Abstract
Background
Since their identification nearly 80 years ago, steroids have played a prominent role in the treatment of many disease states. Many of the clinical roles of steroids are related to their potent antiinflammatory and immune-modulating properties.
Methods
This review summarizes the basic pharmacology, complications, and practice delivery issues regarding steroids.
Results
Clinically relevant side effects of steroids are common and problematic. Side effects can occur at a wide range of doses and vary depending on the route of administration. The full spectrum of side effects can be present even in patients taking low doses.
Conclusions
Practitioners must be aware that these drugs might exacerbate a preexisting condition or present a new medical condition. Knowledge of the clinical implications of prescribing these agents is critical.
Keywords: Adrenal cortex hormones, diabetes mellitus, drug-related side effects and adverse reactions, glucocorticoids, medication therapy management, mineralocorticoids
INTRODUCTION
Since their identification in 1935, steroids have served a wide range of uses. Initially, these isolates from adrenal glands were thought to be useful only in patients suffering from Addison disease.1 Today, many of the clinical roles of steroids are related to their potent antiinflammatory and immune-modulating properties. Clinically relevant side effects of steroids are common and problematic, ranging from a minor case of acne to Cushing syndrome that can result in diabetes mellitus and potentially life-threatening heart disease if untreated.2 Side effects can occur at a wide range of doses and vary depending on the route of administration.1
The term steroid applies to a wide range of molecules with varying physiological effects. More specifically, corticosteroids are a class of chemicals encompassing both laboratory-synthesized and naturally produced hormones. Glucocorticoids, in general, regulate metabolism and inflammation; mineralocorticoids regulate sodium and water levels. Corticosteroids fall along a spectrum from exclusively glucocorticoid effects to exclusively mineralocorticoid effects, and steroid compounds are selected based on their appropriateness for a given treatment. For example, although a compound may possess potent antiinflammatory properties, it may additionally have mineralocorticoid activity that adversely affects blood pressure.
CORTICOSTEROID METABOLISM AND CLINICAL ROLE
Although corticosteroid metabolism is complicated by enzyme induction, protein binding, molecular interconversion, and interaction with endogenous cortisol, corticosteroids are generally metabolized by the hepatic P450 system.3 Direct application (eg, topical, intraarticular, inhaled, or epidural) of these agents to sites of inflammation bypasses the liver and its first-pass effect.
Chronic oral glucocorticoid use is common in patients with rheumatoid arthritis, chronic obstructive pulmonary disease, systemic lupus erythematosus, inflammatory bowel disease, and asthma.4 Side effects of chronic use include bruising, muscle weakness, weight gain, skin changes, sleep disturbances, cataracts, and pathologic fractures.4 Glucocorticoid administration can also have psychiatric side effects: mood disorders, anxiety, delirium, and panic disorder. Psychotropic medication may be required to treat these symptoms, but the prognosis is favorable once the glucocorticoids are reduced or discontinued.5-7 Adverse effects occur in up to 90% of patients who take glucocorticoids for >60 days.4 These side effects, including the more serious fractures and cataracts, occur even in patients taking low (≤7.5 mg/d) dosages.4,8
Glucocorticoids affect bone mineralization by inhibiting calcium absorption in the gastrointestinal tract and shifting signaling-molecule production to favor bone resorption.8 Recommendations for preventing glucocorticoid-induced osteopenia and its subsequent complications and comorbidities include supplementing calcium with vitamin D for glucocorticoid doses ≥5 mg/d and starting bisphosphonates when indicated by densiometric evaluation.8
