shear stress (5.0 dyne/cm2). Cell populations, though heterogeneous within any given sample, were all largely adherent and displayed similar morphologies across the different substrate groups (Figure 2A). Likewise, shear stress responses for the flk1, tie2, and runx1 genes were all similar for the different substrates (Figure 2B). Thus, a range of stress magnitudes and protein coatings seem equally suitable during shear-stress mediated differentiation and this type of mechanical modulation can simultaneously promote both endothelial and hematopoietic phenotypes.
Figure 1. A range of magnitudes induce similar shear stress responses.
Cells were seeded on slides coated with collagen type-IV, cultured under static conditions for two days, and then for two days either exposed to 1.5, 5.0, or 15 dyne/cm2 of shear stress (SHEAR samples) or maintained in culture under static conditions (STATIC controls). flk1, tie2, and runx1 gene expression was assessed for SHEAR samples (■) and normalized to trial-matched STATIC controls (□). Data presented are mean ± SEM (n=7-12), where significant differences between STATIC and SHEAR groups are indicated with asterisks (* p<0.05, ** p<0.01, and *** p<0.001).
Figure 2. Various proteins induce similar shear stress responses.
Cells were seeded on slides coated with collagen type-IV (COL), fibronectin (FN), or laminin (LM), cultured under static conditions for two days, and then for two days either exposed to 5.0 dyne/cm2 of shear stress (SHEAR samples) or maintained in culture under static conditions (STATIC controls). Phase images of cells for both STATIC controls and SHEAR samples on COL, FN, and LM are shown. flk1, tie2, and runx1 gene expression was assessed for SHEAR samples (■) and normalized to trial-matched STATIC controls (□). Data presented are mean ± SEM (n=7-12), where significant differences between STATIC and SHEAR groups are indicated with asterisks (** p<0.01 and *** p<0.001). Scale bar represents 100Lm.
Extended Durations of Shear Stress Promote Endothelial and Hematopoietic Phenotypes
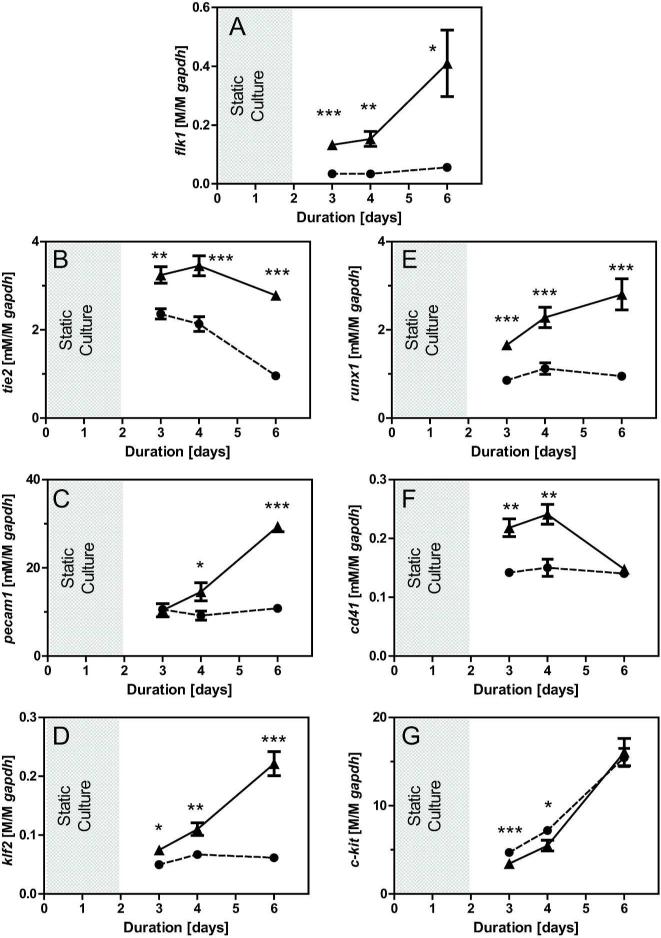
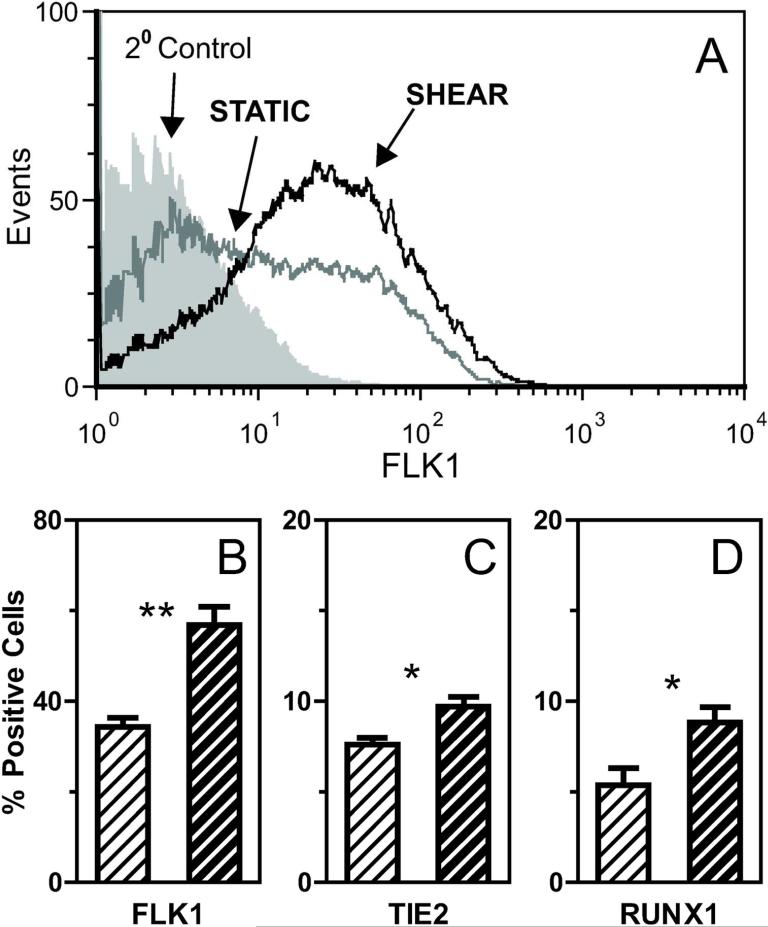
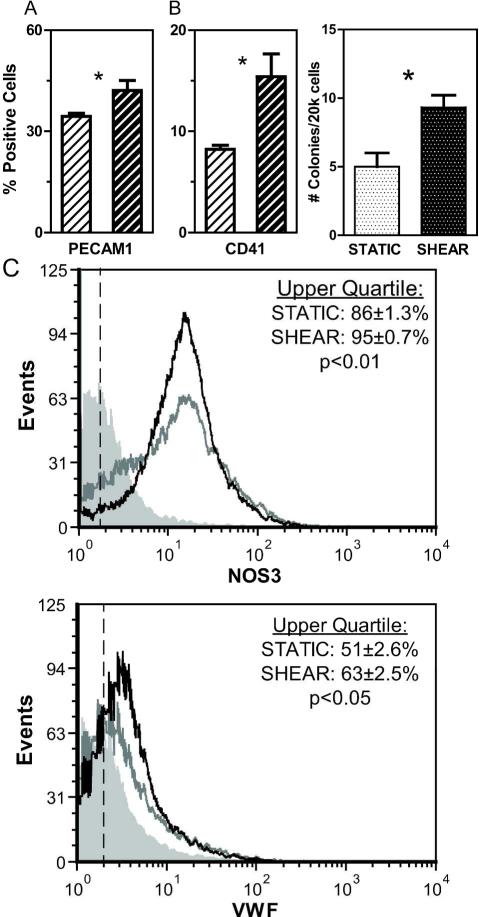
To determine the effects of longer durations of treatment, ESCs were seeded onto protein-coated slides (COL was used in all subsequent studies), cultured under static conditions for two days to allow for cell attachment, and then either continued under STATIC conditions or exposed to SHEAR (5.0 dyne/cm2) for 1, 2, or 4 days. Gene expression (normalized to gapdh) was quantified and SHEAR samples were compared to time-matched STATIC controls (Figure 3). Early differentiation markers flk1, tie2, and runx1 were significantly upregulated for all tested durations (Figure 3A,B,E), with sustained treatment leading to the greatest levels of increase (upregulation after 4 days by 6.8X for flk1 