Abstract
Surviving an infection requires the generation of an immune response that controls the invading pathogen while limiting collateral damage to self tissues that may result from an exuberant immune response. Various populations of regulatory cells, including Foxp3+ Treg, have been shown to play a central role in the establishment of these controlled immune responses. In this review, I discuss current hypotheses and points of polemic associated with the origin, mode of action and antigen specificity of Foxp3+ Treg during infection.
Keywords: Infection, Foxp3, Regulatory T cell
Treg in infection
Pathogens have evolved mechanisms to manipulate the regulatory network of the host to their advantage, thereby generating conditions that secure their survival for an extended period of time. This can be achieved directly through the induction of host immune regulatory mediators, or indirectly through the generation of regulatory cells. Although it has long been recognized that T cells with suppressive or anergic activity could be generated in vivo during infection, it has only recently emerged that a specialized subsets of Treg (Foxp3+ Treg) also contribute to this regulatory network.
Role of Foxp3+ Treg during infection in experimental models
Foxp3+ Treg were initially described as a unique population of CD4+ T cells that prevent the expansion of self-reactive lymphocytes and subsequent auto-immune disease [1]. Some of the earliest studies of Treg emphasized that such cells control the extent of immune-mediated pathology. Activated Treg efficiently control self-reactive T cells and innate responses in mouse models of colitis, thereby minimizing collateral tissue damage [2]. A similar scenario can occur during some chronic infections, whereby Treg are required to monitor the constant immune response by the host and to prevent detrimental tissue damage.
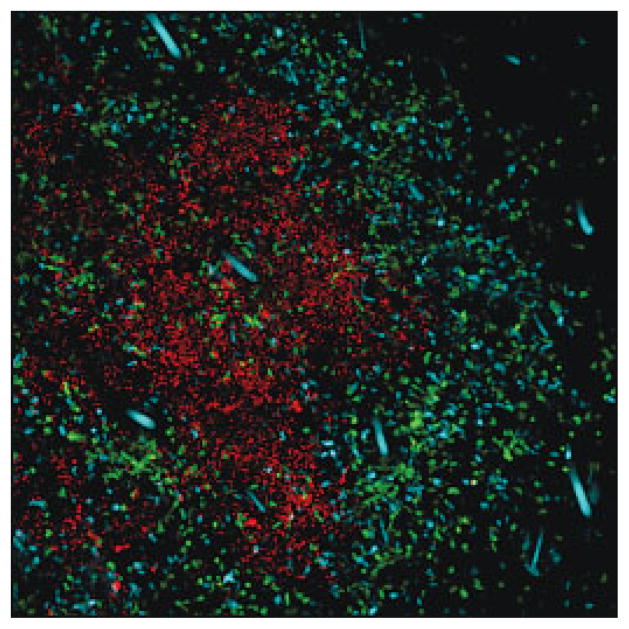
Experimental evidences support the idea that Treg-mediated control of immunopathology may be particularly important for protecting immune-privileged environments or tissues with highly specialized functions, such as the liver or eyes [3, 4]. Even when Treg successfully preserve homeostasis in the host by controlling excessive immune responses, one consequence of such control is enhanced pathogen survival [5] and, in some cases, long-term pathogen persistence. Treg that accumulate at the site of Leishmania infection (Fig. 1) regulate the function of local effector cells, which prevents efficient elimination of the parasite [6]. In this model of infection, parasite persistence, as a result of immune suppression by Treg, is necessary for the maintenance of protective immunity against the parasite [3, 6]. So, in some instance, Treg can control the fine balance that can be established between the pathogen and its host, mediating an equilibrium that can become mutually beneficial. In other cases, regulatory control is excessive, allowing the pathogen to replicate without restraint and overwhelm the host, thereby compromising the survival of the host [7].
Figure 1.
Foxp3+ Treg accumulate at sites of Leishmania infection. TCR−/− mice were injected with CD4+CD25− T cells from cyan fluorescent protein-expressing transgenic mice and CD4+Foxp3+ Treg from Foxp3-GFP-knock-in mice. Leishmania expressing red fluorescent protein were injected intra-dermally into the ear. This image was taken at 1 month post-infection (red: Leishmania; cyan: CD4+CD25− T cells; green: CD4+Foxp3+ Treg). dysregulation of Treg function requires further analysis.
