Abstract
Background
Postoperative vision loss (POVL) after spine surgery is a rare but devastating outcome. We present the first case-control study from a single institution for POVL with the diagnoses of ischemic optic neuropathy or central vision loss after complex spine surgery.
Methods
POVL cases following spine surgeries between December 1995 and December 2010 at the Cleveland Clinic were identified retrospectively using administrative codes. Each instance of POVL was matched to 5 case-control patients based on age, gender, body mass index, systolic blood pressure, diastolic blood pressure, mean arterial pressure, and hematocrit. Duration of anesthesia, fluid volumes, and hemodynamic measurements were then compared between POVL cases and control cases using Wilcoxon rank sum test.
Results
Six patients developed POVL. These patients had significantly greater blood loss (P=0.002, Wilcoxon test) and a significantly greater volume of red blood cells transfused (P=0.006) than the control patients. No other intraoperative measures differed significantly after Bonferroni correction for multiple outcomes.
Conclusion
We found that patients with POVL had significantly greater blood loss and significantly more red blood cell transfusions than their matched controls.
Keywords: Optic neuropathy–ischemic, spinal fusion, surgical procedures–operative, vision–ocular
INTRODUCTION
Postoperative vision loss (POVL) after spine surgery is a rare but devastating outcome. The incidence of POVL after spine fusion surgery has been estimated at 3.1 per 10,000 cases or between 0.03% and 0.2%, based on various national databases and multicenter studies.1,2 The operative causes of POVL are multifactorial and include surgical duration, major blood loss and large-volume fluid resuscitation, hypotension, and anemia.3 Despite the recent publication of a multicenter case-control study for ischemic optic neuropathy (ION) cases,4 to our knowledge, no case-control study has been published to date by a single institution for POVL cases after spine surgery. We present the first case-control study for POVL after complex spine surgeries from a single institution. The primary goal of this retrospective study was to compare intraoperative fluid administration and minimum blood pressure measurements in patients identified as having POVL with similar, matched patients who also underwent complex spine surgery but did not experience POVL.
METHODS
Retrospective analysis of patient discharge International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes via the institution's revenue cycle management software (Allscripts) was used to identify the occurrence of POVL cases following complex spine surgery between December 1995 and December 2010 at the Cleveland Clinic. Specific postoperative secondary diagnoses sought for qualification as POVL included visual disturbances, blindness, and/or low vision. For each identified case of POVL, 5 case-control complex spine surgery patients were selected and matched based on age; sex; height; weight; body mass index; minimum intraoperative systolic, diastolic, and mean arterial blood pressure; and hematocrit. Patient case controls were selected from the Perioperative Health Documentation System (PHDS) registry maintained by the Anesthesiology Institute at Cleveland Clinic; use of the registry was approved by the institutional review board. At the time of case-control matching, the PHDS registry contained data on 2,532 patients who had had complex spine surgery between August 2005 and December 2009. Matching 5 controls to each case was done to improve the accuracy of results and to remove any selection bias for POVL attributable to the previously stated factors.
Patients were considered as potential controls when their records contained at least 1 ICD-9-CM procedure code belonging to the spinal fusion procedure category (as defined by the US Agency for Healthcare Research and Quality's Clinical Classifications Software for ICD-9-CM codes).5
Statistical Methods
Each patient with vision loss was matched to 5 case controls using a multivariate (greedy) distance matching algorithm. Patients were randomly ordered prior to matching to remove any temporal biases. Potential control patients with missing data were excluded from consideration. Successful matches were restricted to common sex and to within 1.5 standard deviations of the case on each of the other matching variables. Balance after matching was evaluated using standard univariable summaries and tests (Pearson chi square test, Student t test, or Wilcoxon rank sum test, as appropriate). Further matching on other potential confounding variables was unfortunately not possible in this study because of the limited sample size.
The following intraoperative measurements were compared between POVL cases and matched controls using the Wilcoxon rank sum test: duration of anesthesia, estimated blood loss, total crystalloid volume, total colloid volume, transfused red blood cell volume, total fluid volume (defined as the sum of crystalloids, colloids, and red blood cells), minimum systolic blood pressure, minimum diastolic blood pressure, and minimum mean arterial pressure. We set the significance level to P≤0.01 to adjust for multiple comparisons.