and 2.9X for both tie2 and runx1). While the downstream endothelial markers klf2 and pecam1 underwent little or no change in expression after just 1 day of shear stress (Figure 3C-D), extending treatment to 4 days led to upregulated expression by 3.7X and 2.7X, respectively. To determine if the effects on gene expression led to actual changes towards an endothelial phenotype, protein expression was evaluated in samples exposed to 4 days of treatment. Flow cytometry analysis showed shear stress significantly (p<0.01) increased the percentage of cells positively expressing FLK1 to 57±4% (compared to 34±2% in the STATIC controls, Figure 4A, B). Shear-treatment also increased the percentage of cells positively expressing TIE2 from 8±0.3% to 10±0.6% (p<0.05) and RUNX1 from 5 ±1% to 9±1% (p<0.05) compared to STATIC controls (Figure 4C, D). Protein expression of mature endothelial markers also increased (Figure 5A, C): the percentage of PECAM1+ cells increased from 34±1% to 42±3% (p<0.05) with slight but significant increases in NOS3 and VWF observed based on quartile analysis (p<0.01 and p<0.05, respectively). Thus, extended application of shear stress elevates gene and protein expression indicative of an endothelial phenotype.
Figure 3. Extending treatment duration promotes endothelial gene expression.
All samples were initially allowed to adhere for two days under static conditions on COL (indicated by gray shading) and then experimental samples were exposed to shear stress (5.0 dyne/cm2) for 1, 2, or 4 days (▲, □—) while time-matched controls were cultured under static conditions (●, - - -). Gene expression normalized to gapdh is shown for flk1 (A), tie2 (B), pecam1 (C), klf2 (D), runx1 (E), cd41 (F), and c-kit (G). Data presented are mean ± SEM with n=9-12 (3-4 independent trials), where significant differences between time-matched STATIC and SHEAR groups are indicated with asterisks (* p<0.05, ** p<0.01, *** p<0.001).
Figure 4. Extended durations of shear stress promote hematopoietic and endothelial phenotypes.
Samples were exposed to four days of either STATIC (white striped bars) or SHEAR treatment (τ=5.0 dyne/cm2: black striped bars). A representative histogram of FLK1 protein expression (A) is shown for an immunostaining 2° antibody-only control (filled gray), a STATIC experimental control (gray line), and a SHEAR experimental sample (black line). Fluorescence of both STATIC and SHEAR samples were assessed and compared to flow cytometry control samples to calculate the percentage of cells positive for markers of early differentiation. Assessed proteins were FLK1 (B), TIE2 (C), and RUNX1 (D). Data presented are mean ± SEM (n=3-4), where significant differences between STATIC and SHEAR groups are indicated by asterisks (* p<0.05, *** p<0.001)
Figure 5. Extended durations of shear stress promote mature and definitive phenotypes.