Foxp3 positive Treg and human infections
In humans, the establishment of a role for Treg during infection has been complicated by the fact that human Foxp3+ Treg constitute a more heterogenous population than in mice (see discussion by Costantino et al.[8]). Furthermore, most published studies in human were conducted by analyzing Treg frequency or function in peripheral blood. However, in some chronic infections such as the one due to HIV, Treg can accumulate in infected tissues [9] potentially depleting the relevant cells from this compartment. Despite these caveats some reports provide solid evidence for a role of Treg in many human infections [10].
In most published studies, the removal of CD4+CD25+ T cells from cultures of peripheral or lymphoid leukocytes from HIV, HCV- or hepatitis B virus-infected patients results in an increase in virus-specific immune responses in vitro [11, 12]. These findings suggest that Treg by suppressing virus-specific immunity may contribute to uncontrolled viral replication, therefore potentially playing a detrimental role in human viral infection. On the other hand, the inverse correlation between HCV-specific TGF-β response by CD4+CD25+ T cells and liver damage strongly support the idea that Treg also have a role in controlling chronic inflammatory responses and liver damage in HCV carriers [13]. Interestingly, patients who are chronically infected with HCV and go on to develop auto-immunity have fewer peripheral Treg [14]. However, the link between chronic infection, autoimmune disorders and
Mechanism of action
Despite extensive studies in various models, the mechanism by which Foxp3+ Treg limit effector responses during infection process remains largely unknown. While IL-10, TGF-β and CTLA4 have been shown to potentially contribute to their function in both experimental models and human infections, a role for molecules recently identified to contribute to Treg function, such as adenosine, cAMP or IL-35, has not yet been evaluated [15–17]. It is important to keep in mind that the “magic bullet” suppressive factor may remain difficult to identify during infection. Firstly, regulation of infected tissues usually arises from the coordinated action of various populations of regulatory cells and varies according to the site of infection or the degree of inflammation. In addition, the mechanisms by which Treg exert their function are likely to be complementary as shown in the context of experimental colitis.
Independently of their direct control of microbe-specific immune responses, following activation, Treg can suppress unrelated immune responses in a non-antigen-specific manner either through cell contact or through the regulatory cytokines they produce – a mechanism known as bystander suppression. A few years ago, the concept of the ‘hygiene hypothesis’ emerged, stating that increasing incidences of allergy and asthma in Western countries are a consequence of reduced infectious stresses during early childhood [18]. The mechanistic explanations appear to be associated with a “counter-regulatory” model involving the induction of various Treg populations during infection. For example, during gastrointestinal infection, helminth-driven Treg suppression of effector function is responsible for protection against subsequent airway inflammation [19].
It is likely that some of this mechanism has evolved as a result of our symbiotic relationship with gut flora. Thus, the presence of symbiotic and pathogenic microorganisms, in the gut or other peripheral tissues, could lead to the maintenance of a pool of activated Treg (both natural and inducible) that would maintain host immune homeostasis and enhance the threshold required for immune activation and induction of an immune response [20]. The benefit of such deactivation would be to decrease the instances of aberrant immune responses, such as allergic and autoimmune disorders.
Antigen specificity of natural Treg
While the antigen specificity of inducible Treg (Tr1 and Th3 cells) is associated with microbial antigens, the nature of the antigens recognized by thymically derived Foxp3+ Treg is less obvious (Fig. 2). Treg are believed to recognize a wide array of self antigens, as a consequence of their development and selection in the thymus [21]. During the onset of acute disease, Treg could recognize self antigens that are released by tissue damage. In a murine model of Leishmania infection, Treg that accumulate at the chronic site of infection are able to recognize parasite-derived antigen [22]. Notably, these cells are restricted to the site of infection and are dependent on antigen for their maintenance [22]. The relative contribution of self versus microbial specificities in the function of Treg and effector T cells during infection needs further exploration.
Figure 2.