SAS statistical software version 9.2 for Windows (SAS Institute) and R statistical software version 2.11.1 for Windows (R Foundation for Statistical Computing) were used for all analyses. The R package “Matching” was used to implement the matching procedure.
RESULTS
Of the patients reviewed who had spine surgery during the period 1995 to 2010, 6 patients developed POVL. Three patients were diagnosed with anterior ischemic optic neuropathy (AION): one patient had bilateral anterior optic neuropathy resulting in bilateral vision loss, the second patient had peripheral field vision loss, and the third patient had complete unilateral vision loss. The other 3 patients had central vision loss that was caused by occipital lobe infarction in 2 patients and by tuberculosis encephalomeningitis in 1 patient. Of the 2 patients with occipital lobe infarct, 1 developed bilateral occipital lobe infarcts and a left mesial temporal infarct resulting in permanent difficulty in focusing and reading, while the other patient suffered complete loss of vision in 1 eye. The patient with tuberculosis encephalomeningitis developed complete bilateral vision loss. None of our patients suffered vision loss because of central retinal artery occlusion. Of note, 2 of the 3 AION cases included in this study occurred after 2006 and therefore were not included in the Postoperative Visual Loss Study Group study for identifying risk factors for ION.4
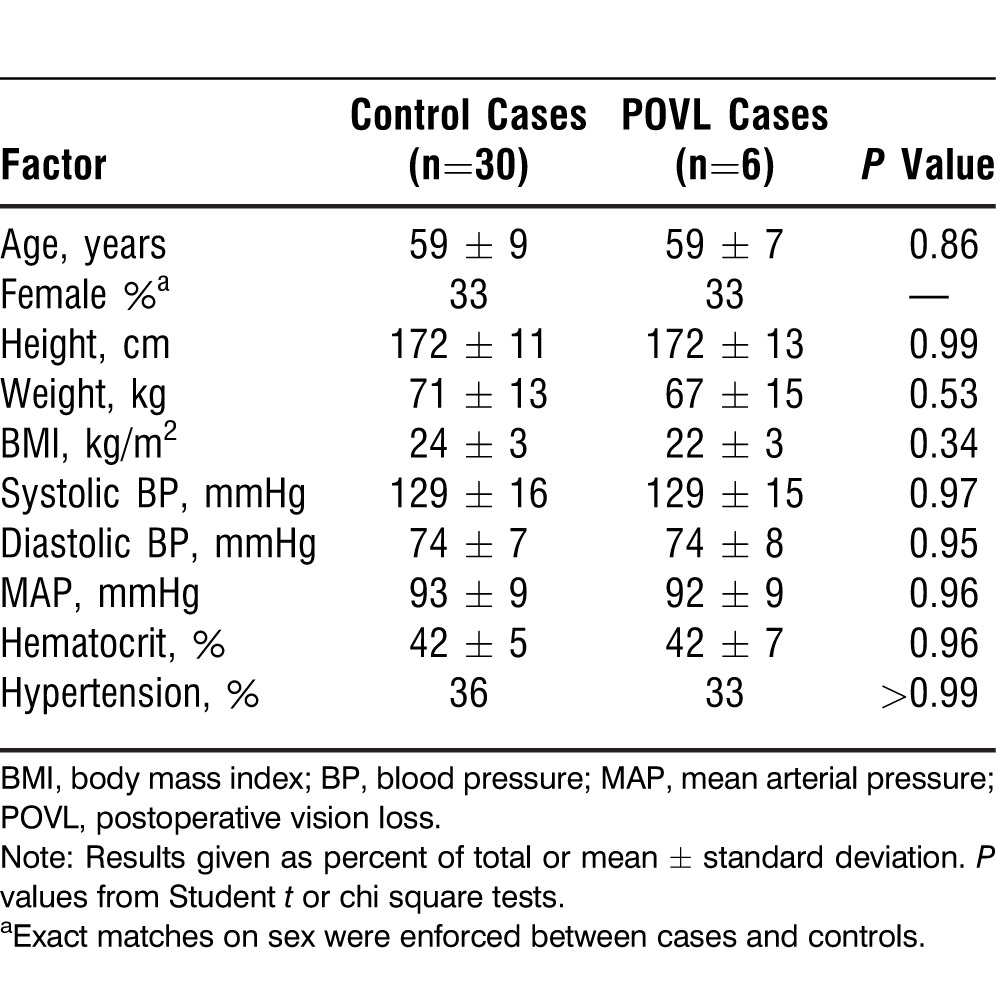
After removing 784 patients who had 1 or more missing variables in the PHDS registry, 1,748 spinal fusion surgery patients were considered as potential control cases. Matching variables were well balanced between cases and controls (Table 1).