Samples were exposed to four days of either STATIC (white bars, striped or dotted) or SHEAR treatment (τ=5.0 dyne/cm2: black bars, striped or dotted). Fluorescence of both STATIC and SHEAR samples were assessed and compared to flow cytometry control samples to calculate the percentage of cells positive for differentiation markers. Assessed proteins were PECAM1 (A) and CD41 (B, LEFT). A representative histogram of NOS3 and VWF protein expression (C) is shown for an immunostaining 2° antibody-only control (filled gray), a STATIC experimental control (gray line), and a SHEAR experimental sample (black line). To detect subtle changes in protein expression, quartile analysis was used to quantify the expression of NOS3 and VWF. Following STATIC and SHEAR treatment, hematopoietic potential was assessed using a colony counting assay (B, RIGHT). Data presented are mean ± SEM (n=3-4), where significant differences between STATIC and SHEAR groups are indicated by asterisks (* p<0.05) or displayed p-value.
The effect of extended durations of shear treatment on hematopoietic differentiation was also evaluated. As discussed above, tie2 and runx1, early markers of endothelial and hematopoietic differentiation, respectively, were similarly upregulated with extended durations of shear treatment (Figure 3B, C). In contrast to gene changes in downstream endothelial markers, however, applied shear stress had a transient effect on gene expression of the downstream hematopoietic marker cd41. After 1 or 2 days of SHEAR treatment, cd41 was upregulated by >1.5-fold (p<0.01). With four days of treatment, however, the effects were lost and levels for SHEAR samples and STATIC controls were almost identical (Figure 3F). Similarly, the expression of the hematopoietic marker c-kit was not detectably different after 4 days of treatment (Figure 3G). To further explore the effects of shear stress on hematopoietic gene expression, additional markers (cd34, scl, cd133, and vav1) were evaluated and it was found that only modest effects were observed overall (Supplemental Figure 4). Although the transient upregulation of cd41 gene expression was over by 4 days of shear stress treatment, protein expression at that time was significantly (p<0.05) increased (Figure 5B). This indication of sustained phenotypic changes prompted evaluation in hematopoietic potential using the colony forming assay. After maturation in rotary-suspension culture for seven days, cells from both SHEAR and STATIC samples generated hematopoietic colonies (Figure 5B), with those from the SHEAR group generating almost twice as many in number (1.9X, p<0.05). While the various colony types formed by cells in the hematopoietic compartment were not discerned, the negligible effects of shear stress on downstream hematopoietic gene markers indicate that our treatment may primarily promote the initial differentiation towards an early hematopoietic lineage. Thus, the application of shear stress for 4 days promotes a functional hematopoietic phenotype, which is correlated to gene expression of runx1 but not other hematopoietic markers.
Shear Stress-mediated Differentiation Depends on Time of Application
The initial attachment period prior to the application of treatment induces early differentiation due to the removal of LIF ((Wolfe et al. 2012); Supplemental Figure 5). Two days was used for the studies described above, but for these studies the attachment period was varied from 1 to 3 days followed by one day of treatment (STATIC vs SHEAR at 15 dyne/cm2). SHEAR treatment after two days of attachment induced a significant increase in gene expression of flk1, tie2, and runx1, consistent with the two-day treatment results described above; however, SHEAR effects were found to be dependent on pre-treatment duration (Figure 6). flk1 was upregulated after 1 (4.4X, p<0.05) or 2 (6.0X, p<0.01) days of pre-treatment, but differences were no longer detectable (p=0.06) after 3 days (Figure 6A). The early endothelial marker tie2 was significantly upregulated for 1, 2, or 3 days of pre-treatment, but the effect dropped from a 1.9X increase after 1 day to <1.6X after 2 or 3 days (Figure 6B). The early hematopoietic marker runx1, in contrast, was only significantly upregulated when treatment was applied after 2 days (3.1-fold, p<0.05) but had no effect outside this brief window (Figure 6C). Thus, the effect of shear stress is dependent on the stage of differentiation.
Figure 6. Delayed exposure dampens the shear-mediated increase in gene expression.
Cells were cultured under static conditions for 1-3 days before one day of STATIC (□) or SHEAR (τ=15 dyne/cm2, ■) treatment. Gene expression (normalized to gapdh) was calculated for flk1 (A), tie2 (B), and runx1 (C). Data presented are mean ± SEM (n=6-8), where significant differences between trial-matched STATIC and SHEAR groups are indicated by asterisks (*=p<0.05, **=p<0.01).