Because of their effects on insulin resistance, glucocorticoids are the most common cause of drug-induced diabetes mellitus.9 Screening guidelines using a fasting glucose ≥126 mg/dL or HbA1c ≥6.5% are suitable for diagnosing steroid-induced diabetes; however, per American Diabetes Association guidelines, results should be confirmed via repeat testing.9 Management is similar to that of type 2 diabetes mellitus; treatment options progress from single agent to double agent to insulin ± another agent, based upon fasting glucose measurements and glucose control.9 In patients with preexisting diabetes, blood sugars should be measured more often than in patients without preexisting diabetes, and medications should be adjusted to maintain adequate control.9
Cushing syndrome and adrenal suppression have been observed in patients taking oral, intraarticular, epidural, inhaled, nasal, ocular, and topical glucocorticoid preparations.8,9 These side effects become more likely with longer durations of treatment and higher dosages.8,9
Mineralocorticoid activity causes the retention of sodium and free water and the excretion of potassium.2 Derangements in mineralocorticoid production can manifest with abnormalities in any of these areas. Hyponatremia, hyperkalemia, and hypotension are present to varying degrees in mineralocorticoid-deficient states (eg, various congenital adrenal hyperplasias and aldosterone synthase deficiency), whereas the inverse is present in mineralocorticoid-excess states (eg, Conn syndrome). Because endogenous glucocorticoids also have activity at mineralocorticoid receptors, signs and symptoms of mineralocorticoid excess can be seen in cases of excess glucocorticoid production (eg, Cushing syndrome).2
CORTICOSTEROID PREPARATIONS
Steroid injections are associated with side effects related to dosage, duration of administration, added ingredients or contaminates, and particle size. Particulate steroids present a theoretical risk of occluding vessels depending on the size of particulate aggregates.10 Common additives in steroid preparations, such as benzyl alcohol and ethylene glycol, have been implicated in case reports and studies of complications following epidural steroid administration.10,11 Dexamethasone and betamethasone sodium phosphate are pure liquids, whereas methylprednisolone, triamcinolone, and betamethasone are solutions, and their particle size depends upon the type of preparation and dosage. Studies have shown that transforaminal dexamethasone is just as effective at 4 mg as it is at 8 mg and 12 mg and that nonparticulate steroid preparations are just as effective as particulate preparations in treating cervical radicular pain.12,13 Methylprednisolone and triamcinolone are the drugs most commonly used for epidural steroid injections. Common side effects of epidural steroid injections are paresthesia, pain on injection, intravascular injection, bleeding, and dysesthesia.12 The most serious complications of epidural steroid injections are related to intravascular injections. Intraarterial injections may occur even with a negative aspirate and have been shown to potentially cause paraplegia.14 Although the use of computed tomography guidance instead of conventional fluoroscopy provides a better image of relevant anatomy, it does not assure avoidance of these adverse events.14
Topical corticosteroids (2.5% ointment, triamcinolone 0.1% ointment, and clobetasol propionate 0.05% foam) achieve more effective skin concentrations than oral prednisone.15 Side effects, including skin thinning, color change, and systemic effects, can be expected with topical application of corticosteroids and increase in a dose-dependent manner.16 Inhaled corticosteroids have evolved into a mainstay of therapy for moderate to severe asthma. Effectiveness and systemic bioavailability vary with each corticosteroid molecule and dosage, but in general, systemic effects are minimized with proper administration.17 Common side effects of inhaled corticosteroids include gingival irritation and oral candidiasis, as well as the many systemic effects associated with corticosteroid use.17,18
Fludrocortisone is a synthetic corticosteroid that has potent mineralocorticoid effects.2 It has been used clinically to achieve the mineralocorticoid effects of sodium and water retention in cases of cerebral salt wasting, orthostatic hypotension, and adrenocortical insufficiency in Addison disease.19-21 Potassium wasting is a common side effect of fludrocortisone administration, and electrolyte levels should be monitored while a patient is undergoing fludrocortisone administration.21
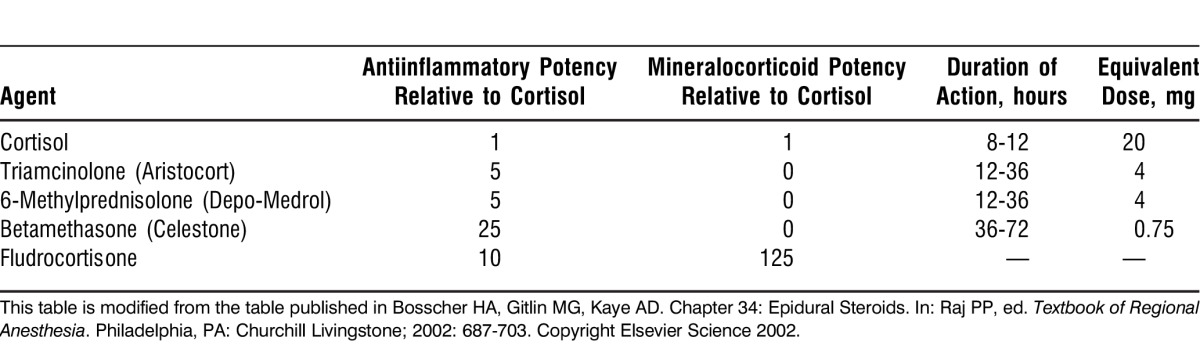
The potencies of corticosteroids vary widely, with synthetic compounds generally retaining greater antiinflammatory potency and weaker salt-retaining properties; these potencies are summarized in the Table.