Origins and specificities of Treg during infections. The origin and antigen specificity of Treg may vary according to the site and the nature of the infection. In acute infection, tissue damage may be associated with enhanced presentation of self antigens. In this case, self-reactive natural Treg may be activated and could, in a bystander manner, limit effector responses against the pathogen. At sites of infection various populations of microbe-specific Treg can be induced (e.g. converted Foxp3+ Treg (cFoxp3+), Th1 cells producing IL-10 or inducible T reg (Tr1 cells)). In some chronic infections, there is evidence that natural Treg may also accumulate at sites of infection and can recognize microbial antigens. In an environment that is rich in TGF-β and the vitamin A metabolite retinoic acid (RA), such as the gut, peripheral conversion of Foxp3− T cells into Foxp3+ Treg may occur in response to food or gut flora antigen or during oral infection. These converted Foxp3+ T cells could potentially limit immune responses. Some of these converted cells may be able to recirculate and could contribute to the control of peripheral homeostasis. Red arrows indicate control of immune responses.
Potential role for converted Foxp3+ Treg during infection
Until recently, the expression of Foxp3 on CD4+ T cells was believed to indicate that these cells had acquired their regulatory properties in the thymus. However, there is mounting evidence that Foxp3+ Treg can also develop extrathymically. In vitro studies have shown that conversion of naive peripheral CD4+CD25− Tcells into Foxp3+ Treg could be achieved through ligation of the TCR in the presence of TGF-β [23]. Such conversion can be mimicked in vivo by delivering antigen under subimmunogenic conditions [24] or by targeting antigen to DC via the regulatory receptor DEC205 [25]. Targeting or manipulating DC, as well as chronic exposure to low dose of antigen, is characteristic of many chronic infections.
During infection, the downstream effects of inflammatory responses are also often associated with anti-inflammatory processes including TGF-β production. Furthermore, some pathogens target sites in which TGF-β is highly produced, such as the gastrointestinal tract, the skin and the eye [26]. TGF-β can be also produced by infected cells or by cells the microorganisms are in contact with, or arise as a result of an inflammatory process. Interestingly, following malaria infection of human, enhanced TGF-β and Foxp3+ Treg responses in peripheral blood correlate with a faster parasitic growth rate [27]. One intriguing observation is that only a fraction of infected individuals displayed this TGF-β burst. Whether there is a genetic predisposition in the capacity of individuals to produce cytokines known to promote Treg induction and how this could correlate with their susceptibility to infectious diseases remains to be addressed.
Acute infection with Listeria monocytogenes in mice failed to induce Foxp3 by conventional CD4+ Tcells [28]. Thus, highly inflammatory environments that will prevail in acute infection may not favor the emergence of Foxp3+ T cells. This hypothesis is supported by the observation that Th1- or Th2-polarizing cytokines can interfere with the induction of these cells [29]. On the other hand, we speculate that chronic infections may require an additional layer of regulation, which would be provided by converted Foxp3+ Treg. For example we found that BCG can specifically induce new populations of Foxp3+ Tcells in vivo that accumulate at the dermal site of infection (Blank and Belkaid, unpublished observation). Some pathogens that target highly regulated environments, such as the gut, may also utilize this pathway to their own advantage.
The gastrointestinal tract requires additional levels of control because it has to maintain the delicate balance between tolerance to commensal bacteria and food products and the capacity to mount an effective immune response against ingested pathogens. Indeed we and others found that the main site in which peripheral conversion can be observed are the gut-associated lymphoid tissues [30, 31]. Such conversion is associated with the observation that antigen-presenting cells from the lamina propria of the small intestine or the mesenteric lymph node have the unique property to generate Treg in vitro via a mechanism that, in addition to TGF-β, is dependent on the vitamin A metabolite retinoic acid [30–32].
A compelling hypothesis would be that these gut-converted Treg could become part of the peripheral Treg pool (Fig. 2). So over time, the gut flora, oral pathogens or food may have an important role in shaping the repertoire of peripheral Foxp3+ Treg. The relative contribution of these converted Treg to peripheral tolerance and the outcome of infections as well as how pathogens can utilize or interfere with this pathway to favor their own survival remains to be addressed. Currently, in absence of definitive markers to distinguish endogenous versus converted Foxp3+ Treg these questions will remain difficult to answer.