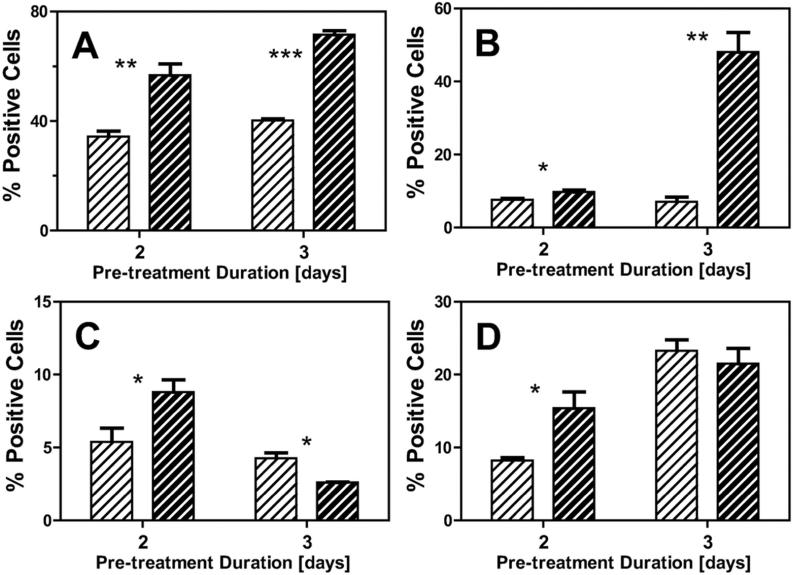
Delayed Application of Shear Stress is Less Effective at Promoting Hematopoietic Differentiation Phenotypes
Endothelial and hematopoietic phenotypic changes were evaluated by protein expression after either 2 or 3 days of attachment followed by four days of treatment (STATIC vs SHEAR at 5.0 dyne/cm2). SHEAR treatment significantly increased the percentage of cells expressing the early mesodermal marker FLK1 (Figure 7A) after either 2 or 3 days of pre-treatment (p<0.01 and p<0.001, respectively). In terms of the endothelial phenotype, the percentage of cells expressing the early marker TIE2 was significantly (p<0.05) increased for SHEAR samples at both pre-treatment durations (Figure 7B). Thus, shear stress applied after 3 days of pre-treatment still promoted an endothelial phenotype. Conversely, the significant upregulation due to SHEAR of the early RUNX1 (Figure 7C) and the downstream CD41 (Figure 7D) hematopoietic markers after 2 days of attachment was lost with the additional day of pre-treatment. Thus, the data after 2 days of pre-treatment confirms that applied shear stress promotes both endothelial and hematopoietic phenotypes, though it seems that application at later time points may be less effective at promoting hematopoietic differentiation.
Figure 7. Delayed exposure to shear stress is less effective at promoting a hematopoietic phenotype.
Cells were cultured under static conditions for 2 or 3 days before four days of either STATIC (striped white bars) or SHEAR (τ=5.0 dyne/cm2, striped black bars) treatment. Fluorescence of both STATIC and SHEAR samples were assessed and compared to flow cytometry control samples to calculate the percentage of cells positive for markers of differentiation. Assessed proteins were FLK1 (A), TIE2 (B), RUNX1 (C), and CD41 (D). Data presented are mean ± SEM (n=3-4), where significant differences between STATIC and SHEAR groups were determined by a t-test and indicated by asterisks (* p<0.05 and *** p<0.001).
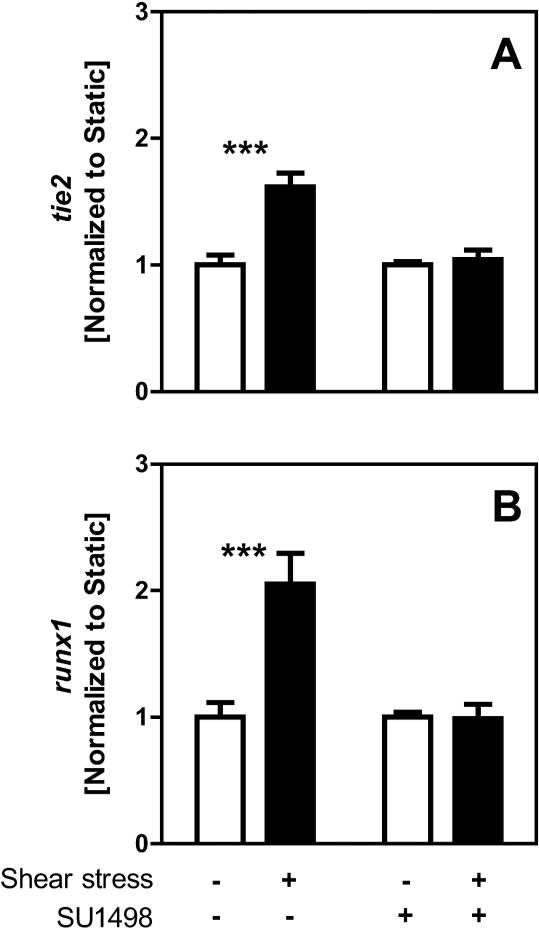
FLK1 is Required for Shear-mediated Differentiation
FLK1, a receptor for the vascular endothelial growth factor, has long been identified as a mechanosensor of shear stress in mature endothelial cells (Chen et al. 1999). To determine the role of FLK1 in shear stress-mediated endothelial and hematopoietic differentiation of ESC's, an inhibitor of FLK1 activation (SU1498) was added to the medium. All samples were first cultured for two days under static conditions to allow for cell attachment, then samples were designated for an additional two days under static (STATIC) or shear stress (SHEAR, τ=5.0 dyne/cm2) conditions, in the presence or absence of SU1498. For the trial-matched controls tie2 and runx1 gene expression were significantly (p<0.001) upregulated in response to shear stress, as was shown earlier, but here it was found that the addition of SU1498 completely prevented (p=0.62 and 0.93, respectively) this response (Figure 8). Thus, these results indicate that FLK1 may be a necessary mediator for early endothelial and hematopoietic differentiation of ESCs due to applied shear stress.