Table 1.
Comparison of Baseline Patient Characteristics Used for Matching
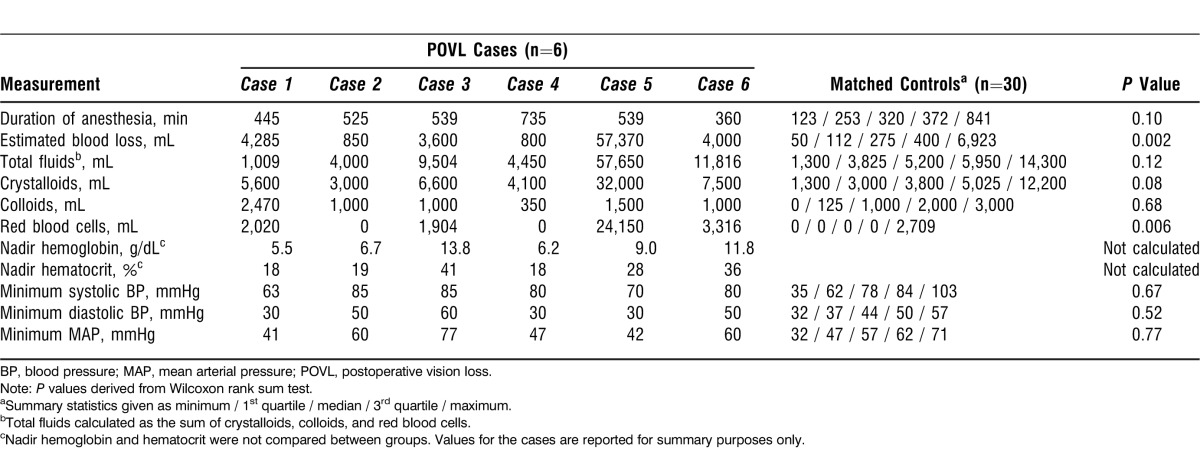
Comparisons between POVL cases and control cases on selected intraoperative measures are provided in Table 2. Patients with vision loss had significantly greater blood loss (P=0.002, Wilcoxon test) and a significantly greater volume of red blood cells transfused (P=0.006) than the control patients. No other intraoperative measures differed significantly after Bonferroni correction for multiple outcomes.
Table 2.