Table.
Basic Potency, Duration of Action, and Equivalent Dose of Typical Steroid Preparations
MECHANISTIC PHARMACOLOGY AND PHYSIOLOGY OF STEROIDS
The antiinflammatory properties of steroids have been attributed to their inhibitory effects on the action of phospholipase A2, an enzyme critical to the production of inflammatory compounds.22 Research has shown that steroids are active in affecting gene expression, translation, and enzyme activity.23 In short, they bring about their physiologic effects through a multitude of biochemical pathways.23 One such pathway is through their induction of the production of proteins called lipocortins. Glucocorticoids stem the production of inflammatory mediators such as leukotrienes and prostaglandins and effectively halt the inflammatory cascade.22,24 As their wide-ranging side effects indicate, glucocorticoids can impact many systems throughout the body. Through negative feedback regulation of the hypothalamic-pituitary-adrenal (HPA) axis, exogenous glucocorticoids can directly induce hypopituitarism (Addison disease).2,25 Their actions on glucose metabolism can increase insulin resistance in tissues and increase fasting glucose levels.2,25 Glucocorticoids can act directly on osteoclasts to affect bone resorption and decrease calcium absorption in the gastrointestinal tract, resulting in osteopenia and osteoporosis.2,25
Because of the wide-ranging effects that glucocorticoids can have on a patient's body and on the HPA axis in particular, a practitioner must be careful when discontinuing their administration. If steroids have been administered for less than 1 week, they can be stopped without tapering. For dosing lasting 1-3 weeks, tapering should be based upon clinical conditions and the illness for which the medication was prescribed.9 When the patient has taken glucocorticoids for more than 3 weeks, the practitioner's goal is a quick tapering to physiologic doses and then a slow decrease in dosage while evaluating adrenal function.4 For patients who are taking equivalent doses of 30 mg of hydrocortisone daily or have established HPA axis dysfunction and are under stress (eg, major surgery, critical illness, trauma), an increased dosing of steroids (intravenous or intramuscular hydrocortisone) is recommended every 6 hours for 24 hours, followed by a tapering to the previous maintenance dose by 50% per day.25
Mineralocorticoids, endogenously represented by aldosterone and deoxycorticosterone, effect physiologic changes by altering electrolyte (sodium and potassium) levels, causing volume changes to occur.2 Rather than being moderated by the HPA axis as glucocorticoid production is, mineralocorticoid production is mainly regulated by the renin-angiotensin-aldosterone system, although adrenocorticotropic hormone, a product of the HPA axis, does have minimal activity in stimulating aldosterone release.2
CONTROVERSY WITH STEROID PREPARATION
Recent developments involving both morbidity (751 total infections in 20 states as of October 2013) and mortality (64 deaths over the same time period) related to steroid compounds manufactured at the New England Compounding Center (NECC) show that the side effects of steroid injections range beyond those that can be explained by the physiologic and pharmacologic properties of glucocorticoids.26 The glucocorticoid preparations implicated in the nationwide fungal meningitis outbreak were manufactured at a compounding pharmacy, a facility that was neither licensed nor inspected by the United States Food and Drug Administration (FDA) for large-scale pharmaceutical manufacturing but was under regulation by the state pharmacy board in Massachusetts.27 Traditionally, physicians turn to local compounding pharmacies to prepare mainstream pharmaceuticals that either are not offered in the concentration required for patient administration or are not compatible with a particular route of administration. Compounding pharmacies historically have been licensed to produce these medications for individual patients in quantities suitable to fill the prescription.27 Physicians also turn to compounding pharmacies to manufacture drugs for individual patient administration when FDA-approved drugs are not available through traditional distribution channels.27 Such pharmaceuticals may contain the same active ingredients as FDA-approved medications, but the potency and concentrations of the active ingredients vary greatly (from 68.5% to 265.4%).27 Although the FDA views compounded pharmaceuticals as unapproved new drugs because of their untested nature, the recent inspections of compounding pharmacies and the enforcement of laws regulating them have focused on the pharmacies effectively operating as drug manufacturing companies that distribute their compounded pharmaceuticals nationwide, rather than those that serve individual patients locally, such as NECC.27
Multiple reports of fungal meningitis occurring after epidural steroid injection prompted an FDA inspection of the NECC pharmacy facilities and revealed a number of problems with manufacturing process and facilities, ranging from stagnant puddles of water in autoclaves to visible discoloration and fungal growth around the facilities.26,28 An examination of 321 recalled vials of methylprednisolone acetate revealed that 100 of these vials contained visible foreign matter.28 This finding shows that although physicians may not play a direct role in the manufacture of the compounds administered to patients, they can play a crucial role in the quality control process by simply looking at the compounds they give to their patients.