Figure 8. FLK1 inhibition negates the effects of shear stress on early differentiation.
Cells were exposed to two days of STATIC (□) or SHEAR (τ=5.0 dyne/cm2, ■) treatment, where media from select STATIC and SHEAR samples was supplemented with a FLK1 inhibitor (SU14-8: 4.5Lg/ml) during treatment. Gene expression was assessed and each value was normalized to the average value of the trial-matched STATIC controls. Genes assessed include tie2 (A) and runx1 (B). Data presented are mean ± SEM (n=3-4), where significant differences between trial-matched STATIC and SHEAR are indicated by asterisks (*** p<0.001).
DISCUSSION
Establishing embryonic stem cell (ESC)-derived cell sources for tissue engineering and therapeutic applications will require methods for promoting differentiation which are not only effective but robust enough for scale up applications. Here, we found that application of two days of shear stress during early ESC differentiation increased the genetic expression of early mesoderm, hematopoietic, and endothelial markers for a range of shear stress magnitudes and underlying protein substrates. Increasing the duration of shear stress further augmented the effect on genetic markers for early mesoderm, endothelial, and hematopoietic differentiation, as well as downstream endothelial differentiation, but the overall effects on downstream hematopoietic markers were less pronounced. Although application of shear stress after an initial two day static pre-differentiation period increased the genetic and protein expression of endothelial and hematopoietic markers, the effects on hematopoietic differentiation diminished when shear treatment was applied after three days. Supplementing the media with SU1498 during treatment abated the shear-mediated upregulation of endothelial and hematopoietic markers, suggesting that this response requires activation of the FLK1 membrane protein. Overall, this process characterization indicates that shear stress is a robust method for promoting ESC differentiation and informs several operating parameters for scale-up bioreactors.
We found that applied shear stress promotes ESC differentiation towards both endothelial and hematopoietic phenotypes in an adherent model system which, in the absence of externally applied forces or chemical cues, allows for very little endothelial and hematopoietic differentiation (Supplemental Figure 5). In our adherent model system, four days of applied shear stress increased the number of mesodermal FLK1+, endothelial PECAM1+, and hematopoietic CD41+ cells by 1.7X, 1.2X, and 1.9X, respectively, though the actual percentage of positive cells still remained fairly low. To better understand the effects of applied mechanical forces, the medium used in the presented studies was not supplemented with growth factors that would favor any particular phenotype. Hence, it may be possible to optimize the medium composition to further increase cell yield or perhaps allow for more specific targeting of a single phenotype. In suspension-cultured EBs, other groups found that medium supplementation with VEGF approximately doubled the number of T-GFP+FLK+ mesodermal cells (Purpura et al. 2008b), increased the number of PECAM1+ endothelial cells by 60-100% (Purpura et al. 2008a; Vittet et al. 1996), and approximately doubled the number of CD41+ cells when additional growth factors were used (Pearson et al. 2008). Although these initial comparisons are across culture paradigms and include medium supplemented with serum, our results indicate that applied shear stress can promote mesoderm, endothelial, and hematopoietic differentiation on a scale similar to those achieved using growth factor-based methods. This raises the meaningful possibility of utilizing mechanical cues that can readily be incorporated into scalable bioreactors to substitute for exogenously added expensive or xenogeneic reagents in clinical production of cells.
Large scale bioprocessing needs to consistently and homogenously ensure a high quality cell product. Chemical gradients that can lead to unsought biological responses can be overcome by use of microcarriers and stir-or mixing-based features. While studies in these systems have explored expansion of ESCs and induced pluripotent cells (Abranches et al. 2007; Fernandes et al. 2007; Kehoe et al. 2010), the effects of the inherently present shear stresses on differentiation had yet not been thoroughly investigated. Using our surrogate 2D adherent model system we found that the underlying protein substrate has little effect on the shear-mediated differentiation, suggesting that the microcarriers can be coated with a variety of proteins not limited to specific lab or manufacturing capabilities. Furthermore, for early endothelial and hematopoietic differentiation, we found that the presence or absence of shear stress was more important than its magnitude. Thus, early differentiation to these phenotypes can be achieved in large volume bioreactors in which shear stress varies in magnitude as a function of the impeller size, speed, and location (Vallejos et al. 2011; Venkat et al. 1996). Interestingly, other studies in sorted populations of differentiating ESCs have found that applied shear stress at much later times points of differentiation have found that FLK1 (Yamamoto et al. 2005) and runx1 (Adamo et al. 2009) expression was highly dependent on the magnitude of shear stress. The acquisition of magnitude-sensitivity suggests that the shear activated mechanisms may increase in complexity with further differentiation.
The mechanisms involved in shear-mediated early ESC differentiation are largely unknown. Studies in terminally differentiated phenotypes have identified several mechanosensors of shear stress, including FLK1 (Chen et al. 1999), PECAM1 (Osawa et al. 2002), heparin sulfate proteoglycans (Florian et al. 2003), G-protein coupled receptors (Makino et al. 2006), and integrins (Wang et al. 2002). But it is unclear if all of these mechanisms are present in ESCs and at which points in differentiation they are acquired or lost. Our findings with the various protein substrates, known to bind to distinct integrin heterodimers, imply that shear-mediated differentiation does not depend on activation of unique integrin subunits. Initial studies by others have indicated that shear-mediated differentiation of ESCs may involve epigenetic changes (Illi et al. 2005) and heparan sulphate proteoglycans (Toh and Voldman 2011). Other studies have suggested that shear stress can promote endothelial differentiation by activation of FLK1 (Zeng et al. 2006) in a ligand-independent manner (Yamamoto et al. 2005). This is consistent with published results that FLK1 activation by shear stress, as opposed to VEGF, employs different signaling pathways in mature endothelial cells (Wang et al. 2007). Here, we used inhibition experiments to show that FLK1 activation is necessary in the early stages of shear-mediated hematopoietic and endothelial differentiation, but the specific downstream signaling pathways still need to be determined.