Comparison of Intraoperative Measurements Between 6 Patients with Vision Loss and 30 Matched Controls
DISCUSSION
The results of this study are consistent with a previous case-control study by Myers et al6 that concluded no statistical difference existed between the patients with vision loss and the matched group in intraoperative blood pressure and hematocrit values. Our findings are also in agreement with a recently published report for risk factors associated with ION following spinal fusion surgery4 that noted increased estimated blood loss and prolonged surgical duration. All of our cases of vision loss occurred in conjunction with major spine surgeries. We did not find that hypotension or anemia were independent risk factors, also in agreement with the report of risk factors after spine surgery. Interestingly, the 3 patients who developed ION in this study were males, and the male sex is considered an independent risk factor for ION.4
All of our patients who developed vision loss had periods of severe hemodynamic instability, and all had systolic blood pressure readings ≤85 mmHg for at least some time during surgery. However, hypotension was not independently associated with vision loss, a finding consistent with a previous case-control study.6 The American Society of Anesthesiologists' (ASA) postoperative visual loss registry showed the lowest systolic blood pressures (SBP) to be ≤90 mmHg in 67% of vision loss cases and ≤80 mmHg in 20% of vision loss cases.3 However, hypotension was found to be a causative factor for vision loss in the US Nationwide Inpatient Sample.7 These 2 findings can be reconciled to some extent by the recent report by Pillunat et al8 indicating that autoregulation might be deficient in some healthy individuals, rendering them unable to maintain normal blood flow to the optic nerve head in the presence of low ocular perfusion pressure. This finding could explain why hypotension was not found to be an independent risk factor for POVL in some case study series. Low ocular perfusion pressure can likely be tolerated in some patients because of their intact ocular autoregulation.8
Mean ocular perfusion pressure is calculated by the difference between mean arterial blood pressure (MAP) and intraocular pressure (IOP). We have demonstrated the decrease in ocular perfusion pressure in spine cases performed in prone position caused by a combined increase in IOP and decrease in MAP.9 Ocular autoregulation maintains retinal blood flow over a wide range of perfusion pressures. The higher range in perfusion pressures or rightward shift in ocular autoregulation in hypertensive patients may explain the apparent increased incidence of vision loss in hypertensive patients compared to normotensive patients.10-12 Our results support the theory that deficient ocular autoregulation in hypertensive patients augments risk because most of our vision loss patients were hypertensive.
Anemia is also associated with POVL after spine surgery.13,14 As might be expected, some of our patients who developed vision loss had severe anemia as a result of massive intraoperative blood loss. For example, 1 patient who developed a central vision defect had a hemoglobin nadir of 5.5 g/dL (hematocrit of 18%) (Table 2) while another who developed bilateral AION had a blood loss of 35 L (blood was continuously replaced during the surgery through resuscitation) with an intraoperative hemoglobin nadir of 6.7 g/dL.13,15 The ASA practice advisory for perioperative visual loss associated with spine surgery recommends keeping the average hemoglobin or hematocrit to 9.4 g/dL or 28%, respectively.16
Choroidal blood flow, which accounts for up to 70% of retinal requirements of oxygen and glucose, decreases during experimental isovolemic hemodilution to a hematocrit of 20%-22%.17 This finding may imply the importance of avoiding anemia during complex spine surgery. Transfusions have been identified as risk factors for vision loss in our analysis consistent with a previous report by Shen et al.2 Multiple blood transfusions are an indicator of massive blood loss and low hematocrit during spine surgery. Periods of hemodynamic instability further compromise ocular perfusion pressure.
The case-control patients were tightly matched to the patients with POVL. Matching required access to electronic intraoperative records that have only been available at the Cleveland Clinic from 2005-2009. In contrast, our 6 POVL cases occurred over a 15-year span. All the central vision loss cases and one AION occurred before the implementation of electronic anesthetic records at our institution, so differing surgical techniques and patient characteristics over time may have confounded our results. Furthermore, our pool of potential control cases excluded a relatively large proportion of spinal fusion cases (31%) because of missing data, leaving the possibility of selection bias due to factors that led to missing data. Finally, the retrospective nature of the study rendered us unable to control for unavailable confounders.
Unanticipated vision loss after inpatient surgery is, fortunately, extremely rare. Because of its rarity, the complication is virtually impossible to study prospectively; even retrospectively, only a few events can be identified for case-control analysis. The lack of significant differences in fluids and blood pressure in our study does not indicate that no association is present. The small sample size could have created a false negative conclusion.
CONCLUSION
We found that patients with POVL had significantly greater blood loss and significantly more red blood cell transfusions than their matched control cases. Multicenter registry-based perioperative outcomes trials with larger data sets are needed to confirm these exploratory findings.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Practice-Based Learning and Improvement.