The laws governing compounding pharmacies and their regulation have recently been revised with the passage of the Drug Quality and Security Act signed on November 27, 2013. With this new law and revisions made to the Federal Food, Drug and Cosmetic Act (section 503A provided the exemptions for compounding pharmacies from compliance with current good manufacturing practices [CGMP], FDA approval prior to marketing, and labeling with adequate directions for use), compounding pharmacies can become “outsourcing facilities” and be placed under FDA regulation.29 The new laws mandate compounding pharmacies to comply with CGMP requirements, to be inspected by the FDA on a risk-based schedule if they are an “outsourcing facility,” and to report adverse events to the FDA.29
CONCLUSION
Since their discovery, steroids have infiltrated nearly every branch of medicine and can be administered in nearly every route available. The effects of steroid use can vary widely, and the full spectrum of side effects can be present even in patients taking low doses. Practitioners must be aware that the drug can possibly exacerbate a preexisting condition or present a new medical condition. Knowledge of the clinical implications of prescribing these agents is critical.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Kendall EC. Nobel lecture: The development of cortisone as a therapeutic agent. Nobelprize.org. 1950 December 11. http://www.nobelprize.org/nobel_prizes/medicine/laureates/1950/kendall-lecture.html. Accessed March 10, 2013. [Google Scholar]
- 2.Stewart PM, Krone NP. The adrenal cortex. In: Melmed S, Polonsky K, Larsen PR, Kronenberg H, editors. Williams Textbook of Endocrinology. 12th ed. Philadelphia, PA: Saunders;; 2011. In. eds. [Google Scholar]
- 3.Xu J, Winkler J, Derendorf H. A pharmacokinetic/pharmacodynamic approach to predict total prednisolone concentrations in human plasma. J Pharmacokinet Pharmacodyn. 2007 Jun;34(3):355–372. doi: 10.1007/s10928-007-9050-8. Epub 2007 Feb 23. [DOI] [PubMed] [Google Scholar]
- 4.Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006 Jun 15;55(3):420–426. doi: 10.1002/art.21984. [DOI] [PubMed] [Google Scholar]
- 5.Kenna HA, Poon AW, de los Angeles CP, Koran LM. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011 Oct;65(6):549–560. doi: 10.1111/j.1440-1819.2011.02260.x. [DOI] [PubMed] [Google Scholar]
- 6.Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006 Oct;81(10):1361–1367. doi: 10.4065/81.10.1361. [DOI] [PubMed] [Google Scholar]
- 7.Patten SB, Neutel CI. Corticosteroid-induced adverse psychiatric effects: incidence, diagnosis and management. Drug Saf. 2000 Feb;22(2):111–122. doi: 10.2165/00002018-200022020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Pereira RM, Carvalho JF, Canalis E. Glucocorticoid-induced osteoporosis in rheumatic diseases. Clinics (Sao Paulo) 2010;65(11):1197–1205. doi: 10.1590/S1807-59322010001100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lansang MC, Hustak LK. Glucocorticoid-induced diabetes and adrenal suppression: how to detect and manage them. Cleve Clin J Med. 2011 Nov;78(11):748–756. doi: 10.3949/ccjm.78a.10180. [DOI] [PubMed] [Google Scholar]
- 10.Benzon HT, Gissen AJ, Strichartz GR, Avram MJ, Covino BG. The effect of polyethylene glycol on mammalian nerve impulses. Anesth Analg. 1987 Jun;66(6):553–559. [PubMed] [Google Scholar]
- 11.Craig DB, Habib GG. Flaccid paraparesis following obstetrical epidural anesthesia: possible role of benzyl alcohol. Anesth Analg. 1977 Mar-Apr;56(2):219–221. [PubMed] [Google Scholar]
- 12.Ahadian FM, McGreevy K, Schulteis G. Lumbar transforaminal epidural dexamethasone: a prospective, randomized, double-blind, dose-response trial. Reg Anesth Pain Med. 2011 Nov-Dec;36(6):572–578. doi: 10.1097/AAP.0b013e318232e843. [DOI] [PubMed] [Google Scholar]