Studies have suggested that the RUNX1 transcription factor can inhibit flk1 gene expression (Sakai et al. 2009) by a DNA binding mechanism (Hirai et al. 2005), but the means by which FLK1 activation may upregulate RUNX1 are unclear. In our studies, shear-induced FLK1 upregulation was associated with upregulation of both TIE2 and RUNX1, except at later stages of differentiation when the FLK1 and TIE2 responses were sustained but the RUNX1 upregulation was lost. While FLK1 and endothelial differentiation have been linked in a variety of stem, progenitor, and differentiated cells, our studies imply that the relationship between FLK1 and shear-induced hematopoietic differentiation may change as differentiation progresses. This is consistent with studies by others that found that only during the first 5 days of EB differentiation did FLK1 activation by VEGF simultaneously promote both hematopoietic and endothelial differentiation (Purpura et al. 2008a). Thus, the role of FLK1 in later stages of hematopoietic specification is still unclear.
The recent identification of the FLK1 positive progenitor to both endothelial and hematopoietic cells has implications not only for the field of development but also stem cell biology. While traditionally identified as an endothelial-specific marker, FLK1 protein is expressed very early on during ESC differentiation (Vittet et al. 1996) and is widely expressed by mesoderm-derived progenitors of several phenotypes (Bautch 2006; Ema et al. 2006), including hematopoietic cells (Shalaby et al. 1997). These new insights suggest that bioprocessing techniques may not differ in targeting early ESC differentiation towards endothelial versus hematopoietic phenotypes. Other studies found that FLK1 activation by VEGF promotes ESC differentiation towards an endothelial phenotype (Nourse et al. 2010; Vittet et al. 1996), but when present with other cues it can promote a hematopoietic phenotype (Chadwick et al. 2003; Pearson et al. 2008). More recent studies have suggested that activation of FLK1 by hypoxia-induced endogenous VEGF can promote both endothelial and hematopoietic phenotypes (Purpura et al. 2008a). Our presented studies are the first to examine the simultaneous effects of an identical mechanical force (in the absence of additional specific growth factors) on differentiation towards both endothelial and hematopoietic phenotypes. It has yet to be determined, however, whether these conditions also favor other clinically-needed mesoderm phenotypes.
We found that applied shear stress in an adherent model system promotes early ESC differentiation towards both endothelial and hematopoietic phenotypes in a robust substrate-independent manner. In addition, we found that during early endothelial and hematopoietic specification, responses to shear stress are magnitude insensitive, which may be beneficial in large-scale bioreactors that inherently have heterogeneous shear stress profiles. As differentiation progresses, however, stress effects may independently modulate endothelial versus hematopoietic maturation. An extension of these findings is that ESCs cultured on the surface of microcarriers within mixing bioreactors is a reasonable approach to large scale production of endothelial and hematopoietic cells. This can be used to build on previous results that pluripotent cells can be expanded on microcarriers in scale up systems (Abranches et al. 2007; Fernandes et al. 2007; Kehoe et al. 2010) and that ESC-seeded microcarriers can be cryopreserved (Nie et al. 2009). Together this insinuates that a microcarrier-based approach may utilize both early stress magnitude -insensitive and later stress magnitude–sensitive stages, along with intermittent storage steps, to develop a flexible modular scale-up production process to yield mesodermal cells from pluripotent stem cells for clinical use. Future efforts to develop this type of paradigm will need to investigate the effects of different shear stress profiles (e.g. oscillatory, pulsatile), as well as optimization of medium composition and exchange rates when in combination with shear stress.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. WT Godbey for the generous access to his flow cytometer. The authors would also like to thank Tulane University, the Louisiana Board of Regents (RW) and the NIH (#P20 GM103629) for supporting this work.
Footnotes
These authors have no conflict of interest.