REFERENCES
- 1.Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976) 1997 Jun 15;22(12):1319–1324. doi: 10.1097/00007632-199706150-00008. [DOI] [PubMed] [Google Scholar]
- 2.Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009 Nov;109(5):1534–1545. doi: 10.1213/ane.0b013e3181b0500b. Epub 2009 Aug 27. [DOI] [PubMed] [Google Scholar]
- 3.Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006 Oct;105(4):652–659. doi: 10.1097/00000542-200610000-00007. quiz 867-868. [DOI] [PubMed] [Google Scholar]
- 4.Postoperative Visual Loss Study Group. Risk factors associated with ischemic optic neuropathy after spinal fusion surgery. Anesthesiology. 2012 Jan;116(1):15–24. doi: 10.1097/ALN.0b013e31823d012a. [DOI] [PubMed] [Google Scholar]
- 5.Clinical Classifications Software (CSS) for ICD-9-CM. Rockville, MD: Agency for Healthcare Research and Quality; 2012. [computer program] Version. 2012. [Google Scholar]
- 6.Myers MA, Hamilton SR, Bogosian AJ, Smith CH, Wagner TA. Visual loss as a complication of spine surgery. A review of 37 cases. Spine (Phila Pa 1976) 1997 Jun 15;22(12):1325–1329. doi: 10.1097/00007632-199706150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976) 2008 Jun 1;33(13):1491–1496. doi: 10.1097/BRS.0b013e318175d1bf. [DOI] [PubMed] [Google Scholar]
- 8.Pillunat LE, Anderson DR, Knighton RW, Joos KM, Feuer WJ. Autoregulation of human optic nerve head circulation in response to increased intraocular pressure. Exp Eye Res. 1997 May;64(5):737–744. doi: 10.1006/exer.1996.0263. [DOI] [PubMed] [Google Scholar]
- 9.Farag E, Sessler DI, Kovaci B, et al. Effects of crystalloid versus colloid and the α-2 agonist brimonidine versus placebo on intraocular pressure during prone spine surgery: a factorial randomized trial. Anesthesiology. 2012 Apr;116(4):807–815. doi: 10.1097/ALN.0b013e3182475c10. [DOI] [PubMed] [Google Scholar]
- 10.Haefliger IO, Meyer P, Flammer J, Lüscher TF. The vascular endothelium as a regulator of the ocular circulation: a new concept in ophthalmology? Surv Ophthalmol. 1994 Sep-Oct;39(2):123–132. doi: 10.1016/0039-6257(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 11.Hayreh SS. Anterior ischemic optic neuropathy. Clin Neurosci. 1997;4(5):251–263. [PubMed] [Google Scholar]
- 12.Graham SL, Drance SM. Nocturnal hypotension: role in glaucoma progression. Surv Ophthalmol. 1999 Jun;43(Suppl 1):S10–S16. doi: 10.1016/s0039-6257(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 13.Brown RH, Schauble JF, Miller NR. Anemia and hypotension as contributors to perioperative loss of vision. Anesthesiology. 1994 Jan;80(1):222–226. doi: 10.1097/00000542-199401000-00033. [DOI] [PubMed] [Google Scholar]
- 14.Katzman SS, Moschonas CG, Dzioba RB. Amaurosis secondary to massive blood loss after lumbar spine surgery. Spine (Phila Pa 1976) 1994 Feb 15;19(4):468–469. doi: 10.1097/00007632-199402001-00018. [DOI] [PubMed] [Google Scholar]
- 15.Shaw PJ, Bates D, Cartlidge NE, et al. Neuro-ophthalmological complications of coronary artery bypass graft surgery. Acta Neurol Scand. 1987 Jul;76(1):1–7. doi: 10.1111/j.1600-0404.1987.tb03535.x. [DOI] [PubMed] [Google Scholar]
- 16.American Society of Anesthesiologists Task Force on Perioperative Visual Loss. Practice advisory for perioperative visual loss associated with spine surgery: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Visual Loss. Anesthesiology. 2012 Feb;116(2):274–285. doi: 10.1097/ALN.0b013e31823c104d. [DOI] [PubMed] [Google Scholar]
- 17.Roth S. The effects of isovolumic hemodilution on ocular blood flow. Exp Eye Res. 1992 Jul;55(1):59–63. doi: 10.1016/0014-4835(92)90092-7. [DOI] [PubMed] [Google Scholar]