- 13.Dreyfuss P, Baker R, Bogduk N. Comparative effectiveness of cervical transforaminal injections with particulate and nonparticulate corticosteroid preparations for cervical radicular pain. Pain Med. 2006 May-Jun;7(3):237–242. doi: 10.1111/j.1526-4637.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 14.Somayaji HS, Saifuddin A, Casey AT, Briggs TW. Spinal cord infarction following therapeutic computed tomography-guided left L2 nerve root injection. Spine (Phila Pa 1976) 2005 Feb 15;30(4):E106–E108. doi: 10.1097/01.brs.0000153400.67526.07. [DOI] [PubMed] [Google Scholar]
- 15.McClain RW, Yentzer BA, Feldman SR. Comparison of skin concentrations following topical versus oral corticosteroid treatment: reconsidering the treatment of common inflammatory dermatoses. J Drugs Dermatol. 2009 Dec;8(12):1076–1079. [PubMed] [Google Scholar]
- 16.Morley KW, Dinulos JG. Update on topical glucocorticoid use in children. Curr Opin Pediatr. 2012 Feb;24(1):121–128. doi: 10.1097/MOP.0b013e32834ef53d. [DOI] [PubMed] [Google Scholar]
- 17.Busse WW, Bleecker ER, Bateman ED, et al. Fluticasone furoate demonstrates efficacy in patients with asthma symptomatic on medium doses of inhaled corticosteroid therapy: an 8-week, randomised, placebo-controlled trial. Thorax. 2012 Jan;67(1):35–41. doi: 10.1136/thoraxjnl-2011-200308. Epub 2011 Aug 9. [DOI] [PubMed] [Google Scholar]
- 18.Doherty DE, Tashkin DP, Kerwin E, et al. Effects of mometasone furoate/formoterol fumarate fixed-dose combination formulation on chronic obstructive pulmonary disease (COPD): results from a 52-week Phase III trial in subjects with moderate-to-very severe COPD. Int J Chron Obstruct Pulmon Dis. 2012; 7:57–71. doi: 10.2147/COPD.S27320. Epub 2012 Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taplin CE, Cowell CT, Silink M, Ambler GR. Fludrocortisone therapy in cerebral salt wasting. Pediatrics. 2006 Dec;118(6):e1904–e1908. doi: 10.1542/peds.2006-0702. [DOI] [PubMed] [Google Scholar]
- 20.Freitas J, Santos R, Azevedo E, Costa O, Carvalho M, de Freitas AF. Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone. Clin Auton Res. 2000 Oct;10(5):293–299. doi: 10.1007/BF02281112. [DOI] [PubMed] [Google Scholar]
- 21.Physicians Total Care, Inc. Fludrocortisone acetate tablet. Daily Med. 2010 September. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bf927cd9-1891-4de4-b4be-743a26363a64. Accessed April 1, 2014. [Google Scholar]
- 22.Wallner BP, Mattaliano RJ, Hession C, et al. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986 Mar 6-12;320(6057):77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]
- 23.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005 Oct 20;353(16):1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 24.Blackwell GJ, Carnuccio R, Di Rosa M, Flower RJ, Parente L, Persico P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980 Sep 11;287(5778):147–149. doi: 10.1038/287147a0. [DOI] [PubMed] [Google Scholar]
- 25.Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003 Feb 20;348(8):727–734. doi: 10.1056/NEJMra020529. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Multistate outbreak of fungal meningitis and other infections. Centers for Disease Control and Prevention. 2013 October 23. http://www.cdc.gov/hai/outbreaks/meningitis.html. Accessed April 1, 2014. [Google Scholar]
- 27.US Food and Drug Administration. 2006 Limited Survey of Compounded Drug Products. 2010 March 22. http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/pharmacycompounding/ucm204237.htm. Accessed March 14, 2013. [Google Scholar]
- 28.US Food and Drug Administration. FDA Form 483: inspectional observations. 2012 http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofGlobalRegulatoryOperationsandPolicy/ORA/ORAElectronicReadingRoom/UCM325980.pdf. Accessed March 14, 2013. [Google Scholar]
- 29.US Food and Drug Administration. Compounding Quality Act, Title I of the Drug Quality and Security Act of 2013. 2014 January 10. http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/pharmacycompounding/default.htm. Accessed April 1, 2014. [Google Scholar]