REFERENCES
- Abranches E, Bekman E, Henrique D, Cabral JM. Expansion of mouse embryonic stem cells on microcarriers. Biotechnology and bioengineering. 2007;96(6):1211–21. doi: 10.1002/bit.21191. [DOI] [PubMed] [Google Scholar]
- Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia9Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, Yoder MC. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459(7250):1131–5. doi: 10.1038/nature08073. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan T, Nerem RM. Fluid shear stress promotes an endothelial-like phenotype during the early differentiation of embryonic stem cells. Tissue engineering. Part A. 2010;16(11):3547–53. doi: 10.1089/ten.tea.2010.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarin SM, Palecek SP. Development of Scalable Culture Systems for Human Embryonic Stem Cells. Biochemical engineering journal. 2010;48(3):378. doi: 10.1016/j.bej.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautch VL. Flk1 expression: promiscuity revealed. Blood. 2006;107(1):3–4. [Google Scholar]
- Bilgen B, Sucosky P, Neitzel GP, Barabino GA. Flow characterization of a wavy-walled bioreactor for cartilage tissue engineering. Biotechnol Bioeng. 2006;95(6):1009–22. doi: 10.1002/bit.20775. For Peer Review. [DOI] [PubMed] [Google Scholar]
- Cameron CM, Hu WS, Kaufman DS. Improved development of human embryonic stem cell-derived embryoid bodies by stirred vessel cultivation. Biotechnology and bioengineering. 2006;94(5):938–48. doi: 10.1002/bit.20919. [DOI] [PubMed] [Google Scholar]
- Carpenedo RL, Sargent CY, McDevitt TC. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem cells. 2007;25(9):2224–34. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- Chadwick K, Wang L, Li L, Menendez P, Murdoch B, Rouleau A, Bhatia M. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102(3):906–15. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JYJ. Mechanotransduction in response to shear stress - Roles of receptor tyrosine kinases, integrins, and Shc. Journal of Biological Chemistry. 1999;274(26):18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- Chisti Y. Hydrodynamic damage to animal cells. Critical reviews in biotechnology. 2001;21(2):67–110. doi: 10.1080/20013891081692. [DOI] [PubMed] [Google Scholar]
- Eilken HM, Nishikawa SI, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457(7231):896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Ema M, Takahashi S, Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood. 2006;107(1):111–7. doi: 10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- Fernandes AM, Fernandes TG, Diogo MM, da Silva CL, Henrique D, Cabral JM. Mouse embryonic stem cell expansion in a microcarrier9based stirred culture system. Journal of biotechnology. 2007;132(2):227–36. doi: 10.1016/j.jbiotec.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Florian JA, Kosky JR, Ainslie K, Pang ZY, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circulation research. 2003;93(10):E1369–E142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Schmid-Schonbein GW. Regulation of CD18 expression on neutrophils in response to fluid shear stress. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(23):13152–7. doi: 10.1073/pnas.2336130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecht-Nir S, Cohen S, Itskovitz-Eldor J. Bioreactor cultivation enhances the efficiency of human embryoid body (hEB) formation and differentiation. Biotechnology and bioengineering. 2004;86(5):4939502. doi: 10.1002/bit.20045. [DOI] [PubMed] [Google Scholar]
- Gerszten RE, Lim YC, Ding HT, Snapp K, Kansas G, Dichek DA, Cabanas C, Sanchez-Madrid F, Gimbrone MA, Jr., Rosenzweig A. Adhesion of monocytes to vascular cell adhesion molecule-1-transduced human endothelial cells: implications for atherogenesis. Circulation research. 1998;82(8):871–8. doi: 10.1161/01.res.82.8.871. others. [DOI] [PubMed] [Google Scholar]
- Gregoriades N, Clay J, Ma N, Koelling K, Chalmers JJ. Cell damage of microcarrier cultures as a function of local energy dissipation created by a rapid extensional flow. Biotechnology and bioengineering. 2000;69(2):171–82. doi: 10.1002/(sici)1097-0290(20000720)69:2<171::aid-bit6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Hirai H, Samokhvalov IM, Fujimoto T, Nishikawa S, Imanishi J. Involvement of Runx1 in the down-regulation For Peer Review of fetal liver kinase91 expression during transition of endothelial cells to hematopoietic cells. Blood. 2005;106(6):1948–55. doi: 10.1182/blood-2004-12-4872. [DOI] [PubMed] [Google Scholar]
- Hua J, Erickson LE, Yiin TY, Glasgow LA. A review of the effects of shear and interfacial phenomena on cell viability. Critical reviews in biotechnology. 1993;13(4):305–28. doi: 10.3109/07388559309075700. [DOI] [PubMed] [Google Scholar]
- Illi B, Scopece A, Nanni S, Farsetti A, Morgante L, Biglioli P, Capogrossi MC, Gaetano C. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circ Res. 2005;96(5):501–8. doi: 10.1161/01.RES.0000159181.06379.63. [DOI] [PubMed] [Google Scholar]
- Kehoe DE, Jing D, Lock LT, Tzanakakis ES. Scalable stirred-suspension bioreactor culture of human pluripotent stem cells. Tissue engineering. Part A. 2010;16(2):405–21. doi: 10.1089/ten.tea.2009.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac DC, Zandstra PW. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3(4):36–981. doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457(7231):892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque MJ, Nerem RM. The elongation and orientation of cultured endothelial cells in response to shear stress. Journal of biomechanical engineering. 1985;107(4):341–7. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- Makino A, Prossnitz ER, Bunemann M, Wang JM, Yao WJ, Schmid-Schoenbein GW. G protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. American Journal of Physiology-Cell Physiology. 2006;290(6):C1633–C1639. doi: 10.1152/ajpcell.00576.2005. [DOI] [PubMed] [Google Scholar]