The importance of preventing the induction/activation of regulatory responses during vaccination has been highlighted by recent findings. Conventional T cells converted into Treg in the periphery under subimmunogenic conditions can be subsequently expanded by the delivery of antigen under immunogenic conditions [25]. So, if not done in optimal conditions, vaccination itself can generate its own set of Treg [33]. These results highlight the necessity to address the potential of each microbial antigen to trigger Treg following vaccination and the importance of defining adjuvants that prevent Treg priming or activation.
To date no vaccines are available against many life-threatening diseases such as malaria, tuberculosis and HIV. It is now clear that Treg can control the intensity of both primary and secondary responses to infection or vaccines. The growing understanding that most pathogens thrive in the presence of regulatory responses supports the idea that efficient protective immune responses have to be initiated under conditions that limit the initiation of regulatory responses.
Conclusion
In some circumstances, the regulation exerted by Treg is excessive preventing the establishment of protective immune responses, whereas in other circumstances, this control is not sufficient to prevent immunopathology. At both extremes, manipulation of Treg has been shown to potentially offer therapeutic potential. Because Treg offer an opportunity for microorganisms to generate favorable conditions for persistence, their induction and survival can also be manipulated by microbes. Indeed some recent reports suggest that some pathogen may provide a survival/proliferative signal to Treg. In addition, microbe-associated DC maturation [34], stimulation of TLR or other pattern recognition receptors [35], induction of cytokine production can all favor Treg activation (or induction) and thereby support survival of the pathogen. These concepts have provided the basis for new therapeutic approaches in which microbial strategies could be utilized to induce or manipulate Treg to control allergic and autoimmune diseases.
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We would like to thank David B. Chow for the confocal image. We apologize to those authors whose work we were unable to cite because of space limitations.
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Shevach EM, et al. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 2.Powrie F, et al. Novartis Found Symp. 2003;252:92–98. [PubMed] [Google Scholar]
- 3.Suvas S, et al. J Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 4.Hesse M, et al. J Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 5.Scott-Browne JP, et al. J Exp Med. 2007;204:2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkaid Y, et al. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 7.Hisaeda H, et al. Nat Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 8.Constantino C, et al. Eur J Immunol. 2008;38 doi: 10.1002/eji.200738104. [DOI] [Google Scholar]
- 9.Andersson J, et al. J Immunol. 2005;174:3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 10.Rouse BT, et al. Immunol Rev. 2006;212:272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 11.Kinter AL, et al. J Exp Med. 2004;200:331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira LE, et al. J Virol. 2007;81:4445–4456. doi: 10.1128/JVI.00026-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolacchi F, et al. Clin Exp Immunol. 2006;144:188–196. doi: 10.1111/j.1365-2249.2006.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyer O, et al. Blood. 2004;103:3428–3430. doi: 10.1182/blood-2003-07-2598. [DOI] [PubMed] [Google Scholar]
- 15.Bopp T, et al. J Exp Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deaglio S, et al. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collison LW, et al. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 18.Wills-Karp M, et al. Nat Rev Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 19.Wilson MS, et al. J Exp Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maizels RM, et al. Curr Opin Immunol. 2005;17:656–661. doi: 10.1016/j.coi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh CS, et al. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Suffia IJ, et al. J Exp Med. 2006;203:777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, et al. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Apostolou I, von Boehmer H. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kretschmer K, et al. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 26.Barnard JA, et al. Gastroenterology. 1993;105:67–73. doi: 10.1016/0016-5085(93)90011-z. [DOI] [PubMed] [Google Scholar]
- 27.Walther M, et al. Immunity. 2005;23:287–296. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Fontenot JD, et al. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Wei J, et al. Proc Natl Acad Sci USA. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun CM, et al. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coombes JL, et al. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denning TL, et al. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 33.Shaw MH, et al. J Immunol. 2006;176:7263–7271. doi: 10.4049/jimmunol.176.12.7263. [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki S, et al. J Exp Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutmuller RP, et al. Trends Immunol. 2006;27:387–393. doi: 10.1016/j.it.2006.06.005. [DOI] [PubMed] [Google Scholar]