- Mollet M, Ma N, Zhao Y, Brodkey R, Taticek R, Chalmers JJ. Bioprocess equipment: characterization of energy dissipation rate and its potential to damage cells. Biotechnol Prog. 2004;20(5):1437–48. doi: 10.1021/bp0498488. [DOI] [PubMed] [Google Scholar]
- Murray PDF. The Development in vitro of the Blood of the Early Chick Embryo. Proceedings of the Royal Society B: Biological Sciences. 1932;111(773):497–521. [Google Scholar]
- Nie Y, Bergendahl V, Hei DJ, Jones JM, Palecek SP. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnology progress. 2009;25(1):20–31. doi: 10.1002/btpr.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebruegge S, Bauwens CL, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, Nanthakumar K, Woodhouse K, Husain M, Kumacheva E. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng. 2009;102(2):493–507. doi: 10.1002/bit.22065. others. [DOI] [PubMed] [Google Scholar]
- Nikmanesh M, Shi ZD, Tarbell JM. Heparan sulfate proteoglycan mediates shear stress-induced endothelial gene expression in mouse embryonic stem cell-derived endothelial cells. Biotechnol Bioeng. 2012;109(2):5839–4. doi: 10.1002/bit.23302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourse MB, Scatena M, Mortisen DJ, Tulloch NL, Hauch KD, Torok-Storb B, Ratner BD, Pabon L, Murry CE. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(1):80–9. doi: 10.1161/ATVBAHA.109.194233. Halpin For Peer Review DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obi S, Yamamoto K, Shimizu N, Kumagaya S, Masumura T, Sokabe T, Asahara T, Ando J. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. Journal of applied physiology. 2009;106(1):203–11. doi: 10.1152/japplphysiol.00197.2008. [DOI] [PubMed] [Google Scholar]
- Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? Journal of Cell Biology. 2002;158(4):773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson S, Sroczynska P, Lacaud G, Kouskoff V. The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to Bmp4, activin A, bFGF and VEGF. Development. 2008;135(8):1525–1535. doi: 10.1242/dev.011767. [DOI] [PubMed] [Google Scholar]
- Purpura KA, George SH, Dang SM, Choi K, Nagy A, Zandstra PW. Soluble Flt-1 regulates Flk-1 activation to control hematopoietic and endothelial development in an oxygen-responsive manner. Stem Cells. 2008a;26(11):2832–42. doi: 10.1634/stemcells.2008-0237. [DOI] [PubMed] [Google Scholar]
- Purpura KA, Morin J, Zandstra PW. Analysis of the temporal and concentration-dependent effects of BMP-4, VEGF, and TPO on development of embryonic stem cell-derived mesoderm and blood progenitors in a defined, serum-free media. Exp Hematol. 2008b;36(9):1186–98. doi: 10.1016/j.exphem.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Sabin FR. Studies on the origin of blood-vessels and of red blood-corpuscles as seen in the living blastoderm of chicks during the second day of incubation: Carnegie Institution of Washington. 1920.
- Sakai E, Kitajima K, Sato A, Nakano T. Increase of hematopoietic progenitor and suppression of endothelial gene expression by Runx1 expression during in vitro ES differentiation. Experimental Hematology. 2009;37(3):334–345. doi: 10.1016/j.exphem.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Sargent CY, Berguig GY, McDevitt TC. Cardiomyogenic differentiation of embryoid bodies is promoted by rotary orbital suspension culture. Tissue engineering. Part A. 2009;15(2):331–42. doi: 10.1089/ten.tea.2008.0145. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89(6):981–90. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Stolberg S, McCloskey KE. Can shear stress direct stem cell fate? Biotechnol Prog. 2009;25(1):10–9. doi: 10.1002/btpr.124. [DOI] [PubMed] [Google Scholar]
- Toh YC, Voldman J. Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate proteoglycans transduction. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25(4):1208–17. doi: 10.1096/fj.10-168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejos JR, Brorson KA, Moreira AR, Rao G. Integrating a 250 mL-spinner flask with other stirred For Peer Review bench-scale cell culture devices: a mass transfer perspective. Biotechnol Prog. 2011;27(3):803–10. doi: 10.1002/btpr.578. [DOI] [PubMed] [Google Scholar]
- Venkat RV, Stock LR, Chalmers JJ. Study of hydrodynamics in microcarrier culture spinner vessels: A particle tracking velocimetry approach. Biotechnology and bioengineering. 1996;49(4):456–66. doi: 10.1002/(SICI)1097-0290(19960220)49:4<456::AID-BIT13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Vittet D, Prandini MH, Berthier R, Schweitzer A, MartinSisteron H, Uzan G, Dejana E. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88(9):3424–3431. [PubMed] [Google Scholar]
- Wang Y, Chang J, Chen KD, Li S, Li JY, Wu C, Chien S. Selective adapter recruitment and differential signaling networks by VEGF vs. shear stress. Proc Natl Acad Sci U S A. 2007;104(21):8875–9. doi: 10.1073/pnas.0703088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Miao H, Li S, Chen KD, Li YS, Yuan S, Shyy JY, Chien S. Interplay between integrins and FLK91 in shear stress-induced signaling. Am J Physiol Cell Physiol. 2002;283(5):C1540–7. doi: 10.1152/ajpcell.00222.2002. [DOI] [PubMed] [Google Scholar]
- Wolfe RP, Leleux J, Nerem RM, Ahsan T. Effects of shear stress on germ lineage specification of embryonic stem cells. Integrative biology : quantitative biosciences from nano to macro. 2012;4(10):1263–73. doi: 10.1039/c2ib20040f. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sokabe T, Watabe T, Miyazono K, Yamashita JK, Obi S, Ohura N, Matsushita A, Kamiya A, Ando J. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288(4):H1915–24. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Takahashi T, Asahara T, Ohura N, Sokabe T, Kamiya A, Ando J. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol. 2003;95(5):2081–8. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- Zeng L, Xiao Q, Margariti A, Zhang Z, Zampetaki A, Patel S, Capogrossi MC, Hu Y, Xu Q. HDAC3 is crucial in shear- and VEGF-induced stem cell differentiation toward endothelial cells. J Cell Biol. 2006;174(7):1059–69. doi: 10.1083/jcb.200605113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.